Abstract
Staphylococcal biofilms on surgical implants are the underlying cause of a lack of response to antimicrobial treatment. We investigated the effects of vancomycin (VAN), daptomycin (DAP), fosfomycin (FOS), tigecycline (TGC), and ceftriaxone (CRX), alone and in combination with azithromycin (AZI), on established biofilms of Staphylococcus epidermidis. Biofilms were studied using the static microtiter plate model with established S. epidermidis biofilms, with an initial inoculum of 106/ml in 96-well polystyrene flat-bottom microtiter plates. Biofilms were inoculated with VAN, DAP, FOS, TGC, or CRX at two concentrations, alone or in combination with AZI (2, 512, or 1,024 mg/liter). To assess the reduction in biomass, the optical density ratio (ODr), calculated as (optical density [OD] of the treated biofilm)/(OD of the untreated biofilm, taken as 1), was used. For antibacterial efficacy, the viable bacterial count was used. Reductions in the biofilm ODr were observed for VAN (15 and 40 mg/liter) and FOS (200 mg/liter) only (ODr [mean ± standard deviation] for VAN at 15 and 40 mg/liter, 0.77 ± 0.32 and 0.8 ± 0.35, respectively; ODr for FOS at 200 mg/liter, 0.78 ± 0.26; P < 0.05), but not for DAP (2 and 5 mg/liter), TGC (0.2 and 2 mg/liter), or CRX (600 and 2,400 mg/liter). The addition of AZI had no further effect on the ODr, but a significant reduction of bacterial growth was achieved with high doses of AZI plus TGC or AZI plus CRX (a 3-log count reduction for AZI at 1,024 mg/liter plus CRX at 600 mg/liter and for AZI at 512 or 1,024 mg/liter plus CRX at 2,400 mg/liter; a 2-log count reduction for AZI at 512 or 1,024 mg/liter plus TGC at 2 mg/liter [P < 0.05]). No significant reduction in bacterial growth was observed for FOS (50 and 200 mg/liter), DAP (2 and 5 mg/liter), or TGC (0.2 mg/liter) in combination with AZI. None of the antibiotics at either concentration reduced the bacterial count of the biofilms when used alone. Thus, the use of a combination of AZI plus TGC, FOS, or CRX at high concentrations has little effect on biofilm density but significantly reduces bacterial growth.
Staphylococcus epidermidis is the leading pathogen causing infections of surgical implants. Given the high incidence of fracture fixation devices, 2 million per annum, the number of implant infections amounts to 100,000 per year in the United States (10). Many of these infections are associated with biofilms formed by staphylococci on implant surfaces (9, 26, 34). The biofilm consists of a structured community of bacterial cells enclosed in a self-produced polymeric matrix and adherent to an inert or living surface. The two consequences of biofilm formation on implant surfaces are increased resistance to antimicrobial agents and frequent failure of conventional antimicrobial therapy. This resistance of bacteria within biofilms is attributed to a possible barrier function of the biofilm, binding of the antimicrobial agents within the matrix, and the metabolic change in the bacterial cells. Thus, infection of medical implants is associated with considerable morbidity and costs due to loss of mobility, nonproductive time, and health care (10, 18, 35).
The antimicrobial agents most widely used for staphylococcal infections are beta-lactam antibiotics, primarily cephalosporins, and in the case of beta-lactam resistance, vancomycin (23, 28). Alternative agents are intravenous fosfomycin, a small-molecule antibiotic with a wide antibacterial spectrum and excellent tissue penetration, and two newer antibiotics, the glycylcycline tigecycline and the cyclic lipopeptide daptomycin (14). Daptomycin, which is highly active against gram-positive cocci resistant to commonly used antibiotics, including methicillin (meticillin)-resistant staphylococci, causes membrane changes in the bacteria, whereas tigecycline inhibits protein synthesis (20). Although macrolides are not commonly used in the treatment of staphylococcal infections, clinical and experimental data suggest that azithromycin decreases biofilm formation and enhances the efficacy of other antibiotics for patients with cystic fibrosis and Pseudomonas infections (17, 19).
The MICs of antimicrobial agents tested on bacterial biofilms are dramatically increased, up to concentrations >1,000 times the MICs for staphylococci under planktonic conditions (5). However, there is some evidence that higher antibiotic concentrations reduce biofilms and bacterial growth, whereas standard concentrations are not effective (24, 30). In a previous study, we demonstrated that antibiotic concentrations equivalent to 100 times the MIC under planktonic conditions did not decrease established staphylococcal biofilms (16). To investigate if even higher concentrations will overcome the bacterial resistance within biofilms and reduce biofilm thickness and bacterial growth, static biofilms of clinical S. epidermidis isolates causing implant infections and catheter-associated bacteremia were incubated with vancomycin, daptomycin, fosfomycin, tigecycline, or ceftriaxone at two concentrations. To explore the additional effect of the macrolide azithromycin (22) on S. epidermidis biofilms, we treated the biofilms with vancomycin, daptomycin, fosfomycin, tigecycline, or ceftriaxone in combination with azithromycin at three concentrations (2, 512, and 1,024 mg/liter).
(Part of this research was presented as a poster at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 2007 [29a].)
MATERIALS AND METHODS
The University Hospital of Vienna is a 2,200-bed primary- and tertiary-care teaching hospital. We tested S. epidermidis isolates identified as pathogens of implant infections (two on a cardiac pacemaker [SE19] or implanted defibrillator [SE25] and three on bone implants [SE32, SE42, and SE99]) or of catheter-related bacteremia (three isolates, SE1, SE4, and SE14). In addition, two S. epidermidis isolates from the skin of non-hospital-associated healthy controls (SEK1 and SEK15) were tested. The characterization of the isolates and the MICs under planktonic conditions are given in Table 1. The biofilm-producing strains Staphylococcus aureus ATCC 29213 and S. epidermidis DSM3269 served as reference strains. Susceptibility testing was performed using routine laboratory methods according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI) (8). Antibiotic susceptibility was determined by the disk diffusion method on cation-adjusted Mueller-Hinton agar (bioMerieux, Marcy L'Etoile, France). Resistance to oxacillin was detected by incubating the plates with disks containing 5 μg of oxacillin at 30°C and 37°C. The isolates were genotyped using the pulsed-field electrophoresis genotyping method as previously described (28).
TABLE 1.
MICs for the S. epidermidis isolates investigated
| Isolate | Type of infection | Antibiotic treatment | MIC (mg/liter) of the following druga:
|
|||||
|---|---|---|---|---|---|---|---|---|
| AZI | VAN | DAP | FOS | TIG | CRX | |||
| SE19 | Pacemaker infection | None | 0.06 | 2 | 0.25 | 32 | 0.125 | 16 |
| SE25 | Implanted defibrillator infection | None | 0.125 | 2 | 0.25 | 4 | 0.125 | 64 |
| SE32 | Hip prosthesis | Ceftriaxone | >256 | 1 | 0.25 | 32 | 0.06 | 16 |
| SE99 | Knee prosthesis | None | >256 | 2 | 0.125 | >256 | 0.25 | 32 |
| SE42 | Hip prosthesis | None | 0.06 | 1 | 0.25 | 16 | 0.06 | 1 |
| SE1 | Intravascular catheter-related bacteremia | Cefuroxime | >256 | 2 | 0.125 | 1 | 0.06 | 16 |
| SE4 | Intravascular catheter-related bacteremia | Amoxicillin-clavulanic acid | >256 | 1 | 0.5 | 32 | 0.5 | 16 |
| SE14 | Intravascular catheter-related bacteremia | Ceftriaxone | 0.125 | 2 | 0.25 | 128 | 0.06 | 1 |
| SEK1 | Skin isolate | None | >256 | 0.5 | 0.025 | 16 | 0.06 | 1 |
| SEK15 | Skin isolate | None | 0.125 | 1 | 0.25 | 2 | 0.06 | 1 |
| ATCC 29213 | 0.25 | 1 | 0.125 | 2 | 0.25 | 4 | ||
| DSM3269 | 0.25 | 2 | 0.25 | 1 | 0.5 | 0.5 | ||
AZI, azithromycin; VAN, vancomycin; DAP, daptomycin; FOS, fosfomycin; TIG, tigecycline; CRX, ceftriaxone.
Antimicrobial agents.
Vancomycin was bought from Lilly (Vienna, Austria), fosfomycin and ceftriaxone from Sandoz (Kundl, Austria), tigecycline from Wyeth-Lederle (Vienna, Austria), daptomycin from Novartis (Vienna, Austria), and azithromycin from Pfizer (Vienna, Austria).
Determination of the MIC.
Before the biofilms were treated with vancomycin, daptomycin, fosfomycin, tigecycline, or ceftriaxone, the MICs of each isolate to be tested were determined. MICs were determined according to the CLSI protocol using the microtiter plate method (8). According to this protocol, cation-adjusted Mueller-Hinton medium and broth containing 50 μg/ml calcium were used for in vitro susceptibility testing of daptomycin. For the testing of susceptibility to fosfomycin, glucose-6-phosphate (25 mg/liter) was added.
Biofilm model.
Biofilms were studied using the static microtiter plate model established by Christensen et al. (7). The S. epidermidis isolates were prepared in Mueller-Hinton broth (MHB) at a McFarland standard of 0.5 and were diluted 1:100 with MHB. Each well of a 96-well polystyrene flat-bottom microtiter plate was filled with 50 μl of diluted bacteria and 50 μl of supplemented MHB (containing calcium) and was incubated for 24 h in ambient air at 35°C. Media and planktonic cells were removed. The biofilms in the wells were fixed with formalin (37%, diluted 1:10) plus 2% natrium acetate, and each well was stained with 150 to 250 μl of 1% crystal violet for 5 min. Then the stained biofilms were washed twice with approximately 300 μl distilled water. Wells were then visually checked for the presence or absence of a biofilm based on the presence of staining at the bottom of the well. The mean optical density (OD) was used for quantification using a routine microtiter plate reader at a wavelength of 550 nm. All biofilm experiments were performed four times for each isolate to minimize the variability in OD measurements. To ascertain biofilm formation, biofilms were grown on cover slides using a 24-well-plate. After 24 h, the biofilms were fixed with 2% glutaraldehyde, and biofilm formation was verified by electron microscope scanning. For the experiments, established biofilms grown for 24 h were used.
Incubation of the biofilms with vancomycin, fosfomycin, tigecycline, daptomycin, or ceftriaxone at low and high doses.
To test the effects of the antimicrobials at high concentrations, the 24-h-old biofilms were incubated for 20 h with vancomycin, fosfomycin, tigecycline, or daptomycin at two concentrations: (i) those representing the trough concentrations in humans in vivo (vancomycin at 15 mg/liter, daptomycin at 2 mg/liter, fosfomycin at 50 mg/liter, and tigecycline at 0.2 mg/liter) and (ii) very high concentrations (vancomycin at 40 mg/liter, daptomycin at 5 mg/liter, fosfomycin at 200 mg/liter, and tigecycline at 2 mg/liter). In addition, the effects of excess concentrations of ceftriaxone were investigated by using two concentrations, 600 and 2,400 mg/liter, because the effects of beta-lactam antibiotics on biofilms are unclear (1, 24, 31).
Combination with azithromycin.
The effects of azithromycin alone at 2 mg/liter, reflecting the average human plasma azithromycin concentrations after the application of two therapeutic doses of 250 mg (13), were investigated. Additionally, because under planktonic conditions, the MICs for some of the isolates to be tested were greater than 256 mg/liter, azithromycin at concentrations of 512 and 1,024 mg/liter was tested on the biofilms. Further, the effects on the biofilms of the combination of azithromycin at 2 mg/liter (as described elsewhere [36]), 512 mg/liter, or 1,024 mg/liter with vancomycin, daptomycin, fosfomycin, tigecycline, or ceftriaxone (at the concentrations given above) were tested.
To correct for the individual biofilm formation of each isolate, the ODr, a ratio of the OD of the biofilm of the isolate incubated with antibiotic to the OD of the untreated biofilm of the same isolate (native biofilm), was calculated. The baseline of the “untreated” biofilm was set as 1. The explanation for this was that using the uncorrected ODs of the biofilms, the intra-assay variability was determined from quadruplicate analysis of the treated biofilms. The median coefficient of variance (CV) for the 10 bacteria was 17.2% (range, 7.6 to 52.9%). The interassay variability (day-to-day variability) was based on bacterial growth without antibiotic (control condition) on two different days: the median CV was 42.5% (range, 8.2 to 91.8%). Due to this relatively high CV, the ODr was used for the calculations. This ODr was used to measure changes in the thickness of the biofilms with increasing concentrations of the antibiotics tested (29). Overall, the intra-assay variability was reduced; the median CV was 19.5% (range, 12.8 to 31%).
Antibacterial efficacies of the antimicrobial agents on the biofilms in all experiments.
To test for viable S. epidermidis in the biofilms in all experiments, the biofilms were not fixed and dyed but scraped off and resuspended in MHB, seeded to Columbia agar, and examined for growth. The numbers of S. epidermidis organisms in suspension were enumerated by serial dilutions, and 0.1 ml of each dilution was inoculated onto blood agar plates. The plates were then incubated at 35°C in ambient air and read after 48 h.
Statistical methods.
The significance of differences was assessed by means of the chi-square test for categorical variables. For subgroups and nonnormally distributed variables, the Mann-Whitney U test was used. To determine the changes due to the different antibiotic combinations and concentrations of every isolate, a general linear model for repeated measurement was calculated. All tests were performed using SPSS for Windows, release 15 (SPSS). A P value of <0.05 was considered significant.
RESULTS
Biofilm density.
Biofilms were incubated with five antimicrobial agents alone: vancomyin (15 and 40 mg/liter), fosfomycin (50 and 200 mg/liter), tigecycline (0.2 and 2 mg/liter), daptomycin (2 and 5 mg/liter), or ceftriaxone (600 and 2,400 mg/liter). The ODs of the biofilms incubated with vancomycin, at concentrations of 15 and 40 mg/liter, were significantly lower than the OD of untreated biofilms, taken as 1 (ODr [mean ± standard deviation], 0.78 ± 0.29 and 0.56 ± 0.45, respectively; P < 0.05). Neither fosfomycin (50 and 200 mg/liter), tigecycline (0.2 and 2 mg/liter), daptomycin (2 and 5 mg/liter), nor ceftriaxone (600 and 2,400 mg/liter) achieved significant reductions in the biofilm OD, as shown in Table 2. Although there were differences between the antibiotic effects for the individual bacterial strains, only vancomycin resulted in a significant decrease in the ODr, particularly for four strains (SE32, SE42, SEK1, and SE14), for which the ODr was reduced below 0.4, although the MIC under planktonic conditions was not particularly low (Tables 1 and 2). With regard to bactericidal efficacy, no antimicrobial employed alone at either concentration achieved any reduction of bacterial growth from that for untreated biofilms.
TABLE 2.
Changes in the ODr of S. epidermidis biofilms grown for 24 h upon incubation with antibiotics used alone or in combination with azithromycin
| Antibiotic and concn (mg/liter) | ODra with the drug used:
|
|||
|---|---|---|---|---|
| As a single agent | In combination with azithromycin at:
|
|||
| 2 mg/liter | 512 mg/liter | 1,024 mg/liter | ||
| Vancomycin | ||||
| 15 | 0.78 ± 0.29* | 0.91 ± 0.16 | 0.77 ± 0.32 | 0.93 ± 0.4 |
| 40 | 0.56 ± 0.45* | 0.96 ± 0.15 | 0.8 ± 0.34 | 0.85 ± 0.26 |
| Daptomycin | ||||
| 2 | 1.22 ± 0.47 | 1.17 ± 0.18 | 1.14 ± 0.61 | 2.03 ± 1.91 |
| 5 | 1.28 ± 0.52 | 1.01 ± 0.2 | 1.41 ± 0.69 | 1.1 ± 0.59 |
| Fosfomycin | ||||
| 50 | 0.9 ± 0.36 | 0.98 ± 0.12 | 1.01 ± 0.39 | 0.9 ± 0.36 |
| 200 | 0.72 ± 0.31 | 1.00 ± 0.23 | 0.78 ± 0.26 | 0.77 ± 0.42 |
| Tigecycline | ||||
| 0.2 | 0.87 ± 0.27 | 1.25 ± 0.30 | 1.29 ± 0.53 | 0.87 ± 0.27 |
| 2 | 1.25 ± 0.65 | 1.26 ± 0.32 | 1.16 ± 0.52 | 1.16 ± 0.52 |
| Ceftriaxone | ||||
| 600 | 1.02 ± 0.51 | 0.85 ± 0.28 | 1.1 ± 0.82 | 1.02 ± 0.51 |
| 2,400 | 1.08 ± 0.68 | 0.94 ± 0.28 | 1.07 ± 0.68 | 1.12 ± 0.74 |
| Azithromycin | ||||
| 2 | 1.09 ± 0.26 | NA | NA | NA |
| 512 | 0.95 ± 0.4 | NA | NA | NA |
| 1,024 | 0.95 ± 0.4 | NA | NA | NA |
The reference ODr of the untreated biofilm is 1. NA, not applicable. *, P < 0.05 compared to the baseline ODr of 1.
Overall, the combination of azithromycin with any of the tested antimicrobial agents did not reduce the biofilm ODr compared to the ODr of biofilms treated with single agents (Table 2). Vancomycin at 15 or 40 mg/liter combined with azithromycin (512 mg/liter) had an effect similar to that of vancomycin at 15 mg/liter as a single agent (mean ODr ± standard deviation, 0.77 ± 0.32 or 0.8 ± 0.35 versus 0.78 ± 0.29, respectively) but reduced bacterial growth less than vancomycin at 40 mg/liter as a single agent (ODr, 0.56 ± 0.45; P < 0.05). The combination of daptomycin or tigecycline with azithromycin resulted in an increase in the ODr. The combination of ceftriaxone at 600 mg/liter and azithromycin at 2 mg/liter decreased the ODr, but not significantly.
Antibacterial effecacy.
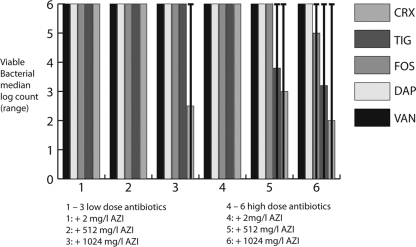
Incubation of the biofilms with any single antimicrobial agent did not result in any reduction of the bacterial count (data not shown). The combination of azithromycin at 2 mg/liter with any antibiotic was not bactericidal at all; however, the combination with ceftriaxone at 2,400 mg/liter resulted in a nonsignificant reduction of the log bacterial count. The bactericidal effects were significantly improved when the biofilms were incubated with a combination of high-dose azithromycin and ceftriaxone, tigecycline (2 mg/liter), or fosfomycin (200 mg/liter): azithromycin at both concentrations (512 and 1,024 mg/liter) led to significant reductions in bacterial growth with ceftriaxone and tigecycline (Fig. 1). The reduction was greater than 3 log counts for ceftriaxone at 600 mg/liter combined with azithromycin at 1,024 mg/liter and for ceftriaxone at 2,400 mg/liter combined with azithromycin at 512 or 1,024 mg/liter (P < 0.05). The range, however, was widely distributed, since some S. epidermidis isolates, in particular the isolates from catheter-associated bacteremia, showed no reduction of the growth rate. Tigecycline at 2 mg/liter combined with azithromycin at 512 or 1,024 mg/liter reduced bacterial growth by more than 2 log counts, an effect that was seen for two isolates from implant infections and two skin isolates (P < 0.05). Azithromycin at 1,024 mg/liter in combination with fosfomycin at 200 mg/liter reduced bacterial growth by <2 log counts (not significant). Only negligible bactericidal effects (lowering the bacterial count by 1 log count for two to three bacterial strains at the highest concentrations only) were observed for the combination of vancomycin or daptomycin with azithromycin.
FIG. 1.
Decrease in bacterial growth with increasing concentrations of vancomycin (VAN), daptomycin (DAP), fosfomycin (FOS), tigecycline (TIG), or ceftriaxone (CRX) in combination with azithromycin (AZI). Low doses of antibiotics are as follows: 2 mg of DAP/liter, 15 mg of VAN/liter, 50 mg of FOS/liter, 0.2 mg of TIG/liter, and 600 mg of CRX/liter. High doses of antibiotics are as follows: 5 mg of DAP/liter, 40 mg of VAN/liter, 200 mg of FOS/liter, 2 mg of TIG/liter, and 2,400 mg of CRX/liter. Columns 1 to 3, low-dose antibiotics plus AZI at 2 mg/liter, 512 mg/liter, and 1,024 mg/liter, respectively; columns 4 to 6, high-dose antibiotics plus AZI at 2 mg/liter, 512 mg/liter, and 1,024 mg/liter, respectively. The colony count of the untreated biofilm is 106 CFU/ml (whiskers represent the range).
DISCUSSION
Biofilm-associated infections of osteosyntheses, endoprostheses, or implanted cardiac devices are most difficult to treat. Removal of the infected material is not feasible in many cases; thus, the infection must be controlled by antimicrobial therapy. In the present study, we used a static microtiter plate biofilm model to mimic the infection of implanted material coated with an established biofilm consisting of densely packed cells. Generally, MICs of standard antistaphylococcal antibiotics, including cephalosporins or vancomycin, are considerably increased when tested on bacterial biofilms (23, 31). Vancomycin diffuses slowly into the deeper layers of bacterial biofilms, risking a promotion of resistance due to the gradual exposure of the bacterial cells to low concentrations (21). In the present study, vancomycin was tested at a higher concentration of 40 mg/liter, achieving a significant decrease of biofilm density as a single agent. No reduction of bacterial growth was achieved. This and the potential nephro- and ototoxicity at high concentrations in serum limit the clinical application of high-dose vancomycin, which results in concentrations of 15 mg/liter in plasma, but with a median protein binding of 65%, thus lowering the active concentration (25). Combining vancomycin with azithromycin had little additional effect. Like vancomycin, fosfomycin effected a significant reduction of biofilm density at a high concentration of 200 mg/liter but did not reduce bacterial growth.
Daptomycin is an alternative agent with excellent in vitro activity against methicillin-resistant S. aureus and S. epidermidis. Employed at concentrations of 2 or 5 mg/liter in the present study, daptomycin reduced neither the biofilm ODr nor the bacterial log count. Although these concentrations were 10 times more than the average MIC for the tested isolates under planktonic conditions, the concentrations of 2 or 5 mg/liter are low compared to those used for vancomycin. There is evidence for using daptomycin at a higher concentration: daptomycin at a dose of 30 mg/kg of body weight was effective against an experimental implant infection (33). Daptomycin was well tolerated up to a daily dose of 12 mg/kg in healthy volunteers (2). Yet, for clinical use, doses up to 6 mg/kg are recommended, achieving much lower concentrations in serum, with a protein binding level as high as 95% (32). The combination of daptomycin with azithromycin resulted in an increase in the ODr that may be due to a chemical effect or to antagonism between these two substances. However, little has been known about daptomycin in combination with other agents until now.
Tigecycline has very low MICs for most clinical isolates, including all S. epidermidis isolates examined in this study. The effects of tigecycline as a single agent on biofilm density and bacterial growth were minimal. However, when tigecycline was combined with azithromycin, a significant reduction of bacterial growth was observed. This observation is consistent with the results of studies of staphylococcal biofilm models showing that the combination of bacteriostatic agents, including tigecycline, rifampin (rifampicin), or linezolid, with cephalosporins or vancomycin reduced bacterial growth in biofilms significantly (30, 31). There is controversy in the literature about the effects of beta-lactam antibiotics on biofilms (1, 24, 31). Generally, beta-lactam antibiotics are considered to kill bacterial cells in the growth phase only. Yet in our study, high concentrations of ceftriaxone reduced the bacterial count, particularly for isolates for which MICs are low under planktonic conditions.
The failure of antibiotic treatment of biofilm-associated infections led to the search for additive measures to eradicate bacteria within biofilms. Successful treatment of biofilm-associated pulmonary infections with Pseudomonas aeruginosa in patients with cystic fibrosis was reported using the macrolide azithromycin (17). Although azithromycin has no bactericidal effect on P. aeruginosa, it retards the formation of biofilms and blocks the bacterial quorum sensing involved in the production of biofilms (15, 19). Incubation of S. epidermidis biofilms with another macrolide, clarithromycin, at a low concentration resulted in the eradication of the slime-like structure and a decrease in the amount of hexose (37). In the present study, the staphylococcal biofilms were incubated with azithromycin alone at the therapeutically used concentration of 2 mg/liter, which was used successfully on biofilms of Haemophilus influenzae previously (36). Further, excess concentrations of 512 and 1,024 mg/liter were investigated because azithromycin was reported not to be effective on biofilms at a concentration of 256 mg/liter (31). However, when azithromycin was used in combination with ceftriaxone, there was a significant reduction of the bacterial count, particularly when a high concentration of ceftriaxone was combined with azithromycin (Fig. 1). Azithromycin also enhanced the bactericidal activity of tigecycline at 2 mg/liter and, to a lesser (not significant) extent, those of vancomycin and fosfomycin, as reported elsewhere (31). Although azithromycin was used in excess concentrations unlikely to be reached in patients when the drug is administered orally or even parenterally, it reduced bacterial growth significantly when combined with ceftriaxone or tigecycline.
Dunne reported that the biofilm ODr increased when growing biofilms were incubated with subinhibitory concentrations of vancomycin and cefamandole, indicating that biofilm production is a kind of defense reaction of the bacteria (11a). However, incubation with a higher concentration resulted in decreased biofilm formation (10). In our study, biofilms with densely packed cells were investigated. Except for vancomycin, excess concentrations of antimicrobial agents had no effect on the biofilm mass. It may be hypothesized that the concentrations of the antibiotics within these biofilms might be so low as to achieve a biofilm-augmenting effect similar to that described by Dunne. A combination of azithromycin with ceftriaxone led to significant reduction of bacterial growth but failed to decrease the biofilm ODr. An explanation of this failure may be that only very low concentrations reach the inner biofilm, thus augmenting the biofilm matrix. The increase in the matrix may be due either to increased production or to accumulation of decaying bacterial cells. For instance, alcohols have a very good bactericidal effect even on established biofilms, as demonstrated in a previous study, but the ODr is increased (29). However, alcohols are cytotoxic and may not be used even locally for rinsing infected protheses.
Successful attempts have been made to use topical azithromycin to treat a biofilm-associated infection such as periodontitis (27); however, applying azithromycin at even higher concentrations used either alone or in combination with other antibiotics did not result in a reduction of the biofilm. Yet excessive concentrations of azithromycin plus a beta-lactam antibiotic reduced bacterial growth. Topical macrolides, e.g., rapamycin, are currently used as antiproliferative drugs with excellent results (3). For this topical use, the mode of drug delivery is pivotal. Coating and bonding of macrolides to polymers on stent surfaces may result in very high local concentrations. Bonding azithromycin to polymer-coated prosthetic surfaces may deliver high local concentrations that may not only prevent colonization and adherence but also—together with the systemically administered antimicrobial substances with excellent tissue penetration and little protein binding—reduce biofilms. This is, so far, a hypothesis only, but new materials and techniques, including nanotechnology, are being developed rapidly (6). Additionally, in the present study, biofilms were treated for 24 h only, and antimicrobial treatment of implant infections is usually administered for a longer period. The combination of azithromycin with fosfomycin, tigecycline, or ceftriaxone had a certain antibacterial effect. Repeated dosing might result in improved bactericidal efficacy, as in a study of a catheter model using biofilms with a lower bacterial burden (30). Yet the action of antimicrobial substances on biofilm density and bacterial growth and the interplay of bacteria, surfaces, antimicrobials at differential concentrations, and the host interfering with leukocytes, platelets, red blood cells, and fibrin—either combating or enhancing the bacterial resistance within the biofilm—is still controversial. Although adhesion may be reduced by coating surfaces with antibiotics or silver, biofilm formation and the subsequent infection of the implant are not predictable, as shown in experimental and clinical studies (4, 11).
In conclusion, bacterial cells within biofilms are highly resistant to antibiotics, even when very low MICs determined in the growing phase predict high susceptibility to the antimicrobial agent. Thus, using a combination of azithromycin plus tigecycline, fosfomycin, or ceftriaxone at high concentrations has little effect on biofilm density as the measure of the biofilm mass but significantly reduces bacterial growth. The combination of azithromycin at 512 mg/liter with a high dose of ceftriaxone has significant bactericidal effects. Although it is hard to believe that such excess concentrations may be used in clinical practice, the design and engineering of materials that may be impregnated or bonded with antimicrobials and thus can release high concentrations, such as in interventional cardiology (3), may be an option for future topical treatment. Further studies are needed to evaluate additional techniques for drug delivery, such as intelligent devices (12) and adjuvant therapeutic measures, which may enhance the effects of antibiotics alone or in combination with azithromycin.
Acknowledgments
We thank Sonja Reichmann for excellent technical work on biofilm testing.
The authors declare no conflict of interest.
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Amorena, B., E. Gracia, M. Monzon, J. Leiva, C. Oteiza, M. Perez, J. L. Alabart, and J. Hernandez-Yago. 1999. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J. Antimicrob. Chemother. 44:43-55. [DOI] [PubMed] [Google Scholar]
- 2.Benvenuto, M., D. P. Benziger, S. Yankelev, and G. Vigliani. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butt, M., D. Connolly, and G. Y. Lip. 2009. Drug-eluting stents: a comprehensive appraisal. Future Cardiol. 5:141-157. [DOI] [PubMed] [Google Scholar]
- 4.Cerca, N., S. Martins, G. B. Pier, R. Oliveira, and J. Azeredo. 2005. The relationship between inhibition of bacterial adhesion to a solid surface by sub-MICs of antibiotics and subsequent development of a biofilm. Res. Microbiol. 156:650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, M. C., H. W. Tsai, C. T. Liu, S. F. Peng, W. Y. Lai, S. J. Chen, Y. Chang, and H. W. Sung. 2009. A nanoscale drug-entrapment strategy for hydrogel-based systems for the delivery of poorly soluble drugs. Biomaterials 30:2102-2111. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A7, 6th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 10.Darouiche, R. O. 2004. Treatment of infections associated with surgical implants. N. Engl. J. Med. 350:1422-1429. [DOI] [PubMed] [Google Scholar]
- 11.Donelli, G., and I. Francolini. 2001. Efficacy of antiadhesive, antibiotic and antiseptic coatings in preventing catheter-related infections: review. J. Chemother. 13:595-606. [DOI] [PubMed] [Google Scholar]
- 11a.Dunne, W. M., Jr. 1990. Effects of subinhibitory concentrations of vancomycin or cefamandole on biofilm production by coagulase-negative staphylococci. Antimicrob. Agents Chemother. 34:390-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrlich, G. D., P. Stoodley, S. Kathju, Y. Zhao, B. R. McLeod, N. Balaban, F. Z. Hu, N. G. Sotereanos, J. W. Costerton, P. S. Stewart, J. C. Post, and Q. Lin. 2005. Engineering approaches for the detection and control of orthopaedic biofilm infections. Clin. Orthop. Relat. Res. 2005:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foulds, G., P. Madsen, C. Cox, R. Shepard, and R. Johnson. 1991. Concentration of azithromycin in human prostatic tissue. Eur. J. Clin. Microbiol. Infect. Dis. 10:868-871. [DOI] [PubMed] [Google Scholar]
- 14.Frossard, M., C. Joukhadar, B. M. Erovic, P. Dittrich, P. E. Mrass, H. M. Van, H. Burgmann, A. Georgopoulos, and M. Muller. 2000. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob. Agents Chemother. 44:2728-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillis, R. J., and B. H. Iglewski. 2004. Azithromycin retards Pseudomonas aeruginosa biofilm formation. J. Clin. Microbiol. 42:5842-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajdu, S., A. Lassnigg, W. Graninger, A. M. Hirschl, and E. Presterl. 24 April 2009. Effects of vancomycin, daptomycin, fosfomycin, tigecycline and ceftriaxone on Staphylococcus epidermidis biofilms. J. Orthop. Res. doi: 10.1002/jor.20902. [DOI] [PubMed]
- 17.Hansen, C. R., T. Pressler, C. Koch, and N. Hoiby. 2005. Long-term azithromycin treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection; an observational cohort study. J. Cyst. Fibros. 4:35-40. [DOI] [PubMed] [Google Scholar]
- 18.Hebert, C. K., R. E. Williams, R. S. Levy, and R. L. Barrack. 1996. Cost of treating an infected total knee replacement. Clin. Orthop. Relat. Res. 1996:140-145. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann, N., B. Lee, M. Hentzer, T. B. Rasmussen, Z. Song, H. K. Johansen, M. Givskov, and N. Hoiby. 2007. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr−/− mice. Antimicrob. Agents Chemother. 51:3677-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, Y. T., C. H. Liao, L. J. Teng, and P. R. Hsueh. 2007. Daptomycin susceptibility of unusual gram-positive bacteria: comparison of results obtained by the Etest and the broth microdilution method. Antimicrob. Agents Chemother. 51:1570-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jefferson, K. K., D. A. Goldmann, and G. B. Pier. 2005. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 49:2467-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandemir, O., V. Oztuna, A. Milcan, A. Bayramoglu, H. H. Celik, C. Bayarslan, and A. Kaya. 2005. Clarithromycin destroys biofilms and enhances bactericidal agents in the treatment of Pseudomonas aeruginosa osteomyelitis. Clin. Orthop. Relat. Res. 2005:171-175. [PubMed] [Google Scholar]
- 23.Monzón, M., C. Oteiza, J. Leiva, M. Lamata, and B. Amorena. 2002. Biofilm testing of Staphylococcus epidermidis clinical isolates: low performance of vancomycin in relation to other antibiotics. Diagn. Microbiol. Infect. Dis. 44:319-324. [DOI] [PubMed] [Google Scholar]
- 24.Olson, M. E., H. Ceri, D. W. Morck, A. G. Buret, and R. R. Read. 2002. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 66:86-92. [PMC free article] [PubMed] [Google Scholar]
- 25.Paterson, D. L. 1999. Reduced susceptibility of Staphylococcus aureus to vancomycin—a review of current knowledge. Commun. Dis. Intell. 23:69-73. [PubMed] [Google Scholar]
- 26.Pfaller, M. A., and L. A. Herwaldt. 1988. Laboratory, clinical, and epidemiological aspects of coagulase-negative staphylococci. Clin. Microbiol. Rev. 1:281-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pradeep, A. R., S. V. Sagar, and H. Daisy. 2008. Clinical and microbiologic effects of subgingivally delivered 0.5% azithromycin in the treatment of chronic periodontitis. J. Periodontol. 79:2125-2135. [DOI] [PubMed] [Google Scholar]
- 28.Presterl, E., A. Lassnigg, B. Parschalk, F. Yassin, H. Adametz, and W. Graninger. 2005. Clinical behavior of implant infections due to Staphylococcus epidermidis. Int. J. Artif. Organs 28:1110-1118. [DOI] [PubMed] [Google Scholar]
- 29.Presterl, E., M. Suchomel, M. Eder, S. Reichmann, A. Lassnigg, W. Graninger, and M. Rotter. 2007. Effects of alcohols, povidone-iodine and hydrogen peroxide on biofilms of Staphylococcus epidermidis. J. Antimicrob. Chemother. 60:417-420. [DOI] [PubMed] [Google Scholar]
- 29a.Presterl, E., S. Reichmann, S. Hajdu, J. Holinka, C. Kratzer, A. M. Hirschl, and W. Graninger. 2007. Azithromycin in combination with highly dosed ceftriaxone and tigecycline is most active against biofilms formed by Staphylococcus epidermidis isolated in implant infections, poster K2069. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 30.Raad, I., H. Hanna, Y. Jiang, T. Dvorak, R. Reitzel, G. Chaiban, R. Sherertz, and R. Hachem. 2007. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 51:1656-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saginur, R., M. StDenis, W. Ferris, S. D. Aaron, F. Chan, C. Lee, and K. Ramotar. 2006. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob. Agents Chemother. 50:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauermann, R., M. Rothenburger, W. Graninger, and C. Joukhadar. 2008. Daptomycin: a review 4 years after first approval. Pharmacology 81:79-91. [DOI] [PubMed] [Google Scholar]
- 33.Schaad, H. J., M. Bento, D. P. Lew, and P. Vaudaux. 2006. Evaluation of high-dose daptomycin for therapy of experimental Staphylococcus aureus foreign body infection. BMC Infect. Dis. 6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulin, T., and A. Voss. 2001. Coagulase-negative staphylococci as a cause of infections related to intravascular prosthetic devices: limitations of present therapy. Clin. Microbiol. Infect. 7(Suppl. 4):1-7. [DOI] [PubMed] [Google Scholar]
- 35.Siegenthaler, M. P., J. Martin, and F. Beyersdorf. 2003. Mechanical circulatory assistance for acute and chronic heart failure: a review of current technology and clinical practice. J. Interv. Cardiol. 16:563-572. [DOI] [PubMed] [Google Scholar]
- 36.Starner, T. D., J. D. Shrout, M. R. Parsek, P. C. Appelbaum, and G. Kim. 2008. Subinhibitory concentrations of azithromycin decrease nontypeable Haemophilus influenzae biofilm formation and diminish established biofilms. Antimicrob. Agents Chemother. 52:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasuda, H., Y. Ajiki, T. Koga, and T. Yokota. 1994. Interaction between clarithromycin and biofilms formed by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 38:138-141. [DOI] [PMC free article] [PubMed] [Google Scholar]