Abstract
The in vitro interaction of antifungals with immunosuppressive drugs was evaluated against zygomycetes. The combination of amphotericin B with cyclosporine, rapamycin, or tacrolimus was synergistic for 90%, 70%, and 30% of the isolates, respectively. For posaconazole, itraconazole, and ravuconazole, synergy was more frequently observed with cyclosporine than with rapamycin or tacrolimus and antagonistic interactions were rarely noted. In summary, calcineurin inhibitors and rapamycin can be synergistic in vitro with amphotericin B and azoles against zygomycetes.
Calcineurin inhibitors (cyclosporine and tacrolimus) and inhibitors of the mTOR pathway (rapamycin) are widely used with solid organ transplant (SOT) patients. Although the use of these immunosuppressive drugs is a risk factor for invasive fungal infections, they also possess antifungal activity against yeasts (3, 4, 36, 39) and filamentous (34) and dimorphic fungi (14). Nevertheless, the activity of immunosuppressive drugs alone or in combination against zygomycetes has been poorly evaluated. The combination of immunosuppressive drugs with antifungal drugs has been evaluated against yeasts (5, 7, 15, 17-21, 25, 26, 37, 38) and filamentous fungi (16, 27, 33, 34). Because zygomycosis is emerging in SOT patients, we studied combinations between amphotericin B or azoles and immunosuppressive agents against zygomycetes.
Combinations were tested against 10 isolates (3 Rhizopus oryzae; 2 Mycocladus corymbiferus, formerly Absidia corymbifera [11]; 2 Mucor circinelloides; 2 Rhizopus microsporus; and 1 Rhizomucor pusillus) according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (2) modified for a broth microdilution checkerboard procedure. Final concentrations were 0.004 to 2 μg/ml for amphotericin B, itraconazole, posaconazole, and ravuconazole; 0.125 to 8 μg/ml for cyclosporine; and 0.06 to 4 μg/ml for tacrolimus and rapamycin. Lower concentrations of 0.001 to 0.06 μg/ml were tested for tacrolimus and rapamycin in additional experiments. More than 70% of the tests were run in duplicate and yielded identical (±1 log2 dilution) MICs between runs in more than 90% of the cases, confirming that the technique was reproducible. The MICs were determined spectrophotometrically after 24 h of incubation at 35°C. MIC endpoints were defined as the lowest drug concentration (tested alone or in combination) that gave 50% of the inhibition except for amphotericin B alone, for which a complete inhibition (as measured by a 90% reduction of growth by spectrophotometric reading) was used. Calculations with two alternative sets of inhibition endpoints ([i] 90% for azoles and 50% for immunosuppressive drugs alone and for all combinations and [ii] 90% for all drugs either alone or in combination) were also performed. Antifungal combinations of amphotericin B or posaconazole with cyclosporine were also evaluated by Etest in RPMI agar after 24 h of incubation at 35°C. Drug combinations were evaluated by the calculation of the fractional inhibitory concentration indices (8) interpreted as previously recommended (24).
All isolates exhibited low MICs for amphotericin B (0.125 to 1 μg/ml). Itraconazole, posaconazole, and ravuconazole showed activity against most of the isolates, with geometric mean MICs of 0.62, 0.57, and 0.44 μg/ml, respectively (data not shown). Immunosuppressive drugs alone also demonstrated activity. Cyclosporine MICs varied between 1 and >8 μg/ml. For tacrolimus and rapamycin, susceptibility depended on the species. Tacrolimus MICs were ≤0.5 μg/ml for R. oryzae, M. circinelloides, and M. corymbiferus and >4 μg/ml for R. microsporus and R. pusillus. For rapamycin, R. microsporus and M. circinelloides exhibited MICs of ≤0.25 μg/ml, whereas R. pusillus and M. corymbiferus had MICs of >4 μg/ml. For the three R. oryzae isolates, variable susceptibilities to rapamycin were observed.
The results of the 12 combinations tested are summarized in Table 1. Synergistic interactions were observed between amphotericin B and cyclosporine (90%), rapamycin (70%), and tacrolimus (30%). For the combinations including azoles, synergy was more frequently observed with cyclosporine (up to 70% for itraconazole and posaconazole) than with rapamycin or tacrolimus. Antagonism was never observed with amphotericin B or posaconazole in combination with any immunosuppressive drugs. However, an antagonism was noted for two R. oryzae isolates for the combination of itraconazole and rapamycin and for one M. circinelloides isolate for the combination of ravuconazole and rapamycin. An equivalent (less than 10% difference) or a higher percentage of synergy was obtained when alternative MIC inhibition endpoints were used (data not shown).
TABLE 1.
Summary of the in vitro interaction of amphotericin B and azoles in combination with immunosuppressive drugs against 10 isolates of Zygomycetes
| Combinationa | Interaction (% of isolates)
|
||
|---|---|---|---|
| Synergy | No interaction | Antagonism | |
| AMB + CyA | 90 | 10 | 0 |
| AMB + Tacro | 30 | 70 | 0 |
| AMB + Rapa | 70 | 30 | 0 |
| ITZ + CyA | 70 | 30 | 0 |
| ITZ + Tacro | 20 | 80 | 0 |
| ITZ + Rapa | 50 | 30 | 20 |
| PSZ + CyA | 70 | 30 | 0 |
| PSZ + Tacro | 0 | 100 | 0 |
| PSZ + Rapa | 40 | 60 | 0 |
| RVZ + CyA | 30 | 70 | 0 |
| RVZ + Tacro | 10 | 90 | 0 |
| RVZ + Rapa | 30 | 60 | 10 |
AMB, amphotericin B; ITZ, itraconazole; PSZ, posaconazole; RVZ, ravuconazole; CyA, cyclosporine; Tacro, tacrolimus; Rapa, rapamycin.
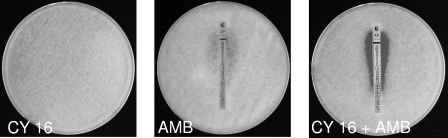
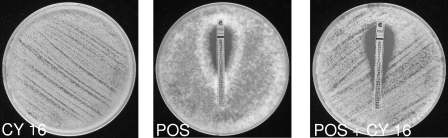
By agar diffusion tests, decreases of ≥2 dilutions of the MIC of the antifungal suggesting synergy were observed for 50% of the isolates for the combination of cyclosporine and either amphotericin B or posaconazole (examples are presented in Fig. 1 and 2).
FIG. 1.
In vitro interaction of amphotericin B (AMB) with cyclosporine (CY 16) against an isolate of Rhizopus oryzae (CNR 03_918) tested by an agar diffusion method. Cyclosporine was incorporated in the agar. The amphotericin B MIC was determined by Etest in the absence or presence of cyclosporine at a concentration of 16 μg/ml.
FIG. 2.
In vitro interaction of posaconazole (POS) with cyclosporine (CY 16) against an isolate of Mycocladus corymbiferus (IP 1279.81) tested by an agar diffusion method. Cyclosporine was incorporated in the agar. The posaconazole MIC was determined by Etest in the absence or presence of cyclosporine at a concentration of 16 μg/ml.
Antifungal combinations showed promising results against zygomycetes either in vitro or in animal models (6, 9, 10, 13, 28, 31), and some combinations between antifungal and nonantifungal drugs have also been evaluated (1, 6, 35). A recent study, however, has shown that the combination of posaconazole and liposomal amphotericin B was not synergistic in murine models of zygomycosis (32).
Because calcineurin inhibitors are associated with improved outcome for SOT patients with some fungal infections such as cryptococcosis (15, 30), we decided to evaluate combinations between antifungals and immunosuppressive drugs against zygomycetes. We found that immunosuppressive drugs alone had antifungal activity against zygomycetes with variable levels of activity depending on the species and on the drug and that combinations of amphotericin B or posaconazole with immunosuppressive drugs could exhibit synergistic interactions. In particular, the synergy was observed with cyclosporine, rapamycin, and, to a lesser extent, tacrolimus. It could be of interest to test a wider panel of zygomycetes including other pathogenic species, such as Cunninghamella.
These results extend previous findings with other fungi such as Cryptococcus neoformans (7, 15, 30), Candida albicans (5, 17-19, 21, 26, 37, 38), and Aspergillus fumigatus (16, 33, 34). More recently, combinations between posaconazole and calcineurin inhibitors evaluated against zygomycetes showed indifference to synergistic interactions (22, 23). Given the recent demonstration of the limited in vivo activity of caspofungin against infection due to R. oryzae and the inhibition of glucan synthase activity (12) and clinical reports of synergy with amphotericin B in animal models (13) and human (29), testing the combination of echinocandins with immunosuppressive drugs should be investigated. Moreover, the combined inhibition of Fks1/2 and homeostatic cell wall stress responses in this fungi by the calcineurin-echinocandin combination would be pharmacologically sound. Similarly, it could be of interest to test if positive interactions could be observed if cells are exposed to H2O2, cycloheximide, or other nonspecific stress molecules.
In summary, we showed that calcineurin inhibitors and rapamycin could enhance the in vitro activity of amphotericin B and posaconazole against zygomycetes. These in vitro results warrant further animal experiments.
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Chamilos, G., R. E. Lewis, and D. P. Kontoyiannis. 2006. Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrob. Agents Chemother. 50:96-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CLSI. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard. Document M-38A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Cruz, M. C., M. Del Poeta, P. Wang, R. Wenger, G. Zenke, V. F. Quesniaux, N. R. Movva, J. R. Perfect, M. E. Cardenas, and J. Heitman. 2000. Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob. Agents Chemother. 44:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz, M. C., A. L. Goldstein, J. Blankenship, M. Del Poeta, J. R. Perfect, J. H. McCusker, Y. L. Bennani, M. E. Cardenas, and J. Heitman. 2001. Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob. Agents Chemother. 45:3162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dannaoui, E., J. Afeltra, J. F. Meis, and P. E. Verweij. 2002. In vitro susceptibilities of zygomycetes to combinations of antimicrobial agents. Antimicrob. Agents Chemother. 46:2708-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Poeta, M., M. C. Cruz, M. E. Cardenas, J. R. Perfect, and J. Heitman. 2000. Synergistic antifungal activities of bafilomycin A1, fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliopoulos, G. M., and R. C. Moellering. 1991. Antimicrobial combinations, p. 432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine. The Williams & Wilkins Co., Baltimore, MD.
- 9.Gomez-Lopez, A., M. Cuenca-Estrella, E. Mellado, and J. L. Rodriguez-Tudela. 2003. In vitro evaluation of combination of terbinafine with itraconazole or amphotericin B against Zygomycota. Diagn. Microbiol. Infect. Dis. 45:199-202. [DOI] [PubMed] [Google Scholar]
- 10.Guembe, M., J. Guinea, T. Pelaez, M. Torres-Narbona, and E. Bouza. 2007. Synergistic effect of posaconazole and caspofungin against clinical zygomycetes. Antimicrob. Agents Chemother. 51:3457-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann, K., S. Discher, and K. Voigt. 2007. Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycol. Res. 111:1169-1183. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim, A. S., J. C. Bowman, V. Avanessian, K. Brown, B. Spellberg, J. E. Edwards, Jr., and C. M. Douglas. 2005. Caspofungin inhibits Rhizopus oryzae 1,3-β-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob. Agents Chemother. 49:721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim, A. S., T. Gebremariam, Y. Fu, J. E. Edwards, Jr., and B. Spellberg. 2008. Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob. Agents Chemother. 52:1556-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkland, T. N., and J. Fierer. 1983. Cyclosporin A inhibits Coccidioides immitis in vitro and in vivo. Antimicrob. Agents Chemother. 24:921-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kontoyiannis, D. P., R. E. Lewis, B. D. Alexander, O. Lortholary, F. Dromer, K. L. Gupta, G. T. John, R. Del Busto, G. B. Klintmalm, J. Somani, G. M. Lyon, K. Pursell, V. Stosor, P. Munoz, A. P. Limaye, A. C. Kalil, T. L. Pruett, J. Garcia-Diaz, A. Humar, S. Houston, A. A. House, D. Wray, S. Orloff, L. A. Dowdy, R. A. Fisher, J. Heitman, N. D. Albert, M. M. Wagener, and N. Singh. 2008. Calcineurin inhibitor agents interact synergistically with antifungal agents in vitro against Cryptococcus neoformans isolates: correlation with outcome in solid organ transplant recipients with cryptococcosis. Antimicrob. Agents Chemother. 52:735-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontoyiannis, D. P., R. E. Lewis, N. Osherov, N. D. Albert, and G. S. May. 2003. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. J. Antimicrob. Chemother. 51:313-316. [DOI] [PubMed] [Google Scholar]
- 17.Li, Y., S. Sun, Q. Guo, L. Ma, C. Shi, L. Su, and H. Li. 2008. In vitro interaction between azoles and cyclosporin A against clinical isolates of Candida albicans determined by the chequerboard method and time-kill curves. J. Antimicrob. Chemother. 61:577-585. [DOI] [PubMed] [Google Scholar]
- 18.Maesaki, S., P. Marichal, M. A. Hossain, D. Sanglard, H. Vanden Bossche, and S. Kohno. 1998. Synergic effects of tacrolimus and azole antifungal agents against azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 42:747-753. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti, O., J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser, and P. Moreillon. 2000. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44:2932-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchetti, O., P. Moreillon, J. M. Entenza, J. Vouillamoz, M. P. Glauser, J. Bille, and D. Sanglard. 2003. Fungicidal synergism of fluconazole and cyclosporine in Candida albicans is not dependent on multidrug efflux transporters encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes. Antimicrob. Agents Chemother. 47:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchetti, O., P. Moreillon, M. P. Glauser, J. Bille, and D. Sanglard. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narreddy, S., E. Manavathu, P. H. Chandrasekar, G. J. Alangaden, and S. G. Revankar. 2008. In vitro interaction of posaconazole with calcineurin inhibitors (CI) against Zygomycetes. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1533. [DOI] [PubMed]
- 23.Narreddy, S., E. Manavathu, P. H. Chandrasekar, G. J. Alangaden, and S. G. Revankar. 2007. In vitro interaction of triazoles with calcineurin inhibitors (CI) against Zygomycetes. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1851. [DOI] [PubMed]
- 24.Odds, F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 25.Onyewu, C., N. A. Afshari, and J. Heitman. 2006. Calcineurin promotes infection of the cornea by Candida albicans and can be targeted to enhance fluconazole therapy. Antimicrob. Agents Chemother. 50:3963-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onyewu, C., E. Eads, W. A. Schell, J. R. Perfect, Y. Ullmann, G. Kaufman, B. A. Horwitz, I. Berdicevsky, and J. Heitman. 2007. Targeting the calcineurin pathway enhances ergosterol biosynthesis inhibitors against Trichophyton mentagrophytes in vitro and in a human skin infection model. Antimicrob. Agents Chemother. 51:3743-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perkhofer, S., M. Locher, M. Cuenca-Estrella, R. Ruchel, R. Wurzner, M. P. Dierich, and C. Lass-Florl. 2008. Posaconazole enhances the activity of amphotericin B against hyphae of zygomycetes in vitro. Antimicrob. Agents Chemother. 52:2636-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed, C., R. Bryant, A. S. Ibrahim, J. Edwards, Jr., S. G. Filler, R. Goldberg, and B. Spellberg. 2008. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin. Infect. Dis. 47:364-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh, N., B. D. Alexander, O. Lortholary, F. Dromer, K. L. Gupta, G. T. John, R. del Busto, G. B. Klintmalm, J. Somani, G. M. Lyon, K. Pursell, V. Stosor, P. Munoz, A. P. Limaye, A. C. Kalil, T. L. Pruett, J. Garcia-Diaz, A. Humar, S. Houston, A. A. House, D. Wray, S. Orloff, L. A. Dowdy, R. A. Fisher, J. Heitman, M. M. Wagener, and S. Husain. 2007. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J. Infect. Dis. 195:756-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spellberg, B., Y. Fu, J. E. Edwards, Jr., and A. S. Ibrahim. 2005. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob. Agents Chemother. 49:830-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spellberg, B., T. Gebremariam, J. Schwartz, J. Edwards, Jr., and A. Ibrahim. 2008. Posaconazole and liposomal amphotericin B therapy of murine mucormycosis. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-3762.
- 33.Steinbach, W. J., W. A. Schell, J. R. Blankenship, C. Onyewu, J. Heitman, and J. R. Perfect. 2004. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 48:1664-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinbach, W. J., N. Singh, J. L. Miller, D. K. Benjamin, Jr., W. A. Schell, J. Heitman, and J. R. Perfect. 2004. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus isolates from transplant and nontransplant patients. Antimicrob. Agents Chemother. 48:4922-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugar, A. M., and X. P. Liu. 2000. Combination antifungal therapy in treatment of murine pulmonary mucormycosis: roles of quinolones and azoles. Antimicrob. Agents Chemother. 44:2004-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugita, T., M. Tajima, T. Ito, M. Saito, R. Tsuboi, and A. Nishikawa. 2005. Antifungal activities of tacrolimus and azole agents against the eleven currently accepted Malassezia species. J. Clin. Microbiol. 43:2824-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun, S., Y. Li, Q. Guo, C. Shi, J. Yu, and L. Ma. 2008. In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob. Agents Chemother. 52:409-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uppuluri, P., J. Nett, J. Heitman, and D. Andes. 2008. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob. Agents Chemother. 52:1127-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vezina, C., A. Kudelski, and S. N. Sehgal. 1975. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. (Tokyo) 28:721-726. [DOI] [PubMed] [Google Scholar]