Abstract
There are currently renewed efforts to develop drugs that could shorten the duration of antituberculosis therapy. This is best achieved by optimizing the sterilizing effect. However, the current pathway for the development of new molecules with the potential to have a sterilizing effect is inefficient. We designed an in vitro pharmacokinetic-pharmacodynamic model in which Mycobacterium tuberculosis replicating slowly at pH 5.8 was exposed to pyrazinamide by use of the concentration-time profiles encountered in patients. The sterilizing effect rates and the time to the emergence of drug resistance were examined. Daily pyrazinamide dosing for 28 days accurately achieved (i) the pyrazinamide pharmacokinetic parameters, (ii) the lack of early bactericidal activity, (iii) a sterilizing effect rate of 0.10 log10 CFU/ml per day starting on day 6 of therapy, and (iv) a time to the emergence of resistance of the from 2 to 3 weeks of monotherapy encountered in patients with tuberculosis. Next, dose-scheduling studies were performed. The sterilizing effect was linked to the pyrazinamide ratio of the area under the concentration-time curve from 0 to 24 h (AUC0-24) to the MIC (r2 = 0.80 to 0.90), with 90% of the maximal effect being achieved by an AUC0-24/MIC of 209.08. Resistance suppression was associated with the percentage of time that the concentration persisted above the MIC (r2 = 0.73 to 0.91). Monte Carlo simulations of 10,000 patients demonstrated that the currently recommended pyrazinamide doses (15 to 30 mg/kg of body weight/day) achieved the AUC0-24/MIC of 209.08 in the epithelial lining fluid of only 15.1 to 53.3% of patients. Doses of >60 mg/kg per day performed better. Our vitro model for the sterilizing effect, together with Monte Carlo simulations, can be used for the faster identification of the clinical doses that are needed to achieve a sterilizing effect and that can then be studied in clinical trials.
Current therapy for tuberculosis (TB) consists of rifampin (rifampicin), isoniazid, and pyrazinamide (5). In order to improve therapy, several studies have examined the pharmacokinetic (PK)-pharmacodynamic (PD) relationship between isoniazid and rifampin treatment and the response of Mycobacterium tuberculosis (22, 25, 29, 30). However, those studies were of short duration and mainly reflected the bactericidal activities of these agents, defined as the killing of bacilli in log-phase growth. No similar studies have examined the sterilizing activities of anti-TB drugs. An anti-TB drug is considered to have a sterilizing effect when it is able to kill one of two subpopulations of M. tuberculosis: slowly growing bacilli in an acidic environment (slowly replicating bacilli [SRB]) and nonreplicating persistent bacilli (5, 26). The sterilizing activity of a drug also reflects its ability to shorten anti-TB therapy duration (41). Pyrazinamide has unique sterilizing activity against SRB, which are estimated to constitute the majority of bacilli in the pulmonary cavities of patients with TB (31, 35). A measure of the size of the SRB population is that the addition of pyrazinamide to isoniazid- and rifampin-containing regimens led to a one-third reduction in the duration of therapy and a two-thirds reduction in the rate of TB relapse (2, 48). Given the size of this population and the central role of pyrazinamide in eradicating it, pyrazinamide PK-PD studies could help with the further optimization of therapy.
After oral administration, pyrazinamide is almost completely systematically absorbed and eventually enters the pulmonary cavities containing M. tuberculosis. Pyrazinamide works in these cavities under acidic conditions (38). There is conflicting evidence as to whether pyrazinamide enters the bacilli by simple diffusion or by active uptake (44, 56). However, once the bacilli are in the cell, bacillary nicotinamidase deaminates the pyrazinamide to pyrazinoic acid (POA), which is transported to the extracellular milieu by an efflux pump and is then protonated to HPOA (34, 47, 56). It is believed that the HPOA reenters the bacillus and accumulates. Microbial killing may occur via the reduction of the intracellular pH, the disruption of membrane transport, or the inhibition of fatty acid synthase type I (7, 57, 59, 60). An unresolved issue is whether the acidic conditions under which pyrazinamide works are intracellular or extracellular. A long-held and popular belief is that pyrazinamide works in the acidic pH environment within macrophages (37). However, it has also been proposed that the bacilli which pyrazinamide kills are extracellular and at the edges of necrotic cavities (5). Arguments for and against each of these viewpoints have recently been summarized (28). PK-PD approaches could help resolve this question. If the bacilli are within macrophages, a desirable property of new drugs would be a high level of intracellular penetration. If, however, they are extracellular, the crucial and desirable chemical property of sterilizing agents would be achievement of a high concentration in the epithelial lining fluid (ELF).
Currently, it is difficult to identify the sterilizing effects of old and new anti-TB drugs in short-term clinical trials because of the difficulty of separating the sterilizing activity of a single drug from that of multiple drugs used for therapy (8). One solution is a preclinical TB model in which the sterilizing effect can be studied and the results can be translated to the treatment of patients. Unfortunately, there are several problems in that regard. To begin with, the exact pH under which SRB survive in patients with TB is unknown. A pH range of 5.0 to 7.4 either has been measured in cavities containing M. tuberculosis or has been utilized for in vitro studies (9, 38). In terms of animal models, pyrazinamide is not active against TB in guinea pigs, while the relevant bacillary metabolic status in mice with TB may be different from that in humans (1, 49). Furthermore, the half-life of pyrazinamide is 10-fold higher in humans than in mice (20, 43, 52, 58). When the difference in interspecies drug half-life is large and the half-life in the animal is very short, the PK-PD index associated with microbial killing often defaults to the percentage of the time that the concentration exceeds the MIC (TMIC), which can lead to erroneous therapeutic conclusions when the findings are translated to humans (15, 17, 18). In the current study, we established an in vitro PK-PD model of TB that mimics the pharmacokinetics of pyrazinamide in humans and that results in pyrazinamide sterilizing activity in patients with TB. We used the model to perform dose-effect and dose-scheduling studies. The results were then utilized to translate the PK-PD findings to humans in order to optimize pyrazinamide therapy.
(The data presented here were presented, in part, as an oral presentation at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy-46th Infectious Diseases Society of America Annual Meeting, Washington, DC, October 2008, abstr. A-1823.)
MATERIALS AND METHODS
Bacterial isolates.
Mycobacterium tuberculosis H37Rv (ATCC 27294) was used in all studies. For each experiment, the cultures were first incubated under 5% CO2 at 37°C in Middlebrook 7H9 broth (herein termed “medium”) enriched with 10% oleic acid, albumin, dextrose, and catalase (OADC) for 4 days to achieve log-phase growth. The pyrazinamide MIC was determined by the methods recommended by the Clinical and Laboratory Standards Institute (12).
Drugs and supplies.
Pyrazinamide (Sigma-Aldrich) was first dissolved in dimethyl sulfoxide (DMSO) and was then subsequently diluted in acidified medium to the desired drug concentrations. The final DMSO concentration in all experiments was less than 0.1%. Hollow-fiber-system components were purchased from FiberCell Systems.
Development of an in vitro PD model of TB for sterilizing activity.
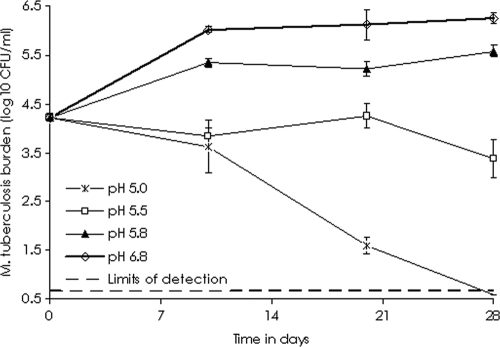
Citric acid was chosen to acidify the medium because it is an excellent buffer at pHs of between 5 and 7 required for use in our studies. The medium was acidified to a pH of 5.0 to 6.8 with no OADC supplementation. Cultures of 5.5 log10 CFU/ml M. tuberculosis were inoculated into triplicate T flasks with medium at pHs of 6.8, 5.8, 5.5, and 5.0; and the flasks were incubated under 5% CO2 at 37°C and 90 rpm shaking. No extra pH points between pH 6.0 and 6.8 were investigated, on the basis of preliminary results which demonstrated no major differences in growth between these two pHs. The flasks were sampled on days 10, 20, and 28 for quantitative cultures. Since SRB in the pulmonary cavities of patients with TB are characterized by a lower rate of replication than bacilli in log-phase growth (5, 31, 40), we were interested in choosing an in vitro pH that was associated with rates of growth consistently lower than those of cultures in log-phase growth but that resulted in a growth rate of >0.
For all studies, pyrazinamide was initially dissolved in DMSO and was then diluted to the desired concentration in Middlebrook 7H9 broth to make a final DMSO concentration of ≤0.1%. The DMSO with citric acid (pH 5.8) had no effect on bacillary killing when DMSO was present at a concentration of up to 1%. After the incubation of identical inocula at 37°C for 21 days, medium with 1% DMSO plus citric acid at pH 5.8 had an M. tuberculosis burden of 5.37 ± 0.07 log10 CFU/ml, whereas medium with citric acid alone (pH 5.8) had an M. tuberculosis burden of 5.24 ± 0.04 log10 CFU/ml. The level of microbial killing by 12.5 mg/liter pyrazinamide initially dissolved in water was no different from that of pyrazinamide initially dissolved in DMSO (final DMSO concentration, 0.1%) at pH 5.8. Therefore, the interaction of pyrazinamide, citric acid, and DMSO with regard to the killing of M. tuberculosis was considered minimal.
Next, hollow-fiber systems were utilized to construct an in vitro PK-PD model of TB for sterilizing activity. The basic construction of this system has been described in detail in our studies of bactericidal activity (21-25). The system allows M. tuberculosis growing in the peripheral compartment to be exposed to concentration-time profiles of drug similar to those achieved in patients with TB. We modified the system by using media that had been acidified to a pH of 5.8, on the basis of the findings of the growth rate studies described above. M. tuberculosis was incubated in medium at pH 5.8 for 4 days and then inoculated into the peripheral compartment of each hollow-fiber system, which used medium acidified to pH 5.8. Given the continuous inflow of fresh acidified medium and the continuous outflow of used medium, as well as citric acid's buffering ability at pH 5.8, the large populations of bacilli in the peripheral compartment were not able to change the pH in their microenvironment. Measurement of the pH at the start of each experiment, as well as every 7 days for up to 28 days, demonstrated no pH change in either the external or the peripheral compartment.
Dose-effect studies.
Starting 24 h after inoculation, pyrazinamide was administered into the central compartment of each hollow-fiber system by computer-controlled syringe pumps. Pyrazinamide was administered daily for 28 days to achieve a half-life of 10 h and mimic human doses of 0, 7.5, 15, 30, 60, 90, and 120 mg/kg of body weight. During the first 48 h, the central compartment of each system was sampled 12 times to validate that the intended drug concentration-time profiles had been achieved. The peripheral compartment was sampled for bacteria for culture on days 0, 1, 2, 4, 6, 10, 14, 21, and 28. The samples were washed twice to prevent drug carryover, as described before (22, 23). The cultures were then serially diluted and plated onto Middlebrook 7H10 agar (herein termed “agar”) with 10% OADC for enumeration of the total microbial population. In order to quantify the pyrazinamide-resistant subpopulation, samples were plated on agar acidified to a pH of 5.8, enriched with 10% fetal bovine serum, and supplemented with 900 mg/liter pyrazinamide, on the basis of previously published methods (27, 55). All cultures were incubated under 5% CO2 at 37°C for 21 days, after which the colonies were counted. In order to validate the clinical relevance of the in vitro PK-PD model of TB, the sterilizing effect patterns and the rates of microbial killing achieved in our hollow-fiber systems were compared to those achieved in patients with TB treated with pyrazinamide monotherapy, as published in the literature (31, 32, 46, 54).
Dose-scheduling studies.
An inoculum of 7.5 log10 CFU/ml M. tuberculosis was used for the dose-scheduling studies. We utilized the results of the dose-effect study to calculate the exposures associated with 20%, 40%, and 60% of maximal killing (20% effective concentration [EC20], EC40, and EC60, respectively). The exposures were chosen partially based on optimal design parameters for Fisher information, and partially as a compromise to include exposures achieved by standard clinical doses.
Each of the three exposures was administered to our in vitro system for 28 days as one of three dose schedules: a single dose administered each week (every 168 h), two equally divided doses administered every 3.5 days, and seven equally divided doses administered every 24 h. On the basis of the results of the dose-effect study described above, the peripheral compartment was sampled for bacteria for culture on days 0, 14, 21, and 28. The samples were washed twice to remove drug carryover, serially diluted, and plated on agar to determine the total M. tuberculosis population as well as the pyrazinamide-resistant subpopulation. The cultures were incubated at 37°C for 21 days.
Measurement of pyrazinamide concentration.
The pyrazinamide concentration in samples from each hollow-fiber system were measured by the liquid chromatography-tandem mass spectrometry method. Acetazolamide was used as an internal standard (14). Samples in Middlebrook 7H9 broth were diluted 1:100 in methanol and injected directly, without further processing, and analyzed on a Shimadzu high-pressure liquid chromatograph with a Supelcosil ABZ+Plus column. The isocratic mobile phase consisted of 80% of 0.1% formic acid and 20% (vol/vol) acetonitrile. Detection was accomplished with an API 3000 mass spectrometer, which was programmed in the multiple-reaction-monitoring mode and monitored the transition of the m/z 124.1 and 223.0 precursor ions to the m/z 80.95 and 180.8 product ions for pyrazinamide and acetazolamide, respectively. The method was linear over the concentration range of 0.1 to 100 mg/liter, the correlation coefficient was 0.998, and the accuracy was within ±5%. The assay bias was −2.7%. The between-day coefficient of variation for the assay was 1.65 to 2.99%. The lower limit of quantification was 0.1 mg/liter.
PK-PD modeling.
PK analysis of the pyrazinamide concentrations measured was performed by using the ADAPT II software of D'Argenio and Schumitzky (16). A one-compartment open model with first-order input and elimination was chosen (52). The PK parameter estimates were then used to calculate the ratio of the pyrazinamide area under the concentration-time curve from 0 to 24 h (AUC0-24) to MIC, the ratio of the maximum concentration in plasma (Cmax) to the MIC, and the percent TMIC. The relationship between the pyrazinamide exposure (AUC0-24/MIC, Cmax/MIC, or TMIC) and the killing of M. tuberculosis in the dose-effect and the dose-scheduling studies was analyzed by using the Hill-type inhibitory sigmoid maximum-effect model, as described in the past (22, 23, 25, 29, 30). The relationship between the level of pyrazinamide exposure and the resistant subpopulation was analyzed by inspection of graphs of the size of the resistant subpopulation versus the level of exposure. Tam et al. have described the relationship between the level of drug exposure and the population of resistant bacteria as an inverted U (50). We have observed that the relationship between the level of drug exposure and the size of the drug-resistant subpopulation of M. tuberculosis is characterized by a system of U-shaped curves which change with an increase in the duration of therapy (25). In the current study, we expressed this relationship as a quadratic function: Y = aX2 + bX + k, where Y is the size of the resistant subpopulation (log10 CFU/ml); X is the anti-TB drug exposure value for Cmax/MIC, AUC0-24/MIC, or TMIC; and k is the drug-resistant subpopulation in the nontreated controls. The level of drug exposure associated with the highest drug-resistant subpopulation in the inverted U or the lowest resistant subpopulation with an upright U-shaped curve was equal to −b/2a, where a and b are coefficients. The leading coefficient, a, starts as a positive value in the upright U-shaped curve but changes to a negative value with an increased duration of therapy, indicating an inverted U-shaped curve. This formula was utilized to examine the relationship between the level of pyrazinamide exposure (independent variable) and the size of the resistant subpopulation (log10 CFU/ml). Weighting was done by least-squares estimation. However, when 1/Y2 weighting or automatic outlier elimination by the program was employed, the results were similar. All PK-PD analyses were performed with Prism (version 5) software (GraphPad Software).
Monte Carlo simulations.
We were interested in using the results of our PK-PD study to help determine if pyrazinamide works in the acidic pH within macrophages. If this is true, then the pyrazinamide concentrations associated with a sterilizing effect must be achieved in infected alveolar macrophages in the majority of patients with TB treated with the standard dose of 2 g a day. On the other hand, the bacilli may be extracellular. If this is true, then the pyrazinamide concentrations associated with a sterilizing effect must be achieved within ELF in the majority of treated patients with TB. Conte et al. have measured the pyrazinamide concentrations in the plasma, alveolar macrophages, and ELF of patients treated with oral pyrazinamide (13). They identified a mean macrophage/plasma ratio of 0.83 (95% confidence interval [CI], 0.52 to 1.14) and a mean ELF concentration/plasma concentration ratio of 17.8 (95% CI, 13.9 to 21.7), on the basis of our calculation of their data. The reasons for these differences are unknown (13). Oral pyrazinamide at 2 g a day is associated with a sterilization rate of 0.11 log10 CFU/ml per day in adult patients (31, 32). On the basis of the inhibitory sigmoid maximum-effect relationship derived in our in vitro dose-effect studies, the level of pyrazinamide exposure (target exposure) associated with this sterilization rate of 0.11 log10 CFU/ml per day was calculated. We then performed Monte Carlo simulations for 10,000 subjects to determine how likely the standard dose of 2 g a day would achieve the AUC0-24/MIC target exposure in patient alveolar macrophages on the basis of the upper 95% confidence bound of the macrophage penetration ratio of 1.14. We next performed similar Monte Carlo simulations based on the ELF concentration/plasma concentration ratios. While pyrazinamide is 15 to 40% protein bound, the concentration of proteins in ELF is low; thus, the effect of ELF protein binding on pyrazinamide is considered negligible (33, 53). Therefore, the total drug concentration in ELF was considered equivalent to the free drug concentration. The pyrazinamide population PK parameter estimates and their covariances, based on the findings of the study of Wilkins et al. (52), were embedded in the subroutine PRIOR of the ADAPT II and ADAPT5 (beta version) programs (16). The PK parameters utilized were an absorption rate constant (Ka) of 3.56 ± 1.84/h, a clearance of 3.42 ± 0.79 liters/h, and a volume of distribution of 29.20 ± 7.69 liters, on the basis of both published and unpublished data (16). Normal and log-normal distributions were evaluated, and the distribution that was best able to recreate the mean parameter estimates and standard deviations in the original population PK model was chosen. The variability in the pyrazinamide MICs among clinical isolates of M. tuberculosis was also incorporated (45).
We also performed a Monte Carlo simulation for 10,000 pediatric patients to determine how likely daily pyrazinamide doses of 15, 25, 30, 50, 60, and 100 mg/kg would achieve the AUC0-24/MIC associated with 90% maximal killing (EC90). The PK parameters for the pediatric population utilized as prior data were a Ka of 1.47 ± 1.65/h, a clearance of 0.13 ± 0.11 liter/h/kg, and a volume of distribution of 0.80 ± 0.43 liter/kg, on the basis of both published and unpublished data from Zhu et al. (58). The MIC distributions were as described above. Similar simulations were performed for 10,000 adult patients treated with 1.5 g, 2 g, 3 g, 4 g, or 5 g pyrazinamide per day. Since Wilkins et al. have demonstrated that patient weight is an important covariate on pyrazinamide clearance and volume of distribution, the clearance was adjusted by 0.545 liter h−1 and the volume of distribution was adjusted by 0.433 liter for every 10-kg increase in weight above 50 kg, on the basis of weight distributions in the United States (39, 52). We also took into account the effect of gender on the volume of distribution, and utilized a male/female ratio of 62/38 to reflect the gender proportions of patients with TB in the United States (10).
RESULTS
Growth rates.
The rates of growth of M. tuberculosis at different pHs are shown in Fig. 1, which demonstrates that there was no net growth at pHs below 5.8. However, at a pH of 5.8, the bacilli grew but at rates lower than those of bacilli in log-phase growth at pH 6.8. Thus, a pH of 5.8 was chosen for use in all subsequent studies. At pH 5.8, the pyrazinamide MIC was 12.5 mg/liter.
FIG. 1.
Growth of Mycobacterium tuberculosis at different pHs.
Dose-effect studies.
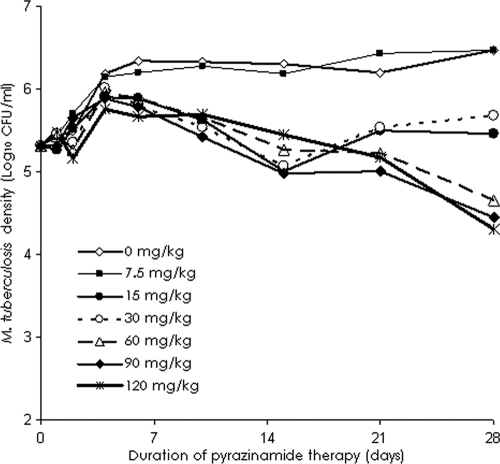
The PK parameters (means ± standard deviations) for pyrazinamide achieved in our in vitro PK-PD model of TB were a Ka of 3.45 ± 0.99 h−1, an elimination rate constant of 0.06 ± 0.02 h−1, a volume of distribution of 1.68 ± 0.40 liters/kg, and a clearance of 0.10 ± 0.02 liters/h/kg. The bacillary responses to pyrazinamide in each of the systems are shown in Fig. 2. During the first 4 days, the bacillary growth in the pyrazinamide-treated systems was −0.10 log10 CFU/ml per day, which was similar to that detected for the nontreated control arm. Thus, pyrazinamide had no early bactericidal activity. In the systems treated with doses of ≥15 mg/kg, microbial killing commenced after 4 days. In the arms treated with doses that mimicked the standard daily doses administered to patients (15 to 30 mg/kg/day), the sterilizing activity rate was 0.09 to 0.10 log10 CFU/ml per day. However, the doses failed between days 14 and 21, despite the continuation of therapy (Fig. 2). On day 28, the relationship between the microbial burden and the level of pyrazinamide exposure was as follows:
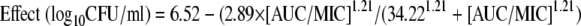 |
(1) |
The r2 value for this regression was 0.93, and the P value was <0.01. From this relationship, the pyrazinamide EC90 was calculated to be an AUC0-24/MIC ratio of 209.08. In addition, the AUC0-24/MIC ratios associated with effects of 0.5, 1.0, and 2.0 log10 CFU/ml were 9.43, 20.26, and 66.67, respectively.
FIG. 2.
Change in the total Mycobacterium tuberculosis density with 28 days of pyrazinamide therapy. Doses of 15 and 30 mg/kg failed between weeks 2 and 3, but higher doses continued to kill the bacilli until the end of the study.
Dose-scheduling studies.
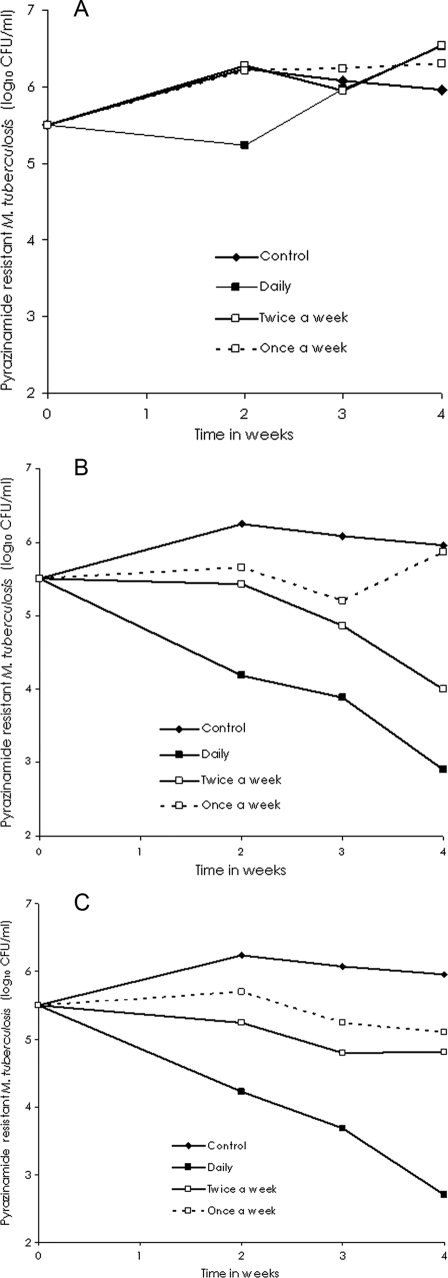
The relationship between the dose schedule and microbial killing is shown in Table 1, which demonstrates that the AUC0-24/MIC ratio best explains the sterilizing effect, on the basis of the r2 values. With regard to resistance suppression, inspection of Fig. 3A to C shows that for each cumulative weekly AUC0-24/MIC (EC20, EC40, or EC60), the schedule of pyrazinamide administration once a week had the worst outcome, while daily therapy had the best result, consistent with an effect linked to TMIC. This was confirmed by modeling the relationship between the size of the resistant subpopulation and the level of drug exposure with the results shown in Table 1. The steepest decline in the size of the drug-resistant subpopulation occurred when TMIC exceeded 66.7%.
TABLE 1.
Relationship between sterilizing effect, resistance suppression, and pyrazinamide exposure
| Wk of treatment |
r2
|
|||||
|---|---|---|---|---|---|---|
| Sterilizing effect
|
Resistance suppression
|
|||||
| Cmax/MIC | AUC/MIC | TMIC | Cmax/MIC | AUC/MIC | TMIC | |
| 2 | 0.56 | 0.9 | 0.76 | 0.16 | 0.27 | 0.73 |
| 3 | 0.63 | 0.89 | 0.78 | 0.17 | 0.66 | 0.91 |
| 4 | 0.61 | 0.8 | 0.75 | 0.09 | 0.56 | 0.86 |
FIG. 3.
Changes in pyrazinamide-resistant Mycobacterium tuberculosis burden with different dosing schedules. Doses that achieved the EC20 (A), EC40 (B), and EC60 (C) were administered by using different dosing schedules for each cumulative weekly dose.
Monte Carlo simulations.
On the basis of equation 1, the level of pyrazinamide exposure associated with a sterilization rate of 0.11 log10 CFU/ml per day was an AUC0-24/MIC ratio of 120. If an alveolar macrophage penetration ratio of 1.14 in Monte Carlo simulations is assumed, pyrazinamide at 2 g a day achieved or exceeded the AUC0-24/MIC of 120 in patient alveolar macrophages in only 0.07% of 10,000 virtual patients. A normal distribution was better able to achieve the original mean PK parameter values and standard deviations. However, the performance of similar simulations with ELF penetration ratios of 13.9 to 21.7 revealed that 2 g a day would achieve an AUC0-24/MIC of 120 in 80 to 90% of all patients. Since clinical studies have repeatedly demonstrated the effectiveness of 2 g a day of pyrazinamide in patients with TB, the extracellular location of SRB is more consistent with the known clinical effects of this dose.
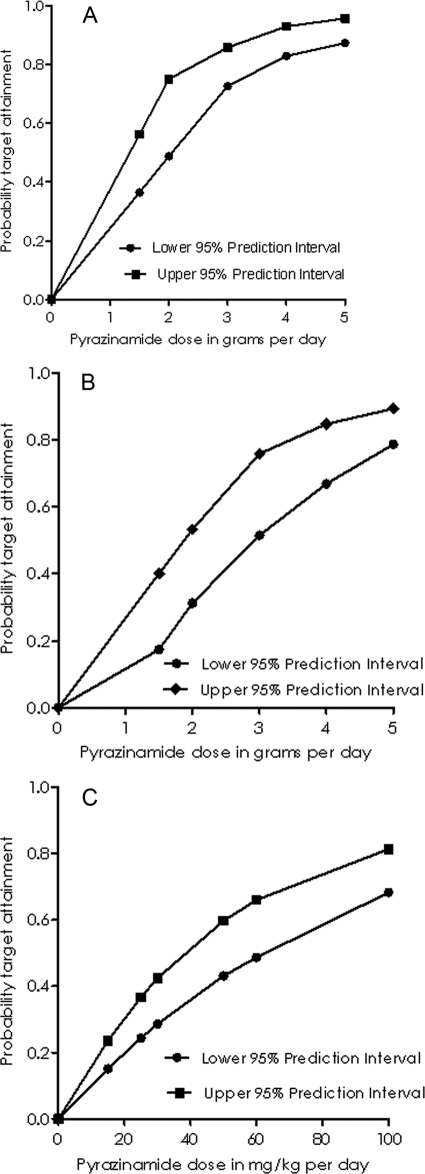
Next, Monte Carlo simulations for dose selection were performed on the basis of the ability of each dose to achieve the EC90 in the ELF of adult patients. The results are shown in Fig. 4. Figure 4A demonstrates that standard doses of 1.5 to 2 g a day are suboptimal and that higher daily doses would optimize the sterilizing effect. After gender as well as the weight distribution was taken into account, the standard doses performed even worse, as shown in Fig. 4B. The probabilities of target attainment for a TMIC of ≥66.7% and, therefore, the suppression of drug resistance were 0.76 to 0.87 for a dose of 1.5 g a day, 0.84 to 0.92 for 2 g a day, and >0.91 for 3 g a day. Simulations for the optimal dose required to achieve the EC90 in children are shown in Fig. 4C, which demonstrates that standard doses of 15 to 30 mg/kg were suboptimal.
FIG. 4.
Proportion of 10,000 patients with TB who attained the AUC0-24/MIC of 209.08, the EC90 after receiving daily pyrazinamide doses. (A) Adult patients with no adjustment for weight and patient gender; (B) adult patients with weight and gender adjusted for those for the U.S. adult population; (C) children.
DISCUSSION
The current pathway for the development of a molecule with the potential to have a sterilizing effect, from the bench to patients with TB, is dated, cumbersome, and inefficient. We would like to propose a more efficient pathway. First, our preclinical PK-PD model can be used to generate data on the relationship between level of drug exposure, time, and sterilizing effect. Second, standard mathematical (PK-PD) models are used to describe the deterministic relationship between the level of drug exposure, the level of microbial killing, and the suppression of resistance. The levels of exposure associated with the maximal effect are then incorporated as the drug exposures that need to be achieved in the ELF of patients in stochastic clinical trial simulations that take into account randomness as well as the MIC and the variability in human PKs. Such Monte Carlo simulations have been extensively utilized for the development of other pharmaceuticals as well as antibiotics (6, 19). In the current studies, we took into account demographic factors, such as gender and weight. In the past we have also incorporated the pharmacogenetics of isoniazid (25). The output is the proportion of patients in whom exposures associated with a sterilizing effect are expected to be achieved with a particular dose. If the PK parameter associated with pyrazinamide toxicity were known, Monte Carlo simulations would also be performed and the maximum dose that results in the least acceptable toxicity would be identified. The dose associated with the optimal sterilizing effect in as large a proportion of patients with TB as desired, without appreciably increased toxicity, is then recommended for use in multiple-drug therapy in randomized controlled clinical trials of the sterilizing effect.
Our in vitro PK-PD model of TB for sterilizing effect is based on the hollow-fiber system, which has been used as a PK model of humans, starting with previous work (3, 4, 36, 50). In the current study, we adapted the model to address the vexing problem of the sterilizing effect in patients with TB. We were interested in determining if the model adequately represented pharmacological events in patients. First, as was our intention, the PK parameters such as Ka, the volume of distribution, and the systemic clearance achieved in the system were similar to those encountered in patients (43, 52, 58). In order to more closely mimic cavitary TB and to take into account the inoculum effect on microbial killing and the emergence of resistance, we established bacillary burdens similar to those measured in the sputum of patients with cavitary TB during studies of pyrazinamide therapy (31). These bacillary burdens did not change the pH in our systems, likely because of the continuous inflow of fresh acidified medium and the outflow of used medium. We then compared the PD results to those achieved with pyrazinamide monotherapy in clinical studies (8, 31, 32, 46, 54). Pyrazinamide monotherapy in patients has an early bactericidal effect of 0.05 ± 0.18 log10 CFU/ml per day, a time to the start of a sterilizing effect of ≥4 days, a sterilizing effect rate of 0.11 log10 CFU/ml per day, and a time to the emergence of resistance of 2.5 to 3 weeks. Thus, our in vitro model achieved (i) the PK parameters of pyrazinamide, (ii) the lack of early bactericidal activity, (iii) the daily rate of sterilization, and (iv) the time to the emergence of resistance in patients with TB. Therefore, our model has adequate clinical relevance, especially with pyrazinamide, and could be of use for the study of the sterilizing effects of old and new anti-TB drugs. In addition, our studies indicate that the model is robust enough that it can be used to perform studies for which the duration of therapy is at least 1 month, which is important when one is studying very slowly replicating bacilli.
The central problem with the development of shorter-duration anti-TB regimens is the eradication of SRB and nonreplicating persistent bacilli. In the development of new drugs that target SRB, it will be crucial to know the histological location where the drug will act. If the bacilli are within macrophages, then a desirable property of the candidate drug would be a high level of intracellular penetration. If the bacilli are extracellular, then the crucial and desirable chemical property of sterilizing agents would be achievement of a high ELF concentration. Our data demonstrate that pyrazinamide exposures high enough to kill SRB are unlikely to be achieved in the pulmonary macrophages of most patients treated with standard doses of pyrazinamide. On the other hand, effective exposures were achieved in the ELF of 80 to 90% simulated patients treated with 2 g pyrazinamide. Even the remaining 10 to 20% patients still attained AUC0-24/MIC ratios of 80, which is associated with some sterilizing effect, although it is suboptimal. Thus, the assumption that SRB are extracellular led to findings more consistent with the known clinical effectiveness of pyrazinamide. This suggests that SRB are extracellular. Our conclusions are tempered somewhat by the fact that the ELF and alveolar macrophage pyrazinamide concentrations in the original study by Conte et al. (13) were determined at a single time point (4 h postdosing) and did not address possible system hysteresis. However, analyses by others have determined that this high ELF pyrazinamide concentration is unlikely due to technical or measurement problems or late sampling times (33).
Even though pyrazinamide is administered as part of combination therapy, it is crucial to study pyrazinamide monotherapy since it is likely that the drugs used in combination with pyrazinamide will change as new treatment regimens become available. Monotherapy studies avoid, in part, the logical problem of inductive generalization of the results achieved with one particular combination. This occurs when observations from one specific pyrazinamide-drug combination regimen are generalized to different drug combination regimens. In addition, exposure-effect relationships can be more easily established in monotherapy studies. We utilized the relationship between the pyrazinamide AUC/MIC as well as TMIC and both sterilizing activity and resistance suppression in Monte Carlo simulations to examine the likely successes of different pyrazinamide doses. The currently recommended doses of 1.0 to 2 g (15 to 30 mg/kg) a day for adults and 15 to 30 mg/kg for children achieved optimal exposures in only a minority of patients. Daily doses higher than 60 mg/kg in children and 4 g in adults were more successful in achieving the exposures associated with optimal sterilization in larger proportions of patients. The higher doses have the extra advantage in that with a daily dose schedule, they are also optimized to minimize the emergence of drug resistance, given that pyrazinamide monoresistance is being reported in diverse parts of the world (11, 42, 51). Unfortunately, pyrazinamide doses of >40 mg/kg have been associated with higher rates of toxicity when they were used in the past. However, the toxicity data are based on old studies that did not account for the duration of pyrazinamide therapy and patient weight and that did not identify the particular PK parameters most closely associated with toxicity. Our results are a call to consider the performance of a clinical study that will examine the relationship between higher doses of pyrazinamide, the sterilizing effect, and the rate of adverse events.
In summary, we established an in vitro PK-PD model that can be used to examine the sterilizing effects of anti-TB drugs. We used the model to examine the PK-PD properties of the pyrazinamide sterilizing effect and established that microbial killing was linked to the AUC/MIC, while resistance suppression was linked to TMIC. Monte Carlo simulations with these data strongly suggest that the use of doses of pyrazinamide higher than those currently used could result in better outcomes. Clinical studies that would examine the efficacy of these higher doses versus potential toxicity are warranted.
Acknowledgments
Funding for the study was provided to Tawanda Gumbo from the Department of Medicine at UT Southwestern Medical Center and from an NIH Director New Innovator Award (award 1 DP2 OD001886-01).
Population PK variability data such as covariances for the published adult data were provided as personal communications to Tawanda Gumbo by Helen McIlleron and Justin Wilkins of the University of Cape Town (Cape Town, South Africa). Pediatric PK covariance data were obtained in a personal communication from Charles Peloquin of the National Jewish Medical and Research Center (Denver, CO).
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Aly, S., K. Wagner, C. Keller, S. Malm, A. Malzan, S. Brandau, F. C. Bange, and S. Ehlers. 2006. Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J. Pathol. 210:298-305. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1981. Controlled trial of four thrice-weekly regimens and a daily regimen all given for 6 months for pulmonary tuberculosis. Lancet i:171-174. [PubMed] [Google Scholar]
- 3.Bilello, J. A., G. Bauer, M. N. Dudley, G. A. Cole, and G. L. Drusano. 1994. Effect of 2′,3′-didehydro-3′-deoxythymidine in an in vitro hollow-fiber pharmacodynamic model system correlates with results of dose-ranging clinical studies. Antimicrob. Agents Chemother. 38:1386-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser, J., B. B. Stone, and S. H. Zinner. 1985. Two compartment kinetic model with multiple artificial capillary units. J. Antimicrob. Chemother. 15(Suppl. A):131-137. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg, H. M., W. J. Burman, R. E. Chaisson, C. L. Daley, S. C. Etkind, L. N. Friedman, P. Fujiwara, M. Grzemska, P. C. Hopewell, M. D. Iseman, R. M. Jasmer, V. Koppaka, R. I. Menzies, R. J. O'Brien, R. R. Reves, L. B. Reichman, P. M. Simone, J. R. Starke, and A. A. Vernon. 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. [DOI] [PubMed] [Google Scholar]
- 6.Bonate, P. L. 2000. Clinical trial simulation in drug development. Pharm. Res. 17:252-256. [DOI] [PubMed] [Google Scholar]
- 7.Boshoff, H. I., V. Mizrahi, and C. E. Barry III. 2002. Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase I. J. Bacteriol. 184:2167-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brindle, R., J. Odhiambo, and D. Mitchison. 2001. Serial counts of Mycobacterium tuberculosis in sputum as surrogate markers of the sterilising activity of rifampicin and pyrazinamide in treating pulmonary tuberculosis. BMC Pulm. Med. 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canetti, G. 1955. The tubercle bacillus in the pulmonary lesion of man. Springer Publishing Company, New York, NY.
- 10.Centers for Disease Control and Prevention. 2006. Reported tuberculosis in the United States, 2005. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, Atlanta, GA. http://www.cdc.gov/tb/surv/surv2005/PDF/TBSurvFULLReport.pdf (accessed 4 October 2008).
- 11.Cheng, S. J., L. Thibert, T. Sanchez, L. Heifets, and Y. Zhang. 2000. pncA mutations as a major mechanism of pyrazinamide resistance in Mycobacterium tuberculosis: spread of a monoresistant strain in Quebec, Canada. Antimicrob. Agents Chemother. 44:528-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2003. Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic Actinomycetes; approved standard. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed]
- 13.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1999. Intrapulmonary concentrations of pyrazinamide. Antimicrob. Agents Chemother. 43:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conte, J. E., Jr., E. Lin, and E. Zurlinden. 2000. High-performance liquid chromatographic determination of pyrazinamide in human plasma, bronchoalveolar lavage fluid, and alveolar cells. J. Chromatogr. Sci. 38:33-37. [DOI] [PubMed] [Google Scholar]
- 15.Craig, W. A. 2007. Pharmacodynamics of antimicrobials: general concepts and applications, p. 1-19. In C. H. Nightangle, P. G. Ambrose, G. L. Drusano, and T. Murakawa (ed.), Antimicrobial pharmacodynamics in theory and practice. Informa Healthcare USA, Inc., New York, NY.
- 16.D'Argenio, D. Z., and A. Schumitzky. 1997. ADAPT II. A program for simulation, identification, and optimal experimental design. User manual. Biomedical Simulations Resource, University of Southern California, Los Angeles.
- 17.Deziel, M. R., H. Heine, A. Louie, M. Kao, W. R. Byrne, J. Basset, L. Miller, K. Bush, M. Kelly, and G. L. Drusano. 2005. Effective antimicrobial regimens for use in humans for therapy of Bacillus anthracis infections and postexposure prophylaxis. Antimicrob. Agents Chemother. 49:5099-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson, J. M., and D. A. Mitchison. 1991. Efficacy of intermittent pyrazinamide in experimental murine tuberculosis. Tubercle 72:110-114. [DOI] [PubMed] [Google Scholar]
- 19.Drusano, G. L., S. L. Preston, C. Hardalo, R. Hare, C. Banfield, D. Andes, O. Vesga, and W. A. Craig. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosset, J., C. Truffot-Pernot, C. Lacroix, and B. Ji. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob. Agents Chemother. 36:548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gumbo, T., A. Louie, M. R. Deziel, and G. L. Drusano. 2005. Pharmacodynamic evidence that ciprofloxacin failure against tuberculosis is not due to poor microbial kill but to rapid emergence of resistance. Antimicrob. Agents Chemother. 49:3178-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gumbo, T., A. Louie, M. R. Deziel, W. Liu, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 51:3781-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumbo, T., A. Louie, M. R. Deziel, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642-1651. [DOI] [PubMed] [Google Scholar]
- 24.Gumbo, T., A. Louie, W. Liu, P. G. Ambrose, S. M. Bhavnani, D. Brown, and G. L. Drusano. 2007. Isoniazid's bactericidal activity ceases because of the emergence of resistance, not depletion of Mycobacterium tuberculosis in the log phase of growth. J. Infect. Dis. 195:194-201. [DOI] [PubMed] [Google Scholar]
- 25.Gumbo, T., A. Louie, W. Liu, D. Brown, P. G. Ambrose, S. M. Bhavnani, and G. L. Drusano. 2007. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob. Agents Chemother. 51:2329-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heifets, L., and P. Lindholm-Levy. 1992. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. Am. Rev. Respir. Dis. 145:1223-1225. [DOI] [PubMed] [Google Scholar]
- 27.Heifets, L., and T. Sanchez. 2000. New agar medium for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Clin. Microbiol. 38:1498-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iseman, M. D. 2000. Tuberculosis chemotherapy, including directly observed therapy, p. 271-321. In A clinician's guide to tuberculosis. Lippincott Williams & Wilkins, Philadelphia, PA.
- 29.Jayaram, R., S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharat, R. K. Shandil, E. Kantharaj, and V. Balasubramanian. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayaram, R., R. K. Shandil, S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharath, E. Kantharaj, and V. Balasubramanian. 2004. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 48:2951-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jindani, A., V. R. Aber, E. A. Edwards, and D. A. Mitchison. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 121:939-949. [DOI] [PubMed] [Google Scholar]
- 32.Jindani, A., C. J. Dore, and D. A. Mitchison. 2003. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am. J. Respir. Crit. Care Med. 167:1348-1354. [DOI] [PubMed] [Google Scholar]
- 33.Kiem, S., and J. J. Schentag. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52:24-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konno, K., H. Nagayama, and S. Oka. 1959. Nicotinamidase in mycobacteria: a method for distinguishing bovine type tubercle bacilli from other mycobacteria. Nature 184(Suppl. 22):1743-1744. [DOI] [PubMed] [Google Scholar]
- 35.Kubendiran, G., C. N. Paramasivan, S. Sulochana, and D. A. Mitchison. 2006. Moxifloxacin and gatifloxacin in an acid model of persistent Mycobacterium tuberculosis. J. Chemother. 18:617-623. [DOI] [PubMed] [Google Scholar]
- 36.Lister, P. D., A. M. Prevan, and C. C. Sanders. 1997. Importance of beta-lactamase inhibitor pharmacokinetics in the pharmacodynamics of inhibitor-drug combinations: studies with piperacillin-tazobactam and piperacillin-sulbactam. Antimicrob. Agents Chemother. 41:721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackaness, G. B. 1956. The intracellular activation of pyrazinamide and nicotinamide. Am. Rev. Tuberc. 74:718-728. [DOI] [PubMed] [Google Scholar]
- 38.McDermott, W., and R. Tompsett. 1954. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am. Rev. Tuberc. 70:748-754. [DOI] [PubMed] [Google Scholar]
- 39.McDowell, M. A., C. D. Fryar, R. Hirsch, and C. L. Ogden. 2005. Anthropometric reference data for children and adults: U.S. population, 1999-2002. Report 361. National Center for Health Statistics, Hyattsville, MD.
- 40.Mitchison, D. A. 1979. Basic mechanisms of chemotherapy. Chest 76:771-781. [DOI] [PubMed] [Google Scholar]
- 41.Mitchison, D. A. 1985. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 66:219-225. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen, D., P. Brassard, J. Westley, L. Thibert, M. Proulx, K. Henry, K. Schwartzman, D. Menzies, and M. A. Behr. 2003. Widespread pyrazinamide-resistant Mycobacterium tuberculosis family in a low-incidence setting. J. Clin. Microbiol. 41:2878-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peloquin, C. A., G. S. Jaresko, C. L. Yong, A. C. Keung, A. E. Bulpitt, and R. W. Jelliffe. 1997. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob. Agents Chemother. 41:2670-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raynaud, C., M. A. Laneelle, R. H. Senaratne, P. Draper, G. Laneelle, and M. Daffe. 1999. Mechanisms of pyrazinamide resistance in mycobacteria: importance of lack of uptake in addition to lack of pyrazinamidase activity. Microbiology 145(Pt 6):1359-1367. [DOI] [PubMed] [Google Scholar]
- 45.Salfinger, M., and L. B. Heifets. 1988. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob. Agents Chemother. 32:1002-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz, W. S., and R. E. Moyer. 1954. The chemotherapy of pulmonary tuberculosis with pyrazinamide used alone and in combination with streptomycin, para-aminosalicylic acid, or isoniazid. Am. Rev. Tuberc. 70:413-422. [DOI] [PubMed] [Google Scholar]
- 47.Scorpio, A., and Y. Zhang. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662-667. [DOI] [PubMed] [Google Scholar]
- 48.Snider, D. E., Jr., J. Rogowski, M. Zierski, E. Bek, and M. W. Long. 1982. Successful intermittent treatment of smear-positive pulmonary tuberculosis in six months: a cooperative study in Poland. Am. Rev. Respir. Dis. 125:265-267. [DOI] [PubMed] [Google Scholar]
- 49.Steenken, W., Jr., and E. Wolinsky. 1954. The antituberculous activity of pyrazinamide in vitro and in the guinea pig. Am. Rev. Tuberc. 70:367-369. [DOI] [PubMed] [Google Scholar]
- 50.Tam, V. H., A. Louie, M. R. Deziel, W. Liu, and G. L. Drusano. 2007. The relationship between quinolone exposures and resistance amplification is characterized by an inverted U: a new paradigm for optimizing pharmacodynamics to counterselect resistance. Antimicrob. Agents Chemother. 51:744-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Victor, T. C., E. M. Streicher, C. Kewley, A. M. Jordaan, G. D. van der Spuy, M. Bosman, H. Louw, M. Murray, D. Young, P. D. van Helden, and R. M. Warren. 2007. Spread of an emerging Mycobacterium tuberculosis drug-resistant strain in the western Cape of South Africa. Int. J. Tuberc. Lung Dis. 11:195-201. [PubMed] [Google Scholar]
- 52.Wilkins, J. J., G. Langdon, H. McIlleron, G. C. Pillai, P. J. Smith, and U. S. Simonsson. 2006. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur. J. Clin. Pharmacol. 62:727-735. [DOI] [PubMed] [Google Scholar]
- 53.Woo, J., W. Cheung, R. Chan, H. S. Chan, A. Cheng, and K. Chan. 1996. In vitro protein binding characteristics of isoniazid, rifampicin, and pyrazinamide to whole plasma, albumin, and alpha-1-acid glycoprotein. Clin. Biochem. 29:175-177. [DOI] [PubMed] [Google Scholar]
- 54.Yeager, R. L., W. G. Munroe, and F. I. Dessau. 1952. Pyrazinamide (aldinamide) in the treatment of pulmonary tuberculosis. Am. Rev. Tuberc. 65:523-546. [PubMed] [Google Scholar]
- 55.Zhang, Y., S. Permar, and Z. Sun. 2002. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J. Med. Microbiol. 51:42-49. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, Y., A. Scorpio, H. Nikaido, and Z. Sun. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 181:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang, Y., M. M. Wade, A. Scorpio, H. Zhang, and Z. Sun. 2003. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J. Antimicrob. Chemother. 52:790-795. [DOI] [PubMed] [Google Scholar]
- 58.Zhu, M., J. R. Starke, W. J. Burman, P. Steiner, J. J. Stambaugh, D. Ashkin, A. E. Bulpitt, S. E. Berning, and C. A. Peloquin. 2002. Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy 22:686-695. [DOI] [PubMed] [Google Scholar]
- 59.Zimhony, O., J. S. Cox, J. T. Welch, C. Vilcheze, and W. R. Jacobs, Jr. 2000. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 6:1043-1047. [DOI] [PubMed] [Google Scholar]
- 60.Zimhony, O., C. Vilcheze, M. Arai, J. T. Welch, and W. R. Jacobs, Jr. 2007. Pyrazinoic acid and its n-propyl ester inhibit fatty acid synthase type I in replicating tubercle bacilli. Antimicrob. Agents Chemother. 51:752-754. [DOI] [PMC free article] [PubMed] [Google Scholar]