Abstract
Maintaining quinolone concentrations outside the mutant selection window (MSW) between the MIC and mutant prevention concentration (MPC) was suggested by in vitro and in vivo studies to prevent the selection of resistant mutants. However, selection also may depend on the presence of resistant bacterial mutants at the start of treatment, which is highly dependent on the initial inoculum size. In this study, a mouse thigh bacterial infection model was used to test the influence of different exposures to marbofloxacin on the selection of resistant bacteria after infection with a low (105 CFU) or high (108 CFU) initial inoculum of Escherichia coli. The inoculum size was shown to influence the exposure to marbofloxacin and the values of pharmacokinetic/pharmacodynamic indices. When the abilities of the indices time within the MSW (TMSW), area under the concentration-time curve of 0 to 24 h divided by the MIC, and the maximum concentration of drug in plasma divided by the MIC to predict the selection of resistant bacteria were compared, only TMSW appeared to be a good predictor of the prevention of resistance for values less than 30%. When the TMSW was higher than 34%, the selection of resistant bacteria occurred less often in thighs initially infected with the low inoculum (11/24; 46%) than in those infected with the high inoculum (30/36; 80%), suggesting that the selection of resistant mutants depends on both the TMSW and inoculum size. The relevance of these results merits further investigation to test different strategies of antibiotic therapy depending on the expected bacterial burden at the infectious site.
Resistances to fluoroquinolones can occur spontaneously in bacterial populations at a frequency of about 10−6 to 10−8 (5) by following a stepwise process that involves mutations in genes coding for the targets DNA gyrase and topoisomerase IV (23, 29). Consequently, if the bacterial load at the infectious site exceeds the inverse of the mutation frequency, it can be presumed that a small resistant subpopulation already coexists with a larger susceptible population before any antimicrobial treatment is administered. Traditionally, in vitro antimicrobial studies and animal infection models have been used to assess the reduction in the total bacterial population at an infectious site while often ignoring the impact of drug pressure on the amplification of the drug-resistant subpopulation (2, 8). Thus, the values of pharmacokinetic/pharmacodynamic (PK/PD) indices determined from these experiments were selected previously to predict the bacteria killing and not the selection of resistant bacteria. The PK/PD indices fAUC/MIC (area under the free-plasma concentration curve divided by the MIC), fCmax/MIC (peak free-plasma concentration divided by the MIC), and fT>MIC (the time the free concentrations are above the MIC) all are expressed as a function of the MIC, which is the pharmacodynamic parameter used to describe the susceptibility of the major drug-susceptible population. From experiments carried out with fluoroquinolones, the mutant prevention concentration (MPC) has been proposed to assess the susceptibility of the resistant subpopulation (12, 24, 30). MIC and MPC then define the boundaries of the so-called mutant selection window (MSW), which is the range of antibiotic concentrations that would favor the selection of first-step mutants (30). First-step mutants of Staphylococcus aureus and Streptococcus pneumoniae were selected by ciprofloxacin, levofloxacin, or moxifloxacin when antibiotic concentrations fell within the MSW in vitro (6, 7, 17, 31). A study carried out in a rabbit lung infection model with Streptococcus pneumoniae showed that the selection of resistant bacteria occurred systematically when concentrations of gatifloxacin were within the MSW (TMSW) for more than 45% of the treatment duration (10). Another experiment in rabbits infected by Staphylococcus aureus also showed that drug concentrations needed to be at the bottom of the MSW (just above the MIC) for only 33% of the time to enrich mutants (11). These in vivo results, which suggest that fluoroquinolone concentrations need to be outside the MSW for most of the time to prevent the selection of resistant mutants, were observed with a large inoculum size (more than 109 CFU). However, the bacterial burdens to be eradicated at the infectious site may be very low or null when antibiotics are used preventively, as in the prophylaxis of gram-negative bacteremia in immunocompromised patients (25) or metaphylaxis in veterinary medicine. For both human and veterinary medicine, prophylaxis is the administration of antimicrobials to exposed individuals who are considered at risk but before the expected onset of disease. Metaphylaxis consists of treating all animals at the herd level when there is a clinical disease in only some animals (27). In the case of prophylaxis and metaphylaxis, the bacterial inocula at the beginning of the treatment presumably are much smaller than those targeted when patients express clinical signs or are critically ill, and therefore the likelihood of a mutant appearing may be low, particularly if the bacterial load is less than the inverse of the mutation rate.
We previously observed in vitro that the emergence of resistance was more frequent when both the TMSW and the bacterial inoculum size increased (15), and the aim of this study was to test, in vivo, the ability of TMSW and also of the area under the concentration-time curve of 0 to 24 h divided by the MIC (AUC0-24/MIC) and Cmax/MIC indices to predict the selection of resistant bacteria in a low and a high inoculum. We assessed the selection of a preexisting resistant subpopulation of Escherichia coli in mouse thighs infected with the two bacterial inoculum sizes after different exposures to marbofloxacin, a fluoroquinolone extensively used in veterinary medicine.
MATERIALS AND METHODS
Bacteria and antibiotic.
Escherichia coli ATCC25922 was used as the susceptible bacteria, and Escherichia coli isolated from a thigh previously infected by E. coli ATCC25922, treated with marbofloxacin, and carrying an S83L mutation on GyrA was used as the resistant bacteria. Marbofloxacin, an expanded-spectrum quinolone, was kindly provided by Vetoquinol, Lure, France.
In vitro susceptibility testing. (i) MIC determination.
MICs were determined in triplicate for the susceptible and mutant bacteria by a broth microdilution method according to CLSI reference methods.
(ii) MPC determination.
The MPC was determined as previously described (5). Briefly, an overnight culture of the tested bacteria in Mueller-Hinton broth (MHB) was concentrated 100 times in 0.9% NaCl to obtain a suspension containing 1010 CFU/ml. One hundred microliters of this suspension then was plated on MH agar containing various concentrations of marbofloxacin obtained by successive twofold dilutions. The MPC was the lowest marbofloxacin concentration preventing the growth of bacterial colonies after incubation for 72 h at 37°C. Determinations were done in triplicate.
PCR amplification of quinolone resistance-determining regions and DNA sequencing.
Genomic DNA was isolated from bacterial strains and used as the template in the PCR amplification of the quinolone resistance-determining regions of gyrA and parC genes. PCR amplification and nucleotide sequencing were carried out with previously described primers (14).
Inoculum preparation.
A few colonies from an overnight culture of susceptible (ATCC25922) and resistant (mutant) Escherichia coli strains were grown at 37°C for 18 h in 50 ml of MHB and in 10 ml of MHB supplemented with 0.128 μg/ml marbofloxacin (the first concentration below the MIC of the resistant bacteria), respectively. Bacteria then were collected by centrifugation at 3,000 × g for 10 min and resuspended in 0.9% NaCl to obtain a final suspension containing 109 CFU/ml of susceptible bacteria and 103 CFU/ml of resistant bacteria. This suspension, which corresponded to the high inoculum, then was diluted 1,000 times to obtain the low inoculum. This method to prepare the bacterial inoculum implied that the low inoculum (6.6 ± 0.21 log10 CFU/ml) contained resistant mutants in about 40% of the experiments depending on the sampling.
Animals.
Female Swiss mice (Charles River Laboratories, L'arbresle, France) were used for all studies. All animal procedures were conducted in accordance with the accepted human standards of animal care under agreement number A 31909 for animal experimentation from the French Ministry of Agriculture.
Neutropenic mouse thigh infection model.
The mouse thigh infection model used was described previously by Andes and Craig (3). Briefly, female Swiss mice were made neutropenic by the intraperitoneal injection of cyclophosphamide (Sigma-Aldrich, Saint Quentin Fallavier, France) at rates of 150 and 100 mg/kg of body weight on days 1 and 4, respectively. On day 5, the mice were infected by intramuscular injection of 100 μl of the high or low inoculum in each thigh (six thighs per dose, dosing schedule, and inoculum size). Marbofloxacin was administered intraperitoneally beginning 2 h after infection. The total doses were 0, 2, 5, 10, and 20 mg/kg marbofloxacin for mice sacrificed at 24 h after infection and 0, 4, 10, 20, and 40 mg/kg marbofloxacin for mice sacrificed at 48 h after infection. For each dose of marbofloxacin, one group of mice received the total dose in a single administration 2 h after infection, and another group received the same total dose divided into two or four administrations for mice sacrificed 24 or 48 h, respectively, after infection (which corresponded to administration twice a day). The mice were sacrificed by an intraperitoneal injection of pentobarbital (DolethalND; Vetoquinol) 2 (noninfected mice), 24, or 48 h after infection. Both thighs were aseptically removed and homogenized in 10 ml of 0.9% NaCl. The homogenates were centrifuged at 3,000 × g for 10 min and washed twice in 10 ml of 0.9% NaCl. Ten microliters of successive 10-fold dilutions of homogenates then were plated in triplicate on MH drug-free agar plates or on MH plates supplemented with 0.128 μg/ml of marbofloxacin. The colonies were counted after overnight incubation at 37°C. If the bacterial counts were less than 1,000 CFU/thigh, 100 μl of the homogenates were plated on agar. If the colonies were too small, incubation was continued for a further 24 h. The lowest level of detection was 100 CFU/thigh, and bacteria were considered eradicated below this level.
Pharmacokinetics.
Female Swiss mice, satellites from the pharmacodynamic assay, were treated by cyclophosphamide as described above. On the fifth day, the mice were infected with either the high or low inoculum in each thigh and 2 h later were administered a single intraperitoneal dose of marbofloxacin. The doses tested were 1 and 5 mg/kg marbofloxacin for the low inoculum and 5 and 20 mg/kg marbofloxacin for the high inoculum. Groups of three or four mice each were anesthetized by the intraperitoneal injection of pentobarbital (DolethalND; Vetoquinol) 0.25, 0.5, 1, 2, 4, 6, 8, or 24 h after dosing. Blood samples (one sample from each animal) were collected by the puncture of the caudal vena cava. Protein binding was assessed by ultrafiltration as previously described (21). Briefly, plasma was spiked with 0.05, 0.1, 0.5, 1, 5, or 10 μg/ml marbofloxacin and was incubated for 30 min at 37°C. Plasma then was transferred to Centrifree devices (Millipore Corporation, Billerica, MA) and centrifuged at ambient temperature for 30 min at 1,200 × g. The volumes of the ultrafiltrates were at least 200 μl. A simple and sensitive high-performance liquid chromatography method, using UV detection at 295 nm to determine the marbofloxacin concentrations in plasma and ultrafiltrates, was adapted from Schneider et al. (28). Briefly, samples were extracted by solid-phase extraction on a C8 100-mg cartridge. Marbofloxacin and the internal standard, ofloxacin, were separated on a reverse-phase C18 Inertsil ODS-3 column and eluted with 25 mM citrate buffer (pH, 3.0) and acetonitrile in an 85:15 ratio. The standard calibration curve for marbofloxacin, using a weighted linear regression model, was linear for concentrations ranging from 0.01 to 2 μg/ml. The intraday and interday precision ranged from 4.58 to 9.27% and from 4.85 to 9.46%, respectively. The accuracy varied from 91 to 108%.
PK/PD analysis.
The data obtained with a single intraperitoneal marbofloxacin injection of 5 mg/kg were analyzed separately for each inoculum size using WinNonlin version 5.2 (Pharsight Corporation, Mountain View, CA). For the two inocula tested (low and high), a naïve pooling approach was used to fit the marbofloxacin data with a biexponential model. The AUC0-∞, AUC0-24, and AUC0-48 were calculated by integrating the equation used to fit the data from zero to infinity, from 0 to 24 h, and from 0 to 48 h, respectively (18). The terminal half-life (T1/2elim) was calculated as the naperian logarithm of 2 divided by the elimination rate constant. Dose proportionality was assessed from the kinetic data obtained with 1 mg/kg marbofloxacin for the low inoculum and with 20 mg/kg marbofloxacin for the high inoculum. Marbofloxacin pharmacokinetic parameters obtained from mice treated with 5 mg/kg then were used to estimate the TMSW, AUC0-24/MIC, and Cmax/MIC for each tested marbofloxacin dosing regimen (single and fractionated doses) and bacterial inoculum size. The AUC0-24/MIC for mice sacrificed 48 h after the infection were determined by dividing the AUC0-48/MIC by 2 to obtain a mean AUC0-24/MIC for each day.
The sigmoid inhibitory Emax models describing the dose-response relationships for the low and high inocula after 24 h of exposure to marbofloxacin were delineated with WinNonlin version 5.2 (Pharsight Corporation, Mountain View, CA).
RESULTS
Susceptibility studies.
The MICs of marbofloxacin were 0.008 and 0.256 μg/ml for the susceptible and mutant strains of Escherichia coli, respectively. The marbofloxacin MPC was 0.256 μg/ml for the susceptible bacteria and corresponded to the MIC for the mutant strain. The MSW for the susceptible bacteria therefore was the range of marbofloxacin concentrations between the MIC (0.008 μg/ml) and the MPC (0.256 μg/ml). The term resistant bacteria in the present paper should be understood as bacteria growing on 0.128 μg/ml marbofloxacin.
Pharmacokinetics.
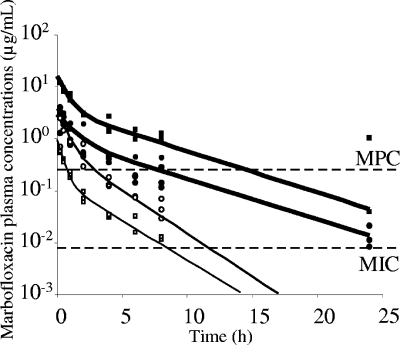
The observed (total) and the predicted (total) plasma concentrations of marbofloxacin following single intraperitoneal doses of 1 and 5 mg/kg in neutropenic mice infected with the low inoculum and doses of 5 and 20 mg/kg in neutropenic mice infected with the high inoculum are shown in Fig. 1. The values for the marbofloxacin pharmacokinetic parameters obtained for each dose are shown in Table 1. Marbofloxacin kinetics in mice differed considerably between the two inoculum sizes, the AUC0-24 (exposure) in mice subjected to the high inoculum being two times greater after the same dose of 5 mg/kg. Dose proportionality was assessed with marbofloxacin doses of 1 mg/kg for the low inoculum and 20 mg/kg for the high inoculum. The dose/AUC0-24 ratios with both doses were similar to those of mice infected with the same inoculum size and treated with 5 mg/kg marbofloxacin, suggesting dose proportionality for the range of concentrations tested. The binding of marbofloxacin to mice plasma proteins, between 0.05 and 10 μg/ml, was less than 10%, and the PK/PD indices were determined from the total plasma concentrations.
FIG. 1.
Plasma marbofloxacin concentrations versus time after the administration of a single intraperitoneal dose of 1 (□) or 5 (○) mg/kg in mice infected with a low inoculum and doses of 5 (•) and 20 (▪) mg/kg in mice infected with a high inoculum. Fitted concentration-time curves for the low (thin line) and the high (boldface line) inoculum also are represented.
TABLE 1.
Marbofloxacin pharmacokinetic parameters for mice infected with a low or high bacterial inoculuma
| Inoculum size | Dose (mg/kg) | T1/2elim (h) | AUC0-∞ (μg·h·ml−1) | Dose/AUC (ml·h−1·kg−1) |
|---|---|---|---|---|
| Low | 1 | 1.98 | 0.78 | 1,282 |
| 5 | 1.82 | 3.59 | 1,393 | |
| High | 5 | 3.91 | 7.7 | 649 |
| 20 | 3.98 | 27.9 | 715 |
The mice were treated with single intraperitoneal doses.
The TMSW, AUC0-24/MIC, and Cmax/MIC, obtained after the administration of each marbofloxacin dosing regimen to mice sacrificed 48 h after infection with the low or high inoculum, are presented in Table 2. Assuming dose proportionality, the calculated AUC0-24 values were directly proportional to the total dose of marbofloxacin for a given inoculum size and were independent of dose fractionation. The Cmaxs also were linked to the total dose, but the values were four times less after the fractionation of the doses into four administrations (twice a day for 2 days) than after the administration of single doses. The influence of the marbofloxacin dosage regimen (both total dose and fractionation) and inoculum size on the TMSW indices was much more complex and resulted in an overlap of TMSW values between the dosage regimens.
TABLE 2.
TMSW, AUC0-24/MIC, and Cmax/MIC after administration of marbofloxacin for mice sacrificed after 48 h of infection with a low or high inoculuma
| Inoculum size | Total dose (mg/kg) | Fractionated dose
|
Single dose
|
||||
|---|---|---|---|---|---|---|---|
| TMSW (%) | AUC0-24/MIC (h) | Cmax/MIC | TMSW (%) | AUC0-24/MIC (h) | Cmax/MIC | ||
| Low | 4 | 57.7 | 175 | 95 | 18.9 | 175 | 375 |
| 10 | 66.6 | 450 | 237 | 19.7 | 450 | 950 | |
| 20 | 65.4 | 900 | 475 | 19.8 | 900 | 1,900 | |
| 40 | 59.4 | 1,800 | 950 | 19.8 | 1,800 | 3,800 | |
| High | 4 | 87.4 | 387 | 75 | 42.3 | 387 | 300 |
| 10 | 63.8 | 962 | 187 | 42.5 | 962 | 750 | |
| 20 | 33.8 | 1,925 | 375 | 42.5 | 1,925 | 1,500 | |
| 40 | 10.9 | 3,850 | 750 | 42.4 | 3,850 | 3,000 | |
The values are given for each dosing regimen (dose administration was a single dose 2 h after the infection or fractionated doses twice a day). The indices were estimated from kinetic studies performed on mice subjected to a low or high inoculum and treated with 5 mg/kg marbofloxacin.
PK-PD analysis. (i) Total bacterial populations.
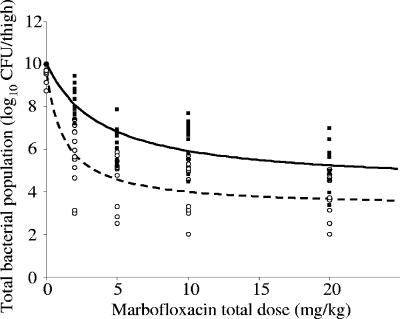
The total bacterial populations after 24 h of exposure to marbofloxacin are shown for each inoculum size and each dosing regimen in Table 3. The lowest tested dose of 2 mg/kg marbofloxacin, corresponding to AUC0-24/MIC values of 175 and 387 h for the low and high inocula, respectively, was bacteriostatic. The dose of 5 mg/kg marbofloxacin, corresponding to AUC0-24/MIC values of 450 and 962 h for the low and high inocula, respectively, reduced the bacterial population by more than one log for both inocula. Figure 2 shows that the dose-response relationship was best described by inhibitory Emax curves for the low (equation 1) and the high inocula (equation 2):
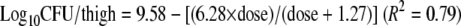 |
(1) |
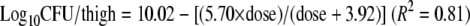 |
(2) |
TABLE 3.
Total bacterial populations after 24 h of exposure to different total doses of marbofloxacin for the initial low and high inoculaa
| Total dose (mg/kg) | Bacterial population (means ± SD) by inoculum and dose type
|
|||
|---|---|---|---|---|
| Low
|
High
|
|||
| Single | Fractionated | Single | Fractionated | |
| Startb | 6.15 ± 0.48 | 8.65 ± 0.17 | ||
| 0 | 9.52 ± 0.35* | ** | ||
| 2 | 6.17 ± 1.12 | 5.07 ± 1.62 | 8.66 ± 0.61 | 7.86 ± 0.61 |
| 5 | 4.36 ± 1.36 | 4.77 ± 1.16 | 6.86 ± 0.58 | 6.03 ± 0.77 |
| 10 | 4.31 ± 1.45 | 4.51 ± 1.07 | 6.39 ± 1.37 | 6.21 ± 0.55 |
| 20 | 4.06 ± 0.83 (1) | 3.56 ± 1.19 (2) | 5.54 ± 1.28 | 4.71 ± 0.93 |
Six thighs were tested for each inoculum, each dose, and each dosing regimen. For the start and at dose 0, 12 thighs were tested per inoculum size. Numbers in parentheses correspond to the number of thighs in which bacteria were eradicated. *, One mouse out of six was dead; **, all six mice were dead.
Total bacterial population 2 h after the time of infection.
FIG. 2.
Total bacterial population in each thigh infected with a low (○) or a high (▪) inoculum 24 h after different total doses of marbofloxacin. Fitted curves for the dose-effect relationship are shown for the low (dashed line) and high (continuous line) inocula. The bacterial populations were assumed to be 10 log10 CFU/thigh for the dead mice.
The 50% effective doses were 1.27 and 3.92 mg/kg for the low and the high inocula, respectively.
By assuming dose proportionality for a given inoculum, the relationships between the dose or AUC0-24/MIC and the reduction of the total bacterial population had exactly the same shape. After 24 h, we observed the same reduction in the total bacterial population whatever the dose fractionation, even though the Cmax values were four times lower and the T>MIC always was longer after fractionated doses than after single doses. As a consequence, no relationship could be found between Cmax/MIC or T>MIC and the reduction of the total bacterial population.
The bacterial populations after 48 h of exposure to marbofloxacin are shown in Table 4 for each inoculum size and each dosing regimen. All control mice (dose 0) and all mice infected by the high inoculum and treated with a total dose of 4 mg/kg marbofloxacin, corresponding to AUC0-24/MIC and AUC0-24/MPC values of 387 and 12 h, respectively, were dead within 48 h postinoculation. At the higher doses and also for the low inoculum, the bacterial population was eradicated in some thighs, the frequency of eradication being greater the higher the total dose. However, no clear relationship between the reduction of the bacterial populations after 48 h of exposure to marbofloxacin and the total dose or AUC0-24/MIC was apparent. After single doses associated with higher Cmax/MIC values, the bacterial populations were lower when the initial inoculum was low but higher when the initial inoculum was high than after the same fractionated doses. This strongly suggested that there was no relationship between the Cmax/MIC and the reduction of the total bacterial population.
TABLE 4.
Total bacterial populations after 48 h of exposure to different total doses of marbofloxacina
| Total dose (mg/kg) | Bacterial population (means ± SD) by inoculum and dose typeb
|
|||
|---|---|---|---|---|
| Low
|
High
|
|||
| Single | Fractionated | Single | Fractionated | |
| 0 | * | * | * | * |
| 4 | 3.63 ± 1.65 | 6.24 ± 1.57 (2) | * | * |
| 10 | 4.92 ± 1.79 | 5.23 ± 1.38 (3) | 7.93 ± 1.50 | 6.23 ± 0.86 |
| 20 | 2.75 ± 0.37 | 5.61 ± 0.44 (4) | 8.28 ± 0.92 | 4.72 ± 1.37 |
| 40 | 4.25 ± 1.59 (4) | 5.21 ± 0.12 (4) | 5.89 ± 0.98 | 3.67 ± 1.16 (1) |
Six thighs were tested for each inoculum, each dose, and each dosing regimen. Numbers in parentheses correspond to the number of thighs in which bacteria were eradicated. There was no clear relationship between the dose and the reduction of the total bacterial population.
*, All three mice were dead.
(ii) Resistant bacterial populations.
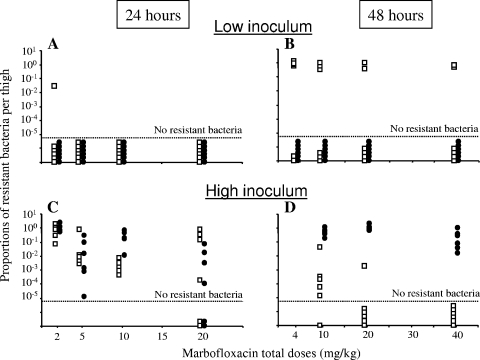
When the treatment was started, no resistant bacteria were detected in mice infected with the low inoculum, while 2.74 ± 0.41 log10 CFU of resistant bacteria were present in mice infected with the high inoculum. Twenty-four hours after infection, no resistant bacteria were recovered from the control mice infected with the low inoculum. The proportions of resistant bacteria after 24 and 48 h versus the dose are shown in Fig. 3. After 24 h, with the low inoculum, resistant bacteria in a proportion of 3.10−2 of the total bacteria count were found only in one thigh of a mouse treated with the fractionated dose of 2 mg/kg marbofloxacin (Fig. 3A). With the high inoculum, the proportion of resistant bacteria in all thighs was always greater than 10−5 except for four thighs of mice treated with a total dose of 20 mg/kg marbofloxacin, in which all resistant bacteria had been eradicated (Fig. 3C).
FIG. 3.
Proportions of resistant bacteria in each thigh infected with a low (A and B) or a high (C and D) inoculum 24 (A and C) or 48 (B and D) h after different total doses of marbofloxacin. Proportions obtained after single doses (•) and after fractionated doses (□) are represented. Six thighs were tested per time, inoculum size, and dosing regimen.
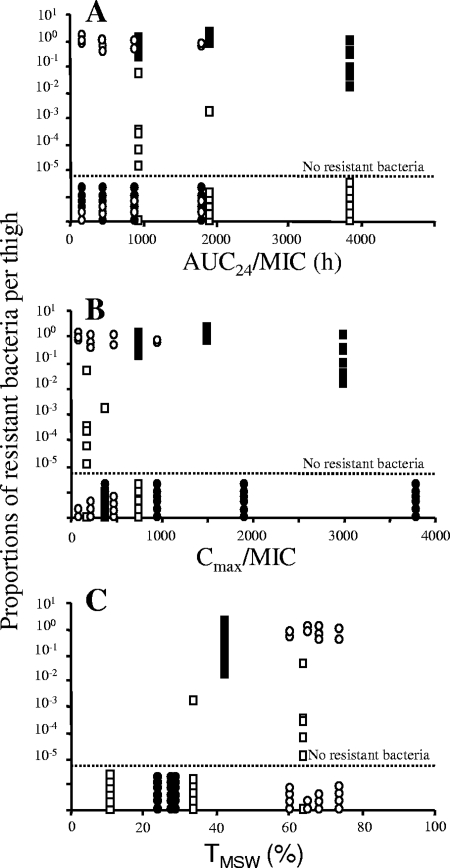
After 48 h, no resistant bacteria were detected in mice infected with the low inoculum and treated with single doses of marbofloxacin, whereas resistant bacteria were recovered in 46% of the thighs treated with fractionated doses. The proportion of resistant bacteria ranged from 10−2 to 1 in these thighs (Fig. 3B). For the high inoculum, resistant bacteria were recovered in all thighs after single doses, whereas resistant bacteria were recovered only in 33% of the thighs treated with fractionated doses. The proportions of resistant bacteria were always greater than 10−2 after single doses and ranged from 10−6 to 10−2 after the administration of fractionated doses (Fig. 3D). As these results indicated a net difference in the selection of resistant bacteria after the administration of single or fractionated doses, it can be presumed that no simple relationship existed between the total dose and the selection of resistant bacteria. We then investigated the ability of TMSW and also AUC0-24/MIC and Cmax/MIC indices that reflected the overall exposure to predict the selection of resistant bacteria. The proportions of resistant bacteria after 48 h of treatment are shown against the PK/PD indices in Fig. 4. The relationship between the proportion of resistant bacteria and the AUC0-24/MIC (R2 < 0.01 for an inhibitory sigmoid Emax model [data not shown]) and Cmax/MIC indices (R2 < 0.01 with a sigmoid Emax model [data not shown]) was poor, whereas a clear relationship existed between TMSW and the prevention of resistance. Indeed, after 48 h of treatment, the marbofloxacin dosing regimen associated with a TMSW of 34 to 82% led to the selection of resistant bacteria in 46% of the thighs with the low inoculum and in 80% with the high inoculum. In contrast, no selection of resistant bacteria occurred when TMSW was less than 30%, whatever the inoculum size. These observations were confirmed by the fitting of the proportion of resistant bacteria versus TMSW using a sigmoid Emax model, those parameters (the 50% effective TMSW and coefficient of sigmoidicity) were characteristic of a threshold value for TMSW ranging from 30 to 40% (R2 = 0.37; data not shown).
FIG. 4.
Proportions of resistant bacteria in each thigh infected with a low (circles) or high (squares) inoculum after 48 h of exposure to marbofloxacin versus the AUC0-24/MIC (A), Cmax/MIC (B), and TMSW (C). Proportions are represented by empty symbols when they were obtained after fractionated doses of marbofloxacin and by filled symbols after single doses of marbofloxacin.
DISCUSSION
Maintaining drug concentrations outside the MSW, which is the range of antibiotic concentrations favoring the selection of resistant mutants, has been suggested to restrict mutant outgrowth (13, 30). Previous in vivo studies that showed an enrichment of mutants when drug concentrations were within the MSW most of the time tested only one inoculum size (1, 9-11, 19). However, the bacterial load at the site of infection, against which antibiotic drugs are prescribed, can vary considerably whether the treatment is prophylactic or curative. The aim of the present study was to investigate the ability of TMSW and also of AUC0-24/MIC and Cmax/MIC indices to predict the selection of resistant bacteria in a mouse thigh infection model. Mice infected with a low or high inoculum of Escherichia coli already containing a proportion of 10−6 resistant mutants were treated with different regimens of marbofloxacin to assess the influence of dose, actual exposure (as determined by the AUC0-24/MIC, Cmax/MIC, and TMSW), and inoculum size on the selection of resistant bacteria.
Pharmacokinetic studies in which 5 mg/kg marbofloxacin was administered to infected animals revealed a large difference between the rates of the elimination of marbofloxacin with the two tested inoculum sizes. Higher elimination half-lives and decreased systemic clearance were observed previously in infected calves (21) and horses (26) that were treated with marbofloxacin. Explanations for the alteration of the drug elimination pathways may be the production by gram-negative bacteria of endotoxins that were shown to decrease the activity of hepatic metabolism, the renal blood flow, and the glomerular filtration rate (20).
Whatever the regimen, a given total dose of marbofloxacin produced a similar reduction in bacterial population size after 24 h of treatment, thereby suggesting that the AUC0-24/MIC index, which was proportional to the total dose, was an appropriate predictor of bacterial killing. In addition, after 48 h of exposure to marbofloxacin, the only PK/PD index that was identical for all mice that died after infection with the high inoculum and a treatment with 4 mg/kg marbofloxacin for 48 h also was the AUC0-24/MIC, since the Cmax/MIC and TMSW indices differed between mice treated with single doses and mice treated with fractionated doses. In a previous study, an AUC/MIC of 70 h for gatifloxacin was suggested to have a bacteriostatic effect in a thigh infection of Klebsiella pneumoniae in neutropenic mice (4). In our study, the value of AUC0-24/MPC (corresponding to the AUC0-24/MIC for the resistant bacteria) was 12.1 h for a dose of 4 mg/kg marbofloxacin, which explains the inability of this dose to slow the growth of the resistant subpopulation enough to prevent death. For the susceptible bacteria, the lowest AUC0-24/MIC observed in our study, i.e., 175 and 387 h for the low and high inocula, respectively, always were sufficient to prevent bacterial growth for 24 h. For the low inoculum, in which the initial resistant population was assumed to be very small (the probability of the presence of at least one resistant mutant was 0.4), an AUC/MIC of 175 h was associated with the prevention of the emergence of a resistant population for 24 h in 11 thighs out of 12. This is in agreement with the AUC/MIC of 157 h found by Jumbe et al. (22) to prevent the amplification of mutant strains in a large population of Pseudomonas aeruginosa treated with levofloxacin. However, after 48 h of treatment, the AUC0-24/MIC became a poor predictor of the selection of resistant bacteria. For example, no resistant bacteria were selected in the low inoculum after a single dose of marbofloxacin corresponding to an AUC0-24/MIC of 175 h, whereas resistant bacteria were selected in four thighs out of six after fractionated doses corresponding to the same AUC0-24/MIC. For the challenge with the high inoculum, the opposite was observed. Indeed, for the same AUC0-24/MIC and AUC0-24/MPC values of 3,850 and 120 h, respectively, resistant bacteria were selected only after single doses. This could not be explained by the PK/PD parameter Cmax/MIC, because the value of Cmax that prevented the selection of resistant bacteria after dose fractionation was four times lower than that after a single dose that did lead to the selection of resistant bacteria. Therefore, in our study the lack of consistency in the selection of resistance, regarding inoculum size and the dosing regimen after 48 h of treatment, could not be explained by the AUC0-24/MIC and Cmax/MIC indices. Indeed, no relationship was found in these cases between the AUC0-24/MIC or Cmax/MIC and the prevention of the selection of resistant bacteria.
In contrast, when the marbofloxacin exposures expressed as TMSW for each inoculum size were examined, a TMSW of less than 30% was found to predict the prevention of resistance selection for both inoculum sizes. This result suggested that TMSW could be a good predictor of resistance prevention in vivo, although a previous in vitro model with Staphylococcus aureus showed no correlation between the TMSW and the selection of resistant bacteria (6). However, recently it was suggested that the discrepancies observed in the ability of TMSW to predict selection can be explained by an additional influence of the position of antimicrobial concentrations within the MSW on the selection of resistant bacteria (11, 16). Indeed, in vivo it was shown in a local infection of rabbits with Staphylococcus aureus that the TMSW leading to the emergence of resistance could be 30 or 80% depending on the area of the MSW in which drug concentrations fluctuated (11). In our study, we found the same minimal time of 30% associated with the selection of resistance. By relating marbofloxacin exposure to the TMSW, we clearly showed that all mice in which a selection of resistant bacteria occurred after 48 h of marbofloxacin treatment were exposed to a TMSW of greater than 34% for 48 h irrespective of the inoculum size. Similar results were reported in a rabbit model of human therapy with Streptococcus pneumoniae and moxifloxacin or gatifloxacin in which selection occurred when the TMSW ranged from 72.5 to 93.5% or when the TMSW was greater than 45% (9, 10). However, in these experiments, the rabbits were infected with a high inoculum containing more than 109 CFU/lung. By testing 106 and 109 CFU/thigh, we were able to show that resistance was less frequently selected in the low inoculum than in the high inoculum. After 48 h of exposure to marbofloxacin, we showed that for a TMSW of 34% or more, resistant bacteria were selected in 80% (30/36) of the thighs exposed to the high inoculum but in only 46% of thighs (11/24) in mice infected by the low inoculum. Interestingly, this percentage of 46% corresponded to the probability of resistant bacteria being present at the start of treatment with our method of inoculum preparation. The less frequent selection of resistant bacteria in the low inoculum, which is associated with marbofloxacin concentrations remaining within the MSW for a long time, seemed to be due to the fact that these bacteria were rarer from the start in the case of the low inoculum.
In conclusion, our results fully support the concept of MSW in vivo in a mouse thigh model, since we showed that maintaining the antibiotic concentrations outside the MSW prevented the selection of resistant mutants. We also showed in our pharmacokinetic study in animals infected with either a high or low inoculum that the size of the bacterial population at the start of treatment could influence both the exposure to the antibiotic and the selection of resistance. Indeed, the PK/PD study indicated that, for approximately the same TMSW, the selection of resistant bacteria occurred less often with the low inoculum than with the high inoculum, probably due to the less frequent presence of mutants in the low inoculum. This study also highlights the importance of assessing antibiotic exposure in critically ill patients or animal models when establishing fluoroquinolone dosing strategies in relation to the MSW concept. Finally, due to the good relationship between PK/PD animal studies and data from infected patients (2), the doses of fluoroquinolones for human and veterinary uses could be improved to provide exposures in agreement with the PK/PD target for the prevention of the emergence of resistant bacteria in animal models. The relevance of these results merits further investigation with pathogens of interest and in a clinical context because of the common uses of fluoroquinolones to treat a variety of bacterial infections, including urinary bacterial infection, and to prevent febrile neutropenia in cancer patients.
Acknowledgments
We thank Sylvie Puel for performing analytical assays and Nathalie Arpaillange for technical support.
Footnotes
Published ahead of print on 1 June 2009.
REFERENCES
- 1.Almeida, D., E. Nuermberger, S. Tyagi, W. R. Bishai, and J. Grosset. 2007. In vivo validation of the mutant selection window hypothesis with moxifloxacin in a murine model of tuberculosis. Antimicrob. Agents Chemother. 51:4261-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 3.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D., and W. A. Craig. 2002. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob. Agents Chemother. 46:1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blondeau, J. M., X. Zhao, G. Hansen, and K. Drlica. 2001. Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campion, J. J., P. Chung, P. J. McNamara, W. B. Titlow, and M. E. Evans. 2005. Pharmacodynamic modeling of the evolution of levofloxacin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 49:2189-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campion, J. J., P. J. McNamara, and M. E. Evans. 2004. Evolution of ciprofloxacin-resistant Staphylococcus aureus in in vitro pharmacokinetic environments. Antimicrob. Agents Chemother. 48:4733-4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 9.Croisier, D., M. Etienne, E. Bergoin, P. E. Charles, C. Lequeu, L. Piroth, H. Portier, and P. Chavanet. 2004. Mutant selection window in levofloxacin and moxifloxacin treatments of experimental pneumococcal pneumonia in a rabbit model of human therapy. Antimicrob. Agents Chemother. 48:1699-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croisier, D., M. Etienne, L. Piroth, E. Bergoin, C. Lequeu, H. Portier, and P. Chavanet. 2004. In vivo pharmacodynamic efficacy of gatifloxacin against Streptococcus pneumoniae in an experimental model of pneumonia: impact of the low levels of fluoroquinolone resistance on the enrichment of resistant mutants. J. Antimicrob. Chemother. 54:640-647. [DOI] [PubMed] [Google Scholar]
- 11.Cui, J., Y. Liu, R. Wang, W. Tong, K. Drlica, and X. Zhao. 2006. The mutant selection window in rabbits infected with Staphylococcus aureus. J. Infect. Dis. 194:1601-1608. [DOI] [PubMed] [Google Scholar]
- 12.Dong, Y., X. Zhao, B. N. Kreiswirth, and K. Drlica. 2000. Mutant prevention concentration as a measure of antibiotic potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drlica, K., and X. Zhao. 2007. Mutant selection window hypothesis updated. Clin. Infect. Dis. 44:681-688. [DOI] [PubMed] [Google Scholar]
- 14.Everett, M. J., Y. F. Jin, V. Ricci, and L. J. Piddock. 1996. Contributions of individual mechanisms to fluoroquinolone resistance in 36 Escherichia coli strains isolated from humans and animals. Antimicrob. Agents Chemother. 40:2380-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferran, A., V. Dupouy, P. L. Toutain, and A. Bousquet-Melou. 2007. Influence of inoculum size on the selection of resistant mutants of Escherichia coli in relation to mutant prevention concentrations of marbofloxacin. Antimicrob. Agents Chemother. 51:4163-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firsov, A. A., M. V. Smirnova, E. N. Strukova, S. N. Vostrov, Y. A. Portnoy, and S. H. Zinner. 2008. Enrichment of resistant Staphylococcus aureus at ciprofloxacin concentrations simulated within the mutant selection window: bolus versus continuous infusion. Int. J. Antimicrob. Agents 32:488-493. [DOI] [PubMed] [Google Scholar]
- 17.Firsov, A. A., S. N. Vostrov, I. Y. Lubenko, K. Drlica, Y. A. Portnoy, and S. H. Zinner. 2003. In vitro pharmacodynamic evaluation of the mutant selection window hypothesis using four fluoroquinolones against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1604-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, vol. 15. Marcel Dekker, Inc., New York, NY.
- 19.Ginsburg, A. S., R. Sun, H. Calamita, C. P. Scott, W. R. Bishai, and J. H. Grosset. 2005. Emergence of fluoroquinolone resistance in Mycobacterium tuberculosis during continuously dosed moxifloxacin monotherapy in a mouse model. Antimicrob. Agents Chemother. 49:3977-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa, T., K. Takagi, and K. Kitaichi. 1999. Effects of bacterial endotoxin on drug pharmacokinetics. Nagoya J. Med. Sci. 62:11-28. [PubMed] [Google Scholar]
- 21.Ismail, M., and Y. A. El-Kattan. 2007. Comparative pharmacokinetics of marbofloxacin in healthy and Mannheimia haemolytica infected calves. Res. Vet. Sci. 82:398-404. [DOI] [PubMed] [Google Scholar]
- 22.Jumbe, N., A. Louie, R. Leary, W. Liu, M. R. Deziel, V. H. Tam, R. Bachhawat, C. Freeman, J. B. Kahn, K. Bush, M. N. Dudley, M. H. Miller, and G. L. Drusano. 2003. Application of a mathematical model to prevent in vivo amplification of antibiotic-resistant bacterial populations during therapy. J. Clin. Investig. 112:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khodursky, A. B., E. L. Zechiedrich, and N. R. Cozzarelli. 1995. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc. Natl. Acad. Sci. USA 92:11801-11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcusson, L. L., S. K. Olofsson, P. Komp Lindgren, O. Cars, and D. Hughes. 2005. Mutant prevention concentrations of ciprofloxacin for urinary tract infection isolates of Escherichia coli. J. Antimicrob. Chemother. 55:938-943. [DOI] [PubMed] [Google Scholar]
- 25.Moon, S., S. Williams, and M. Cullen. 2006. Role of prophylactic antibiotics in the prevention of infections after chemotherapy: a literature review. Support Cancer Ther. 3:207-216. [DOI] [PubMed] [Google Scholar]
- 26.Peyrou, M., M. Y. Doucet, A. Vrins, D. Concordet, M. Schneider, and A. Bousquet-Melou. 2004. Population pharmacokinetics of marbofloxacin in horses: preliminary analysis. J. Vet. Pharmacol. Ther. 27:283-288. [DOI] [PubMed] [Google Scholar]
- 27.Phillips, I., M. Casewell, T. Cox, B. De Groot, C. Friis, R. Jones, C. Nightingale, R. Preston, and J. Waddell. 2004. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 53:28-52. [DOI] [PubMed] [Google Scholar]
- 28.Schneider, M., V. Thomas, B. Boisrame, and J. Deleforge. 1996. Pharmacokinetics of marbofloxacin in dogs after oral and parenteral administration. J. Vet. Pharmacol. Ther. 19:56-61. [DOI] [PubMed] [Google Scholar]
- 29.Sugino, A., C. L. Peebles, K. N. Kreuzer, and N. R. Cozzarelli. 1977. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc. Natl. Acad. Sci. USA 74:4767-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao, X., and K. Drlica. 2001. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin. Infect. Dis. 33(Suppl. 3):S147-S156. [DOI] [PubMed] [Google Scholar]
- 31.Zinner, S. H., I. Y. Lubenko, D. Gilbert, K. Simmons, X. Zhao, K. Drlica, and A. A. Firsov. 2003. Emergence of resistant Streptococcus pneumoniae in an in vitro dynamic model that simulates moxifloxacin concentrations inside and outside the mutant selection window: related changes in susceptibility, resistance frequency and bacterial killing. J. Antimicrob. Chemother. 52:616-622. [DOI] [PubMed] [Google Scholar]