Abstract
We evaluated the efficacies of posaconazole and voriconazole in comparison with that of amphotericin B in a systemic murine infection by Candida krusei. Posaconazole at 50 mg/kg/day and voriconazole at 40 and 60 mg/kg/day prolonged survival and reduced the fungal tissue burden in the kidneys of mice similarly to amphotericin B at 1.5 mg/kg/day and liposomal amphotericin B at 10 mg/kg/day. None of the treatments tested completely resolved the infection.
Candidiasis has become one of the most frequent causes of nosocomial infections. Fluconazole (FLC) is the recommended drug, but several non-albicans Candida species such as Candida krusei have an intrinsic resistance to FLC. This species is the fifth most common Candida species to cause candidemia. An ideal therapy does not yet exist for C. krusei infections, and a high mortality rate is reported (15). Currently recommended antifungals for the treatment of disseminated C. krusei infections are amphotericin B (AMB) and echinocandins, with voriconazole (VRC) being regarded as an alternative (11, 24). Posaconazole (PSC) is a promising drug, though not yet explored enough in vivo, that shows in vitro MICs against C. krusei similar to or lower than those of VRC (12). In this study, we have tested the triazoles VRC and PSC, comparing their efficacies with those of two different formulations of AMB, in an immunocompromised murine model of disseminated infection by C. krusei.
Two clinical strains of C. krusei, FMR 9728 and FMR 9729, were used. The inocula containing ≥99% of the viable cells for both the in vitro and in vivo studies were adjusted to the desired concentration by counting them with a hemocytometer. The in vitro susceptibilities of both strains were determined using a reference method (7). The minimal fungicidal concentration (MFC) was defined as a 99.9% or greater reduction in the number of CFU/ml (2) (Table 1).
TABLE 1.
In vitro antifungal activity of AMB, VRC, and PSC against the two strains of C. kruseia
| Strain | AMB MIC-0 (μg/ml) | VRC
|
PSC
|
||
|---|---|---|---|---|---|
| MIC-2 (μg/ml) | MFC (μg/ml) | MIC-2 (μg/ml) | MFC (μg/ml) | ||
| 9728 | 1 | 0.125 | 16 | 0.125 | 1 |
| 9729 | 1 | 0.25 | >16 | 0.25 | 2 |
MIC-0 corresponds to a 100% inhibition of growth and MIC-2 to a 50% inhibition of growth. MFC corresponds to a 99.9% or greater reduction in the CFU/ml count.
Male OF1 mice were immunosuppressed by a single intraperitoneal (i.p.) injection of 200 mg of cyclophosphamide/kg of body weight, plus a single intravenous (i.v.) injection of 150 mg of 5-fluorouracil/kg on the same day of infection. For the survival studies, the mice received an additional dose of 5-fluorouracil (75 mg/kg) on day 5 after infection, which in previous tests yielded a mortality rate of 100% within 10 days after infection (data not shown). For the survival studies, the mice were challenged with 5 × 107 CFU in 0.2 ml of sterile saline into the lateral tail vein. For the tissue burden studies, the mice were inoculated with 5 × 106 CFU in 0.2 ml of sterile saline, and all the animals survived during the observation period. The procedure standards were approved by the Animal Welfare Committee of the Rovira i Virgili University.
Groups of 10 mice were randomly established for the survival and tissue burden studies. The different groups were treated once daily as follows: AMB deoxycholate (D-AMB) at 1.5 mg/kg of body weight/dose given i.p. (6); liposomal AMB (L-AMB) at 10 mg/kg given i.v. (9); VRC at 10 or 20 mg/kg i.v. (20) and at 40 or 60 mg/kg given orally (p.o.) (22); and PSC at 50 or 100 mg/kg p.o. (19). From 3 days prior to infection, the mice that received VRC were given diluted (50%) grapefruit juice instead of water. The selected doses of VRC have previously been shown to deliver adequate plasma levels in mice when coadministered with grapefruit juice (5, 18, 25). All treatments began 24 h after challenge, and the therapy lasted for 5 days. For the survival studies, the mice were checked daily for 15 days. For the tissue burden studies, the mice were killed 1 day after the completion of the treatment. The spleens and kidneys were aseptically removed, and the entire organs were homogenized in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenates were plated and incubated at 35°C for 72 h. The mean survival time was estimated by the Kaplan-Meier method and compared among groups using the log rank test. Colony counts for the tissue burden studies were analyzed using the Mann-Whitney U test. A P value of <0.05 was considered statistically significant.
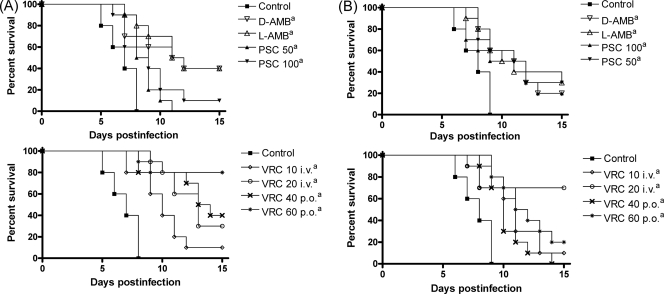
For both strains tested, all the treatments significantly prolonged survival relative to the control group (P < 0.05) (Fig. 1). No statistically significant differences were observed between the treatments.
FIG. 1.
Cumulative mortality of mice infected with C. krusei FMR 9728 (A) and FMR 9729 (B). D-AMB, D-AMB at 1.5 mg/kg/day i.p.; L-AMB, L-AMB at 10 mg/kg/day i.v.; VRC 10 i.v. and VRC 20 i.v., VRC at 10 and 20 mg/kg/day i.v., respectively; VRC 40 p.o. and VRC 60 p.o., VRC at 40 and 60 mg/kg/day p.o., respectively; PSC 50 and PSC 100, PSC at 50 and 100 mg/kg/day p.o., respectively. a, P < 0.05 versus control.
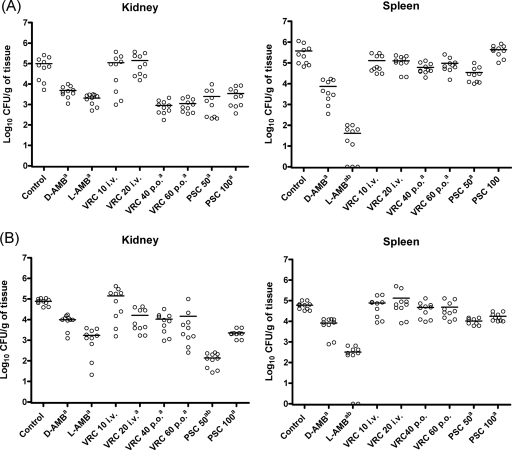
For strain 9728, all the drugs except i.v. administered VRC were effective in reducing the fungal burden in the kidneys relative to that of the control group (Fig. 2). In the spleens, the two formulations of AMB, the lower dose of PSC, and both of the p.o. administered doses of VRC were able to reduce the CFU counts relative to that of the control group. For strain 9729, D-AMB, L-AMB, and PSC were effective in reducing the fungal burdens in both organs, while VRC even at high doses was only able to reduce the fungal load in the kidneys. In addition, PSC at 50 mg/kg was able to significantly reduce the fungal burden in the kidneys relative to the other therapies. L-AMB was clearly more effective than the other therapies in reducing the tissue burden in the spleens for both strains.
FIG. 2.
Effects of the antifungal treatments on the tissue burden of C. krusei FMR 9728 (A) and FMR 9729 (B) in the kidneys and spleens of mice. D-AMB, D-AMB at 1.5 mg/kg/day i.p.; L-AMB, L-AMB at 10 mg/kg/day i.v.; VRC 10 i.v. and VRC 20 i.v., VRC at 10 and 20 mg/kg/day i.v., respectively; VRC 40 p.o. and VRC 60 p.o., VRC at 40 and 60 mg/kg/day p.o., respectively; PSC 50 and PSC 100, PSC at 50 and 100 mg/kg/day p.o., respectively. a, P < 0.05 versus control. b, P < 0.05 versus the rest of the therapies. Horizontal lines of scatter plots indicate mean values.
Despite the relatively high MICs that AMB showed against both strains tested, this drug was effective in vivo. Overall, in our murine model, L-AMB performed slightly better than VRC and PSC, while D-AMB did not outperform the azole treatments.
Although C. krusei shows an intrinsic resistance to FLC, no cross-resistance to other azoles has been observed (13, 14). VRC remains active against most strains of C. krusei (12, 17), its efficacy being demonstrated in vitro and in clinical trials (3, 10). Several authors have reported a fungistatic effect of VRC (16, 26), while others have stated a fungicidal effect of this drug (1, 21) against Candida. Despite the high MFCs observed for both strains, in our study, VRC improved the survival of mice for the two strains tested and reduced the tissue burden greatly for strain 9728 and modestly for strain 9729.
In our murine model, PSC has demonstrated efficacy in the treatment of C. krusei-disseminated infection with similar or even improvement of the results obtained with currently recommended treatments such as D-AMB, L-AMB, and VRC. Surprisingly, the lower dose of PSC proved to be slightly more effective than 100 mg/kg in tissue burden clearance. A lack of a dose-effect relationship for this drug has been previously reported with different fungi in mice (4, 19). A decrease in the absorption of PSC at doses higher than 50 mg/kg in mice (8) could easily correlate with a lack of effect increase, although a decrease in drug efficacy is puzzling and merits further investigation. The low MFCs observed and the efficacy of PSC in the survival and fungal burden studies agree with the reported fungicidal activity of this compound against C. krusei (23). Despite the lower MFCs observed for PSC with respect to those for VRC, no statistical differences were observed in vivo between the two compounds with the exception of tissue burden reduction in the kidneys for strain 9729.
In conclusion, our results suggest that PSC could be a therapeutic alternative to AMB and VRC for the treatment of disseminated infections by C. krusei. Further experimental studies are warranted to confirm our results.
Acknowledgments
This work was supported by a grant from Fondo de Investigaciones Sanitarias from the Ministerio de Sanidad y Consumo of Spain (PI 050031).
Footnotes
Published ahead of print on 26 May 2009.
REFERENCES
- 1.Espinel-Ingroff, A., E. Cantón, J. Pemán, and M. Gobernado. 2002. Fungicidal activity of voriconazole against Candida krusei by time-kill curves. Proceedings of the 8th Congress of the European Confederation of Medical Mycology, Budapest, Hungary.
- 2.Isham, N. C., and M. A. Ghannoum. 2007. Voriconazole and caspofungin cidality against non-albicans Candida spp. Infect. Dis. Clin. Pract. 15:250-253. [Google Scholar]
- 3.Kullberg, B. J., J. D. Sobel, M. Ruhnke, P. G. Pappas, C. Viscoli, J. H. Rex, J. D. Cleary, E. Rubinstein, L. W. Church, J. M. Brown, H. T. Schlamm, I. T. Oborska, F. Hilton, and M. R. Hodges. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 366:1435-1442. [DOI] [PubMed] [Google Scholar]
- 4.Lozano-Chiu, M., S. Arikan, V. L. Paetznick, E. J. Anaissie, D. Loebenberg, and J. H. Rex. 1999. Treatment of murine fusariosis with SCH 56592. Antimicrob. Agents Chemother. 43:589-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majithiya, J., A. Sharp, A. Parmar, D. W. Denning, and P. A. Warn. 2009. Efficacy of isavuconazole, voriconazole and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei. J. Antimicrob. Chemother. 63:161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariné, M., C. Serena, B. Fernández-Torres, F. J. Pastor, and J. Guarro. 2005. Activities of flucytosine, fluconazole, amphotericin B, and micafungin in a murine model of disseminated infection by Candida glabrata. Antimicrob. Agents Chemother. 49:4757-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility of yeasts. Approved standard M27-A2, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 8.Nomeir, A. A., P. Kumari, M. J. Hilbert, S. Gupta, D. Loebenberg, A. Cacciapuoti, R. Hare, G. H. Miller, C. C. Lin, and M. N. Cayen. 2000. Pharmacokinetics of SCH 56592, a new azole broad-spectrum antifungal agent, in mice, rats, rabbits, dogs, and cynomolgus monkeys. Antimicrob. Agents Chemother. 44:727-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortoneda, M., J. Capilla, F. J. Pastor, I. Pujol, and J. Guarro. 2002. Efficacy of liposomal amphotericin B in treatment of systemic murine fusariosis. Antimicrob. Agents Chemother. 46:2273-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrosky-Zeichner, L., M. A. Oude Lashof, B. J. Kullberg, and J. H. Rex. 2003. Voriconazole salvage treatment of invasive candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 22:651-655. [DOI] [PubMed] [Google Scholar]
- 11.Pastor, F. J., and J. Guarro. 2007. The role of voriconazole in the treatment of emerging mycoses. Rev. Iberoam. Micol. 24:228-232. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., D. J. Diekema, D. L. Gibbs, V. A. Newell, J. F. Meis, I. M. Gould, W. Fu, A. L. Colombo, E. Rodriguez-Noriega, and the Global Antifungal Surveillance Study. 2007. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 45:1735-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., D. J. Diekema, D. L. Gibbs, V. A. Newell, E. Nagy, S. Dobiasova, M. Rinaldi, R. Barton, A. Veselov, and the Global Antifungal Surveillance Group. 2008. Candida krusei, a multidrug-resistant opportunistic fungal pathogen: geographic and temporal trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J. Clin. Microbiol. 46:515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Cross-resistance between fluconazole and ravuconazole and the use of fluconazole as a surrogate marker to predict susceptibility and resistance to ravuconazole among 12,796 clinical isolates of Candida spp. J. Clin. Microbiol. 42:3137-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., P. G. Pappas, and J. R. Wingard. 2006. Invasive fungal pathogens: current epidemiological trends. Clin. Infect. Dis. 43:S3-S14. [Google Scholar]
- 16.Pfaller, M. A., D. J. Sheehan, and J. H. Rex. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quindós, G., L. O. Sánchez-Vargas, M. Villar-Vidal, E. Eraso, M. Alkorta, and J. L. Hernández-Almaraz. 2008. Activities of fluconazole and voriconazole against bloodstream isolates of Candida glabrata and Candida krusei: a 14-year study in a Spanish tertiary medical centre. Int. J. Antimicrob. Agents 31:266-271. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez, M. M., E. Calvo, C. Serena, M. Mariné, F. J. Pastor, and J. Guarro. 2009. Effect of double and triple combinations of antifungal drugs in a murine model of disseminated infection by Scedosporium prolificans. Antimicrob. Agents Chemother. 53:2153-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez, M. M., F. J. Pastor, C. Serena, and J. Guarro. 2009. Posaconazole efficacy in a murine disseminated infection caused by Paecilomyces lilacinus. J. Antimicrob. Chemother. 63:361-364. [DOI] [PubMed] [Google Scholar]
- 20.Roffey, S. J., S. Cole, P. Comby, D. Gibson, S. G. Jezequel, A. N. Nedderman, D. A. Smith, D. K. Walker, and N. Wood. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 31:731-741. [DOI] [PubMed] [Google Scholar]
- 21.Rubio, M. C., I. R. de Ocáriz, J. Gil, R. Benito, and A. Rezusta. 2005. Potential fungicidal effect of voriconazole against Candida spp. Int. J. Antimicrob. Agents 25:264-267. [DOI] [PubMed] [Google Scholar]
- 22.Serena, C., F. J. Pastor, M. Mariné, M. M. Rodríguez, and J. Guarro. 2007. Efficacy of voriconazole in a murine model of cryptococcal central nervous system infection. J. Antimicrob. Chemother. 60:162-165. [DOI] [PubMed] [Google Scholar]
- 23.Sóczó, G., G. Kardos, P. M. McNicholas, E. Balogh, L. Gergely, I. Varga, B. Kelentey, and L. Majoros. 2007. Correlation of posaconazole minimum fungicidal concentration and time kill test against nine Candida species. J. Antimicrob. Chemother. 60:1004-1009. [DOI] [PubMed] [Google Scholar]
- 24.Spellberg, B. J., S. G. Filler, and J. E. Edwards, Jr. 2006. Current treatment strategies for disseminated candidiasis. Clin. Infect. Dis. 42:244-251. [DOI] [PubMed] [Google Scholar]
- 25.Sugar, A. M., and X. P. Liu. 2000. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Med. Mycol. 38:209-212. [DOI] [PubMed] [Google Scholar]
- 26.Theuretzbacher, U., F. Ihle, and H. Derendorf. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 45:649-663. [DOI] [PubMed] [Google Scholar]