Abstract
We determined the mode of action of nukacin ISK-1. It did not cause membrane potential dissipation or the efflux of ATP or K+ ions from the cells of a sensitive bacterial strain; however, it blocked the membrane depolarization activity of nisin. Nukacin ISK-1-treated cells had single arrangements of cells without the formation of a complete septum. A remarkable reduction in cell wall width was also observed, but cytoplasmic content was unaffected. We concluded that nukacin ISK-1 is bacteriostatic.
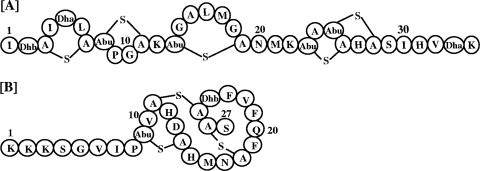
On the basis of their structural topologies, lantibiotics are classified as types A and B (12). Type A lantibiotics are further divided into 2 subtypes, namely, type A(I) and type A(II). The mode of action of lantibiotics is largely based on a variety of cell-killing mechanisms, and some lantibiotics exhibit a combination of these mechanisms. For example, the prototypic lantibiotic nisin [type A(I)] (Fig. 1A) acts against bacteria and causes multiple abnormalities, such as leakage of the cytoplasmic content, reduction in the cell wall thickness, and minicell formation; these events lead to cell death (9). Nisin uses lipid II as a docking molecule and binds to it with high affinity, leading to pore formation and the inhibition of cell wall biosynthesis (14). This unique mechanism of action renders nisin highly potent at nanomolar concentrations (6, 8). The well-studied type B lantibiotic mersacidin is also bactericidal (5). Nukacin ISK-1 (Fig. 1B) is a type A(II) lantibiotic that is produced by Staphylococcus warneri ISK-1 and consists of 27 amino acids, including a dehydrobutyrine, a 3-methyllanthionine, and two lanthionine residues (13). Dufour et al. (7) referred to type A(II) as the largest group among the lantibiotics. Because the available information regarding the mode of action of type A(II) lantibiotics is insufficient, we aimed to elucidate the mode of action of nukacin ISK-1. In this regard, we have previously established that three lysine-oriented charges located at the N-terminal end are crucial for the binding of nukacin ISK-1 to the cell membrane through electrostatic interactions (1). Here, we report that the mode of action of nukacin ISK-1 is distinct from that of the two major groups of lantibiotics [type A(I) and type B].
FIG. 1.
Structure of nisin A (A) and nukacin ISK-1 (B). A-S-A, lanthionine; Abu-S-A, 3-methyllanthionine; Abu, aminobutyrate; Dha, dehydroalanine; Dhb, dehydrobutyrine.
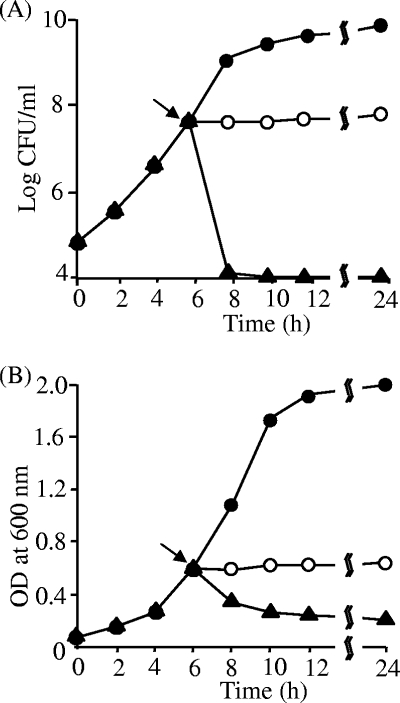
Nukacin ISK-1 was purified according to the method developed by Aso et al. (2). Commercial nisin A (Sigma-Aldrich, St. Louis, MO) was purified by using a reverse-phase high-performance liquid chromatography column. The antibacterial activities of nukacin ISK-1 and nisin A were determined by the spot-on-lawn method (13). The antibacterial activity of nisin A was considerably higher (8- to 32-fold lower than the MIC) than that of nukacin ISK-1 (Table 1). It has been reported that nisin exerts its antibacterial activity at nanomolar concentrations (3). However, both lantibiotics showed similar antibacterial spectra against the tested indicator strains. The antibacterial activity of nukacin ISK-1 was evaluated on the basis of its bacteriostatic or bactericidal mode of action. Treatment with nukacin ISK-1 resulted in a cessation of growth of the indicator strain (Fig. 2A); this suggested that nukacin ISK-1 has a bacteriostatic mode of action. Concomitant measurement of the turbidity of the aliquots revealed that the optical density was stable after treatment with nukacin ISK-1 (Fig. 2B); this result further confirmed its bacteriostatic nature. In contrast, the activity of nisin could easily be distinguished by a sharp decline in the viable cell counts (Fig. 2A). Moreover, the decrease in cell viability correlated well with the decrease in optical density, further evidence of the lytic action of nisin A (Fig. 2B). These results indicated that nukacin ISK-1 possesses bacteriostatic activity, in contrast to the well-studied bactericidal activity of nisin A.
TABLE 1.
Comparison of antibacterial activities of nukacin ISK-1 and nisin A
| Indicator strain | MICa (μM)
|
|
|---|---|---|
| Nukacin ISK-1 | Nisin A | |
| Bacillus subtilis JCM 1465T | 0.26 | 0.008 |
| Micrococcus luteus IFO 12708 | 2.08 | 0.032 |
| Listeria innocua ATCC 33090T | 16.64 | 0.065 |
| Lactococcus lactis ATCC 19435T | 0.52 | 0.065 |
| Enterococcus faecalis TUA 1344L | 4.16 | 0.260 |
| Lactobacillus plantarum ATCC 14917T | ND | 1.040 |
| Lactobacillus sakei JCM 1157T | 0.26 | 0.008 |
| Staphylococcus carnosus TM 300 | 0.26 | 0.016 |
| Escherichia coli JM 109 | ND | ND |
Antibacterial activity was determined at least twice for each lantibiotic. ND, not detected.
FIG. 2.
Bacteriostatic or bactericidal nature of nukacin ISK-1. CFU were counted (A) and optical density (OD) determined (B). The indicator bacterial strain L. sakei JCM 1157T was grown to the mid-log phase, and the culture was divided into three aliquots. Control cells (•) were not treated, and cells in the other two aliquots were treated with either nukacin ISK-1 (○) or nisin (▴). The lantibiotics (10× MIC) were added, the turbidity (OD) measured, and the CFU determined by the spread plate method after plating the cells on MRS agar in 10-fold serial dilutions. The arrow indicates the point at which nukacin ISK-1/nisin was added. This experiment was performed twice with high reproducibility. Representative data were used for the figure.
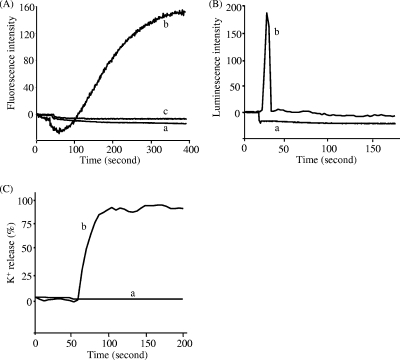
Membrane depolarization and pore formation induced by nukacin ISK-1 were determined by the methods described in the legend of Fig. 3. The ability of nukacin ISK-1 to dissipate the membrane potential of bacterial cells was determined using B. subtilis JCM 1465T as the indicator strain. Nukacin ISK-1 treatment failed to dissipate the membrane potential in this highly sensitive bacterial indicator (Fig. 3A). However, nisin A caused a high degree of membrane depolarization. This result suggested that nukacin ISK-1 was ineffective in disrupting the membrane potential of this indicator strain. Further, the ability of nukacin ISK-1 to block the activity of nisin A was also evaluated by monitoring the effect of nisin A on the membrane potential of nukacin ISK-1-treated B. subtilis JCM 1465T cells. Nukacin ISK-1-treated cells were found to resist the effects of dissipation of membrane potential by nisin A (Fig. 3A); this indicated that nukacin ISK-1 exerts its structure-specific antibacterial action by binding to the cells at the same site as nisin A. The results shown in Fig. 3B reveal that ATP efflux was not observed when cells of the sensitive indicator strain B. subtilis were treated with nukacin ISK-1. In contrast, treatment with nisin A, which efficiently induces pore formation, rapidly resulted in ATP efflux. This finding indicated that nukacin ISK-1 did not form pores large enough to permit the efflux of molecules like ATP. Therefore, we evaluated the possibility that nukacin ISK-1 creates small pores or ion channels. The results (Fig. 3C) suggested that nukacin ISK-1 did not induce K+ efflux from the cells, indicating that nukacin ISK-1 did not form pores of a size to efflux even monovalent cations like K+. In contrast, K+ efflux was clearly observed after treatment with nisin A. The formation of pores by various lantibiotics differs considerably; for example, nisin forms large pores that are highly stable and permit the rapid efflux of ATP molecules and ions (4), whereas other lantibiotics, such as the type A(II) lantibiotic SA-FF22, form short-lived pores that minimally permit the efflux of molecules or ions (11).
FIG. 3.
Antibacterial action of nukacin ISK-1. (A) Dissipation of the cell membrane potential by nukacin ISK-1 in normal cells (a), nisin A in normal cells (b), and nisin A in nukacin ISK-1-treated cells (c). The dissipation was examined by observing the fluorescence emitted by DiS-C3 (3,3′-dipropylthiadicarbocyanine iodide). (B) Efflux of ATP molecules from nukacin ISK-1-treated cells (a) and nisin A-treated cells (b), detected by using a Lucifer-HS ATP detection kit. (C) Efflux of K+ ions from nukacin ISK-1-treated cells (a) and nisin A-treated cells (b), detected by using potassium-binding benzofuran isophthalate-AM (PBFI). K+ efflux is expressed in relation to the total amount of K+ released after the addition of 1 μM nisin (100% value). These experiments were carried out with the lantibiotics at 10× MIC, using B. subtilis JCM 1465T as an indicator. These experiments were performed twice with high reproducibility. Representative data were used for the figure.
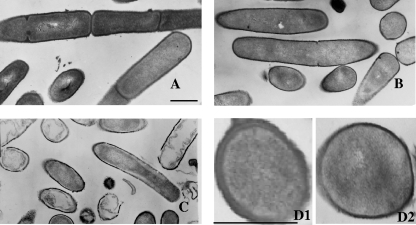
Next, B. subtilis JCM 1465T cells were prepared for transmission electron microscopy according to the protocol described by Iida et al. (10). To elucidate the specific effects that nukacin ISK-1 exerted on the cells, we analyzed the longitudinal and cross sections of cells. The comparison of the micrographs obtained for the untreated (Fig. 4A) and nukacin ISK-1-treated cells (Fig. 4B) showed that the plasma membrane was not detached from the cell wall. No abnormalities were observed in the interior structural distribution of the internal components in any of the bacterial sections analyzed. The internal structure of the nukacin ISK-1-treated cells was thus similar to that of the control cells. On the other hand, the micrographs of the nisin-treated cells (Fig. 4C) showed cell lysis, evidenced by the detachment of the cell wall, abnormalities in the cytoplasmic content, and disruption of cells. Hyde et al. (9) also reported the multiple morphological abnormalities in bacterial cells caused by nisin. Surprisingly, the cells that were exposed to nukacin ISK-1 for long periods exhibited the initial stages of septal orientation, and the bacterial cells were found in single arrangements (Fig. 4B). A striking feature of the cross sections of the cells that was noted was that although the overall shape and size of the cells were not affected, the cell wall thickness was dramatically reduced in the nukacin ISK-1-treated cells (Fig. 4D2) in comparison with that of the control cells (Fig. 4D1).
FIG. 4.
Overview of transmission electron micrographs of B. subtilis JCM 1465T cells. (A) The control cells were not treated with any lantibiotics. (B and C) Micrographs of cells that were treated with nukacin ISK-1 (B) and nisin A (C) at 10× MIC for 90 min are shown. (D1 and 2) Enlarged cross sections of control cells (D1) and nukacin ISK-1-treated cells (D2). Bars in panels A and D1, 500 nm.
Nukacin ISK-1 differs greatly from type A(I) and type B lantibiotics. It neither dissipates the membrane potential of its target cells nor forms pores permitting the efflux of molecules or ions. It halted the growth of bacterial cells cultured in a liquid medium. Nukacin ISK-1 did not affect the morphology or the cytoplasmic content of the bacterial cell. However, it significantly reduced the width of the cell wall, and the treated cells did not show complete septum formation. Considering all these observations together, we concluded that the mode of action of nukacin ISK-1 is bacteriostatic. As far as we are aware, this is the first report describing a lantibiotic with bacteriostatic properties.
Acknowledgments
This work was supported by grants from the Japan Society for the Promotion of Science (JSPS), the Japan Science Society, the Novartis Foundation (Japan) for the Promotion of Science, the Novozymes Japan Research Fund, and the Nagase Science and Technology Foundation.
Footnotes
Published ahead of print on 8 June 2009.
REFERENCES
- 1.Asaduzzaman, S. M., J. Nagao, Y. Aso, J. Nakayama, and K. Sonomoto. 2006. Lysine-oriented charges trigger the membrane binding and activity of nukacin ISK-1. Appl. Environ. Microbiol. 72:6012-6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aso, Y., K. Okuda, J. Nagao, Y. Kamenasa, N. T. B. Phuong, H. Koga, K. Shioya, T. Sashihara, J. Nakayama, and K. Sonomoto. 2005. A novel type of immunity protein, NukH, for the lantibiotic nukacin ISK-1 produced by Staphylococcus warneri ISK-1. Biosci. Biotechnol. Biochem. 69:1403-1410. [DOI] [PubMed] [Google Scholar]
- 3.Breukink, E., H. E. van Heusden, P. J. Vollmerhaus, E. Swiezewska, L. Brunner, S. Walker, A. J. Heck, and B. de Kruijff. 2003. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J. Biol. Chem. 278:19898-19903. [DOI] [PubMed] [Google Scholar]
- 4.Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H.-G. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. [DOI] [PubMed] [Google Scholar]
- 5.Brötz, H., G. Bierbaum, A. Markus, E. Molitor, and H.-G. Sahl. 1995. Mode of action of the lantibiotic mersacidin: inhibition of peptidoglycan biosynthesis via a novel mechanism? Antimicrob. Agents Chemother. 39:714-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brötz, H., M. Josten, I. Wiedemann, U. Schneider, F. Götz, G. Bierbaum, and H.-G. Sahl. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 30:317-327. [DOI] [PubMed] [Google Scholar]
- 7.Dufour, A., T. Hindre, D. Haras, and J.-P. Le Pennec. 2007. The biology of lantibiotics from the lacticin 481 group is coming of age. FEMS Microbiol. Rev. 31:134-167. [DOI] [PubMed] [Google Scholar]
- 8.Hasper, H. E., N. E. Kramer, J. L. Smith, J. D. Hillman, C. Zachariah, O. P. Kuipers, B. de Kruijff, and E. Breukink. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636-1637. [DOI] [PubMed] [Google Scholar]
- 9.Hyde, A. J., J. Parisot, A. McNichol, and B. B. Bonev. 2006. Nisin-induced changes in Bacillus morphology suggest a paradigm of antibiotic action. Proc. Natl. Acad. Sci. USA 103:19896-19901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iida, H., L. Wang, K. Nishii, A. Ookuma, and Y. Shibata. 1996. Identification of rab12 as a secretory granule-associated small GTP-binding protein in atrial myocytes. Circ. Res. 78:343-347. [DOI] [PubMed] [Google Scholar]
- 11.Jack, R., R. Benz, J. Tagg, and H.-G. Sahl. 1994. The mode of action of SA-FF22, a lantibiotic isolated from Streptococcus pyogenes strain FF22. Eur. J. Biochem. 219:699-705. [DOI] [PubMed] [Google Scholar]
- 12.Jung, G. 1991. Lantibiotics: a survey, p. 1-34. In G. Jung and H.-G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM, Leiden, The Netherlands.
- 13.Kimura, H., H. Matsusaki, T. Sashihara, K. Sonomoto, and A. Ishizaki. 1998. Purification and partial identification of bacteriocin ISK-1, a new lantibiotic produced by Pediococcus sp. ISK-1. Biosci. Biotechnol. Biochem. 62:2341-2345. [DOI] [PubMed] [Google Scholar]
- 14.Wiedemann, I., E. Breukink, C. van Kraaij, O. P. Kuipers, G. Bierbaum, B. de Kruijff, and H.-G. Sahl. 2001. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276:1772-1779. [DOI] [PubMed] [Google Scholar]