Abstract
NB-002 is an oil-in-water emulsion designed for use for the treatment of skin, hair, and nail infections. The activity of NB-002 was compared to the activities of the available antifungal drugs against the major dermatophytes responsible for cutaneous infections, Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum spp., as well as 12 other genera of filamentous fungi. NB-002 consistently displayed fungicidal activity against all dermatophytes. The comparator compounds were either fungistatic or fungicidal, and for some strain-drug combinations, tolerance was observed. Assessment of the development of spontaneous resistance to NB-002 in different dermatophyte species yielded few stably resistant mutants. For filamentous nondermatophyte fungi, the MIC range varied from 0.06 to 0.5 μg/ml for Alternaria spp. to 2 to 8 μg/ml for Paecilomyes spp. NB-002 had activity against both azole-susceptible and -resistant Candida albicans yeast isolates, with MIC90s of 2 μg/ml, respectively, and minimum fungicidal concentrations at which 90% of isolates are inhibited of 4 and 8 μg/ml, respectively. The kinetics of the fungicidal activity of NB-002 against T. rubrum isolates were compared to those of the other antifungal drugs. NB-002 killed both mycelia and microconidia even when the fungal forms were dormant or not actively growing. Electron micrographs of mycelia and spores treated with NB-002 showed the significant disruption of the fungal structure. The in vitro broad coverage of NB-002 against filamentous fungi, dermatophytes, and C. albicans, as well as its rapid fungicidal activity, warrants further investigations to ascertain if NB-002 would be useful for the treatment of cutaneous mycoses.
Superficial fungal infections are found in the top layers of the skin and mucous membranes, the hair, and nails. Examples of fungal infections of the skin and other external surfaces include athlete's foot, jock itch, ringworm, and other tinea infections. Most of these infections are caused by three genera of dermatophytes: Trichophyton, Epidermophyton, and Microsporum spp. (3, 4, 20, 29, 45, 47).
Filamentous fungi that are normal soil saprophytes have also emerged as major opportunistic fungi, especially in immunosuppressed patients (34, 53). Such organisms include Aspergillus spp. (1, 18, 34, 42, 49), Fusarium spp. (18, 22, 28, 34, 36, 49), Scedosporium spp. (18, 34), Paecilomyces spp. (23, 24), Scopulariopsis spp. (18), Scytalidium spp. (11), Chaetomium spp. (13), Alternaria spp. (2, 18, 49), Acremonium spp. (18), and Curvularia spp. (49). Yeasts such as Candida albicans also cause skin infections, generally at sites between the fingers and toes, around the anus, and on the penis or at sites of abrasion where the skin is continuously moist (46).
Most cutaneous infections are treated with topical antifungals containing naftidine, tolnaftate, terbinafine, or itraconazole. Oral therapies of griseofulvin, terbinafine, and itraconazole are used to treat tinea capitis. Nail infections can be treated with orally administered agents as well as the topical agent ciclopirox. Terbinafine and azole-like compounds carry the risk of liver and cardiac side effects and drug-drug interactions (14, 31, 38). Topical therapies for inflammatory dermatomycoses often combine an azole and a corticosteroid to rapidly reduce inflammatory symptoms and to increase the bioavailability of the antifungal agent (30).
Antimicrobial nanoemulsions are highly stable oil-in-water emulsions composed of nanometer-sized, positively charged droplets that have broad-spectrum activity against enveloped viruses, fungi, and bacteria (5, 17, 25-27, 35, 43, 48). NB-002 contains the cationic quaternary ammonium compound cetylpyridinium chloride (CPC) oriented at its oil-water interface, which stabilizes the nanoemulsion droplets, contributes to the anti-infective activity, and serves as a marker of activity.
Studies with NB-002 containing fluorescein have shown that the nanodroplets permeate human cadaver skin by a transfollicular route (6). By the use of a modified Franz cell apparatus, NB-002 was also shown to diffuse laterally from hair follicles and sebaceous glands along tissue planes to reach concentrations in excess of 200 μg/g in human cadaver epidermis as far as 1 cm from the site of application (7). This concentration is significantly above the MIC90 and the minimum fungicidal concentration at which 90% of isolates are inhibited (MFC90) or the ranges of MICs and MFCs determined in this work: ≤4 μg/ml for Trichophyton spp., Microsporum spp., and Epidermophyton floccosum.
The studies described here assessed the microbiological activity of NB-002 against fungal pathogens that cause cutaneous infections. Furthermore, we show that the fungicidal activity of NB-002 is rapid and that it kills both the microconidia and mycelia of the dermatophyte Trichophyton rubrum. Consistent with this kill-on-contact mechanism of action, the MICs for the majority of dermatophyte isolates spontaneously resistant to NB-002 were plus or minus twofold of the initial MIC.
MATERIALS AND METHODS
Nanoemulsion manufacturing and potency.
NB-002 is an oil-in-water emulsion manufactured from ingredients that are on the FDA list of recognized inactive ingredients in drug substances. The emulsion is formed from highly purified oil, ethanol, polysorbate 20, CPC, and water. The average nanoemulsion droplet size is 180 nm, as measured by dynamic light scattering with a Zetasizer Nano 3600 instrument (Malvern Instruments Ltd., Worcestershire, United Kingdom). The relative activity of NB-002 is expressed in terms of the concentration of the cationic surfactant present, as we have previously done in clinical trials with NB-001, a formulation of the same components, for the treatment of herpes labialis (32). Thus, the antifungal activity of NB-002 is expressed in μg CPC/ml.
Sources of comparator compounds.
Amphotericin B, ciclopirox, itraconazole, terbinafine, and tolnaftate were purchased from Sigma Chemical Co. (catalog numbers A4888, C0415, I6657, T8826, and T6638, respectively.) Naftifine (catalog number 1450404) was obtained from United States Pharmacopeia.
Sources of fungal isolates.
The dermatophytes T. rubrum, Trichophyton mentagrophytes, and E. floccosum were obtained from specimens from clinical trials. The specimens were either from untreated subjects under evaluation for participation in clinical trials or from subjects in ongoing trials undergoing treatment in blinded studies with an azole or allylamine for onychomycosis, tinea pedis, tinea corporis, and tinea unguium (Mycology Consultants Laboratory, Holland, MI). The terbinafine-resistant T. rubrum isolates were from the Center for Medical Mycology, Case Western Reserve University, Cleveland, OH (19). The Microsporum sp. isolates (five isolates each of Microsporum canis, Microsporum nanum, and Microsporum gypseum) were from the American Type Culture Collection. A total of 65 clinical isolates of filamentous fungi included 5 Aspergillus sp. isolates (Aspergillus sydowii [n = 1], Aspergillus terreus [n = 3], Aspergillus niger [n = 1]), 4 Paecilomyces lilacinus isolates, 4 Fusarium oxysporum isolates, 5 Fusarium solani isolates, 1 Fusarium semitectum isolate, 3 Trichophyton soudanense isolates, 3 Trichopyton verrucosum isolates, and 3 Epicoccum nigrum isolates. Other isolates that were tested but that were identified to the genus level only included 5 Acremonium sp. isolates, 5 Scopulariopsis sp. isolates, 5 Scedosporium sp. isolates, 10 Scytalidium sp. isolates, 3 Alternaria sp. isolates, 3 Curvularia sp. isolates, 3 Phoma sp. isolate, and 3 Chaetomium sp. isolate. All these filamentous fungi were recovered from dermatologic sources by the Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio. C. albicans isolates were obtained from David Stevens (Stanford University, Stanford, CA) and have been described by White et al. (51).
MIC and MFC determinations.
MICs were determined by the microtiter broth dilution methodology in RPMI 1640 medium, as described by the CLSI method for filamentous fungi (9). For the dermatophytes, a hemacytometer count of conidia was done to check the initial inoculum count. In most of the studies with dermatophytes, the inoculum level was increased from 1 × 103 to 3 × 103 CFU/ml to 1 × 104 to 3 × 104 CFU/ml to allow the determination of MFCs by accepted statistical methods (8, 44). Premade microtiter plates containing each of the drugs at twice the desired final concentration and serially diluted as described in the CLSI document for the testing of filamentous fungi were inoculated with twice the final desired inoculum. The final volume in each well was 200 μl. The plates were incubated at 35°C for 4 days and were then examined visually for 80% growth inhibition endpoints (100% growth inhibition endpoints for NB-002). T. rubrum MYA-4498 and T. mentagrophytes MYA-4439, the two dermatophyte quality control strains recommended for use in CLSI document M38-A2 (9), were included on each day of testing.
For the molds, antifungal susceptibility testing was done by the CLSI methodology, which used RPMI 1640 with glutamine and without bicarbonate, an inoculum size of 0.4 × 104 to 5 × 104, and incubation at 35°C for 48 h (9, 10). Paecilomyces variotii ATCC MYA-360-3630 was the quality control isolate. Testing of amphotericin B was conducted in antibiotic medium 3 (Difco, Detroit, MI). The MIC was defined as the lowest concentration that resulted in an 80% reduction in turbidity compared to the turbidity of a drug-free control for each isolate-drug combination for terbinafine and ciclopirox; for nondermatophyte species, the MICs of NB-002, amphotericin B, and itraconazole were the lowest concentrations that resulted in optically clear wells. The MIC50 and the MIC90 were the lowest concentrations of antifungal agent that collectively inhibited the growth of 50% and 90% of the isolates tested, respectively.
For MFC determinations, 100 μl was removed from each well at the MIC and at least four concentrations above the MIC and plated onto Sabouraud dextrose agar (SDA). To avoid antifungal carryover, the aliquots were allowed to soak into the agar and were then streaked for isolation once it was dry, thus removing the cells from the drug source. The MFC was defined as the lowest concentration of drug that reduced the initial inoculum by ≥3 log units. A compound works by a fungicidal mechanism if the MFC/MIC ratio is ≤4; if the compound is fungistatic, the MFC/MIC ratio will be >4 (8). The MFC50 and the MFC90 are the lowest concentrations of antifungal agent that collectively kill (≥3-log-unit reduction) 50% and 90% of the isolates tested, respectively. Tolerance was defined as a minimum bactericidal concentration/MIC ratio of ≥32 (8, 44).
Susceptibility studies with C. albicans isolates were determined by the microtiter broth dilution methodology, as specified by the CLSI (10). A change to a denser inoculum (1 × 104 to 4 × 104 CFU/ml) was done to allow determination of the MFC upon removal of 100 μl from each well where there was no growth (at the MIC) and at least four concentrations above the MIC (8, 44). Colony counts were determined on SDA after incubation at 35°C for 4 days. Both Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were included as quality control isolates; and the results of the tests were validated from the ranges of the MICs of fluconazole, amphotericin B, and itraconazole for the quality control isolates, as described in the CLSI guidance (10).
Susceptibility of terbinafine-resistant T. rubrum to NB-002.
Clinical isolates with decreased susceptibility to terbinafine were part of the Center for Medical Mycology collection (Case Western Reserve University) (19) and were tested in the laboratory of Mahmoud Ghannoum for their susceptibilities to NB-002 and terbinafine, according to recent CLSI standards (9).
Determination of spontaneous resistance frequencies.
RPMI 1640 agar plates containing 4×, 8×, or 16× the MIC of NB-002, itraconazole, terbinafine, or ciclopirox were inoculated with 107 to 108 CFU of E. floccosum (two isolates), T. mentagrophytes (five isolates), and T. rubrum (five isolates) and incubated for 7 to 8 days at 35°C. Colonies recovered from the plates were cloned onto RPMI 1640 agar plates containing the compound at the selecting concentration. Microtiter broth- or agar-based MICs for the isolates recovered were determined by the CLSI methodology (9), except that a higher inoculum (1 × 104 to 4 × 104 CFU/ml) was used. To ensure that drug selective pressure was maintained, putative drug-resistant isolates were cultured on RPMI 1640 agar plates containing the drug at the concentration with which they were initially selected during the outgrowth of the frozen cultures. The percentage of spontaneously resistant isolates for an isolate-drug combination was calculated from the number of colonies that grew on plates containing drug versus the number of colonies that grew on unsupplemented agar.
Time-kill studies and electron microscopy.
For time-kill experiments and electron micrographs of the mechanism of action, microconidia were harvested from 7-day-old cultures of T. rubrum growing on potato dextrose agar with sterile distilled water and adjusted to a concentration of 106 conidia/ml (33, 44). Part of the conidial suspension was pelleted and resuspended in RPMI 1640 medium and grown for 16 to 18 h overnight at room temperature to allow the germination of the microconidia (33). After germination, the hyphae were collected by centrifugation and resuspended in distilled water. After the microconidia and mycelia were mixed with different concentrations of NB-002 or a comparator compound, the rate of killing of microconidia and mycelia was monitored for up to 24 h by plating 0.1 ml of 10−1, 10−2, and 10−3 dilutions onto SDA. The numbers of CFU were counted after 4 days of incubation at 35°C. Control experiments determined that samples containing NB-002 had to be diluted 1:100 to remove residual activity (data not shown).
For scanning electron microscopy, samples (450 μl) obtained at different time points during the time-kill study with NB-002 were mixed with 113 μl of fixative (10% aqueous solution of glutaraldehyde). The mixtures were vortexed and placed at 4°C for at least 18 h. Samples were fixed in 1.0% OsO4 in Sorenson's buffer, taken through a gradual series of dehydration steps with ethanol, and mounted on scanning electron microscopy stubs by using a mixture of colloidal graphite and Duco cement. Samples for electron microscopy were sputter coated with gold by using a Polaron sputter coater (Quorum Technologies, United Kingdom), examined on an Amray 1910 FE scanning electron microscope, and digitally imaged with Xstream imaging software (SEMTech Solutions, Inc. North Billerica, MA).
RESULTS
NB-002 is fungicidal for dermatophytes.
The antifungal activities of NB-002, its topically administered comparator compounds (ciclopirox, tolnaftate, and naftifine), and its orally administered comparator compounds (terbinafine, itraconazole, and griseofulvin) were evaluated by using clinical isolates of dermatophytes. NB-002 was the only antifungal that was consistently fungicidal against isolates of T. rubrum, T. mentagrophytes, Trichophyton tonsurans, E. floccosum, and Microsporum spp. (Table 1). Ciclopirox, itraconazole, tolnaftate, naftifine, and griseofulvin had MICs that ranged from 0.125 to 2 μg/ml, 0.031 to 0.5 μg/ml, 0.016 to 1.0 μg/ml, 0.016 to 0.25 μg/ml, and 0.25 to 4 μg/ml, respectively; but none were fungicidal for any species. Terbinafine was fungicidal only for T. rubrum isolates. Fungicidal activity was not determined for T. soundanense, but the two compounds most active against that organism were NB-002 and terbinafine, with the MIC ranges being 0.06 μg/ml and ≤0.004 μg/ml, respectively.
TABLE 1.
MICs and MFCs of NB-002 and comparator compounds against dermatophytes
| Active compound | Activity (μg/ml) by organism (no. of isolates)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
T. rubrum (n = 15)
|
T. mentagrophytes (n = 13)
|
Microsporum spp. (n = 15)
|
MIC/MFC range for E. floccosum (n = 6) | MIC/MFC range for T. tonsurans (n = 6) | MIC range for T. soudanense (n = 3) | MIC range for T. verrucosum (n = 3) | MIC/MFC range for terbinafine-resistant T. rubrum (n = 5) | |||||||
| MIC90/MFC90 | MIC50/MFC50 | MIC/MFC range | MIC90/MFC90 | MIC50/MFC50 | MIC/MFC range | MIC90/MFC90 | MIC50/MFC50 | MIC/MFC range | ||||||
| NB-002 | 2/2 | 2/2 | 1-2/1-2 | 2/4 | 2/4 | 2-4/2-4 | 2/4 | 2/2 | 1-2/1-4 | 2-4/2-4 | 2/4-8 | 0.06 | ≤0.03-0.06 | 2-4/2-8 |
| Ciclopirox | 2/16 | 0.5/1 | 0.25-2/0.25-16 | 2/16 | 1/4 | 0.5-2/0.5->32 | 1/16 | 0.5/0.5 | 0.125-2/0.125-16 | 0.25-2/0.25-2 | NDa | 0.125-0.25 | ≤0.06-0.125 | ND |
| Terbinafine | 0.125/0.5 | 0.063/0.063 | 0.016-0.125/0.031-2 | 0.063/>8 | 0.016/0.25 | 0.016-0.125/0.125->8 | 0.125/>8 | 0.063/4 | 0.031-2/0.031->8 | 0.063/0.063-2 | ND | ≤0.004 | ≤0.004-0.015 | >8/>8 |
| Itraconazole | 0.25/>16 | 0.25/>16 | 0.125-0.5/0.5->16 | 0.5/>16 | 0.25/>16 | 0.063-0.5/2->16 | 0.25/>16 | 0.125/>8 | 0.031-0.5/0.25->16 | 0.125-0.25/1->16 | ND | 0.125-0.25 | 0.06-0.125 | ND |
| Amphotericin B | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.125-0.25 | 0.125-0.25 | ND |
| Tolnaftate | 0.125/1 | 0.063/0.125 | 0.016-0.125/0.016-8 | 0.5/>8 | 0.063/>8 | 0.031-1/0.5->8 | 0.25/>8 | 0.125/>8 | 0.031-0.125/0.125->8 | 0.125-0.5/0.5-8 | ND | ND | ND | ND |
| Naftifine | 0.063/0.5 | 0.031/0.125 | 0.016-0.063/0.031-4 | 0.031/>8 | 0.016/0.125 | 0.016-0.031/0.031->8 | 0.25/>8 | 0.125/2 | 0.016-0.25/0.125->8 | 0.063-0.25/0.125->4 | ND | ND | ND | ND |
| Griseofulvin | 4/>16 | 2/2 | 0.25-4/1->16 | 2/>16 | 0.5/8 | 0.25-4/0.25->16 | ND | ND | ND | 1-2/1-16 | ND | ND | ND | ND |
ND = not done.
NB-002 was tested against five clinical isolates of T. rubrum characterized as having elevated terbinafine MICs by the Center for Medical Mycology (Case Western Reserve University) (Table 1) (19). The MIC and MFC ranges of NB-002 were 2 to 4 μg/ml and 2 to 8 μg/ml, respectively. The terbinafine MICs and MFCs were >8 μg/ml for all isolates.
Antibiotic susceptibilities of filamentous fungi.
The antifungal activity of NB-002 against 12 genera of filamentous fungi was assessed (Table 2). Nearly all isolates were susceptible to ≤4 μg/ml NB-002, with an overall range of MICs of 0.06 to 8 μg/ml. Against certain genera, other compounds were either uniformly inactive or had mixed potency. NB-002 distinguished itself from amphotericin B, itraconazole, and terbinafine because of its potency against Scopulariopsis spp. and Scedosporium spp. and was superior to ciclopirox because of its activity against Fusarium spp. and Paecilomyces spp.
TABLE 2.
MIC ranges of NB-002 and comparator compounds for filamentous fungi
| Species | No. of isolates | MIC range (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| NB-002 | Amphotericin B | Itraconazole | Terbinafine | Ciclopirox | ||
| Aspergillus sydowii | 1 | 1 | 1 | 0.06 | 0.25 | 2 |
| Aspergillus terreus | 3 | 0.5-1 | 2-4 | 0.25-1 | 0.03-0.25 | 0.5-1 |
| Aspergillus niger | 1 | 1 | 1 | 1 | 0.03 | 0.5 |
| Paecilomyces lilacinus | 4 | 2-8 | >16 | 1->8 | 0.25-0.5 | 8-16 |
| Fusarium oxysporum | 4 | 1-2 | 4->16 | >8 | ->2 | 1-2 |
| Fusarium solani | 5 | 0.5-2 | 8->16 | >8 | >2 | 16 |
| Fusarium senitectum | 1 | 2 | 4 | 2 | 2 | 4 |
| Acremonium spp. | 5 | 0.5-2 | 0.5->16 | >8 | 0.125-1 | 0.5-4 |
| Scopulariopsis spp. | 5 | 0.5-1 | >16 | 4->8 | 1->2 | 0.5-2 |
| Scedosporium spp. | 5 | 0.25-1 | >16 | 4->8 | >2 | 0.5-8 |
| Scytalydium spp. | 10 | 1-2 | 0.5-1 | 4->8 | 0.125-1 | 0.5-1 |
| Alternaria spp. | 3 | 0.06-0.5 | 1 | 0.25-0.5 | 1-2 | 0.25-0.5 |
| Epicoccum nigrum | 3 | 0.06-1 | 0.25-1 | 0.25-0.5 | 0.03-0.06 | 0.125-2 |
| Curvularia spp. | 3 | 0.5 | 0.125-1 | 0.125-0.25 | 0.03-1 | 0.5-1 |
| Phoma spp. | 3 | 0.5-1 | 0.5-2 | 0.06-0.5 | 0.03 | 0.5-1 |
| Chaetomium spp. | 3 | 0.25 | 0.5-4 | 0.5-1 | 1-2 | 0.25-0.5 |
Antibiotic susceptibilities of C. albicans to NB-002 and comparators.
NB-002 was unvaryingly fungicidal against the panel of C. albicans clinical isolates (Table 3). The MIC90s and MFC90s for the 34 isolates were 2 and 8 μg/ml, respectively. The strains were susceptible to amphotericin B, with the MIC90s and MFC90s being 2 μg/ml. The majority of the isolates were resistant to the two azoles tested, fluconazole and itraconazole, with the MIC90s being >64 and >16 μg/ml, respectively. Ciclopirox had a MIC90 of 1 μg/ml, but its MFC90 and MFC90 range indicated that it was uniformly fungistatic against the isolates. NB-002 had nearly identical MIC90s and MFC90s for both azole-susceptible isolates (2 and 4 μg/ml, respectively) and azole-resistant isolates (2 and 8 μg/ml, respectively).
TABLE 3.
Susceptibilities of C. albicans isolates that are fluconazole or itraconazole resistant and azole susceptible to NB-002 and the comparator drugs
| Susceptibility and active compound | Value (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| MIC90 | MFC90 | MIC50 | MFC50 | MIC range | MFC range | |
| Fluconazole or itraconazole resistant (n = 24) | ||||||
| NB-002 | 2 | 8 | 2 | 4 | 1-2 | 2-8 |
| Fluconazole | >64 | >64 | >64 | >64 | 0.125->64 | >64 |
| Ciclopirox | 1 | >32 | 0.25 | >32 | 0.25-2 | 8->32 |
| Terbinafine | >1 | >1 | >1 | >1 | >1 | >1 |
| Itraconazole | >16 | >16 | 2 | >16 | 0.125->16 | >16 |
| Amphotericin B | 2 | 4 | 1 | 2 | 0.125-2 | 0.25-4 |
| Azole susceptible (n = 10) | ||||||
| NB-002 | 2 | 4 | 2 | 4 | 2 | 4-8 |
| Fluconazole | 0.5 | >64 | 0.5 | >64 | 0.25-64 | >64 |
| Ciclopirox | 4 | >32 | 1 | >32 | 0.25-4 | 16->32 |
| Terbinafine | >1 | >1 | >1 | >1 | >1 | >1 |
| Itraconazole | 0.5 | >16 | 0.25 | >16 | 0.031-0.5 | >16 |
| Amphotericin B | 2 | 2 | 1 | 2 | 0.25-2 | 0.25-4 |
Spontaneous resistance frequency.
Twelve isolates of dermatophytes were evaluated for the frequency of development of spontaneous resistance on drug agar plates containing 4×, 8×, or 16× the MIC of NB-002, ciclopirox, terbinafine, or itraconazole. Both isolates of E. floccosum, all isolates of T. mentagrophytes, and three of the five isolates of T. rubrum yielded one to three colonies on agar plates containing one of the antifungals. Overall, the frequency of recovery of colonies was low and was generally between 1.5 × 10−6 and 2.3 × 10−8 for all compounds with any dermatophyte.
Despite growth on agar plates containing higher concentrations of the selecting compound, most of the phenotypically resistant isolates had MICs plus or minus twofold the initial MIC for all compounds when they were retested on agar or in broth. Two isolates had a 4-fold increase in the MIC for NB-002 (8 μg/ml), and one isolate had a 32-fold increase in the MIC for terbinafine. The MICs of NB-002, itraconazole, terbinafine, and ciclopirox for the recovered isolates tested by the microtiter broth method ranged from ≤1 to 8 μg/ml, ≤0.125 to 0.25 μg/ml, ≤0.063 to 2 μg/ml, and 0.5 to 4 μg/ml, respectively.
Time-kill and mechanism-of-action studies.
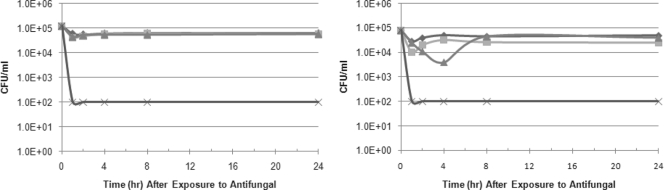
The kinetics of the fungicidal activities of NB-002 and the comparator compounds were evaluated against microconidia and mycelia from three isolates of T. rubrum. Figure 1 shows the reduction in colony counts of representative isolate NBD031 over 24 h for either mycelia or microconidia suspended in water (nongrowth conditions) containing either 4× MIC (16 μg/ml) of NB-002 or 16× MIC of itraconazole (16 μg/ml), terbinafine (4 μg/ml), or ciclopirox (16 μg/ml). In 2 h, NB-002 reduced the colony counts by ≥3 log units for both the mycelia and the microconidia of isolates NBD031 and NBD030; one isolate (isolate NBD032) required incubation for 4 h with NB-002 for a 3-log-unit reduction in colony counts (data not shown). None of the other compounds significantly reduced the colony counts for either dermatophyte form at any time point; an exception was a 3-log-unit reduction by ciclopirox (16× MIC, or 8 μg/ml) after 8 h against nongrowing mycelia, but not microconidia, from T. rubrum NBD032.
FIG. 1.
Impact of NB-002 (4× MIC) and comparators (16× MIC) on the viability of T. rubrum NBD031 mycelia (left) and microconidia (right). ♦, itraconazole; ▪, terbinafine; ▴, ciclopirox; ×, NB-002. The lower limit of detection was 100 CFU because a 1:100 dilution was necessary to neutralize the antifungal carryover.
Scanning electron microscopy was used to explore the morphology of T. rubrum NBD030 treated with NB-002 under nongrowth conditions. Figure 2 shows the mycelia 1 h after incubation with 100 μg/ml of NB-002 at room temperature. The hyphae are distorted, and there are multiple blebs along the surface of the mycelia. The untreated mycelia or mycelia treated with vehicle (the nanoemulsion without CPC) appeared as long septate filaments that were smooth and that had no detectable extrusions on the cell surface. After 1 h of treatment with NB-002, the treated mycelia were nonviable and incapable of forming any detectable colonies on SDA (data not shown). The microconidia (Fig. 2C and D) appeared to be broken, empty shells after 1 h of treatment with 12.5 μg/ml NB-002 (≈6× MIC). NB-002 effectively killed both microconidial spores and mycelia, despite the differences in their cell wall structures (37, 52).
FIG. 2.
Scanning electron micrographs of T. rubrum NBD030 after treatment with NB-002 for 1 h at room temperature. (A) Control mycelia (no treatment or treatment with vehicle); (B) treatment of mycelia with 100 μg NB-002; inset, higher magnification to show the differential size of the nanoemulsion droplets (white arrow, NB-002) compared to the various sizes of the extrusions (blebs); (C) control microconidia (no treatment or treatment with vehicle; white arrow, microconidial spore); (D) treatment of microconidial spores with 12.5 μg NB-002, with white arrows indicating broken spores.
DISCUSSION
NB-002 was developed as a topical treatment for cutaneous mycoses, including tinea infections of the skin, hair, and nails. This work demonstrated the broad, uniformly fungicidal activity of NB-002 against dermatophyte species and the yeast C. albicans. T. rubrum, T. mentagrophytes, and E. floccosum primarily cause onychomycosis, tinea pedis, and tinea cruris (12, 20, 49), while T. tonsurans, T. soudanense, and Microsporum spp. are the major causative pathogens for tinea capitis and tinea corporis worldwide (15, 16, 21, 39-41, 47).
While NB-002 was consistently fungicidal for all dermatophyte species, the orally administered comparator compounds itraconazole, terbinafine, and griseofulvin, which are used for the treatment of tinea capitis and onychomycosis, showed marked heterogeneity in their microbiological and fungicidal activities. Terbinafine was fungicidal for T. rubrum isolates, but the remaining species had a fungistatic response to these drugs. Topically administered drugs like naftifine and tolnaftate, which are used for the treatment of superficial tinea infections, e.g., tinea cruris, tinea pedia, and tinea corporis (12, 50), were fungistatic.
NB-002 was also active against multiple filamentous mold genera that are known to be extremely multidrug resistant and that are increasingly isolated from the growing population of immunocompromised patients (2, 22-24, 34, 49, 53). Since carriage is often the precursor to invasive disease, it is important that at-risk patients be treated for superficial infections. NB-002 distinguished itself from the other antifungals tested by its impressive activity against Paecilomyces lilacinus, Fusarium spp., Scedosporium spp., and Scopulariopsis spp.
NB-002 offers a topical, empirical alternative to the oral azoles that are used to treat C. albicans infections, e.g., intertrigo, diaper candidiasis, paronychia, and fingernail onychomycosis. The rate of resistance to azoles is high among Candida spp. due to one or more upregulated multidrug-resistant pumps, CDR1, CDR2, or MDR1, and/or target-based mutations in ERG11, the gene encoding lanosterol 14α-demethylase (46). Regardless of the mechanism(s) of azole resistance, NB-002 successfully eliminated azole-resistant isolates while retaining its fungicidal activity against azole-susceptible isolates.
Consistent with its physical mechanism of action of interacting with the fungal cell surface, NB-002 had no apparent cross-resistance to known antifungals. NB-002 was equally potent against terbinafine-resistant T. rubrum isolates, azole-resistant C. albicans isolates, and multidrug-resistant molds. Furthermore, stably resistant isolates from cultures of T. rubrum, T. mentagrophytes, and E. floccosum cultured on plates containing 4×, 8×, or 16× the MIC of NB-002 were difficult to recover and were not stable. Two isolates selected with NB-002 had MICs no greater than 8 μg/ml. Spontaneous resistance to the comparator compounds ciclopirox, terbinafine, and itraconazole was seen only in a single isolate that had a 32-fold increase in the terbinafine MIC, the drug with which it was selected.
NB-002 was the only antifungal agent that killed both mycelia and microconidia. The fungicidal activity was rapid, even for fungal cell forms that were dormant (resting or nongrowing). The results of the time-kill studies were consistent with a physical or kill-on-contact mechanism of action for NB-002. Electron microscopy revealed disruption of the fungal cell surface and subsequent fungal lysis. Following a short exposure to NB-002, no hyphae or microconidial spores could be recovered. This mechanism is in contrast to the mechanisms of the comparator agents, which require cells that are actively growing to inhibit fungal metabolism (ciclopirox) or ergosterol biosynthesis (itraconazole and terbinafine). Even under conditions that support active growth (MIC and MFC studies), itraconazole, terbinafine, and ciclopirox were found to have mixed fungistatic and fungicidal activities, depending on the drug-isolate combination.
NB-002 is a broad-spectrum topical antifungal that is being developed for the treatment of diseases of the skin, hair, and nails. Previous work with human cadaver skin samples demonstrated that NB-002 uses a transfollicular route to enter the epidermal and dermal layers and that lateral diffusion occurs along tissue planes to sites distal from the application site (6, 7). NB-002 has fungicidal activity against both microconidial spore and mycelial forms of dermatophytes. NB-002 was not cross-resistant to the other antifungal agents tested, and little to no resistance development was shown with NB-002. Clinical studies of NB-002 for the treatment of herpes labialis confirm that there is no systemic exposure in patients, eliminating worry about drug-drug interactions and undesired systemic side effects with this topical treatment (32). Thus, future animal and clinical studies with NB-002 could prove its value for the treatment of cutaneous mycoses.
Acknowledgments
We acknowledge the laboratory of Mahmoud Ghannoum, director of the Center for Medical Mycology (Case Western Reserve University), for determining the antifungal activities of NB-002 and terbinafine against the isolates of T. tonsurans and terbinafine-resistant T. rubrum.
Footnotes
Published ahead of print on 11 May 2009.
REFERENCES
- 1.Alley, M. R., S. J. Baker, K. R. Beutner, and J. Plattner. 2007. Recent progress on the topical therapy of onychomycosis. Expert Opin. Investig. Drugs 16:157-167. [DOI] [PubMed] [Google Scholar]
- 2.Anandan, V., V. Nayak, S. Sundaram, and P. Srikanth. 2008. An association of Alternaria alternata and Scopulariopsis brevicaulis in cutaneous phaeohyphomycosis. Indian J. Dermatol. Venereol. Leprol. 74:244-247. [DOI] [PubMed] [Google Scholar]
- 3.Asticcioli, S., A. Di Silverio, L. Sacco, I. Fusi, L. Vincenti, and E. Romero. 2008. Dermatophyte infections in patients attending a tertiary care hospital in northern Italy. New Microbiol. 31:543-548. [PubMed] [Google Scholar]
- 4.Awadalla, F., D. A. Rosenbaum, F. Camacho, A. B. Fleischer, Jr., and S. R. Feldman. 2008. Dermatologic disease in family medicine. Fam. Med. 40:507-511. [PubMed] [Google Scholar]
- 5.Boivin, G., N. Goyette, and J. Sutcliffe. 2008. NB-001, a novel nanoemulsion active against acyclovir (ACV)- and foscarnet (FOS)-resistant herpes simplex virus (HSV) 1 and 2, abstr. V-3538. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 6.Ciotti, S., R. Eisma, L. Ma, and J. R. Baker, Jr. 2008. Novel nanoemulsion NB-001 permeates skin by the follucular route, abstr. A-1898. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 7.Ciotti, S., R. Eisma, L. Vengroff, L. Ma, and J. R. Baker, Jr. 2008. Mechanism of skin penetration and distribution of a novel antimicrobial nanoemulsion, abstr. M-2135. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 8.CLSI. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. CLSI document M26-A. CLSI, Wayne, PA.
- 9.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, approved standard, 2nd ed. CLSI document M38-A2, vol. 28. CLSI, Wayne, PA.
- 10.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed. CLSI document M27-A3. CLSI, Wayne, PA.
- 11.Elewski, B. E. 1996. Onychomycosis caused by Scytalidium dimidiatum. J. Am. Acad. Dermatol. 35:336-338. [DOI] [PubMed] [Google Scholar]
- 12.Elewski, B. E. 1998. Onychomycosis: pathogenesis, diagnosis, and management. Clin. Microbiol. Rev. 11:415-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falcon, C. S., M. Falcon Mdel, J. D. Ceballos, V. D. Florencio, V. C. Erchiga, and S. C. Ortega. 2009. Onychomycosis by Chaetomium spp. Mycoses 52:77-79. [DOI] [PubMed] [Google Scholar]
- 14.FDA. 2001. FDA Public Health Advisory. The safety of Sporanox capsules and Lamisil tablets for the treatment of onychomycosis. Center for Drug Evaluation and Research, FDA, Rockville, MD. http://www.fda.gov/cder/drug/advisory/sporanox-lamisil/advisory.htm.
- 15.Fernandes, N. C., F. Lamy, T. Akiti, and M. Barreiros. 1998. Microsporum gypseum infection in AIDS patient: a case report. An. Bras. Dermatol. 73:39-41. [Google Scholar]
- 16.Foster, K. W., M. A. Ghannoum, and B. E. Elewski. 2004. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J. Am. Acad. Dermatol. 50:748-752. [DOI] [PubMed] [Google Scholar]
- 17.Fritsche, T., D. Biedenbach, R. Jones, and J. Sutcliffe. 2008. Antimicrobial activity of nanoemulsions tested against seven gram-negative species, abstr. F1-3945. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 18.Garcia-Martos, P., I. Dominguez, P. Marin, M. Linares, J. Mira, and J. Calap. 2000. Onychomycoses caused by non-dermatophytic filamentous fungi in Cadiz. Enferm. Infecc. Microbiol. Clin. 18:319-324. [PubMed] [Google Scholar]
- 19.Ghannoum, M. A., B. Arthington-Skaggs, V. Chaturvedi, A. Espinel-Ingroff, M. A. Pfaller, R. Rennie, M. G. Rinaldi, and T. J. Walsh. 2006. Interlaboratory study of quality control isolates for a broth microdilution method (modified CLSI M38-A) for testing susceptibilities of dermatophytes to antifungals. J. Clin. Microbiol. 44:4353-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghannoum, M. A., R. A. Hajjeh, R. Scher, N. Konnikov, A. K. Gupta, R. Summerbell, S. Sullivan, R. Daniel, P. Krusinski, P. Fleckman, P. Rich, R. Odom, R. Aly, D. Pariser, M. Zaiac, G. Rebell, J. Lesher, B. Gerlach, G. F. Ponce-De-Leon, A. Ghannoum, J. Warner, N. Isham, and B. Elewski. 2000. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J. Am. Acad. Dermatol. 43:641-648. [DOI] [PubMed] [Google Scholar]
- 21.Ghannoum, M. A., L. A. Wraith, B. Cai, J. Nyirady, and N. Islam. 2008. Susceptibility of dermatophyte isolates obtained from a large worldwide terbinafine tinea capitis clinical trial, abstr. 1708. Abstr. 66th Annu. Meet. Am. Acad. Dermatol. [DOI] [PubMed]
- 22.Guilhermetti, E., G. Takahachi, C. S. Shinobu, and T. I. Svidzinski. 2007. Fusarium spp. as agents of onychomycosis in immunocompetent hosts. Int. J. Dermatol. 46:822-826. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez-Rodero, F., M. Moragon, V. Ortiz de la Tabla, M. J. Mayol, and C. Martin. 1999. Cutaneous hyalohyphomycosis caused by Paecilomyces lilacinus in an immunocompetent host successfully treated with itraconazole: case report and review. Eur. J. Clin. Microbiol. Infect. Dis. 18:814-818. [DOI] [PubMed] [Google Scholar]
- 24.Hall, V. C., S. Goyal, M. D. Davis, and J. S. Walsh. 2004. Cutaneous hyalohyphomycosis caused by Paecilomyces lilacinus: report of three cases and review of the literature. Int. J. Dermatol. 43:648-653. [DOI] [PubMed] [Google Scholar]
- 25.Hamouda, T., and J. R. Baker, Jr. 2000. Antimicrobial mechanism of action of surfactant lipid preparations in enteric gram-negative bacilli. J. Appl. Microbiol. 89:397-403. [DOI] [PubMed] [Google Scholar]
- 26.Hamouda, T., M. M. Hayes, Z. Cao, R. Tonda, K. Johnson, D. C. Wright, J. Brisker, and J. R. Baker, Jr. 1999. A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus species. J. Infect. Dis. 180:1939-1949. [DOI] [PubMed] [Google Scholar]
- 27.Hamouda, T., A. Myc, B. Donovan, A. Y. Shih, J. D. Reuter, and J. R. Baker, Jr. 2001. A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria, enveloped viruses and fungi. Microbiol. Res. 156:1-7. [DOI] [PubMed] [Google Scholar]
- 28.Hattori, N., A. Shirai, Y. Sugiura, W. Li, K. Yokoyama, Y. Misawa, K. Okuzumi, and K. Tamaki. 2005. Onychomycosis caused by Fusarium proliferatum. Br. J. Dermatol. 153:647-649. [DOI] [PubMed] [Google Scholar]
- 29.Havlickova, B., V. A. Czaika, and M. Friedrich. 2008. Epidemiological trends in skin mycoses worldwide. Mycoses 51(Suppl. 4):2-15. [DOI] [PubMed] [Google Scholar]
- 30.Havlickova, B., and M. Friedrich. 2008. The advantages of topical combination therapy in the treatment of inflammatory dermatomycoses. Mycoses 51(Suppl. 4):16-26. [DOI] [PubMed] [Google Scholar]
- 31.Janssen Pharmaceutica Products. 2001. Sporanox (itraconazole) capsules package insert. Janssen Pharmaceutica Products, L.P., Titusville, NJ.
- 32.Jarrat, M., M. M. Ijzerman, M. R. Flack, and J. R. Baker, Jr. 2008. Safety, tolerability and tharmacokinetics of NB-001 in a phase 2 dose ranging trial in subjects with recurrent herpes labialis, abstr. V-3539. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 33.Lass-Florl, C., M. Nagl, C. Speth, H. Ulmer, M. P. Dierich, and R. Wurzner. 2001. Studies of in vitro activities of voriconazole and itraconazole against Aspergillus hyphae using viability staining. Antimicrob. Agents Chemother. 45:124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malani, A. N., and C. A. Kauffman. 2007. Changing epidemiology of rare mould infections: implications for therapy. Drugs 67:1803-1812. [DOI] [PubMed] [Google Scholar]
- 35.Myc, A., T. Vanhecke, J. J. Landers, T. Hamouda, and J. R. Baker, Jr. 2002. The fungicidal activity of novel nanoemulsion (X8W60PC) against clinically important yeast and filamentous fungi. Mycopathologia 155:195-201. [DOI] [PubMed] [Google Scholar]
- 36.Ninet, B., I. Jan, O. Bontems, B. Lechenne, O. Jousson, D. Lew, J. Schrenzel, R. G. Panizzon, and M. Monod. 2005. Molecular identification of Fusarium species in onychomycoses. Dermatology 210:21-25. [DOI] [PubMed] [Google Scholar]
- 37.Nishiyama, Y., Y. Asagi, T. Hiratani, H. Yamaguchi, N. Yamada, and M. Osumi. 1992. Morphological changes associated with growth inhibition of Trichophyton mentagrophytes by amorolfine. Clin. Exp. Dermatol. 17(Suppl. 1):13-17. [DOI] [PubMed] [Google Scholar]
- 38.Novartis Pharmaceuticals Corporation. 2005. Lamisil tablets package insert. Novartis Pharmaceuticals Corporation, East Hanover, NJ.
- 39.O'Keeffe, M. F. 1973. A report of three human infections due to Microsporum nanum. Aust. J. Dermatol. 14:73-74. [DOI] [PubMed] [Google Scholar]
- 40.Onsberg, P. 1978. Human infections with Microsporum gypseum in Denmark. Br. J. Dermatol. 99:527-530. [DOI] [PubMed] [Google Scholar]
- 41.Onsberg, P. 1978. Human infections with Microsporum persicolor in Denmark. Br. J. Dermatol. 99:531-536. [DOI] [PubMed] [Google Scholar]
- 42.Onsberg, P., D. Stahl, and N. K. Veien. 1978. Onychomycosis caused by Aspergillus terreus. Sabouraudia 16:39-46. [PubMed] [Google Scholar]
- 43.Pannu, J., A. McCarthy, A. Martin, T. Hamouda, and J. Sutcliffe. 2008. NB-002, a novel nanoemulsion with anti-dermatophyte activity, abstr. M-2134. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 44.Pfaller, M. A., D. J. Sheehan, and J. H. Rex. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seebacher, C., J. P. Bouchara, and B. Mignon. 2008. Updates on the epidemiology of dermatophyte infections. Mycopathologia 166:335-352. [DOI] [PubMed] [Google Scholar]
- 46.Segal, E. 2005. Candida, still number one—what do we know and where are we going from there? Mycoses 48(Suppl. 1):3-11. [DOI] [PubMed] [Google Scholar]
- 47.Suh, D. C., S. F. Friedlander, M. Raut, J. Chang, L. Vo, H. C. Shin, and A. Tavakkol. 2006. Tinea capitis in the United States: diagnosis, treatment, and costs. J. Am. Acad. Dermatol. 55:1111-1112. [DOI] [PubMed] [Google Scholar]
- 48.Sutcliffe, J., D. Biedenbach, R. Jones, and T. Fritsche. 2008. Novel nanoemulsion antimicrobials tested against nine gram-positive species, abstr. F1-3944. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 49.Veer, P., N. S. Patwardhan, and A. S. Damle. 2007. Study of onychomycosis: prevailing fungi and pattern of infection. Indian J. Med. Microbiol. 25:53-56. [DOI] [PubMed] [Google Scholar]
- 50.Weinstein, A., and B. Berman. 2002. Topical treatment of common superficial tinea infections. Am. Fam. Physician 65:2095-2102. [PubMed] [Google Scholar]
- 51.White, T. C., S. Holleman, F. Dy, L. F. Mirels, and D. A. Stevens. 2002. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 46:1704-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu-Yuan, C. D., and T. Hashimoto. 1977. Architecture and chemistry of microconidial walls of Trichophyton mentagrophytes. J. Bacteriol. 129:1584-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, A. Y., W. L. Camp, and B. E. Elewski. 2007. Advances in topical and systemic antifungals. Dermatol. Clin. 25:165-183. [DOI] [PubMed] [Google Scholar]