Abstract
Background
Pulmonary arterial hypertension (PAH) is an important determinant of morbidity and mortality in children. In this study we aimed to investigate the value of Brain Natriuretic Peptide (BNP) in a cohort of children with PAH, with respect to monitoring disease severity as assessed by hemodynamic and echocardiographic parameters.
Methods
We performed a prospective study to determine if BNP varies over time in this population and if these changes track with hemodynamic or echocardiographic parameters. The population included a group of 78 pediatric patients from January 2005 until April 2008. All patients were diagnosed with PAH and had serum BNP, catheterization and echocardiographic variables collected longitudinally.
Results
The median BNP level, for all observations, was 36.0pg/ml (interquartile range [IQR], 18.0 to 76pg/ml). There was no strong correlation found between commonly used echocardiographic or hemodynamic data and BNP. However, using a bivariate model, the change in BNP measurements over time significantly correlated with the change in the hemodynamic and echocardiographic parameters. Patients with a BNP value greater than 180pg/ml had a decreased survival rate.
Conclusions
There is indication that BNP could be a useful marker to monitor disease severity in pediatric pulmonary hypertension. We show that simple correlations between variables and BNP are not likely to illustrate its usefulness due to variations in the normative levels. Instead, we propose that patient’s BNP levels should be monitored over time, as changes in BNP within a patient are likely to be more informative.
Keywords: Pediatrics, pulmonary arterial hypertension, biomarkers, brain natriuretic peptide, longitudinal changes
Introduction
Pulmonary arterial hypertension (PAH) is an elevation in pulmonary artery pressure that is associated with a spectrum of diseases and causes. At this time, there is no cure for PAH and without appropriate management, PAH is often progressive and fatal. It is an important determinant of morbidity and mortality in children and its clinical severity and presentation are widely varied, making the accurate assessment of prognosis in patients with PAH difficult.1–4
Currently, hemodynamic parameters are the basis for characterizing disease progression and prognosis of patients with PAH.5,6 These measurements are obtained invasively by cardiac catheterization in pediatric patients and are associated with specific risks.7 Additionally, echocardiography is helpful in the monitoring of disease severity in PAH, yet has its own inherent limitations. Therefore, alternative parameters to monitor disease severity and prognosis which are noninvasive, reliable and relatively quick are highly needed in pediatric patients with PAH.
The release of brain natriuretic peptide (BNP) results in improved myocardial relaxation and is targeted at protecting the cardiovascular system from the effects of volume overload. The initiation of synthesis of proBNP results from wall stress caused by volume expansion or pressure overload. Subsequently, the peptide is cleaved to produce the biologically active hormone BNP and the inactive NTproBNP. Both BNP and NTproBNP can be detected and measured in the circulation. In general, these peptide levels are reasonably correlated, and either can be used in patient care, although the absolute levels are not interchangeable. Normal values vary depending on the assay method used and on the demographics of the patient.8 Even though appropriate reference values are lacking in children, preliminary data suggest that BNP levels are useful in diagnosing and managing pediatric heart failure.9–13
Several studies have been performed in the adult population to determine if elevated serum levels of natriuretic peptides can be used as a prognostic indicator of disease severity in pulmonary hypertension.14–19 In one study, survival was strikingly worse for patients with a supramedian value of BNP above 180 pg/ml than those with an inframedian value.17 However, related studies in pediatric PAH patients, specifically involving some form of BNP, are few. It is unknown whether BNP, specifically, can be used as a prognostic tool in the same way as their adult counterparts.
Towards this goal, we performed a prospective study in children with PAH who presented to The Children’s Hospital of Denver from 2005 to the present. This investigation aims to determine the relation between serum levels of BNP and currently monitored hemodynamic and echocardiographic variables in PAH.
Methods and Materials
Seventy-eight pediatric patients with PAH (26 with idiopathic PAH (IPAH), 52 with associated PAH (APAH), 42 females, ages at first sample 6 months-22 years with a median age of 9) were subsequently enrolled in the IRB approved Prospective Evaluation of Adolescents and Children with Pulmonary Arterial Hypertension (PEACH) study at our institution beginning January 2005. IPAH and APAH were defined based on the criteria of the WHO Classification.5 There was an average of 3.4 blood samples drawn per patient (range: 1–12) over the course of the study. Serum BNP levels were measured as part of routine clinical practice at our institution starting in 2005. In addition, information was collected on hemodynamic parameters from cardiac catheterizations and transthoracic echocardiograms from each patient during this time.
Measurement of BNP
BNP levels were determined by using the Beckman Coulter Unicel® DxI 800 kit. The BNP test is a two-site immunoenzymatic sandwich assay. Samples can be accurately measured within the analytic range of the lower limit of detection and the highest calibrator value (approximately 1–5000pg/mL).
Echocardiographic measurements
Transthoracic echocardiography (Vivid 5 and Vivid 7 GE) was used in a standard fashion to obtain right ventricular end-diastolic dimension (RVDd), left ventricular end-diastolic dimension (LVDd), fractional shortening (FS) and tricuspid regurgitation velocity (TR). Tricuspid regurgitation pressure gradient (TRp) was defined by the formula 4TR2.20
Hemodynamic measurements
Hemodynamic parameters were measured in the cardiac catheterization laboratory during a routine pulmonary hypertension study. The data was obtained at baseline with the patient breathing room air, prior to testing pulmonary reactivity with the use of vasodilator therapy. For right-heart catheterization we used a Swan-Ganz catheter in the femoral vein or internal jugular vein to determine mean right atrial pressure (mRAP), mean pulmonary artery pressure (mPAP), pulmonary capillary wedge pressure (PCWP), cardiac index (CI) and pulmonary vascular resistance index (PVRi). Invasive arterial monitoring was used to measure mean aortic pressure (mAoP) and systemic vascular resistance index (SVRi). The method of Fick with measured oxygen consumption was used to determine pulmonary and systemic flow in patients with intracardiac shunting.
Catheterization and laboratory values were obtained while patients were on therapy for pulmonary arterial hypertension. Patients were receiving single or multiple therapeutic agents including any of the following: calcium channel blockers, phosphodiesterase type-5 inhibitors, intravenous epoprostenol or treprostinil, inhaled iloprost or an endothelin receptor antagonist. In this study, we did not analyze results based on the different groups of therapeutic agents due to the small number of patients.
Statistical Analysis
Descriptive statistics were calculated using medians and interquartile ranges (IQR) for continuous variables and percentages for categorical variables. A variable describing time was created in which a patient’s time of first BNP measurement was denoted as time 0, all other measurements within a patient use this time as a reference. Log transformation (base 10) was used to normalize the distribution of variables and all continuous outcome variables were assumed to have a linear relationship with both age and time, expressed in years. Simple correlations between variables were evaluated by Pearson correlation coefficients and were only used for observations in which any two measurements were conducted on the same day. To avoid the inclusion of repeated measurements, which violates the independence assumption of the Pearson coefficient, only the first pair of measurements for a subject was included.
To estimate the change in the BNP biomarker over time, a random coefficient model with an intercept and slope fit for each patient with at least two measurements was used.21 This model included an unstructured covariance matrix for the random coefficients. In addition, a bivariate version of the random coefficients model was fit to address the question of whether the outcome variable trends over time are associated with changes in BNP or, in other words, whether the random slopes of two variables are correlated. This modeling approach is achieved by simultaneously fitting two univariate mixed effects models, one for each outcome, and specifying a joint multivariate distribution on the random effects. A benefit of this method is that it does not require the outcomes to be measured at the same time.22,23
To assess the predictive ability of the last observed BNP measurement, the Kaplan-Meier product-limit estimator was used to approximate the survival functions for high and normal BNP which were compared with the log-rank test. A logistic regression model was fit to assess the predictive ability of the last observed log transformed BNP measurement and the survival event, adjusted for the length of the time interval between the measurement and the survival status. All models were fit using SAS version 9.1 software (SAS Institute Inc.: Cary, NC, 2004), proc MIXED was used for all mixed models including the multivariate mixed models.
Results
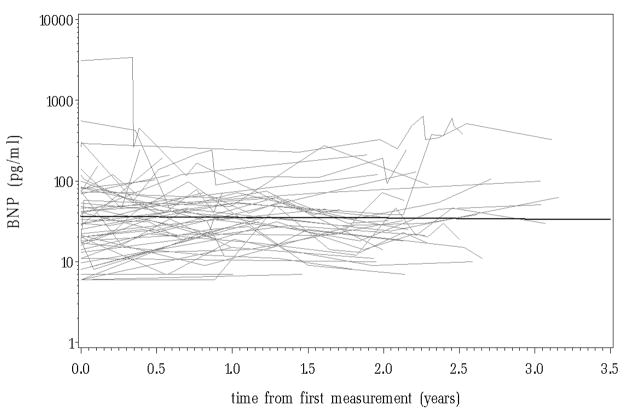
Demographic information and descriptions of the baseline measurements are displayed in table 1. The patients had an elevated median baseline mPAP of 38 mmHg (IQR, 29 – 59 mm Hg) and mRAP of 6 mm Hg (IQR, 4 – 8 mmHg). Initial BNP measurements were not significantly related to age, gender or PAH diagnosis. There were a total of 267 BNP measurements collected. Figure 1 displays the patient specific profiles and the overall trend over time in serum BNP which was not significant (p = 0.83) and illustrates an initial overall BNP estimate of 36.3 pg/ml.
Table 1.
Patient Demographics and Baseline Clinical Measurements (N = 78)
| Median Age, years | 9.3 (5.2 – 14.2) |
| Female | 42 (53.9%) |
| IPAH | 26 (33.3%) |
| Associated Pulmonary Arterial Hypertension | |
| Congenital Heart Disease | 41 (52.5%) |
| Repaired | Repaired/Unrepaired |
| Ventricular Septal Defect | 10/3 |
| Atrial Septal Defect | 6/2 |
| Atrioventricular Septal Defect | 6/2 |
| Patent Ductus Arteriosus | 2/1 |
| Transposition of the Great Arteries | 2/0 |
| Double Outlet Right Ventricle | 1/0 |
| Total Anomalous Pulmonary Venous Return | 2/0 |
| Tetralogy of Fallot | 1/0 |
| “Absent Pulmonary Artery” | 0/3 |
| Portopulmonary Hypertension | 2 (2.5%) |
| Connective Tissue Disease | 3 (3.8%) |
| Sickle Cell Disease | 1 (1.2%) |
| Bronchopulmonary Dyslpasia | 5 (6.4%) |
| Baseline Cath measurements - median (IQR) | |
| Right Atrial Pressure, mm Hg | 6 (4 – 8) N = 77 |
| Pulmonary Artery Pressure, mm Hg | 38 (29 – 59) N = 79 |
| Pulmonary Capillary Wedge Pressure, mm Hg | 9 (7 – 10) N = 73 |
| Cardiac Index, L/min × m2 | 3.5 (2.8 – 4.4) N = 63 |
| Pulmonary Vascular Resistance Index (PVR), Wood units × m2 | 6.5 (4.7 – 14.7) N = 74 |
| Systemic Arterial Pressure, mm Hg | 64 (58 – 70) N = 78 |
| Systemic Vascular Resistance Index (SVR), Wood units × m2 | 14.8 (11.2 – 19.2) N = 72 |
| PVR/SVR | 0.5 (0.4 – 0.8) N = 72 |
| Baseline Echo measurements - median (IQR) | |
| Right Ventricular Diastolic Dimension, cm | 2.7 (2.1 – 3.2) N = 47 |
| Left Ventricular Diastolic Dimension, cm | 3.6 (3.0 – 4.2) N = 70 |
| Fractional Shortening of the left ventricle, % | 40.0 (35.5 – 46.5) N = 69 |
| Tricuspid Regurgitation Velocity, m/s | 4.2 (3.5 – 4.8) N = 64 |
| Tricuspid Regurgitation Pressure Gradient, mm Hg | 70.1 (49.2 – 91.9) N = 64 |
| No. caths per patient (range) | 0 – 8 |
| No. patients with 1 or no cath | 41 |
| No. patients with 2 or more caths | 37 |
| No. echos per patient (range) | 0 – 27 |
| No. patients with 1 or no echo | 7 |
| No. patients with 2 or more echos | 71 |
Values are given as the median (IQR) or No. (%), unless otherwise indicated.
Figure 1.
The patient specific profiles and the overall trend over time in serum BNP
Table 2 displays the Pearson correlation coefficients testing the association between the specified hemodynamic and echocardiographic parameters with BNP. Serum BNP levels were significantly correlated with cath variables RAP (r = 0.34; p = 0.01), PCWP (r = 0.26; p = 0.05) and with the echo variable LVDd (r = −0.52; p <.01). Even though these correlations are statistically significant, the magnitude of these associations is still relatively weak. These low correlations are consistent with other previously reported results for NT-proBNP.10,11,17 Given, however, that extensive studies of BNP levels in varying populations have revealed that absolute levels change with different co-morbidities and patient demographics,8 these results are expected. For this reason, we proposed, instead, to test the correlations between changes in the markers in an individual patient.
Table 2.
Pearson correlation coefficients for association with BNP
| r | n | |
|---|---|---|
| Cath | ||
| Right Atrial Pressure | 0.34* | 56 |
| Pulmonary Artery Pressure | 0.16 | 57 |
| Pulmonary Capillary Wedge Pressure | 0.26* | 56 |
| Cardiac Index | −0.08 | 52 |
| Pulmonary Vascular Resistance Index | 0.06 | 56 |
| Systemic Arterial Pressure | −0.10 | 57 |
| Systemic Vascular Resistance Index | 0.05 | 56 |
| PVR/SVR | 0.01 | 55 |
| Echo | ||
| Right Ventricular Diastolic Dimension | 0.23 | 42 |
| Left Ventricular Diastolic Dimension | −0.52* | 45 |
| Fractional Shortening of the left ventricle | 0.23 | 45 |
| Tricuspid Regurgitation Velocity | 0.24 | 46 |
| Tricuspid Regurgitation Pressure Gradient | 0.23 | 47 |
estimate was significantly different from 0, p-value < 0.05
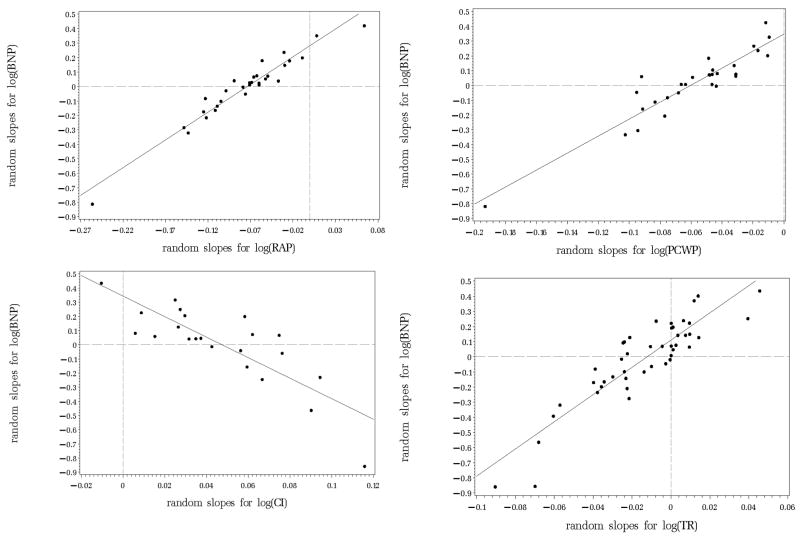
The bivariate random coefficient model was used to simultaneously estimate and correlate the random slopes of BNP with each echocardiographic and hemodynamic variable separately. Table 3 lists the correlations between the BNP random slopes and the random slopes of the clinical variables and the strength and direction of the overall slope for each variable. Trends in serum BNP levels are significantly correlated with trends in almost all hemodynamic parameters and with TR and FS (echocardiography variables). Illustrations of the associations of the random slopes for selected variables are displayed in figure 2. The random slopes were estimated after the log transformation of the variables and should therefore be interpreted as changes in log BNP over one year. Most of the random slopes are clustered near the zero axes (dotted lines) indicating only subtle changes in BNP and the other parameters. More important, is the agreement in the extreme values. For those patients with large deviations over time observed for a variable, there is an equally extreme change observed in BNP.
Table 3.
Correlations between random slopes of specified variables and random slopes for BNP
| r | Slope | |
|---|---|---|
| Cath | ||
| Right Atrial Pressure | 0.95* | −0.07* |
| Pulmonary Artery Pressure | 0.94* | −0.04* |
| Pulmonary Capillary Wedge Pressure | 0.93* | −0.06* |
| Cardiac Index | −0.71 | 0.05* |
| Pulmonary Vascular Resistance Index | 0.89* | −0.04 |
| Systemic Arterial Pressure | −0.45 | 0.02 |
| Systemic Vascular Resistance Index | 0.50 | <.01 |
| PVR/SVR | 0.66 | −0.02 |
| Echo | ||
| Right Ventricular Diastolic Dimension | 0.21 | 0.02 |
| Left Ventricular Diastolic Dimension | −0.28 | 0.02* |
| Fractional Shortening of the left ventricle | 0.80* | −0.02 |
| Tricuspid Regurgitation Velocity | 0.81* | −0.01* |
| Tricuspid Regurgitation Pressure Gradient | 0.37* | −0.05* |
estimate was significantly different from 0, p-value < 0.05
Figure 2.
Correlations between the random slopes for log BNP with; Top left: log RAP. Top right: log PCWP. Bottom left: log CI. Bottom right: log TR.
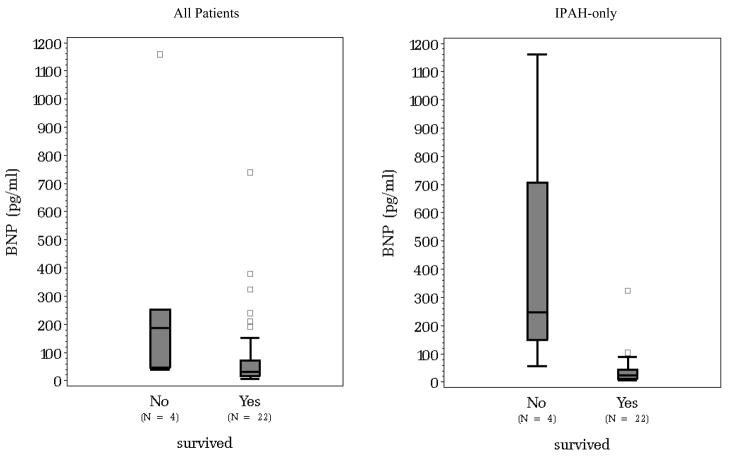
Of the 78 patients included in the study, 7 did not survive. Five of those 7 only had one BNP measurement, for this reason, the value of the last observed BNP measurement was used instead of the change in BNP over time. It was found, that on average those who did not survive had higher BNP values (p < 0.01). Figure 3 displays the difference in the distribution of BNP between those who survived and those who did not, first for all patients and second for the IPAH subgroup.
Figure 3.
Difference in the last observed BNP distributions between survivors and non-survivors for all patients (left) and IPAH patients (right).
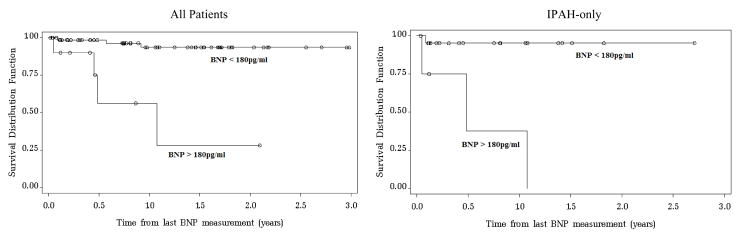
Using the cut-off of 180pg/ml suggested by Nagaya et al.17, there were 10 patients classified as having a high BNP. Of those, 60% survived, compared to a 96% survival rate in the group with BNP < 180pg/ml. In contrast, 4 of the 26 IPAH patients had a high BNP, only one of which survived (25%) compared to a 95% survival rate in the normal IPAH subgroup. Figure 4 displays the significantly different (p < 0.01) survival curves for those with and without a high BNP value using the 180pg/ml cutoff.
Figure 4.
Kaplan-Meier curves estimating cumulative survival for all patients (left) and for the subgroup of IPAH patients (right) categorized with either high BNP (BNP > 180pg/ml) or normal BNP (< 180pg/ml).
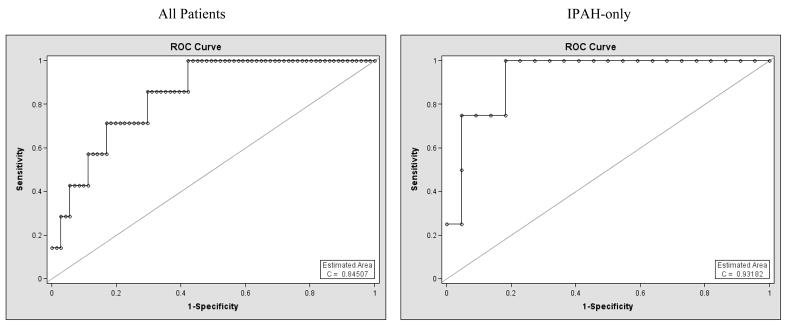
The resulting ROC curves from the logistic regressions fit to the last observed BNP value for all patients and to the IPAH subgroup are shown in figure 5. The area under the curve (c = 0.84 and c = 0.93 for all patients and the IPAH subgroup, respectively) indicates promising discriminative ability of log transformed BNP level to classify survivors and non-survivors.
Figure 5.
ROC curves for last observed log transformed serum BNP level predicting death, adjusted for time between last measurement and survival status. Left: all patients. Right: subgroup of patients with IPAH.
Discussion
In recent years, serum biomarkers are being considered as possible tools for the assessment of disease severity and patient prognosis in pulmonary hypertension. Studies have been performed in the adult population to determine if these biomarkers can be used as prognostic indicators or if a correlation can be established between biomarkers and the severity of disease.10,19,24–26
Studies in children are more challenging than in adults and remain limited, with one reason being the lack of normative biomarker values in children. It has been shown that BNP values vary with certain demographics and specific co-morbidities (i.e., renal function, age, gender and previous heart failure) and even across commercially available assay kits.8,27 Regardless of these limitations, higher BNP values have been associated with an increase in mortality and morbidity, however, specifying a cutoff based on this information will likely depend on various conditions.9,10,13,19
Our data indicates that there is no strong correlation between commonly used echocardiographic variables or hemodynamic data and BNP, which is likely due to the variability in BNP levels discussed previously and in Conen et al.19 However, we found strong evidence that changes over time in these variables were highly correlated with hemodynamic variation, which have been shown to be predictive of outcomes in PAH and are considered indicators for disease severity.6 Specifically, BNP changes correlated inversely with CI and positively with mRAP, mPAP, PCWP and PRVi. This suggests that BNP may have additional value in the management of children with pulmonary arterial hypertension if tracked over time. Additionally, we observed that a high BNP is indicative of poor survival, more so in IPAH patients. Given the small number of mortality events in this dataset, however, a more thorough investigation of the predictive ability of a high BNP value, or a significant increase in BNP level, is needed.
The BNP values presented in this work were similar to previously published values of left ventricular systolic dysfunction in children who had not experienced an adverse event within 90 days as described in Price et al.13 Yet, our BNP values are lower than those reported in adults with PAH.14,17,28
Van Albada et al.11 investigated the correlations between several serum markers in children with PAH with functional and hemodynamic parameters. They found that NTproBNP did not significantly correlate with hemodynamic variables but was weakly correlated with functional class. Here, we correlated BNP with both hemodynamic and echocardiographic parameters in a similar population. Similarly, we found weak associations between BNP levels and the other clinical variables. However, we found that serial changes in BNP were predictive of changes in hemodynamic parameters.
BNP is now recognized as an essential part of adult cardiologic evaluation. A limited but expanding knowledge base exists in the pediatric age group. Towards the goal of incorporating this marker into the clinical assessment of disease severity and prognosis, BNP levels should be monitored and information regarding changes from the patient specific normative value should be utilized versus comparison with a general cutoff.
Acknowledgments
This study was supported in part by:
UO1-HL081335, Clinical Proteomics Center in Lung Disease, NIH
M01-RR00069, General Clinical Research Center, National Center for Research Resources, NIH
SCCOR, P50 HL084923, NIH
Colorado CTSA grant 1 UL1 RR025780 from NCRR/NIH
Footnotes
The authors have no conflicts of interest to disclose
References
- 1.Badesch DB, Abman SH, Simonneau G, et al. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131:1917–1928. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 2.Rashid A, Ivy D. Pulmonary hypertension in children. Current Pediatrics. 2006;16:237–247. [Google Scholar]
- 3.Rosenzweig EB, Barst RJ. Idiopathic pulmonary arterial hypertension in children. Curr Opin Pediatr. 2005;17:372–380. doi: 10.1097/01.mop.0000163356.51027.c1. [DOI] [PubMed] [Google Scholar]
- 4.Rosenzweig EB, Widlitz AC, Barst RJ. Pulmonary arterial hypertension in children. Pediatr Pulmonol. 2004;38:2–22. doi: 10.1002/ppul.20051. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 6.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 7.Carmosino MJ, Friesen RH, Doran A, et al. Perioperative complications in children with pulmonary hypertension undergoing noncardiac surgery or cardiac catheterization. Anesth Analg. 2007;104:521–527. doi: 10.1213/01.ane.0000255732.16057.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Davis GK, Bamforth F, Sarpal A, et al. B-type natriuretic peptide in pediatrics. Clin Biochem. 2006;39:600–605. doi: 10.1016/j.clinbiochem.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Nir A, Nasser N. Clinical value of NT-ProBNP and BNP in pediatric cardiology. J Card Fail. 2005;11:S76–80. doi: 10.1016/j.cardfail.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Van Albada ME, Loot FG, Fokkema R, et al. Biological serum markers in the management of pediatric pulmonary arterial hypertension. Pediatr Res. 2008;63:321–327. doi: 10.1203/PDR.0b013e318163a2e7. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds EW, Ellington JG, Vranicar M, et al. Brain-type natriuretic peptide in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatrics. 2004;114:1297–1304. doi: 10.1542/peds.2004-0525. [DOI] [PubMed] [Google Scholar]
- 13.Price JF, Thomas AK, Grenier M, et al. B-type natriuretic peptide predicts adverse cardiovascular events in pediatric outpatients with chronic left ventricular systolic dysfunction. Circulation. 2006;114:1063–1069. doi: 10.1161/CIRCULATIONAHA.105.608869. [DOI] [PubMed] [Google Scholar]
- 14.Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol. 2004;43:764–770. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 15.Leuchte HH, Holzapfel M, Baumgartner RA, et al. Characterization of brain natriuretic peptide in long-term follow-up of pulmonary arterial hypertension. Chest. 2005;128:2368–2374. doi: 10.1378/chest.128.4.2368. [DOI] [PubMed] [Google Scholar]
- 16.Nagaya N, Nishikimi T, Okano Y, et al. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol. 1998;31:202–208. doi: 10.1016/s0735-1097(97)00452-x. [DOI] [PubMed] [Google Scholar]
- 17.Nagaya N, Nishikimi T, Uematsu M, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 18.Fijalkowska A, Kurzyna M, Torbicki A, et al. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129:1313–1321. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 19.Conen D, Pfisterer M, Martina B. Substantial intraindividual variability of BNP concentrations in patients with hypertension. J Hum Hypertens. 2006;20:387–391. doi: 10.1038/sj.jhh.1001988. [DOI] [PubMed] [Google Scholar]
- 20.Mourani PM, Sontag MK, Younoszai A, et al. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121:317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 22.Thiebaut R, Jacqmin-Gadda H, Chene G, et al. Bivariate linear mixed models using SAS proc MIXED. Computer Methods and Programs in Biomedicine. 2002;69:249–256. doi: 10.1016/s0169-2607(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 23.Fieuws S, Verbeke G. Pairwise fitting of mixed models for the joint modeling of multivariate longitudinal profiles. Biometrics. 2006;62:424–431. doi: 10.1111/j.1541-0420.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 24.Koch A, Zink S, Singer H. B-type natriuretic peptide in paediatric patients with congenital heart disease. Eur Heart J. 2006;27:861–866. doi: 10.1093/eurheartj/ehi773. [DOI] [PubMed] [Google Scholar]
- 25.Koch AM, Rauh M, Zink S, et al. Decreasing ratio of plasma N-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide according to age. Acta Paediatr. 2006;95:805–809. doi: 10.1080/08035250500466647. [DOI] [PubMed] [Google Scholar]
- 26.Nir A, Bar-Oz B, Perles Z, et al. N-terminal pro-B-type natriuretic peptide: reference plasma levels from birth to adolescence. Elevated levels at birth and in infants and children with heart diseases. Acta Paediatr. 2004;93:603–607. doi: 10.1111/j.1651-2227.2004.tb02984.x. [DOI] [PubMed] [Google Scholar]
- 27.Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–878. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leuchte HH, Holzapfel M, Baumgartner RA, et al. Characterization of Brain Natriuretic Peptide in Long-term Follow-up of Pulmonary Arterial Hypertension. Chest. 2005;128:2368–2374. doi: 10.1378/chest.128.4.2368. [DOI] [PubMed] [Google Scholar]