Abstract
Alzheimer’s disease (AD) is a major threat for the rapidly aging world population. AD is the leading cause of dementia and a major cause of death in developed countries. The disease puts a tremendous practical, emotional and financial burden on individuals and governments. Clinicians and researchers in the AD field face great challenges: the pathophysiological processes that cause AD are not well understood, definite diagnosis of AD requires autopsy, and therapeutic options are limited to treating the symptoms rather than the cause of the disease. Nevertheless, new insights into the earliest events that lead to development of AD increase hope that reliable diagnostics and efficacious therapies may emerge.
Keywords: Aβ, Alzheimer’s disease, amyloid, dementia, diagnosis, mild cognitive impairment, therapy
Alzheimer’s disease (AD) is the most common form of late-life dementia, accounting for approximately two-thirds of all dementia cases [1]. AD is a major cause of death and a tremendous financial and emotional problem, the magnitude of which is predicted to increase steeply in the next few decades if no cure is found. Here, we review the current knowledge of the processes that lead to AD and their implications on recent advances in diagnostics and therapeutics for AD. To this end, we have chosen a stepwise approach of increasing complexity in each step. We discuss how molecular events affect cells, cellular insults accumulate into tissue damage and tissue deterioration eventually affects the whole person. This stepwise approach is directed at both clinicians and basic researchers working in the AD field.
The early stages of AD are characterized by progressive loss of cognitive abilities whereas overall alertness, sensory and motor functions, and social skills are preserved. Individuals with moderate-to-severe AD experience progressive loss of mental faculties and pronounced changes in behavior. Eventually, patients reach a bedridden, noncommunicative stage. AD causes death within 8 years, on average, from the time of first diagnosis [2].
Pathophysiologically, AD is characterized by two hallmark lesions – extracellular amyloid plaques and intracellular neurofibrillary tangles (NFTs) [3]. The main component of amyloid plaques is the amyloid β-protein (Aβ), a small protein produced by post-translational proteolysis from the Aβ-protein precursor (APP), a type I integral membrane glycoprotein. NFTs are composed of abnormally hyperphosphorylated versions of the microtubule-associated protein tau and are not specific to AD [4,5]. In AD, formation of NFTs is believed to be secondary to the neurotoxic effects of Aβ [6]. Therefore, although both types of lesions are important pathogenetic contributors in AD. We focus here on Aβ.
Evidence demonstrating that mutations in the genes that encode either APP or the enzymes responsible for the generation of Aβ from APP cause early onset familial forms of AD [7] have supported the formulation of the amyloid cascade hypothesis [8], a central paradigm that has guided AD research for almost two decades. The hypothesis suggests that “…deposition of Aβ protein, the main component of the … plaques, is the causative agent of Alzheimer’s pathology and … the NFTs, cell loss, vascular damage, and dementia follow as a direct result of this deposition”[8,9]. However, certain clinical and pathological observations have been difficult to reconcile with this hypothesis, in particular, poor correlation between the temporal and spatial courses of amyloid plaque accumulation in the brain of patients with AD and the progression of clinical manifestations of AD. In recent years, mounting evidence has pointed to a primary causative role in AD for prefibrillar, oligomeric assemblies of Aβ [10], leading to revisions of the amyloid cascade hypothesis and a shift in focus from aggregated polymeric forms of Aβ to Aβ oligomers [11].
Premortem diagnosis of AD is based on the patient’s clinical history, cognitive assessment and neuroimaging tests. Cerebrospinal fluid (CSF) biomarker assays are occasionally used, mostly in research settings. The clinical diagnoses of possible and probable AD have a sensitivity of approximately 85% [12]. Definite AD is a postmortem pathological diagnosis based on the identification of amyloid plaques and NFTs.
Currently, approved therapies for AD treat symptoms rather than cause. Approved drugs include acetylcholinesterase inhibitors (AChEIs), which address the deficit in cholinergic transmission associated with AD, and an N-methyl-d-aspartate (NMDA) receptor antagonist, which decreases glutamatergic excitotoxicity [13]. These therapies temporarily and moderately defer cognitive decline. Novel approaches to developing mechanism-based drugs are discussed herein.
Even when effective drugs become available, preventing or curing AD likely will require detection of the disease at an early stage [14]. The earliest clinical manifestation of AD is mild episodic memory dysfunction, a stage often characterized as amnestic mild cognitive impairment (MCI). (Not all MCI patients develop AD. Patients may develop other neurological diseases, remain at the MCI level, or even go back to normal aging. Here, unless otherwise stated, the term MCI refers to those patients who will eventually develop AD). However, the disease may begin as early as 15 years before appearance of the first clinical symptoms [15]. Thus, even though at the time the diagnosis of MCI is made the clinical symptoms are mild, AD neuropathology may be fairly advanced. Autopsy of patients diagnosed with MCI often reveals the presence of senile plaques, NFTs and brain atrophy [16]. Therefore, substantial efforts currently are directed towards very early characterization of MCI and development of rigorous diagnostic criteria for this condition based on neuropsychological evaluation, brain imaging and biomarker detection in the CSF [14,17].
AD development
Molecular level: assembly of Aβ & tau
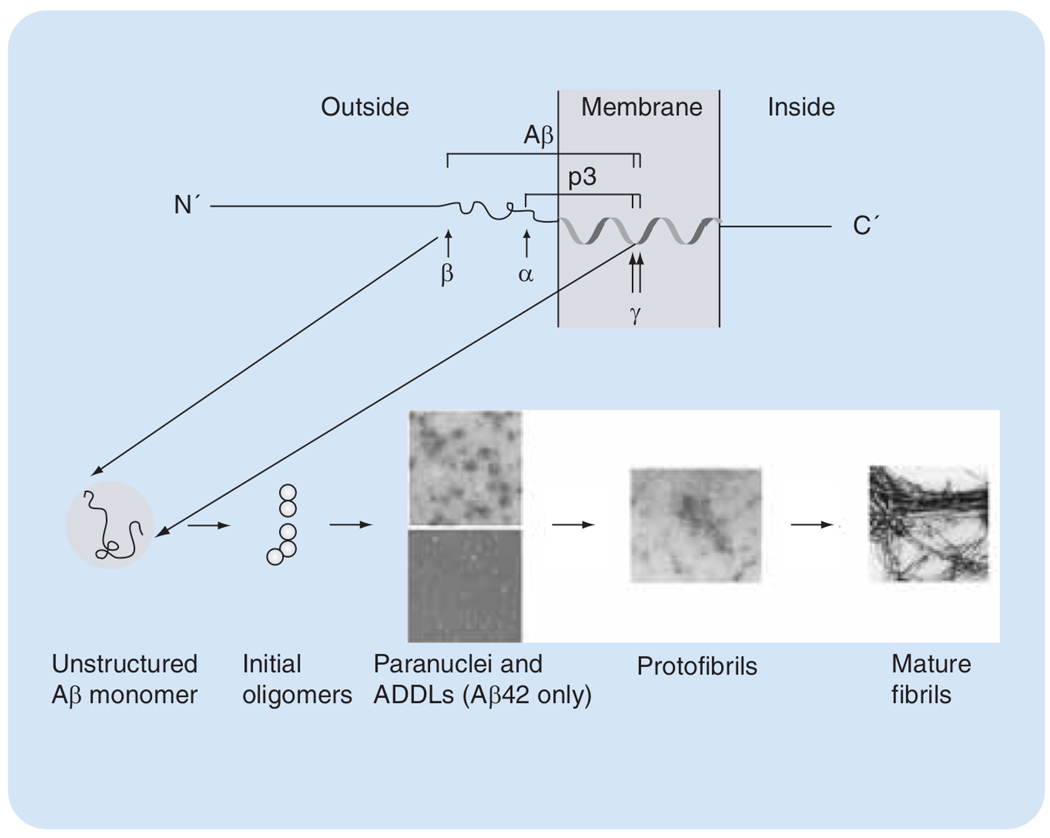
Endoproteolysis of APP is mediated by three proteases termed α, β- and γ-secretases.(FIGURE 1). Aβ is produced by sequential cleavage of APP by β- and γ-secretase predominantly in two forms comprising 40 (Aβ40) or 42 (Aβ42) amino acid residues. Aβ42 is linked particularly strongly to the etiology of AD. Sequential cleavage by α- and γ-secretases produces a shorter peptide, termed p3 (FIGURE 1), which is not associated with disease.
Figure 1. Production and self-assembly of Aβ.
Sequential cleavage of APP by β- and γ-secretases releases Aβ as an unstructured monomer. Aβ40 and Aβ42 monomers self-assemble initially into dimers and trimers. Aβ42 forms quasi-stable paranuclei (upper image, reproduced with permission from [18]) and ADDLs (lower image, reproduced with permission from [25]). Further assembly of both alloforms gives rise to protofibrils, which eventually mature into fibrils (both images reproduced with permission from [29]).
Aβ: Amyloid β; ADDL: Aβ-derived diffusible ligand.
Early findings suggested that assembly into fibrillar polymers was responsible for the neurotoxicity of Aβ. More recent research has led to the discovery of several soluble, low molecular-weight Aβ assemblies, which have higher neurotoxicity than Aβ fibrils [10]. During the very early stages of assembly, Aβ40 and Aβ42 form distinct oligomers [18]. As the assembly process progresses, both alloforms form protofibrils (vide infra) and eventually fibrils. Protofibrils and fibrils of Aβ40 and Aβ42 form with different kinetics but are conformationally and morphologically indistinguishable.
Before its cleavage from APP, the C-terminal region of Aβ resides partially in the membrane and is predicted to have an α-helical conformation. Following Aβ production and subsequent release from the membrane, this conformation likely is destabilized. Nuclear magnetic resonance (NMR) studies of monomeric Aβ in aqueous solutions, in the absence of conformation-inducing agents, such as detergents or fluorinated alcohols, have found little ordered structure for Aβ40 and Aβ42 [19,20]. Other studies indicate the existence of irregular, quasi-stable structures, in particular regions of the Aβ monomer [21], that may nucleate further folding and self-association. However, the precise relationship between Aβ folding and self-assembly is not clear. Most studies indicate that assembly (and toxicity) involve formation of abundant β-sheet structures in Aβ. Alternative models suggest that an activated monomer, which does not have high β-sheet content, is the earliest toxic species and the nucleus for further aggregation and fibril formation [22]. Dimers and trimers of Aβ secreted from cultured Chinese hamster ovary cells expressing high levels of mutant human APP have been shown to be neurotoxic in vitro and in vivo [23]. The conformation of Aβ in these small oligomers is unknown. Similar low-molecular-weight oligomers of synthetic Aβ40 and Aβ42 in aqueous buffers are largely unstructured [18]. Under these conditions, Aβ40 does not form species larger than tetramers [24], whereas Aβ42 gives rise to pentameric/hexameric paranuclei [18]. When synthetic Aβ42 is incubated in cell culture medium at 4°C, small, globular oligomers termed Aβ-derived diffusible ligands (ADDLs) form [10]. ADDLs and paranuclei are prepared from synthetic Aβ42 using different protocols but are indistinguishable morphologically and, in fact, may be identical. Paranuclei form immediately after dissolution and self-assemble into larger oligomers, which likely are predecessors of protofibrils [18]. ADDLs are stabilized by unknown ingredients of cell culture media and cold temperature and are highly neurotoxic [25]. ADDL-like structures have been found in the brain of AD patients and their concentration showed significant correlation with progression of cognitive decline [26]. In vitro-prepared, 60 kDa oligomers of Aβ42 showed similar neurotoxicity to ADDLs [27]. Several other globular oligomers of both Aβ40 and Aβ42 were found under nonphysiological conditions (e.g., βamy balls, amylospheroids), which are not discussed here (for more details, see [28]).
Aβ protofibrils are short, curvilinear structures, typically approximately 5 nm in diameter and less than 150 nm long, comprising 24–700 molecules (100–3000 kDa). Protofibril formation involves conformational rearrangements and formation of significant levels of β-sheet structure [29]. Further maturation of protofibrils presumably yields single protofilaments, which eventually self-associate into mature fibrils [30]. Fibrils are the end product of the assembly process and the form of Aβ found in amyloid plaques. High structural polymorphism has been found among fibrils in different preparations [31], or even in the same preparation [32], and has been linked to significant differences in toxicity [31].
Injection of Aβ into the brain of transgenic mice expressing a mutant form of human tau (P301L tau) has been shown to accelerate the formation of NFTs [33]. In addition, NFT formation was enhanced in double-transgenic mice expressing mutant human tau and APP compared with mice expressing mutant tau alone [34]. Injection of antibodies specific for Aβ oligomers into the hippocampus of triple-transgenic mice expressing mutant human tau, APP and presenilin-1 (an integral membrane protein believed to contain the active site of γ-secretase) not only reduced Aβ accumulation but also decreased tau pathology [35]. These data indicate that Aβ-induced brain insults precede the formation of hyperphosphorylated, aggregated tau.
Aggregation of tau results in the formation of twisted filaments, termed paired helical filaments (PHFs), that exhibit high conformational and morphological similarity to Aβ (and other protein) fibrils. Recent studies of tau assembly intermediates have led to the discovery of a granular oligomer of 40 tau molecules in the brain of AD patients, which forms PHF in a concentration-dependent manner [36]. Thus, similar to Aβ oligomers, neurotoxic tau oligomers may play an important role early in the pathogenesis of AD.
Cellular level: Aβ assembly & toxicity
Multiple factors contribute to the neurodegenerative processes in the AD brain, including inflammation [37], oxidative damage [38], apoptosis [39] and excitotoxicity [40]. These processes are thought to occur secondary to neuronal injury and compromise of synaptic transmission caused by Aβ oligomers [41], although other models hypothesize that oxidative injury coincides with, or even precedes, the earliest Aβ-mediated neurotoxicity [42].
Although the mechanisms of the earliest neurotoxic events occurring in AD are unclear, study of AD brain has suggested what the effectors of these events might be. Using polyclonal antibodies prepared either against Aβ40 attached to gold particles [43] or against ADDLs [26], oligomers recognized by these antibodies were observed in brains from AD patients. In brains from age-matched healthy individuals, similar oligomers were absent or found at substantially lower levels [26,43]. (The oligomers visualized by the anti-ADDL antibody [26] are likely to be ADDL-like Aβ oligomers, whereas those recognized by the antibody prepared against Aβ40 attached to gold particles may be oligomers of other amyloidogenic proteins [43]). In contrast, polymeric Aβ is commonly found in the brains of healthy individuals in the form of diffuse plaques [44]. These data support the hypothesis that early, nonfibrillar Aβ oligomers are the proximal neurotoxins acting in AD.
Aβ protofibrils have been shown to be neurotoxic to cultured cells [29]. Mechanisms involving specific inhibition of K+ channels and excitotoxic effects mediated by NMDA receptors have been implicated in protofibril neurotoxicity [45,46]. In a family in northern Sweden in which affected individuals display autosomal, dominant, early onset familial AD (FAD), a Glu22→Gly mutation in Aβ causes increased protofibril production [47]. In contrast to all other FAD cases, decreased total Aβ levels and Aβ42:Aβ40 concentration ratios were found in this kindred. Based on these observations, protofibrils have been postulated to be the key effectors of the neurodegenerative phenotype in this family and, possibly, in other amyloid-related diseases [48]. Additional insight into specific Aβ assemblies that may inflict the earliest neuronal injury and lead to the onset of AD has come from studies of animal models and cultured neurons [49,50]. One interpretation of the data presented in these studies is that the onset of AD is related to disruption of neuronal communication and compromise of synaptic plasticity, which occur before overt neurodegeneration and neuronal death are observed. At these early stages, soluble, oligomeric assemblies of Aβ may be present in the brain, but amyloid deposits are not yet detected. One such model is the PDAPP mouse, which overexpresses the FAD-linked, Indiana form of APP, APP(V717F). These mice develop AD-like brain changes, including abundant amyloid plaques, dystrophic neurites and gliosis, by 8 months of age. However, the density of presynaptic terminals in the brains of these mice decreases substantially at 2 months of age, well before the development of amyloid plaques [51]. In addition, long-term potentiation (LTP), a common proxy for memory function, is largely impaired in the hippocampus of PDAPP mice several months prior to plaque deposition [51]. In older mice, poor correlation was found between plaque load and the density of synaptophysin-immunoreactive presynaptic terminals [52], a strong correlator of AD progression.
Another common model of AD is the Tg2576 mouse, which expresses the Swedish form (K670N, M671L) of FAD-linked APP. As with PDAPP mice, the cognitive decline of Tg2576 mice was originally believed to be associated with plaque formation (albeit without overt neuronal loss). However, recent studies have shown that the mice develop memory deficits correlating with reduced LTP and low levels of synaptophysin many months prior to plaque deposition [53] and that these deficits may be caused by soluble, low-molecular-weight oligomers of Aβ42, including dodecamers and certain smaller oligomers [53,54]. Interestingly, ADDLs isolated from AD brains may also be dodecamers [26]. Thus, Aβ dodecamers may be key effectors of neurotoxicity in early, possibly presymptomatic, AD. The observation that in vitro Aβ42 forms predominantly hexameric paranuclei that self-assemble into dodecamers supports this postulation (FIGURE 2).
Figure 2. Aβ dodecamers are putative early effectors of neurotoxicity.
A) 2D gel bands of ADDLs extracted from AD brain correspond to an apparent molecular mass of an Aβ dodecamer (reproduced with permission from ref. [26]). B) SDS-PAGE bands of purified Aβ oligomers designated Aβ*56 isolated from brains of Tg2576 mice correspond to an apparent molecular mass of an Aβ dodecamer. The bands are visualized either by silver staining or by Western blot using monoclonal antibody 4G8 [LESNÉ S. AND ASHE KH, PERS. COMM.]. C) SDS-PAGE of cross-linked Aβ42 shows an intensity maximum at dodecamer (arrow), which presumably forms by association of two hexamers (reproduced with permission from [18]).
Aβ: Amyloid β; AD: Alzheimer’s disease; ADDL: Aβ-derived diffusible ligand; SDS-PAGE: Sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Despite progress in understanding the relationship between Aβ assembly and neurotoxicity, the mechanisms by which adverse interactions between Aβ and brain cells, including neurons, microglia and astrocytes, and/or particular cellular compartments occur, and the identity of the species that cause toxicity in the AD brain, are not known. Among current theories are interaction with specific cell membrane receptors [55], aberrant formation of ion channels and disruption of membrane potential [56,57], permeabilization of the cell membrane without pore formation [58], and neurotoxic activity of intracellular Aβ oligomers [59].
Tissue level: the Alzheimer’s brain
Diffuse amyloid deposits are ubiquitous in normal aging. In contrast, neuritic plaques are seen almost exclusively in cognitively impaired subjects [60]. The first neuritic plaques form in the temporo–occipital cortex and subsequently spread to the temporal, parietal and, later, the frontal cortex. Generally, highest plaque densities are seen in temporal and occipital, followed by the parietal and frontal association cortices [61,62]. Neuritic plaques are occasionally seen in the limbic regions of cognitively normal elderly individuals [60]. MCI patients display highly variable amounts of amyloid deposition, ranging from minimal amyloid burden to pathological severity that is typical of advanced AD [63,64].
NFT deposition begins in the transentorhinal area and spreads to the entorhinal and parahippocampal cortex and the hippocampus. The hippocampal subfields that are connected to the entorhinal cortex (i.e., CA1 and subiculum) are affected earlier than the rest of the hippocampus. Cognitively normal elderly and MCI subjects frequently show NFT pathology restricted to the entorhinal and hippocampal areas [60,65]. In contrast, neocortical NFTs are characteristic of more advanced AD. The trajectory of NFTs in the cortex progresses from the medial and inferior, to the lateral temporal, followed by the parietal and occipital, and finally the frontal cortical regions [61,62].
Several competing theories attempt to explain the selective vulnerability of specific neuronal populations and the trajectory of AD pathology in the human brain. The plasticity theory posits that areas most capable of neuroplasticity with resulting high metabolic requirements, such as the limbic regions, are at highest risk and affected early in the disease process, while those least capable of neuroplasticity, such as the primary motor and sensory cortices, are relatively preserved [62,66]. The connectivity theory considers the disease-specific pathological spread to follow the long corticocortical projections; NFT accumulation occurs inside neuronal cell bodies where these fibers originate, whereas neuritic plaques accumulate first in the cell layers where the fibers terminate [67]. The myelination theory proposes that neocortical myelination influences regional susceptibility. According to this theory, late-myelinating regions (e.g., association cortices), which are subjected to higher levels of metabolic demands relative to early myelinating regions (e.g., primary motor and sensory cortices), accumulate more pathological changes [68,69]. Finally, the laminar predilection of AD pathology conceivably could result from an innate cell-specific predisposition encoded genetically and further modified by epigenetic factors.
Cognitive level: from MCI to AD
Retrospective comparison between neuropsychological data of AD patients and cognitively normal elderly individuals demonstrates significant performance discrepancies as many as 15 years prior to AD diagnosis [15]. Such data provide strong evidence for the insidious yet relentless progression of AD and have stimulated enormous research interest in early diagnosis and intervention.
MCI is a clinically useful but somewhat arbitrary concept. It defines a heterogeneous cognitive state where patients are neither cognitively normal nor demented. Its incidence among the elderly is 10–26/10,000 [70,71]. MCI patients usually present with subjective cognitive complaints, demonstrate objective cognitive decline on neuropsychological testing, and are capable of an independent lifestyle [72].
In most patients, the cause of MCI is prodromal AD. However, other etiologies have also been implicated. MCI has been subclassified into four major cognitive subtypes. Patients with single-domain amnestic MCI present with isolated memory impairment and show poor learning and retention of newly learned information. These patients are thought to harbor incipient AD or hippocampal sclerosis. Multiple-domain amnestic MCI patients have additional impairment in nonmemory cognitive domains. They are thought to have underlying AD, but dementia with Lewy bodies and vascular dementia (in particular, mixed vascular dementia/AD) can also be the cause. Single- and multiple-domain nonamnestic MCI patients show cognitive decline in domains other than memory and the MCI may evolve into frontotemporal dementia, vascular dementia or dementia with Lewy bodies [72]. The two amnestic subtypes are well-recognized risk factors for future diagnosis of AD [73].
The most pervasive cognitive symptom even in the mildest stages of AD is short-term declarative memory loss. Deficits are seen in both encoding and spontaneous retrieval of newly learned information. Semantic, phonemic, or multiple-choice cues initially help but as the disease progresses, these recognition strategies gradually fail. Other noticeable deficits in mild AD are loss of abstract thinking, inefficient planning and execution of complex tasks, failing judgment, word-finding deficits (anomia), and mild disturbance of visuospatial skills [74]. At this stage, patients lose their ability to drive, control their financial assets and plan complex events. Behavioral manifestations in the early stages include apathy, irritability, depression and anxiety [75].
Diagnosis of MCI & early AD
An important contemporary research focus is the reliable identification of preclinical AD within the heterogeneous MCI cohort. Such diagnostic capacity would be invaluable for accurate counseling, family planning and therapeutic intervention. In the following section, we discuss recent progress in the early detection of MCI and early AD, in particular, using neuropsychometric screening tests, neuroimaging techniques and biomarker assays.
Cognitive level: neuropsychiatric examination
The diagnosis of dementia requires evidence of cognitive decline in memory and at least one other cognitive domain, and impaired activities of daily living. Criteria established by Petersen are the most frequently used for the diagnosis of MCI [72]. These criteria include cognitive complaint, preferably corroborated by an informant, intact general intellectual function, largely normal activities of daily living, and absence of dementia. In general neurology practice, the most widely used dementia-screening tool is the Mini Mental State Examination (MMSE). This instrument is also widely used for tracking disease progression. However, the MMSE does not reliably capture the subtle cognitive changes of MCI (sensitivity 18–69%) [76,77]. Recently, other short instruments that may help general neurology practice in assessment of MCI have been designed. The Montreal Cognitive Assessment (MoCA) has a sensitivity of 90% and a specificity of 87% for distinguishing MCI from normal aging [76]. Another test, termed DemTect, was reported to perform with a sensitivity of 80% and a specificity of 92% [77].
In academic centers, the diagnosis of AD and MCI, in addition to clinical evaluation and neuroimaging, typically entails a detailed, formal neuropsychological exam. The latter consists of multiple instruments assessing in detail each cognitive domain (i.e., verbal and visual memory, attention, language, executive function, visuospatial abilities and praxis). Most test scores are then normalized, removing confounding influences of increasing age and high or low education.
Tissue level: neuroimaging
Brain imaging provides in vivo evidence of the impact of AD pathology on the brain. Recent technological advances in neuroimaging have enabled exploration of the structural and functional abnormalities associated with AD and MCI. Presence of hippocampal and cortical atrophy with predilection for the parietal lobes, demonstrated by computed tomography (CT) or magnetic resonance imaging (MRI), is considered consistent with the diagnosis of AD. Hippocampal atrophy occurs in normal aging at 0.05–1.7% of hippocampal volume loss per year [78,79]. The rate of hippocampal atrophy in MCI varies. MCI patients who remain stable have on average 1.8% hippocampal atrophy, whereas those who convert to AD experience 3.3% annual hippocampal volume loss. Within the amnestic MCI subgroup, smaller hippocampi increase the risk for conversion to AD by 75% [80]. Recently developed 3D surface reconstruction techniques have emphasized the differential subregional hippocampal involvement in vivo [81,82]. In agreement with pathological data, subicular and CA1 involvement occurs early, while CA2 and CA3 involvement occur later in the disease course [81,83].
The entorhinal cortex is affected prominently even at the MCI stage. The annual atrophy rates increase from 0.04–1.4% volume losses in normal aging to 7% in AD [79,84]. Baseline entorhinal and hippocampal volumes and their annual atrophy rates are predictive of clinical conversion of MCI to AD [85].
Innovative 3D semi-automated techniques for brain analyses have enabled researchers to explore the entire cortex in patients with dementia. Voxel-based morphometry studies have demonstrated the presence of pervasive cortical atrophy in AD and, to a lesser extent, in MCI [86]. New surface-based computational anatomy techniques allow for better localization, statistical power and quantitative characterization of subtle structural changes. These studies have shown that cortical atrophy occurs at a rate of 5%/year throughout the course of AD [87] and that mild AD patients have substantially greater cortical atrophy relative to patients with amnestic MCI (FIGURE 3) [88].
Figure 3. 3D cortical gray matter ratio maps.
The maps show the areas of the cortex where mild Alzheimer’s disease (AD) patients had greater gray-matter atrophy (percentage) relative to amnestic mild cognitive impairment (MCI) patients. Both the extent and severity of cortical atrophy in mild AD patients is substantially greater relative to patients with amnestic MCI. Most pronounced differences (>20%) are seen in the entorhinal, parahippocampal, precuneus, lateral temporal and parietal regions.
Two other magnetic resonance techniques – magnetic resonance spectroscopy and diffusion tensor imaging, were also applied to MCI and AD subjects. N-acetyl aspartatexreatine (NAA:Cr) ratios were found to be lower in patients with MCI and AD [89], and high baseline hippocampal diffusivity may be predictive of conversion of MCI to AD [90]. The first finding has been attributed to neuronal loss and the latter to white-matter degeneration in AD.
Functional MRI (fMRI) is a relatively new technique that relies on the paramagnetic properties of deoxyhemoglobin and indirectly measures the metabolic activity of the brain. AD patients asked to perform visual memory encoding tasks while monitored by fMRI showed decreased activation of the hippocampus and parahippocampal gyrus relative to control subjects [91]. In mild AD, decline in face–name associative memory was found to correlate with decreased hippocampal activation using fMRI [92], whereas MCI patients showed a compensatory increase in hippocampal activation [93].
Additional imaging tools used for AD diagnosis are 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) and single-photon emission computed tomography (SPECT), which measure brain uptake of radiolabeled glucose and cerebral blood flow (CBF), respectively. Regions found to have a decrease either in uptake of glucose or CBF are considered as areas where neuronal death, dysfunction or both have occurred. Early AD and MCI patients tend to show parietal, temporal and posterior cingulate cortical changes [94]. In patients with cognitive and/or behavioral complaints, PET evidence suggestive of neurodegenerative disorders has been shown to increase the likelihood of a progressive cognitive decline process by 18-fold [95].
The most recent addition to AD brain imaging is PET amyloid imaging using radiolabeled Congo red or thioflavin derivatives that bind to amyloid plaques. The two leading compounds – Pittsburg Compound-B (PIB) and fluoro-2-dialkylamino-6-acylmalononitrile substituted naphthalenes (FDDNP) – have demonstrated greater binding in patients with AD compared with normal individuals. Binding has been shown to negatively correlate with cognitive function and cerebral glucose metabolism as measured by FDG-PET [96].
Molecular level: biomarker assays
At the present time, biomarker assays are not used as part of the routine diagnosis of MCI or AD. These assays analyze disease markers in the plasma or, more commonly, in the CSF The CSF is in direct contact with the brain, and thus reflects biochemical changes due to pathological processes in the CNS. Analysis of biomolecules in the CSF is used to determine various infectious, inflammatory and degenerative conditions of the CNS [97]. Leading candidate biomarkers for MCI and AD are the proteins that form the pathological lesions characteristic of AD, namely Aβ, tau and hyperphosphorylated tau (p-tau). The clinical diagnosis of AD was shown to correlate with increased CSF levels of tau and p-tau, as well as decreased levels of Aβ42 [97]. The reduction of Aβ42 levels in the CSF was found to correlate with an increase in the number of plaques in the hippocampus [98], suggesting that retention of insoluble Aβ42 in the brain leads to the observed decrease in Aβ42 concentration in the CSF.
The mean sensitivities of the CSF assays are 85% for Aβ42, and 80% for tau and p-tau, whereas the overall specificity is approximately 90% [99]. The ratio of Aβ42:Aβ40 was shown to differentiate AD patients more accurately from normal controls than Aβ42 alone, with a sensitivity of 94%. The trends shown in AD, namely, increased CSF levels of tau and p-tau, and decreased levels of Aβ42, can be used for the detection of AD pathology in MCI with sensitivity figures slightly lower compared with AD [99]. In longitudinal studies, patients who converted from MCI to AD were distinguished from patients with stable MCI by increased levels of tau and decreased Aβ42 levels. The combination of these markers did not improve the sensitivity and specificity significantly [100].
Current quantitative analyses of CSF or serum biomarkers for MCI and AD diagnosis use enzyme-linked immunosorbent assay (ELISA). Since changes in levels of Aβ, tau and p-tau are not specific for AD, this method is insufficient for differentiating AD from other forms of dementia, such as Lewy body dementia or vascular dementia. This problem is partly inherent to the pathological processes that lead to dementia and partly stems from limitations of the ELISA technique [97]. Since the current average sensitivity of CSF biomarker detection is approximately 85%, these assays do not offer a considerable increase in predictive value over existing algorithms comprising neuropsychological and imaging modalities [97] and, therefore, do not justify the invasive lumbar puncture required for CSF analysis.
To avoid a lumbar puncture, plasma levels of AD-related biomarkers may be studied. However, although high levels of plasma Aβ42 have been suggested to be a risk factor for development of AD, sporadic AD and control cases could not be differentiated using this technique. Secondary pathophysiological and metabolic alterations in AD, including those related to inflammation (interleukins), cholesterol metabolism (cholesterol, apolipoprotein E and homocysteine) and oxidative stress (antioxidants and lipid peroxides) have been studied as potential biomarkers for the disease. Although serum and plasma levels of these biomarkers are altered in AD relative to nondemented, age-matched individuals, they do not offer sufficient discriminatory power to allow reliable diagnosis [101].
Biomarker detection techniques that do not rely on ELISA and/or detect specific neurotoxic Aβ assemblies may offer increased sensitivity and specificity. Using fluorescence correlation spectroscopy, soluble Aβ was detected in the the CSF of AD patients by allowing it to react with fluorescently labeled, synthetic Aβ42. This method allowed researchers to distinguish between AD patients and patients with other neurological disorders, including epilepsy, multi-infarct dementia and cerebral infarction [102]. Recently, using a ‘bio-barcode’ assay, ADDLs were detected with femtomolar sensitivity in the CSF of AD patients but not in age-matched, healthy individuals [103]. The bio-barcode assay is based on ADDL recognition with conformation-specific antibodies linked to oligonucleotide-modified nanoparticles. Several hundred copies of the bio-barcode DNA are released from the nanoparticle and amplified to provide a highly sensitive signal for antigen identification. A similar method based on DNA coding and amplification has recently been used to detect Aβ protofibrils with picomolar sensitivity [104]. These methods rely on the specificity of antibodies to particular Aβ assemblies and are not free from technical issues [105]. Nevertheless, they offer significant advantages over current methods by offering sensitive detection of particular Aβ oligomers. Aβ oligomers are more specific biomarkers for AD than total Aβ levels in the CSF and, therefore, may allow for the differentiation between normal aging, MCI and AD with high levels of accuracy.
Treatment of AD
Approved treatments
Current therapy for AD is largely restricted to symptomatic treatments. Three drugs are considered standard AD therapy, including donepezil (Aricept®, Eisei Co), rivastigmine (Exelon®, Novartis Pharmaceuticals Co.) and galantamine (Razadyne®, Jansen-Cilag Ltd). These are AChEIs. Their use in AD is based on early observations that the disease causes a decrease in acetylcholine levels. These drugs provide moderate and temporary relief in patients with mild-to-moderate AD [13]. The fourth common therapy is the noncompetitive NMDA receptor antagonist memantine (Namenda®, Forrest Laboratories). Memantine was shown to delay cognitive deterioration in patients with moderate-to-severe AD [106]. Its mechanism of action is thought to involve protection of hippocampal neurons from glutamatergic excitotoxicity. Similar to AChEIs, memantine targets the symptoms rather than the cause of AD, thus its effect is moderate and temporary.
A fifth compound commonly used for the treatment of AD is vitamin E. An antioxidant, vitamin E targets AD-associated oxidative stress in the brain. Although vitamin E did not show an improvement of cognitive abilities in patients with AD, it was found to delay the progression of the disease in subjects with fully developed AD compared with placebo [13].
In clinical practice, patients with incipient AD are treated with the therapeutic strategies outlined above. Several clinical trials designed to elicit the effects of AD-relevant drugs on patients with amnestic MCI have recently ended [107]. The AChEIs donepezil and galantamine were found to slightly lower the annual conversion rate from MCI to AD compared with placebo groups. In comparison, vitamin E showed no effect on progression from amnestic MCI to AD. Trials investigating the benefits of the nonsteroidal COX-2 inhibitor rofecoxib and the nootrop piracetam did not show slowing of cognitive decline in patients with mild-to-moderate AD. Currently, methodological heterogeneity in trial design does not allow for meaningful comparisons among trials. Larger, standardized trials and more precise markers for the progression from MCI to AD are required to evaluate the benefits of potential MCI treatments reliably [107].
New therapeutic strategies
If the initial events that precipitate the neuropathological processes in AD, presumably through disruption of synaptic transmission and neuronal injury, are related to accumulation of Aβ in the brain, any efficacious therapy against AD must target these events. Thus, strategies for treating the cause, rather than the symptoms, of AD have focused on decreasing Aβ production, enhancing Aβ clearance or inhibiting Aβ self-association. Effective treatment using any of these approaches will be the ultimate test of the amyloid cascade hypothesis.
Inhibition of Aβ production
Inhibition of β- and/or γ-secretases is a reasonable strategy for decreasing Aβ monomer levels in order to avoid self-assembly and thereby prevent the toxicity of Aβ oligomers and polymers. The process of inhibitor discovery for β- and γ-secretases has been facilitated by identification of the proteins responsible for these enzymatic activities. The membrane-anchored aspartic protease, BACE-1 (β-site APP cleaving enzyme 1) is the predominant β-secretase [108]. Some β-secretase activity has also been assigned to the cysteine protease, cathepsin B [109]. Both of these enzymes are targets for the development of inhibitors as a means to block Aβ production [110]. A major breakthrough that has facilitated drug discovery for β-secretase was obtaining a high-resolution crystal structure of the BACE-1 protease domain complexed with a peptide inhibitor. The structure revealed that the active site of BACE-1 was unusually large and polar. These characteristics increase the difficulty in obtaining potent BACE-1 inhibitors. Most of the inhibitors discovered thus far are relatively large peptides, which do not cross the blood–brain barrier and are metabolically unstable [111].
γ-secretase is a complex of integral membrane proteins, including presenilin (1 or 2), nicastrin, Aph1, Pen2 and, possibly, TMP21 [112]. The catalytic subunit of the γ-secretase complex is an aspartyl protease function located within presenilin. The particular role of each of the other γ-secretase components is the subject of active research. The complex composition of γ-secretase and the fact that all of its components are membrane-bound proteins make obtaining high-resolution structures of γ-secretase difficult. Despite these difficulties, several potent inhibitors have been discovered, both by academic laboratories and pharmaceutical companies [113].
Major challenges in obtaining inhibitors for either β- or γ-secretase that would be useful in vivo include selectivity problems and multiple physiologically important substrates other than APP. In addition, although studies of knockout mice suggested that APP was not an essential protein [114], all the cleavage products of APP, including the large soluble N-terminus, Aβ and the C-terminus, have been shown to have physiological roles [28]. Thus, inhibition of β- or γ-secretase may interfere with physiological processes and cause significant adverse side effects [110,115]. Approaches addressing this issue include redirecting the search from inhibitors of the active sites of the β- or γ-secretases to compounds that reduce Aβ production by specifically binding to APP [116] or modulating secretase activity without blocking the active site. R-flurbiprofen (Flurizan™, Myriad Pharmaceuticals, Inc.) reduces Aβ42 levels specifically by modulating γ-secretase activity without binding to the active site. The drug was well tolerated in a Phase II clinical trial in patients with mild-to-moderate AD and showed retardation of cognitive decline in a subgroup of patients with mild dementia [117]. Another route to reducing Aβ production is by enhancing α-secretase-mediated, nonamyloidogenic processing of APP (FIGURE 1). Recently, Caccamo and colleagues have shown that activation of a disintegrin and metalloproteinase (ADAM)-17, a putative α-secretase, using a selective M1 muscarinic acetylcholine receptor agonist, decreased Aβ-related pathology in a mouse model transgenic for APP, presenilin-1 and tau, demonstrating the feasibility of this approach [118].
Enhancement of Aβ clearance
Following pioneer studies by Solomon and coworkers demonstrating that monoclonal antibodies could suppress Aβ aggregation and inhibit Aβ neurotoxicity in cultured cells [119], studies by Schenk and colleagues showed that active immunization with pre-aggregated Aβ42 reduced AD-like pathology and reversed cognitive deficits in transgenic mice [120]. Despite these encouraging results, a Phase II clinical trial of immunization therapy using aggregated Aβ42 (AN-1792; Elan Pharmaceuticals) was halted because 18 out of 372 patients participating in the trial developed severe meningoencephalitis. Postmortem analysis of two deceased patients from this trial indicates that senile plaque densities were notably reduced in many areas of the neocortex, suggesting that the induced immune response was effective in clearing Aβ deposits [121]. In follow-up studies, a subgroup of patients with an Aβ42-specific immune response showed improvement in cognitive and functional tests compared with the placebo groups, suggesting that immunotherapy may be a useful approach for the treatment of AD [122].
Although the exact cause of the meningoencephalitis that occurred in some of the patients is unknown, it is suggested that patients may have developed an autoimmune response to the immunogen. Synthetic, aggregated, full-length human Aβ42 was used in the AN-1792 trial together with QS21, a T-helper (Th)-1 response-inducing adjuvant [123]. In order to reduce the risk of a T-cell-mediated immune response, novel Aβ peptide immunogens and adjuvants are currently being tested. In humans, T cells were shown to recognize an epitope in the region Aβ (16–33), whereas the majority of antibodies generated in mice, monkeys and humans recognized epitopes in the N-terminal region Aβ (1–16) [124]. Vaccination with N-terminal Aβ fragments may lead to a predominantly humoral response, thereby avoiding the T-cell-associated reactions, which are believed to have caused meningoencephalitis in the AN-1972 trial. In addition, new strategies for the development of safe AD immunotherapy are aimed at downregulating the proinflammatory Th1 response through the optimization of the adjuvant formulation. Current research efforts aim at development of Th2-specific adjuvants that promote humoral responses and limit Th1-type cellular immunogenicity [125].
Regulation of Aβ levels in the brain also may be achieved by increasing Aβ proteolysis. Three enzymes have been shown to mediate the majority of Aβ degradation, insulin degrading enzyme (IDE), angiotensin-converting enzyme and neprilysin (NEP) [126]. NEP, a membrane-bound zinc metallopeptidase, is considered the primary enzyme for in vivo degradation of Aβ [127]. Recently, somatostatin, which acts via a G-protein-coupled receptor, has been identified as a modulator that increases brain NEP activity, resulting in a decrease in Aβ levels [127]. Thus, it may be possible to pharmacologically control brain Aβ levels with somatostatin receptor agonists.
Inhibition of Aβ assembly
Inhibition of Aβ assembly targets a purely pathological process and therefore is not prone to risks of side effects arising from interference with the potential positive physiological roles of Aβ. However, relative to development of enzyme active-site inhibitors, inhibition of protein–protein interactions is a more difficult task due to the higher complexity of the interaction. In the case of Aβ, major efforts have been dedicated to development of fibrillogenesis inhibitors [128]. Since Aβ aggregation is essentially a homotypic protein–protein interaction reaction, rational design strategies used Aβ fragments known to be essential for these interactions as inhibitors. Several strategies to extend the function of Aβ fragment-derived inhibitors have been developed, including attaching several Lys residues acting as a ‘disrupting element’ [129] and incorporation of N-methylated amino acids [130]. The latter inhibitors bind to growth sites of Aβ assemblies and prevent the propagation of β-sheets by disrupting the hydrogen bonds that mediate β-sheet formation [131]. N-methylated peptide inhibitors show improved solubility and metabolic stability relative to nonmethylated peptides, suggesting that they may be effective in vivo [130].
With the recent understanding that Aβ oligomers, rather than fibrils, are likely the most important effectors of neurotoxicity in AD, the direction of inhibitor design efforts has begun to shift towards inhibition of oligomer formation. To our knowledge, five studies have reported inhibitors of Aβ oligomerization to date. Three of these studies used oligomer-specific antibodies to evaluate oligomerization inhibition by unrelated molecules including curcumin [132], monocyclic phenol derivatives [133] and cyclodextrin derivatives [134]. Reliability of these data depends on the specificity of the antibodies used for oligomeric Aβ. If the antibodies cross-react with monomeric or fibrillar Aβ, the results are not specific for inhibition of oligomerization. Avoiding these potential pitfalls, Walsh and colleagues reported a decrease in formation of neurotoxic Aβ oligomers, with no change in production of Aβ monomer, in conditioned cell-culture medium following incubation with hydroxyanaline derivatives, suggesting that these compounds interact directly with Aβ and inhibit its oligomerization [135]. Recently, certain cyclohexanehexol stereoisomers have been reported to interfere with Aβ assembly, decrease brain levels of Aβ and plaques, and reverse cognitive impairment in an AD mouse model [136].
Another approach to inhibit Aβ assembly is based on the observation that metal ions, such as copper and zinc, facilitate Aβ assembly [137,138]. Metal-chelating molecules have been shown to block fibril formation in vitro and inhibit Aβ accumulation in vivo. Consequently, the metal chelator clioquinol was shown to stabilize cognitive abilities of patients in a clinical trial compared with a placebo group and to reduce the levels of plasma Aβ42 [139], suggesting that clioquinol and other chelators may hold promise as therapeutic agents for AD.
Conlusions
Developed countries in today’s world can also be regarded as aged countries [140]. For the people and the governments of these countries, AD is a major threat and a tremendous economic burden. For developing (aging) countries, the same problems await as their economies improve and life expectancy increases.
Recent studies on the molecular basis of AD have increased our understanding of the neurotoxic species that injure and kill the neurons responsible for memory and learning. However, major questions remain unanswered, including how these Aβ species affect the neurons and what mechanisms initiate the neurotoxic processes that give rise to the complex pathology in the AD brain. Answers to these basic questions are now needed to improve currently available diagnostic and therapeutic methods.
Five-year view
The success of therapeutic interventions depends on early detection of AD and MCI. The early aspect cannot be overemphasized, particularly as the beginning of neurodegeneration may precede detection of cognitive deterioration by more than a decade. To date, the gold standard for the diagnosis of AD is the pathological examination of the brain. In day-today clinical practice, the diagnosis is based largely on neuropsychological testing. Brain imaging provides supportive evidence. Structurally, either CT or MRI can be used to exclude alternative etiologies. It is expected that in the next 5 years, functional neuroimaging (fMRI, PET or SPECT) will become increasingly routine tests for clinical diagnosis. Novel technologies, including methods for in vivo visualization of amyloid plaques and NFTs, and high-sensitivity detection of CSF biomarkers, are promising directions for improved early diagnosis. Focus on translational research in the near future, likely will bring the first applications of these techniques from the bench to the clinic.
Most current mechanism-based therapeutic efforts for AD are directed at inhibition of Aβ production, oligomerization and aggregation, and enhancement of Aβ clearance and degradation. Given the complexity of the disease process, it is unlikely that one ‘magic bullet’ will be the ultimate answer. Rather, in the next few years, a combination of therapeutic strategies tailored specifically for individual patients is likely to emerge. The large number of studies currently being performed at different levels, including basic science experiments, translational research and clinical trials, and the diversity of the strategies pursued, raise hopes that sensitive early diagnosis and effective therapy will become available in the near future.
Expert commentary
MCI is an intermediate cognitive state between normal aging and dementia that conveys a high risk for Alzheimer’s dementia. MCI can be reliably diagnosed following detailed clinical examination, neuropsychological testing and a structural brain scan. Not all MCI patients will develop dementia. A prediction model composed of selected cognitive and behavioral tests and questionnaires, brain imaging, and specific CSF biomarkers may be needed to predict AD pathology in MCI patients with high accuracy. Currently, there is no US FDA-approved therapy for MCI. Until mechanism-based drugs for AD become available, AD patients should be treated with AChEIs and/or memantine.
Key issues
Currently, approved therapies for Alzheimer’s disease (AD) (acetylcholinesterase inhibitors and a N-methyl-d-aspartic acid [NMDA] receptor antagonist) treat symptoms rather than the cause of AD, and provide only moderate and temporary relief.
New insights into the molecular mechanisms underlying AD pathophysiology posit Aβ oligomerization as a key causative event in the etiology of AD.
A number of new drug discovery approaches are based on the increased understanding of AD pathophysiology. Novel, mechanism-based drugs targeting Aβ production, oligomerization or aggregation, or enhancement of Aβ clearance and degradation, are currently being developed.
Effective treatment of AD requires diagnosis at an early stage, often characterized as amnestic mild cognitive impairment (MCI). Development of rigorous diagnostic criteria is essential, yet highly challenging, due to the heterogeneity of MCI and AD.
In current clinical practice, diagnosis of MCI and AD largely is based on testing of cognitive abilities. Recent advances in brain imaging techniques and specific biomarker detection hold promise for earlier and more accurate diagnosis.
Acknowledgements
Space limitations and journal policies regarding the number of references allowed preclude citation of all the significant contributions of our colleagues. We acknowledge these contributions here. We thank Drs Sylvain Lesné and Karen H Ashe for making FIGURE 2B available to this review. We thank Drs Jeffrey Cummings, Noel Lazo, David Teplow and Paul Thompson for critical reading of the manuscript. This work was supported by American Federation for Aging Research (AFAR) (NY, USA) grant A04084 (GB), Larry L Hillblom Foundation grant 20052E (GB), Turken Family Award (GB), NIA K23 award AG026803 (jointly sponsored by NIA, AFAR, The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation and an anonymous donor) (LGA), The Kassel Pakinson’s Disease Foundation (LGA) and NIA P50 grant AG16570 (LGA).
Contributor Information
Bernhard H Monien, Department of Neurology, David Geffen School of Medicine, University of California at Los Angeles, Neuroscience Research Building 1, Room 455, 635 Charles E. Young Drive South, Los Angeles, CA 90095-7334, USA, Tel.: +1 310 206 2300, Fax: +1 310 206 1700, monien@ucla.edu.
Liana G Apostolova, Tichi Wilkerson-Kassel Dementia Scholar, UCLA Alzheimer’s Disease Center, 10911 Weyburn Ave., 2nd Floor, Los Angeles, CA 90095-7226, USA, Tel.: +1 310 794 2551, Fax: +1 310 794 3148, lapostolova@mednet.ucla.edu.
Gal Bitan, Department of Neurology, David Geffen School of Medicine, University of California at Los Angeles, Neuroscience Research Building 1, Room 451, 635 Charles E. Young Drive South, Los Angeles, CA 90095-7334, USA, Tel.: +1 310 206 2082, Fax: +1 310 206 1700, gbitan@mednet.ucla.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Hoyert DL, Kung HC, Smith BL. Deaths preliminary data for 2003. Natl Vital Stat. Rep. 2005;53(15):1–48. [PubMed] [Google Scholar]
- 2.Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science. 1987;235:1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer A. Über einen eigenartigen schweren Erkrankungsprozeβ der Hirnrinde. Neurologisches Centralblatt. 1906;23:1129–1136. [Google Scholar]
- 4.Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid β-peptides and APO E in Down’s syndrome: implications for initial events in amyloid plaque formation. Neurobiol. Dis. 1996;3(1):16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 5.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 6.Morishima-Kawashima M, Hara Y. Alzheimer’s disease: β-amyloid protein and tau. J. Neurosci. Res. 2002;70(3):392–401. doi: 10.1002/jnr.10355. [DOI] [PubMed] [Google Scholar]
- 7.Kowalska A. Genetic basis of neurodegeneration in familial Alzheimer’s disease. Pol. J. Pharmacol. 2004;56(2):171–178. [PubMed] [Google Scholar]
- 8. Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. • First formulation of the amyloid cascade hypothesis.
- 9.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 10.Klein WL, Stine WB, Jr, Teplow DB. Small assemblies of unmodified amyloid β-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol. Aging. 2004;25(5):569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-β oligomers: their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 2002;30(4):552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- 12. Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. doi: 10.1212/wnl.56.9.1143. •• American Academy of Neurology (AAN) practice guidelines for the diagnoses of Alzheimer’s disease (AD), dementia with Lewy bodies, fronto-temporal dementia, vascular dementia and Creuzfeldt–Jacob’s disease.
- 13.Cummings JL. Alzheimer’s disease. N. Engl. J. Med. 2004;351(1):56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 14.Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer’s disease. Nat. Med. 2004;10 Suppl.:S34–S41. doi: 10.1038/nrn1433. [DOI] [PubMed] [Google Scholar]
- 15. Kawas CH, Corrada MM, Brookmeyer R, et al. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60(7):1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. • Earliest detection of AD predictors.
- 16.Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch. Neurol. 2001;58(9):1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 17.Feldman HH, Jacova C. Mild cognitive impairment. Am. J. Geriatr. Psychiatry. 2005;13(8):645–655. doi: 10.1176/appi.ajgp.13.8.645. [DOI] [PubMed] [Google Scholar]
- 18. Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ342 oligomerize through distinct pathways. Proc. Natl Acad. Sci. USA. 2003;100(1):330–335. doi: 10.1073/pnas.222681699. • Structural explanation of the biological differences between Aβ40 and Aβ42.
- 19.Riek R, Guntert R, Döbeli H, Wipf B, Wüthrich K. NMR studies in aqueous solution fail to identify significant conformational differences between the monomeric forms of two Alzheimer peptides with widely different plaque-competence, Aβ(1–40)OX and Aβ(1–2)OX. Eur. J. Biochem. 2001;268(22):5930–5936. doi: 10.1046/j.0014-2956.2001.02537.x. [DOI] [PubMed] [Google Scholar]
- 20.Hou L, Shao H, Zhang Y, et al. Solution NMR studies of the Aβ(1–40) and Aβ(1–42) peptides establish that the Met35 oxidation state affects the mechanism of amyloid formation. J. Am. Chem. Soc. 2004;126(7):1992–2005. doi: 10.1021/ja036813f. [DOI] [PubMed] [Google Scholar]
- 21.Lazo ND, Grant MA, Condron MM, Rigby AC, Teplow DB. On the nucleation of amyloid β–protein monomer folding. Protein Sci. 2005;14:1581–1596. doi: 10.1110/ps.041292205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun XD, Mo ZL, Taylor BM, Epps DE. A slowly formed transient conformer of Aβ(1–40) is toxic to inward channels of dissociated hippocampal and cortical neurons of rats. Neurobiol. Dis. 2003;14(3):567–578. doi: 10.1016/j.nbd.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 23. Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. • Demonstration of neurotoxicity of Aβ dimers and trimers in vivo.
- 24.Bitan G, Lomakin A, Teplow DB. Amyloid β-protein oligomerization: prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. J. Biol. Chem. 2001;276(37):35176–35184. doi: 10.1074/jbc.M102223200. [DOI] [PubMed] [Google Scholar]
- 25.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxin. Proc. Natl Acad. Sci. USA. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gong Y, Chang L, Viola KL, et al. Alzheimer’s disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl Acad. Sci. USA. 2003;100(18):10417–10422. doi: 10.1073/pnas.1834302100. •• Demonstration of the presence of neurotoxic Aβ oligomers in the brains of AD patients.
- 27.Barghorn S, Nimmrich V, Striebinger A, et al. Globular amyloid β-peptide oligomer – a homogenous and stable neuropathological protein in Alzheimer’s disease. J. Neurochem. 2005;95(3):834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- 28.Lazo ND, Maji SK, Fradinger EA, Bitan G, Teplow DB. The amyloid β-protein. In: Sipe JD, editor. Amyloid Proteins - The β-Sheet Conformation and Disease. Germany: Wiley-VCH, Weinheim; 2005. pp. 385–448. [Google Scholar]
- 29.Walsh DM, Hartley DM, Kusumoto Y, et al. Amyloid β-protein fibrillogenesis – structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 1999;274(36):25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 30.Serpell LC. Alzheimer’s amyloid fibrils: structure and assembly. Biochim. Biophys. Acta. 2000;1502(1):16–30. doi: 10.1016/s0925-4439(00)00029-6. [DOI] [PubMed] [Google Scholar]
- 31. Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science. 2005;307(5707):262–265. doi: 10.1126/science.1105850. • First experiment-based, high-resolution structure of Aβ fibrils.
- 32.Anderson M, Bocharova OV, Makarava N, Breydo L, Salnikov VV, Baskakov IV. Polymorphism and ultrastructural organization of prion protein amyloid fibrils: an insight from high resolution atomic force microscopy. J. Mol. Biol. 2006;358(2):580–596. doi: 10.1016/j.jmb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P3011 tau transgenic mice induced by Aβ42 fibrils. Science. 2001;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 34.Lewis J, Dickson DW, Lin WL, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 35.Oddo S, Caccamo A, Tran L, et al. Temporal profile of amyloid-β (Aβ) oligomerization in an in vivo model of Alzheimer disease. A link between Aβ and tau pathology. J. Biol. Chem. 2006;281(3):1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- 36.Maeda S, Sahara N, Saito Y, Murayama S, Ikai A, Takashima A. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer’s disease. Neurosci. Res. 2006;54(3):197–201. doi: 10.1016/j.neures.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 37.McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol. Aging. 2001;22(6):799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 38.Tuppo EE, Forman LJ. Free radical oxidative damage and Alzheimer’s disease. J. Am. Osteopath. Assoc. 2001;101(Pt 1) Suppl. 12:S11–S15. [PubMed] [Google Scholar]
- 39.Takuma K, Yan SS, Stern DM, Yamada K. Mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis in Alzheimer’s disease. J. Pharmacol. Sci. 2005;97(3):312–316. doi: 10.1254/jphs.cpj04006x. [DOI] [PubMed] [Google Scholar]
- 40.Barger SW. An unconventional hypothesis of oxidation in alzheimer’s disease: intersections with excitotoxicity. Front. Biosci. 2004;9:3286–3295. doi: 10.2741/1481. [DOI] [PubMed] [Google Scholar]
- 41.Glabe CC. Amyloid accumulation and pathogensis of Alzheimer’s disease: significance of monomeric, oligomeric and fibrillar Aβ. Subcell. Biochem. 2005;38:167–177. doi: 10.1007/0-387-23226-5_8. [DOI] [PubMed] [Google Scholar]
- 42.Perry G, Nunomura A, Hirai K, et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic. Biol. Med. 2002;33(11):1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 43. Kayed R, Head E, Thompson JL, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300(5618):486–489. doi: 10.1126/science.1079469. • Demonstration of the presence of oligomers in AD brains and not in healthy, aged-matched control brains.
- 44.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 45.Ye CP, Selkoe DJ, Hartley DM. Protofibrils of amyloid β-protein inhibit specific K+ currents in neocortical cultures. Neurobiol. Dis. 2003;13(3):177–190. doi: 10.1016/s0969-9961(03)00068-8. [DOI] [PubMed] [Google Scholar]
- 46.Ye C, Walsh DM, Selkoe DJ, Hartley DM. Amyloid β-protein induced electrophysiological changes are dependent on aggregation state: N-methyl-D-aspartate (NMDA) versus non-NMDA receptor/channel activation. Neurosci. Lett. 2004;366(3):320–325. doi: 10.1016/j.neulet.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 47. Nilsberth C, Westlind-Danielsson A, Eckman CB, et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat. Neurosci. 2001;4(9):887–893. doi: 10.1038/nn0901-887. •• Evidence for the causative role of Aβ protofibrils in AD.
- 48.Haass C, Steiner H. Protofibrils, the unifying toxic molecule of neurodegenerative disorders? Nat. Neurosci. 2001;4(9):859–860. doi: 10.1038/nn0901-859. [DOI] [PubMed] [Google Scholar]
- 49.Rowan MJ, Klyubin I, Cullen WK, Anwyl R. Synaptic plasticity in animal models of early Alzheimer’s disease. Philos. Trans. R. Soc. Lond. Ser. B-Biol. Sci. 2003;358(1432):821–828. doi: 10.1098/rstb.2002.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan D. Learning and memory deficits in APP transgenic mouse models of amyloid deposition. Neurochem. Res. 2003;28(7):1029–1034. doi: 10.1023/a:1023255106106. [DOI] [PubMed] [Google Scholar]
- 51. Hsia AY, Masliah E, McConlogue L, et al. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc. Natl Acad. Sci. USA. 1999;96(6):3228–3233. doi: 10.1073/pnas.96.6.3228. •• First demonstration of plaque-independent Aβ-induced neurotoxicity in vivo.
- 52.Mucke L, Masliah E, Yu GQ, et al. High-level neuronal expression of Aβ(1–42) in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobsen JS, Wu C-C, Redwine JM, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2006;103(13):5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lesné S, Koh MT, Kotilinek L, et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–357. doi: 10.1038/nature04533. • Demonstration of temporal correlation between cognitive decline and concentration levels of Aβ oligomers in a mouse model of AD.
- 55.Wang HY, Lee DHS, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. β-amyloid(1–42) binds to α7 nicotinic acetylcholine receptor with high affinity – implications for Alzheimer’s disease pathology. J. Biol. Chem. 2000;275(8):5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 56.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 57.Kagan BL, Hirakura Y, Azimov R, Azimova R, Lin MC. The channel hypothesis of Alzheimer’s disease: current status. Peptides. 2002;23(7):1311–1315. doi: 10.1016/s0196-9781(02)00067-0. [DOI] [PubMed] [Google Scholar]
- 58.Kayed R, Sokolov Y, Edmonds B, et al. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J. Biol. Chem. 2004;279(45):46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 59.Crowther DC, Kinghorn KJ, Miranda E, et al. Intraneuronal Aβ, non-amyloid aggregates and neurodegeneration in a Drosophila model of Alzheimer’s disease. Neuroscience. 2005;132(1):123–135. doi: 10.1016/j.neuroscience.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 60.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 61.Duyckaerts C, Dickson DW. Neuropathology of Alzheimer’s disease. In: Dickson DW, editor. Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. Basel, Switzerland: ISN Neuropath Press; 2003. pp. 47–65. [Google Scholar]
- 62.Mesulam MM. Alzheimer’s disease and dementia. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. Oxford, UK: Oxford University Press; 2000. pp. 439–510. [Google Scholar]
- 63.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 64.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia Arch. Neurol 2006635674–681. •• Postmortem investigation of a clinical cohort of amnestic mild cognitive impairment (MCI) patients who evolved into dementia. [DOI] [PubMed] [Google Scholar]
- 65.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J. Neuropathol. Exp. Neurol. 2003;62(11):1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 66.Arendt T. Neurodegeneration and plasticity. Int. J. Dev. Neurosci. 2004;22(7):507–514. doi: 10.1016/j.ijdevneu.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Giannakopoulos P, Hof PR, Bouras C. Selective vulnerability of neocortical association areas in Alzheimer’s disease. Microsc. Res. Tech. 1998;43(1):16–23. doi: 10.1002/(SICI)1097-0029(19981001)43:1<16::AID-JEMT3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 68.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol. Aging. 2004;25(1):5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Braak H, Del Tredici K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol. Aging. 2004;25(1):19–23. doi: 10.1016/j.neurobiolaging.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59(10):1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 71.Tervo S, Kivipelto M, Hanninen T, et al. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement. Geriatr. Cogn. 2004;17(3):196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- 72. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. •• Detailed review of the concept of MCI.
- 73.Petersen RC. Aging, mild cognitive impairment, and Alzheimer’s disease. Neurol. Clin. 2000;18(4):789–806. doi: 10.1016/s0733-8619(05)70226-7. [DOI] [PubMed] [Google Scholar]
- 74.Pasquier F. Early diagnosis of dementia: neuropsychology. J. Neurol. 1999;246(1):6–15. doi: 10.1007/s004150050299. [DOI] [PubMed] [Google Scholar]
- 75.Cummings JL. Alzheimer’s disease. In: Cummings JL, editor. The Neuropsychiatry of Alzheimer’s Disease and Related Dementias. London, UK: Martin Dunitz Ltd; 2003. pp. 57–116. [Google Scholar]
- 76.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 77.Kalbe E, Kessler J, Calabrese P, et al. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int. J. Geriatr. Psychiatry. 2004;19(2):136–143. doi: 10.1002/gps.1042. [DOI] [PubMed] [Google Scholar]
- 78.Jack CR, Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Du AT, Schuff N, Chao LL, et al. White matter lesions are associated with cortical atrophy more than entorhinal and hippocampal atrophy. Neurobiol. Aging. 2005;26(4):553–559. doi: 10.1016/j.neurobiolaging.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 80. Jack CR, Jr, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62(4):591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. •• Large neuroimaging study of cognitively normal elderly, AD and MCI subjects, investigating the role of several structural magnetic resonance imaging (MRI) measures (whole brain, hippocampus gray matter) for surrogate markers of disease progression.
- 81.Apostolova LG, Dutton RA, Dinov ID, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch. Neurol. 2006;63:693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 82.Thompson PM, Hayashi KM, De Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Nemoimage. 2004;22(4):1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 83. Apostolova LG, Dinov ID, Dutton RA, et al. 3D comparison of hippocampal atrophy in mild cognitive impairment and Alzheimer’s disease. Neurology. 2006;66 5 Suppl. 2 5:A60. doi: 10.1093/brain/awl274. • Imaging study describes the hippocampal differences between amnestic MCI and AD subjects and confirms that subregional hippocampal atrophy in AD is stage specific.
- 84.Du AT, Schuff N, Kramer JH, et al. Higher atrophy rate of entorhinal cortex than hippocampus in AD. Neurology. 2004;62(3):422–427. doi: 10.1212/01.wnl.0000106462.72282.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.deToledo-Morrell L, Stoub TR, Bulgakova M, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol. Aging. 2004;25(9):1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 86. Karas GB, Scheltens P, Rombouts SA, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2004;23(2):708–716. doi: 10.1016/j.neuroimage.2004.07.006. • Cross-sectional study comparing the cortical atrophy pattern between normal elderly, MCI and AD subjects using the voxel-based morphometry technique.
- 87.Thompson PM, Hayashi KM, de Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer’s disease. J. Neurosci. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Apostolova LG, Steiner CA, Akopyan GG, Toga AW, Cummings JL, Thompson PM. Gray matter atrophy in mild cognitive impairment and mild Alzheimer’s disease. Neurology. 2006;66(5) doi: 10.1001/archneur.64.10.1489. Suppl. 2(5) • Cross-sectional study reports striking differences between amnestic MCI and mild AD subjects using an advanced computational anatomy technique.
- 89.Kantarci K, Reynolds G, Petersen RC, et al. Proton MR spectroscopy in mild cognitive impairment and Alzheimer disease: comparison of 1.5 and 3 T. AJNR Am. J. Neuroradiol. 2003;24(5):843–849. [PMC free article] [PubMed] [Google Scholar]
- 90.Kantarci K, Petersen RC, Boeve BF, et al. DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2005;64(5):902–904. doi: 10.1212/01.WNL.0000153076.46126.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rombouts SA, Barkhof F, Veltman DJ, et al. Functional MR imaging in Alzheimer’s disease during memory encoding. AJNR Am. J. Neuroradiol. 2000;21(10):1869–1875. [PMC free article] [PubMed] [Google Scholar]
- 92. Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2003;74(1):44–50. doi: 10.1136/jnnp.74.1.44. • Functional MRI study reporting that hippocampal, parietal and posterior cingulate hyperactivation could reliably differentiate mild AD patients from elderly controls.
- 93.Dickerson BC, Salat DH, Bates JF, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 2004;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging. 2005;32(4):486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 95.Silverman DH, Truong CT, Kim SK, et al. Prognostic value of regional cerebral metabolism in patients undergoing dementia evaluation: comparison to a quantifying parameter of subsequent cognitive performance and to prognostic assessment without PET. Mol. Genet. Metab. 2003;80(3):350–355. doi: 10.1016/S1096-7192(03)00139-2. [DOI] [PubMed] [Google Scholar]
- 96.Nordberg A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol. 2004;3(9):519–527. doi: 10.1016/S1474-4422(04)00853-1. [DOI] [PubMed] [Google Scholar]
- 97.Andreasen N, Blennow K. CSF biomarkers for mild cognitive impairment and early Alzheimer’s disease. Clin. Neurol. Neurosurg. 2005;107(3):165–173. doi: 10.1016/j.clineuro.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 98.Strozyk D, Blennow K, White LR, Launer LJ. CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60(4):652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- 99.Blennow K. CSF biomarkers for mild cognitive impairment. J. Intern. Med. 2004;256(3):224–234. doi: 10.1111/j.1365-2796.2004.01368.x. [DOI] [PubMed] [Google Scholar]
- 100.Hampel H, Teipel SJ, Fuchsberger T, et al. Value of CSF β-amyloid1–42 and tau as predictors of Alzheimer’s disease in patients with mild cognitive impairment. Mol. Psychiatry. 2004;9(7):705–710. doi: 10.1038/sj.mp.4001473. [DOI] [PubMed] [Google Scholar]
- 101.Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226–234. doi: 10.1602/neurorx.1.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pitschke M, Prior R, Haupt M, Riesner D. Detection of single amyloid β-protein aggregates in the cerebrospinal fluid of Alzheimer’s patients by fluorescence correlation spectroscopy. Nat. Med. 1998;4(7):832–834. doi: 10.1038/nm0798-832. • First demonstration of differences in oligomer concentration levels between AD patients and age-matched healthy individuals.
- 103.Georganopoulou DG, Chang L, Nam JM, et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2005;102(7):2273–2276. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kamali-Moghaddam M, Englund H, Schallmeiner E, et al. Proximity ligation-based detection of biomarkers of protein folding disorders. Cambridge Healthtech Institute’s 10th Annual Transmissible Spongiform Encepalopathies Meeting; Baltimore, MD, USA. 2006. [Google Scholar]
- 105.Fradinger EA, Bitan G. En route to early diagnosis of Alzheimer’s disease – are we there yet? Trends Biotechnol. 2005;23(11):531–533. doi: 10.1016/j.tibtech.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 106.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 107.Jelic V, Kivipelto M, Winblad B. Clinical trials in mild cognitive impairment: lessons for the future. J. Neurol. Neurosurg. Psychiatry. 2006;77(4):429–438. doi: 10.1136/jnnp.2005.072926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vassar R. BACE1: the β-secretase enzyme in Alzheimer’s disease. J. Mol. Neurosci. 2004;23(1–2):105–114. doi: 10.1385/JMN:23:1-2:105. [DOI] [PubMed] [Google Scholar]
- 109.Hook V, Toneff T, Bogyo M, et al. Inhibition of cathepsin B reduces β-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: evidence for cathepsin B as a candidate β-secretase of Alzheimer’s disease. Biol. Chem. 2005;386(9):931–940. doi: 10.1515/BC.2005.108. [DOI] [PubMed] [Google Scholar]
- 110.Schmidt B, Baumann S, Braun HA, Larbig G. Inhibitors and modulators of β- and γ-secretase. Curr. Top. Med. Chem. 2006;6(4):377–392. doi: 10.2174/156802606776287027. [DOI] [PubMed] [Google Scholar]
- 111.Citron M. β-secretase inhibition for the treatment of Alzheimer’s disease – promise and challenge. Trends Pharmacol. Sci. 2004;25(2):92–97. doi: 10.1016/j.tips.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 112.Chen F, Hasegawa H, Schmitt-Ulms G, et al. TMP21 is a presenilin complex component that modulates γ-secretase but not ε-secretase activity. Nature. 2006;440(7088):1208–1212. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- 113.Churcher I, Beher D. γ-secretase as a therapeutic target for the treatment of Alzheimer’s disease. Curr. Pharm. Des. 2005;11(26):3363–3382. doi: 10.2174/138161205774370771. [DOI] [PubMed] [Google Scholar]
- 114.Spires TL, Hyman BT. Transgenic models of Alzheimer’s disease: learning from animals. NeuroRx. 2005;2(3):423–437. doi: 10.1602/neurorx.2.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tomita T, Iwatsubo T. γ-secretase as a therapeutic target for treatment of Alzheimer’s disease. Curr. Pharm. Des. 2006;12(6):661–670. doi: 10.2174/138161206775474206. [DOI] [PubMed] [Google Scholar]
- 116.Espeseth AS, Xu M, Huang Q, et al. Compounds that bind APP and inhibit Aβ processing in vitro suggest a novel approach to Alzheimer disease therapeutics. J. Biol. Chem. 2005;280(18):17792–17797. doi: 10.1074/jbc.M414331200. [DOI] [PubMed] [Google Scholar]
- 117.Wilcock GK, Black S, Haworth J, et al. A placebo-controlled, double-blind trial of the selective Aβ-42 lowering agent, flurizan (MPC-7869, (R)-flurbiprofen) in patients with mild to moderate Alzheimer’s disease. Alzheimer’s Association International Conference on Prevention of Dementia; Washington, DC, USA. 2005. [Google Scholar]
- 118.Caccamo A, Oddo S, Billings LM, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49(5):671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 119. Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proc. Natl Acad. Sci. USA. 1997;94(8):4109–4112. doi: 10.1073/pnas.94.8.4109. • Disaggregation of Aβ by antibodies in vitro.
- 120. Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. doi: 10.1038/22124. •• Demonstration that antibody-mediated disassembly of Aβ aggregates in vivo leads to reversal of cognitive decline in a mouse model of AD.
- 121.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat. Med. 2003;9(4):448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 122.Gilman S, Koller M, Black RS, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64(9):1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 123.Cribbs DH, Ghochikyan A, Vasilevko V, et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with β-amyloid. Int. Immunol. 2003;15(4):505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lemere CA, Maier M, Jiang L, Peng Y, Seabrook TJ. Amyloid-β immunotherapy for the prevention and treatment of Alzheimer disease: lessons from mice, monkeys, and humans. Rejuvenation Res. 2006;9(1):77–84. doi: 10.1089/rej.2006.9.77. [DOI] [PubMed] [Google Scholar]
- 125.Ghochikyan A, Mkrtichyan M, Petrushina I, et al. Prototype Alzheimer’s disease epitope vaccine induced strong Th2-type anti-Aβ antibody response with Alum to Quil A adjuvant switch. Vaccine. 2006;24(13):2275–2282. doi: 10.1016/j.vaccine.2005.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morelli L, Bulloj A, Leal MC, Castano EM. Amyloid β degradation: a challenging task for brain peptidases. Subcell. Biochem. 2005;38:129–145. doi: 10.1007/0-387-23226-5_6. [DOI] [PubMed] [Google Scholar]
- 127.Iwata N, Higuchi M, Saido TC. Metabolism of amyloid-β peptide and Alzheimer’s disease. Pharmacol. Ther. 2005;108(2):129–148. doi: 10.1016/j.pharmthera.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 128.Soto C, Estrada L. Amyloid inhibitors and β-sheet breakers. Subcell. Biochem. 2005;38:351–364. doi: 10.1007/0-387-23226-5_18. [DOI] [PubMed] [Google Scholar]
- 129.Lowe TL, Strzelec A, Kiessling LL, Murphy RM. Structure–function relationships for inhibitors of β-amyloid toxicity containing the recognition sequence KLVFF. Biochemistry. 2001;40(26):7882–7889. doi: 10.1021/bi002734u. [DOI] [PubMed] [Google Scholar]
- 130.Gordon DJ, Sciarretta KL, Meredith SC. Inhibition of β-amyloid(40) fibrillogenesis and disassembly of β-amyloid(40) fibrils by short β-amyloid congeners containing N-methyl amino acids at alternate residues. Biochemistry. 2001;40(28):8237–8245. doi: 10.1021/bi002416v. [DOI] [PubMed] [Google Scholar]
- 131.Mason JM, Kokkoni N, Stott K, Doig AJ. Design strategies for anti-amyloid agents. Curr. Opin. Struct. Biol. 2003;13(4):526–532. doi: 10.1016/s0959-440x(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 132.Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 133.De Felice FG, Vieira MN, Saraiva LM, et al. Targeting the neurotoxic species in Alzheimer’s disease: inhibitors of Aβ oligomerization. FASEB J. 2004;18(12):1366–1372. doi: 10.1096/fj.04-1764com. [DOI] [PubMed] [Google Scholar]
- 134.Wang Z, Chang L, Klein WL, Thatcher GR, Venton DL. Per-6-substituted-per-6-deoxy β-cyclodextrins inhibit the formation of β-amyloid peptide derived soluble oligomers. J. Med. Chem. 2004;47(13):3329–3333. doi: 10.1021/jm034224e. [DOI] [PubMed] [Google Scholar]
- 135.Walsh DM, Townsend M, Podlisny MB, et al. Certain inhibitors of synthetic amyloid β-peptide (Aβ) fibrillogenesis block oligomerization of natural Aβ and thereby rescue long-term potentiation. J Neurosci. 2005;25(10):2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McLaurin J, Kierstead ME, Brown ME, et al. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat. Med. 2006;12(7):801–808. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- 137.Atwood CS, Moir RD, Huang X, et al. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J. Biol. Chem. 1998;273(21):12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- 138.Maynard CJ, Bush AI, Masters CL, Cappai R, Li QX. Metals and amyloid-β in Alzheimer’s disease. Int. J. Exp. Pathol. 2005;86(3):147–159. doi: 10.1111/j.0959-9673.2005.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ritchie CW, Bush AI, Mackinnon A, et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Aβ amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch. Neurol. 2003;60(12):1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 140.Takeda M, Tanaka T, Cacabelos R. In: Molecular Neurobiology of Alzheimer Disease and Related Disorders. Takeda M, Tanaka T, Cacabelos R, editors. Basel, Switzerland: Karger AG; 2004. pp. X–XII. [Google Scholar]