Abstract
Colistin is used to treat infections caused by multidrug-resistant gram-negative bacteria (MDR-GNB). It is administered intravenously in the form of colistin methanesulfonate (CMS), which is hydrolyzed in vivo to the active drug. However, pharmacokinetic data are limited. The aim of the present study was to characterize the pharmacokinetics of CMS and colistin in a population of critically ill patients. Patients receiving colistin for the treatment of infections caused by MDR-GNB were enrolled in the study; however, patients receiving a renal replacement therapy were excluded. CMS was administered at a dose of 3 million units (240 mg) every 8 h. Venous blood was collected immediately before and at multiple occasions after the first and the fourth infusions. Plasma CMS and colistin concentrations were determined by a novel liquid chromatography-tandem mass spectrometry method after a rapid precipitation step that avoids the significant degradation of CMS and colistin. Population pharmacokinetic analysis was performed with the NONMEM program. Eighteen patients (6 females; mean age, 63.6 years; mean creatinine clearance, 82.3 ml/min) were included in the study. For CMS, a two-compartment model best described the pharmacokinetics, and the half-lives of the two phases were estimated to be 0.046 h and 2.3 h, respectively. The clearance of CMS was 13.7 liters/h. For colistin, a one-compartment model was sufficient to describe the data, and the estimated half-life was 14.4 h. The predicted maximum concentrations of drug in plasma were 0.60 mg/liter and 2.3 mg/liter for the first dose and at steady state, respectively. Colistin displayed a half-life that was significantly long in relation to the dosing interval. The implications of these findings are that the plasma colistin concentrations are insufficient before steady state and raise the question of whether the administration of a loading dose would benefit critically ill patients.
The worldwide increase in the incidence of antimicrobial resistance among gram-negative bacteria poses a serious threat to the management of infections, particularly in hospital settings. Infections caused by multidrug-resistant gram-negative bacteria (MDR-GNB), including Acinetobacter baumannii, Pseudomonas aeruginosa, and members of the family Enterobacteriaceae, are becoming increasingly difficult to treat due to accumulating resistance through extended-spectrum beta-lactamases, carbapenemases, and alterations in permeability and efflux.
In this setting, colistin, a polypeptide antibiotic available since the 1960s but rarely used due to efficacy and safety concerns, has reemerged as an alternative for the management of MDR-GNB infections (14). Colistin is a peptide antibiotic consisting of two main components, colistin A (polymyxin E1) and colistin B (polymyxin E2). It is systemically administered in the form of colistin methanesulfonate (CMS), which is less toxic and which is hydrolyzed to colistin and a number of intermediate species both in vitro and in vivo. CMS is a prodrug of colistin and has no intrinsic antibacterial activity (2). Pharmacodynamically, colistin is a potent antimicrobial agent that causes rapid bacterial killing in a concentration-dependent manner (6, 15, 24). The Clinical and Laboratory Standards Institute (CLSI) has published MIC interpretation guidelines only for Pseudomonas aeruginosa and Acinetobacter baumannii, the main pathogens targeted by colistin. In Europe, the respective breakpoints for both these organisms and the Enterobacteriaceae are published by the European Committee on Antimicrobial Susceptibility Testing (EUCAST). According to both the CLSI (3) and EUCAST (5), susceptibility for P. aeruginosa and A. baumannii is defined as a MIC of ≤2 mg/liter.
The optimal dosage of colistin is unclear due to a lack of accurate pharmacokinetic and pharmacodynamic information. Most of the available pharmacokinetic data are based on the results of microbiological assays (22) that are erroneous for colistin due to degradation and diffusion problems. Only a few studies have studied the pharmacokinetics of colistin by high-pressure liquid chromatography (HPLC)-based methods (16, 23, 25), and two of these were carried out with patients with cystic fibrosis, in whom the pharmacokinetics of colistin may differ from those in critically ill patients.
The aim of the present study was to examine the pharmacokinetics of colistin after the administration of intravenous doses of CMS in a population of critically ill patients. We describe a population pharmacokinetic analysis in which all available pharmacokinetic data are modeled simultaneously and, thereby, data are shared during the analysis among the individual study subjects. Typical trends in the population are characterized, and variability between patients and between occasions is described. An advantage of model-based analysis is that the model that is developed can easily be used to predict and simulate various dosing regimens.
MATERIALS AND METHODS
Subjects.
Patients admitted to the Critical Care Unit and the 4th Department of Internal Medicine of Attikon University General Hospital in Athens, Greece, were eligible for the study if they fulfilled the following inclusion criteria: (i) the patients were age 18 years and older and (ii) the patients were receiving colistin treatment as part of their standard care due to the presence of a probable or a documented infection caused by MDR-GNB. Patients were excluded if they received continuous venovenous hemodiafiltration as renal replacement therapy. For each patient, the following were recorded on the first day of colistin administration: age; body weight; serum creatinine, serum albumin, hemoglobin, and hematocrit levels; and APACHE II score. Creatinine clearance was calculated by use of the formula of Cockcroft-Gault (4).
The study was approved by the Ethics Committee of the hospital (regulation no. 3/30-3-07).
Colistin administration.
CMS (colistin; Norma, Greece) was administered at a dose of 3 million units (MU; equivalent to 240 mg) dissolved in 100 ml of normal saline every 8 h by intravenous infusion over 15 min. Due to the lack of clear and evidence-based recommendations on the CMS dosage adjustment required for patients with renal failure (14), the dose was empirically reduced to 160 mg every 8 h for patients with a calculated creatinine clearance of less than 50 ml/min.
Sampling.
Venous blood was collected immediately before the first and fourth infusions and at 15, 30, 60, 90, 120, 240, 360, and 465 min after the end of the first and fourth infusions. For selected patients, sampling was performed after the sixth or seventh infusion because it was uncertain if steady state had been reached after administration of the fourth dose. In addition, samples were obtained from additional patients immediately before and at 60, 240, 480, 720, and 1440 min after the end of the last infusion, when colistin therapy was discontinued. All blood samples were immediately chilled and centrifuged, and the plasma was collected and stored at −70°C until it was assayed.
Colistin concentration determination.
Plasma colistin A and colistin B concentrations were determined by a novel liquid chromatography-tandem mass spectrometry method (8). In brief, the samples were thawed in an ice bath, and after protein precipitation with acetonitrile (ACN) containing 0.1% trifluoroacetic acid (TFA), the supernatants were diluted with 0.03% TFA. After this quick sample preparation step, separation was carried out with an Ultrasphere C18 column and a mobile phase consisting of 25% ACN in 0.03% TFA, and detection was performed by tandem mass spectrometry. The lower limits of quantification for 100 μl plasma were 19.4 and 10.5 ng/ml for colistin A and B, respectively; the coefficient of variation (CV) was <6.2%, and the accuracy was <±12.6%.
Plasma CMS concentrations and hydrolysis intermediates were determined by the hydrolysis of CMS to colistin by the method described by Li et al. (17), with some modifications (8). Sulfuric acid (1 M) was mixed with the plasma sample, and after 15 to 20 min, sodium hydroxide (1 M) was added. Thereafter, proteins were precipitated with ACN containing TFA, as described above. CMS concentrations were determined by subtracting the concentration of colistin determined in the samples before hydrolysis from the colistin concentration determined after hydrolysis.
Population pharmacokinetic modeling.
A nonlinear mixed-effects model analysis was performed in which all concentration-time data were modeled simultaneously. The mean tendencies in the population (typical values) were described, as were the random effects, including the variability between subjects (i.e., interindividual variability [IIV]), the variability between occasions (i.e., interoccasion variability [IOV]), and the residual variability.
The time course of the concentrations of CMS A and colistin A and of CMS B and colistin B showed similar shapes, and the data for the A and the B forms were therefore added to the data set used for pharmacokinetic analysis. The B forms made up approximately 21% of the total concentration. One-, two-, and three-compartment models with linear and nonlinear elimination were evaluated for CMS and colistin. First, the model for CMS was built, and thereafter, the colistin data were added and the colistin model was constructed. Colistin was assumed to be formed by CMS, and as the fraction of CMS that forms colistin cannot be determined from these data alone, all colistin pharmacokinetic parameters were scaled by the (unknown) fraction of CMS metabolized to colistin (referred to as the colistin formed [fm]).
The residual error was modeled by using an additive, a proportional, or a combined additive and proportional error model with the log-transformed concentration data in molar units (molar masses are, on average, 1.743 g/mol for CMS and 1,163 g/mol for colistin). The IIV and the IOV in the model parameters were assumed to be log-normally distributed. For parameters for which IOV was of importance, we tested if there were systematic differences, e.g., because of improvements in patient disease status, in the values of the parameters during the first dose or during the last dose compared to the values on other occasions when the drug concentrations were determined. The covariance between parameters was also investigated.
Covariate model building was performed in a stepwise fashion with forward inclusion and backward deletion. Because of the limited number of subjects, only body weight, ideal body weight, age, creatinine clearance, and hemoglobin and hematocrit levels were evaluated as covariates.
To keep a more complex structural model, to include IIV and IOV in the parameters, and for a covariate to remain in the model, a statistical significance level with a P value of <0.001 (corresponding to a reduction in the objective function value of at least 10.83 for 1 degree of freedom) was required. A visual predictive check was performed to evaluate the model in which 500 replicates were simulated from the model developed by using the original data set as a template. The average steady-state concentrations achieved by different dosing regimens were predicted for a typical patient on the basis of the model that was developed with the aim of achieving the same average steady-state concentrations achieved with the current dosing regimen.
Software.
The data were analyzed by using the first-order conditional estimation method with the interaction option in the population analysis software NONMEM (version VI; Icon Development Solutions, Ellicott City, MD). The Xpose program (version 4) was used to check the data set and graphical evaluations (9). The Perl speaks NONMEM (PsN) toolkit (19) was used for stepwise covariate model building and to compute the extent of shrinkage (10) in empirical Bayes estimates (eta shrinkage) and individual predictions (epsilon shrinkage).
RESULTS
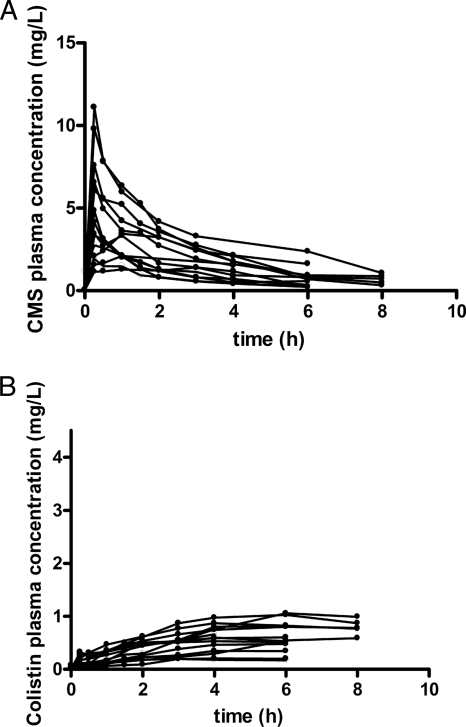
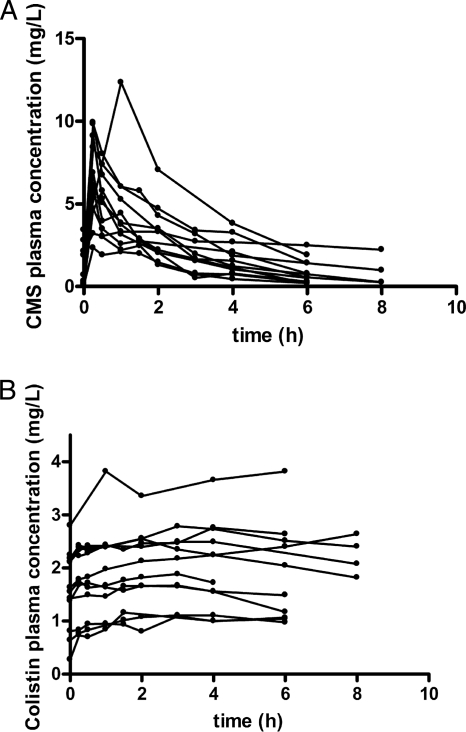
Eighteen patients (12 males, 6 females) were enrolled in this study. The mean age was 63.6 years (age range, 40 to 83 years), and the mean ± standard deviation calculated creatinine clearance was 82.3 ± 24.35 ml/min on the first day of treatment. Demographic and clinical data for each patient are shown in Table 1. For three patients (Table 1, patients 24, 28, and 29), samples were collected only after the last dose, following the termination of treatment; and for two patients (Table 1, patients 4 and 16), no samples were available after the fourth dose or the sixth dose. For four patients (Table 1, patients 11, 12, 13, and 15), samples were collected after the sixth dose. The observed individual plasma CMS and colistin concentrations versus time after the first dose are presented in Fig. 1. The respective concentrations after the fourth dose are shown in Fig. 2.
TABLE 1.
Demographic and clinical data of the enrolled patientsa
| Patient no. | Gender | Age (yr) | Body wt (ideal body wt) (kg) | Dose (MU) | Serum creatinine concn (mg/dl) | Creatinine clearance (ml/min) | Serum albumin concn (g/dl) | Apache II score | Diagnosis | Reason for colistin administration |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 52 | 70 (65) | 9 | 0.8 | 84 | 2.0 | 17 | Breast cancer | VAP |
| 2 | Μ | 69 | 100 (75) | 9 | 1.3 | 57 | 2.9 | 15 | PH | VAP |
| 3 | Μ | 71 | 110 (75) | 9 | 0.8 | 90 | 3.2 | 17 | COPD | VAP |
| 4 | F | 66 | 65 (65) | 9 | 0.6 | 94 | 2.9 | 12 | Breast cancer | VAP |
| 5 | Μ | 79 | 90 (75) | 9 | 0.8 | 79 | 2.7 | 18 | Colon cancer | Sepsis |
| 6 | F | 62 | 110 (70) | 9 | 0.7 | 92 | 2.1 | 10 | Wound infection | Sepsis |
| 7 | Μ | 46 | 85 (75) | 9 | 1.0 | 98 | 2.0 | 13 | Trauma | VAP |
| 8 | F | 67 | 65 (65) | 9 | 1.0 | 66 | 2.4 | 5 | Heat stroke | VAP |
| 9 | Μ | 70 | 90 (75) | 6 | 1.8 | 41 | 2.1 | 20 | Wound infection | Sepsis |
| 10 | F | 70 | 85 (65) | 9 | 0.7 | 77 | 4.1 | 12 | Epilepsy | VAP |
| 11 | F | 49 | 70 (60) | 9 | 0.6 | 126 | 4.1 | 12 | ICH | VAP |
| 12 | Μ | 40 | 80 (75) | 9 | 1.2 | 87 | 2.4 | 9 | Pneumonia | Bacteremia |
| 13 | Μ | 70 | 80 (70) | 9 | 1.3 | 52 | 2.5 | 15 | MVR | Bacteremia |
| 14 | M | 49 | 75 (70) | 9 | 0.7 | 126 | 3.2 | 12 | Pancreatic cancer | Sepsis |
| 15 | M | 74 | 75 (75) | 9 | 0.9 | 77 | 2.5 | 9 | CVA | Bacteremia |
| 16 | M | 83 | 85 (85) | 9 | 0.8 | 84 | 2.5 | 12 | Parkinson's disease | Bacteremia |
| 17 | M | 48 | 70 (70) | 9 | 0.8 | 112 | 2.8 | 6 | Multiple sclerosis | VAP |
| 18 | M | 80 | 75 (70) | 6 | 1.4 | 42 | 2.8 | 17 | Encephalopathy | VAP |
F, female; M, male; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ICH, intracranial hemorrhage; MVR, mitral valve regurgitation; PH, pulmonary hypertension; VAP, ventilator-associated pneumonia.
FIG. 1.
Observed individual concentrations of CMS (A) and colistin (B) in plasma after the administration of the first dose of CMS. Data for patients 14, 15, 17, and 18 (Table 1) were not available after the first dose.
FIG. 2.
Observed individual concentrations of CMS (A) and colistin (B) in plasma after the administration of the fourth dose of CMS. Data for patients 4, 14, 15, 16, 17, and 18 (Table 1) were not available after the fourth dose.
Data analysis.
Both CMS and colistin displayed linear pharmacokinetics. For CMS, a two-compartment model fit the data the best, and for colistin, a one-compartment model was sufficient. Parameter estimates are presented in Table 2. Combined additive and proportional residual error models were used for both CMS and colistin. IIV was included for the clearance (CL) of CMS and the CL of formed colistin (CL/fm), and IIV was included in the residual error magnitude of colistin. The inclusion of covariance between parameters did not improve the model fit; however, IOV was significant in the CL of CMS, the peripheral volume of distribution of CMS, and the (unknown) fraction that formed colistin; i.e., the same IOV parameter was included for both CL/fm and the volume of distribution of formed colistin. No systematic change in any of the pharmacokinetic parameters was detected over time. Hemoglobin and hematocrit levels were the only statistically significant covariates and were estimated to affect the intercompartmental CL of CMS (changes in the objective function value, −18.8 and −18.4, respectively, in the univariate analysis). However, there was no drop in the estimates of IIV or IOV, indicating that the inclusion of these covariates did not explain the between-subject variability. Therefore, these covariates were not included in the final model.
TABLE 2.
Estimated population pharmacokinetic parametersa
| Parameter | CMS
|
Colistin
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CL (liters/h) | V1 (liters) | Q (liters/h) | V2 (liters) | Residual error
|
CL/fm (liters/h) | V/fm (liters) | Residual error
|
|||
| Proportional (%) | Additive (nmol/liter) | Proportional (%) | Additive (nmol/liters) | |||||||
| Typical value (% RSE) | 13.7 (10) | 13.5 (45) | 133 (35) | 28.9 (22) | 22.0 (5.4) | 9.11 (11) | 9.09 (19) | 189 (12) | 7.19 (9.1) | 4.98 (12) |
| IIV (% CV) | 37 (15) | 59 (36) | 35b (34) | 35b (34) | ||||||
| IOV (% CV | 28 (12) | 58 (25) | 43 (16)c | 43 (16)c | ||||||
V1, volume of distribution of the central compartment for CMS; V2, volume of distribution of the peripheral compartment for CMS; Q, intercompartmental CL for CMS; CL, clearance of CMS; CL/fm, clearance of formed colistin; V/fm, volume of distribution of formed colistin; RSE, relative standard error.
A common IIV for the residual error terms was used.
For colistin, a common IOV for CL/fm and the volume of distribution of formed colistin was used.
Goodness-of-fit plots for each of the individuals at each of the observed occasions are presented for the CMS and colistin data in Fig. S1 in the supplemental material. The visual predictive check (Fig. 3) showed that the model explained the observed data well. The observed medians of the data were well included within the model-predicted 95% confidence intervals of the medians for both the first and the fourth doses and for colistin and CMS. The relatively small number of patients limited our evaluation of how well the model can characterize the general trend (median) of the data; i.e., evaluation of the outer percentiles would not be meaningful.
FIG. 3.
Visual predictive check of the model following the first dose (top row) and fourth dose (bottom row) for CMS (left) and colistin (right). The dark gray solid lines are the medians of the simulated data, with their 95% confidence interval (uncertainty) being indicated with areas shaded gray. The black dashed lines are the medians of the observed data (⋄) and should be within the 95% confidence intervals for a perfect model.
Omission of the data for three patients when samples were taken following the last colistin dose resulted in a maximum change in any fixed-effect parameter of −8.1% (CL/fm) and a maximum change in a variance parameter of 16% (IIV in the residual error for colistin). When these patients were allowed to have values of one or several pharmacokinetic parameters different from those for the other patients, there was no significant improvement in the model (P > 0.05).
The half-lives of the two phases of CMS disposition were 0.046 and 2.3 h, respectively, for a typical individual, and the half-life of colistin was determined to 14.4 h. The predicted maximum concentrations in plasma (Cmaxs) were 0.60 mg/liter for the first dose of 3 MU CMS and 2.3 mg/liter following repeated administration of 3 MU CMS every 8 h (Fig. 4).
FIG. 4.
Model-predicted CMS (A) and colistin (B) concentrations in a typical patient following the use of the current dosing regimen (3 MU as a 15-min infusion of CMS every 8 h [q8h]) and alternative dosing regimens with loading doses of 9 or 12 MU CMS as infusions of 15 min or 2 h and a maintenance dose of 4.5 MU CMS every 12 h (q12h).
The predicted plasma CMS and colistin concentrations on the basis of the model developed for the currently used dosage regimen (3 MU as 15-min infusions every 8 h) and for dosage regimens with a loading dose and a maintenance dose of 4.5 MU every 12 h (with infusion lengths of 15 min or 2 h) are shown in Fig. 4. A 2-h infusion resulted in lower peak concentrations of CMS but a similar concentration-time profile for colistin as a 15-min infusion of the same total dose.
DISCUSSION
Colistin is a reemerging antimicrobial due to the increased incidence of infections caused by gram-negative pathogens resistant to most available classes of antimicrobial agents. However, pharmacokinetic data on colistin are scarce. The results of the present study indicate that when it is administered to critically ill patients, colistin has kinetics different from those described earlier.
In contrast to previously published data, the rate of formation of colistin from CMS in plasma appeared to be much lower in the present study, although the estimated half-life of CMS was similar to what has been reported earlier in patients with cystic fibrosis (16, 25). This might partly be because of the nonenzymatic hydrolysis of CMS to colistin ex vivo in earlier studies. Even a low percentage of CMS degradation after sampling and during the workup procedure can have a pronounced influence on the colistin concentrations at time points when the CMS concentration is high and the colistin concentration is low (12; Jansson et al., submitted). The quick chilling of blood samples and the use of a fast workup procedure in a cool environment, such as the one used in the current study, may be crucial for minimizing the degradation of CMS to colistin and avoiding falsely high colistin concentrations. The preparation steps that earlier pharmacokinetic studies of colistin have applied (21) might be the cause for the relatively high colistin concentrations determined at time points when the CMS concentrations are high. A falsely high colistin concentration at early time points would have two consequences: first, the half-life would appear to be shorter than the true value; and second, Cmax would be attained earlier. In the present study, the estimated typical half-life was 14.4 h when a model in which colistin is formed by a first-order process from CMS was used. The typical Cmax was estimated to 2.3 mg/liter at steady state and to occur at approximately 7 h after the start of the infusion.
Previous data that are based on the results of microbiological assays (22) are inaccurate for colistin due to the erroneous diffusion of CMS in agar and the instability of CMS in aqueous media (14). Two more recent studies have employed HPLC methods to measure the concentrations of colistin and its prodrug, CMS, in patients with cystic fibrosis. In those studies, colistin was studied after at least two days of treatment, and the colistin half-lives were determined to 4.2 h and 3.4 h, respectively (16, 25). The significant difference in the values of the pharmacokinetic parameters compared to those in patients with cystic fibrosis may have additional explanations. It is well known that the half-lives of drugs are typically shorter in patients with cystic fibrosis (26).
Only one previous report has described the pharmacokinetics of colistin in critically ill patients (23). Fourteen patients were studied after at least 2 days of CMS administration (3 MU, three times daily). The mean Cmax was 2.93 mg/liter, which occurred 15 min after the end of the 30-min infusion. The half-life was determined to 7.1 h. However, the CMS concentration was not measured and pharmacokinetic analysis of the concentrations after the first dose was not performed (23).
In the present study there was no indication of a correlation between colistin kinetics and creatinine clearance. This finding is in agreement with the results of previous studies demonstrating that colistin CL is mainly effected by nonrenal routes (13, 18). However, the nature of these mechanisms remains unknown. There was neither a significant correlation between the CL of CMS and creatinine clearance in the current study, although CMS has been reported to be partly renally excreted in rats (13). The limited number of patients and the relatively small range of creatinine clearance values may have precluded such a finding. The lack of a correlation between colistin kinetics and creatinine clearance raises the issue of the colistin dose that should be administered to critically ill patients with renal failure. CMS has been administered at a reduced dose in this setting, but this approach might be associated with subtherapeutic concentrations of colistin, further compromising the outcomes for the patients.
The typical trends of the CMS and colistin concentrations were well described by the model (Fig. 3), and the concentration-time profiles for each individual were adequately characterized (see Fig. S1 in the supplemental material). However, the estimated variability between patients and between occasions was relatively high, and since there were only 18 patients in this population analysis, these variability estimates should be treated with caution.
Data on the concentration of colistin after the first dose have not previously been available for the population of critically ill patients. The results from this study indicate that for a typical patient, colistin concentrations are below the MIC breakpoints (2 mg/liter) after the first few doses of the currently used dosing regimen, in effect signifying a delay in appropriate treatment. The significance of these observations cannot be overemphasized, as the delayed initiation of appropriate antimicrobial therapy is associated with increased mortality in critically ill patients (7, 11, 20).
Even at steady state, the plasma concentrations, as measured in the present study, are, in many cases, below the MIC breakpoints for the Enterobacteriaceae and P. aeruginosa, frequent multiresistant pathogens in critically ill patients (3, 5). These observations are a cause for concern, as they may indicate the suboptimal efficacy of the current colistin regimen for the treatment of infections caused by pathogens with MICs in the upper range of the susceptibility breakpoint for colistin, as well as the selection of resistant strains. Consequently, these data should be taken into account in the definition of susceptibility breakpoints of colistin for gram-negative pathogens.
On the basis of the findings of the present study, a reevaluation of the CMS dosage appears to be warranted. The use of a loading dose and longer dosing intervals might be more appropriate and deserves further study. From predictions from the model developed in this study, it is obvious that without the administration of a loading dose, it will take 2 to 3 days before the steady-state concentration of colistin is obtained for a typical individual (Fig. 4B). A loading dose of 9 MU or even 12 MU CMS and a maintenance dose of 4.5 MU CMS every 12 h would result in the same average steady-state concentration of colistin achieved with the current dosing schedule but would achieve the target concentration faster and lead to the need for less frequent administration (Fig. 4B). These or similar dosing regimens remain to be tested clinically, to ascertain whether the efficacy is better without a concomitant increase in toxicity. Concerns over renal toxicity with the administration of high doses have been expressed on the basis of the findings of experiments with animals (27), but such renal toxicity with the administration of high doses has not yet been confirmed in humans. On the basis of the model described here, the colistin concentrations would not exceed those already attained at steady state. On the other hand, even though colistin is considered more toxic than CMS (1), it is not clear whether high CMS concentrations, attained with the higher loading and maintenance doses, would confer the potential of increased toxicity. However, the CMS Cmax can be lowered by increasing the infusion time of the loading dose. A 2-h infusion will not compromise the time required for colistin to reach efficacious levels (Fig. 4A); however, the potential degradation of CMS to colistin in the infusion solution must be considered.
In conclusion, this is the first report of a population pharmacokinetic analysis of colistin after intravenous administration in critically ill patients. The results suggest that a change in the dosing strategy for colistin may be needed. The proper use of this antimicrobial is important, as it remains one of the steadily diminishing options for the treatment of infections caused by MDR-GNB.
Supplementary Material
Acknowledgments
We thank Britt Jansson for skillful technical assistance.
Norma Hellas S.A. provided financial support for the study. L. E. Friberg was supported by the Knut & Alice Wallenberg Foundation of Sweden.
Footnotes
Published ahead of print on 11 May 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Barnett, M., S. R. Bushby, and S. Wilkinson. 1964. Sodium sulphomethyl derivatives of polymyxins. Br. J. Pharmacol. Chemother. 23:552-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergen, P. J., J. Li, C. R. Rayner, and R. L. Nation. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing. Sixteenth informational supplement. Document M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 5.European Committee on Antimicrobial Susceptibility Testing. 19 June 2008. Clinical breakpoints. http:/www.srga.org/eucastwt/MICTAB/MICmiscellaneous.html/. European Committee on Antimicrobial Susceptibility Testing.
- 6.Gunderson, B. W., K. H. Ibrahim, L. B. Hovde, T. L. Fromm, M. D. Reed, and J. C. Rotschafer. 2003. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 47:905-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iregui, M., S. Ward, G. Sherman, V. J. Fraser, and M. H. Kollef. 2002. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 122:262-268. [DOI] [PubMed] [Google Scholar]
- 8.Jansson, B., M. Karvanen, O. Cars, D. Plachouras, and L. E. Friberg. 2009. Quantitative analysis of colistin A and colistin B in plasma and culture medium using a simple precipitation step followed by LC/MS/MS. J. Pharm. Biomed. Anal. 49:760-767. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson, E. N., and M. O. Karlsson. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51-64. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson, M. O., and R. M. Savic. 2007. Diagnosing model diagnostics. Clin. Pharmacol. Ther. 82:17-20. [DOI] [PubMed] [Google Scholar]
- 11.Kumar, A., D. Roberts, K. E. Wood, B. Light, J. E. Parrillo, S. Sharma, R. Suppes, D. Feinstein, S. Zanotti, L. Taiberg, D. Gurka, A. Kumar, and M. Cheang. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34:1589-1596. [DOI] [PubMed] [Google Scholar]
- 12.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, and K. Coulthard. 2003. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob. Agents Chemother. 47:1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, T. C. Smeaton, and K. Coulthard. 2004. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J. Antimicrob. Chemother. 53:837-840. [DOI] [PubMed] [Google Scholar]
- 14.Li, J., R. L. Nation, J. D. Turnidge, R. W. Milne, K. Coulthard, C. R. Rayner, and D. L. Paterson. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect. Dis. 6:589-601. [DOI] [PubMed] [Google Scholar]
- 15.Li, J., J. Turnidge, R. Milne, R. L. Nation, and K. Coulthard. 2001. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, J., K. Coulthard, R. Milne, R. L. Nation, S. Conway, D. Peckham, C. Etherington, and J. Turnidge. 2003. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J. Antimicrob. Chemother. 52:987-992. [DOI] [PubMed] [Google Scholar]
- 17.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, K. Coulthard, and J. Valentine. 2002. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob. Agents Chemother. 46:3304-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, T. C. Smeaton, and K. Coulthard. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 47:1766-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindbom, L., J. Ribbing, and E. N. Jonsson. 2004. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput. Methods Programs Biomed. 75:85-94. [DOI] [PubMed] [Google Scholar]
- 20.Luna, C. M., P. Aruj, M. S. Niederman, J. Garzon, D. Violi, A. Prignoni, F. Rios, S. Baquero, and S. Gando. 2006. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur. Respir. J. 27:158-164. [DOI] [PubMed] [Google Scholar]
- 21.Ma, Z., J. Wang, J. P. Gerber, and R. W. Milne. 2008. Determination of colistin in human plasma, urine and other biological samples using LC-MS/MS. J. Chromatogr. B 862:205-212. [DOI] [PubMed] [Google Scholar]
- 22.Mackay, D. N., and D. Kaye. 1964. Serum concentrations of colistin in patients with normal and impaired renal function. N. Engl. J. Med. 270:394-397. [DOI] [PubMed] [Google Scholar]
- 23.Markou, N., S. L. Markantonis, E. Dimitrakis, D. Panidis, E. Boutzouka, S. Karatzas, P. Rafailidis, H. Apostolakos, and G. Baltopoulos. 2008. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin. Ther. 30:143-151. [DOI] [PubMed] [Google Scholar]
- 24.Owen, R. J., J. Li, R. L. Nation, and D. Spelman. 2007. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J. Antimicrob. Chemother. 59:473-477. [DOI] [PubMed] [Google Scholar]
- 25.Reed, M. D., R. C. Stern, M. A. O'Riordan, and J. L. Blumer. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J. Clin. Pharmacol. 41:645-654. [DOI] [PubMed] [Google Scholar]
- 26.Touw, D. J. 1998. Clinical pharmacokinetics of antimicrobial drugs in cystic fibrosis. Pharm. World Sci. 20:149-160. [DOI] [PubMed] [Google Scholar]
- 27.Wallace, S. J., J. Li, R. L. Nation, C. R. Rayner, D. Taylor, D. Middleton, R. W. Milne, K. Coulthard, and J. D. Turnidge. 2008. Subacute toxicity of colistin methanesulfonate in rats: comparison of various intravenous dosage regimens. Antimicrob. Agents Chemother. 52:1159-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.