Abstract
We established a murine model of Candida albicans central nervous system (CNS) infection and evaluated the efficacy of anidulafungin. Ten milligrams/kg/day anidulafungin, amphotericin B, or voriconazole significantly reduced mortality and fungal burden in brain tissue, although amphotericin B and 10 mg/kg/day anidulafungin reduced fungal burden in brain tissue to a greater extent than did voriconazole. This suggests a potential role for anidulafungin in the treatment of candidal CNS infection.
Prolonged candidiasis can affect the central nervous system (CNS), inducing diffuse encephalopathy with microabscesses (9, 11). Hematogenous candidal meningoencephalitis is a relatively common and serious manifestation of disseminated candidiasis in premature infants (5). Echinocandins have excellent clinical activity in the treatment of invasive candidiasis (10, 15). Little is known regarding the activity of anidulafungin for the treatment of candidal CNS infection. We established a murine model of candidal CNS infection and evaluated the activity of anidulafungin in candidal CNS infection.
(This study was presented in part at the 7th International Symposium on Antimicrobiol Agents and Resistance, Bangkok, Thailand, 18 to 20 March 2009.)
Candida albicans IDRL-5319 (a blood culture isolate) was prepared as previously described (14). Pathogen-free, 6- to 8-week-old, female, immunocompetent hairless mice (crl:SKH1[hrhr]br) nonpedigreed from an albino background (20 to 25 g; Charles River Laboratories, Wilmington, MA) were studied based on previous experience with experimental pneumococcal meningitis (8). Experiments were performed in accordance with local IACUC guidelines.
Anidulafungin and voriconazole (Pfizer Pharmaceuticals, Inc., New York, NY) and amphotericin B deoxycholate (X-Gen Pharmaceuticals Inc., Big Flats, NY) were studied; stock solutions were prepared as previously described (1, 2). Single-dose pharmacokinetics of anidulafungin and voriconazole were determined. Blood samples were collected by cardiac puncture and assayed at the Fungus Testing Laboratory, University of Texas Health Science Center (San Antonio, TX), by high-performance liquid chromatography (16).
A total of 5 × 105 CFU of C. albicans in 20 μl of phosphate-buffered saline was injected transcutaneously into the cisterna magna under anesthesia (ketamine [100 mg/kg] plus xylazine [10 mg/kg] intraperitoneally [i.p.]) based on the method described in previous studies (6, 8). On day 2, treatment was initiated and continued for 8 days.
Sixteen mice were allocated to one of the following conditions: high-dose anidulafungin (10 mg/kg/day i.p.), low-dose anidulafungin (5 mg/kg/day i.p.), voriconazole (60 mg/kg/day orally), amphotericin B (1.5 mg/kg/day i.p.), or no treatment. Beginning 3 days prior to treatment, mice to receive voriconazole were given grapefruit juice (Ocean Spray, Inc., Lakeville-Middleboro, MA) instead of water to inhibit voriconazole metabolism (2, 3, 12). Ten days after infection and 24 h after the last treatment, mice were sacrificed by i.p. injection of 100 mg/kg pentobarbital. The brain and one kidney were aseptically harvested, weighed, homogenized with 2 ml phosphate-buffered saline, and quantitatively cultured, with results expressed as CFU/g of C. albicans. Three mice that were unable to move or eat at the time of treatment initiation were sacrificed and excluded from further study. Their burden of C. albicans (mean ± standard deviation) was 5.43 ± 0.09 log10 CFU/g and 5.04 ± 0.56 log10 CFU/g in the brain and kidney, respectively.
Animals challenged with candidal inoculum were followed for up to 10 days to assess survival using Kaplan-Meier methodology. We also compared quantitative brain culture results between each group of mice using the Kruskal-Wallis test. Considering that death is a worse outcome than is survival, missing data due to death were imputed using the mean log10 CFU/gram of brain from untreated mice dying at 4 days in our preliminary studies, as previously described (2, 7, 13). Comparisons of categorical variables were made using Fisher's exact test, due to small sample sizes. Tests were two-tailed with an alpha level of 0.05.
The C. albicans isolate studied had MICs of 0.25, 0.03, and 0.06 μg/ml for amphotericin B, anidulafungin, and voriconazole, respectively. Plasma concentrations after single-dose administration of anidulafungin and voriconazole are shown in Table 1.
TABLE 1.
Plasma concentrations after single-dose administration of antifungal agents in micea
| Agent | Plasma concn (μg/ml) at indicated time (h) after single-dose administrationb
|
||||
|---|---|---|---|---|---|
| 1 | 3 | 6 | 12 | 24 | |
| Anidulafungin, 5 mg/kg i.p. | 2.50 ± 0.06 | 1.73 ± 0.32 | 1.66 ± 0.22 | 1.18 ± 0.03 | 0.18 ± 0.32 |
| Anidulafungin, 10 mg/kg i.p. | 4.02 ± 1.92 | 4.91 ± 0.41 | 3.18 ± 1.11 | 2.73 ± 0.14 | 1.39 ± 0.05 |
| Voriconazole, 60 mg/kg orally | 7.41 ± 4.66 | 7.28 ± 3.33 | 9.47 ± 2.80 | 5.37 ± 4.88 | 0.16 ± 0.14 |
Pharmacokinetic profiles of anidulafungin were determined by injecting 5 mg/kg or 10 mg/kg i.p. and obtaining plasma samples at 1, 3, 6, 12, and 24 h postinjection. Those of voriconazole were determined following administration of 60 mg/kg orally.
Data at each time point are average concentrations (μg/ml) ± standard deviation of the results for three mice.
Two days after infection (day 2), all mice appeared to be ill (i.e., thin skin, reduced activity). Preliminary studies of seven untreated mice sacrificed on day 4 showed that the candidal burden in brain tissues (mean [95% confidence interval]) was 4.39 (4.01 to 4.77) log10 CFU/g. Of seven untreated mice, four had fungal burdens in the kidney below the detectable level (2.1 log10 CFU/g), and the renal fungal burden of the remaining three was 2.71 (2.26 to 3.16) log10 CFU/g. The fungal burden in the cerebrospinal fluid was also below the detectable level of 2.1 log10 CFU/g. In a survival analysis, 11 of 16 untreated mice died within 6 days after injection of the candidal inoculum. When fungal burdens (mean [95% confidence interval]) in brain tissues were evaluated in 12 untreated mice which survived through the time of sacrifice, they declined over time (4.39 [4.01 to 4.77] on day 4; 3.83 [3.25 to 4.41] on day 6; 3.92 [1.82 to 6.01] on day 7; and 3.49 [3.25 to 3.73] on day 10).
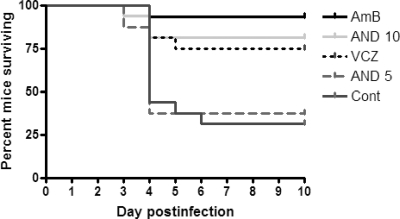
All treatments except low-dose anidulafungin (5 mg/kg/day) reduced mortality of mice infected with C. albicans, compared with untreated controls (P < 0.05) (Fig. 1). Eight days after initiation of antifungal treatment, all treatments except low-dose anidulafungin also reduced the fungal load in brain tissue (Fig. 2). No significant differences in fungal burdens in the brain were observed between mice treated with high-dose anidulafungin (10 mg/kg/day) and amphotericin B, but the fungal burden in the voriconazole-treated group was higher than that in the amphotericin B- or high-dose-anidulafungin-treated group (P < 0.05 for both). When only surviving mice were analyzed, high-dose anidulafungin and amphotericin B (P < 0.001 for both) as well as voriconazole (P = 0.006) also significantly reduced fungal burden in the brain, compared with untreated controls.
FIG. 1.
Cumulative mortality of mice in the different treatment groups shown using Kaplan-Meier survival curves. Treatment was initiated on day 2 and continued for 8 days. The mortality rates of each group were as follows: amphotericin B, 6.7% (1/15); 10 mg/kg anidulafungin, 18.8% (3/16); voriconazole, 25.0% (4/16); 5 mg/kg anidulafungin, 62.5% (10/16); and untreated control, 68.8% (11/16). All treatments except low-dose anidulafungin (5 mg/kg/day) reduced mortality compared with untreated controls (P < 0.05). No significant differences in survival were observed between mice treated with high-dose anidulafungin (10 mg/kg/day), amphotericin B, or voriconazole. AmB, amphotericin B deoxycholate; AND 10, 10 mg/kg/day anidulafungin; VCZ, voriconazole; AND 5, 5 mg/kg/day anidulafungin; Cont, untreated control.
FIG. 2.
Candidal burden in brains of mice according to treatment regimen. Log10 CFU/g of Candida albicans in brain in the amphotericin B (closed circles) (n = 15), anidulafungin 10 mg/kg (triangles) (n = 16), anidulafungin 5 mg/kg (open circles) (n = 16), voriconazole (squares) (n = 16), and control (diamonds) (n = 16) arms are shown. Bars represent median values. A value of 4.39 log10 CFU/g was assigned to mice dying prior to the end of the 10-day follow-up (2, 7, 13). Compared with untreated controls (median, 4.39 log10 CFU/g; interquartile range, 3.69 to 4.39 log10 CFU/g), amphotericin B (median, 2.57 log10 CFU/g; interquartile range, 2.12 to 2.83 log10 CFU/g; P < 0.001), high-dose anidulafungin (10 mg/kg/day) (median, 2.65 log10 CFU/g; interquartile range, 2.38 to 3.01 log10 CFU/g; P < 0.001) and voriconazole (median, 3.23 log10 CFU/g; interquartile range, 2.86 to 3.99 log10 CFU/g; P = 0.002) significantly reduced the fungal burden in brain tissues. The fungal burden in the voriconazole-treated group was higher than that in amphotericin B- or high-dose-anidulafungin-treated group (P < 0.05 for both). No significant difference was found between the amphotericin B- and high-dose-anidulafungin (10 mg/kg/day)-treated groups. AmB, amphotericin B deoxycholate; AND 10, 10 mg/kg/day anidulafungin; VCZ, voriconazole; AND 5, 5 mg/kg/day anidulafungin; Cont, untreated control.
The inclusion of voriconazole was dependent on the concomitant use of grapefruit juice to inhibit rapid voriconazole metabolism (2, 3, 12). Even though voriconazole levels were attainable in mice given grapefruit juice, those at 24 h after single-dose administration were below 1 μg/ml, which might be inadequate for treatment of serious candidal infection. The median fungal burden in the voriconazole-treated group was higher than that in the amphotericin B- or high-dose-anidulafungin-treated group, possibly related to rapid metabolism of voriconazole by the mice.
Our study had several limitations. First, we were unable to detect the antifungal drugs studied in cerebrospinal fluid or the brain. However, anidulafungin levels in plasma were in agreement with a previous pharmacokinetic-pharmacodynamic study of mice, and were comparable to those in humans (1, 4, 15). Second, for analyses of comparative brain burdens of C. albicans, samples missing due to death were assigned an arbitrary value, since we assumed that death was a worse outcome than survival with any amount of fungal burden (2, 7, 13). For further evaluation, a less lethal model may be needed.
Despite the shortcomings, we believe that our data suggest a potential role of anidulafungin as an alternative choice for the treatment of candidal CNS infection. Given the safety and efficacy of anidulafungin and its novel pharmacokinetic characteristics, further investigation is warranted to assess clinical relevance.
Acknowledgments
We thank Melissa J. Jacobson for technical support.
The study was funded by Pfizer Pharmaceuticals, Inc. (New York, NY). C.-I.K. was supported by funding from Samsung Medical Center (Seoul, South Korea).
Footnotes
Published ahead of print on 8 June 2009.
REFERENCES
- 1.Andes, D., D. J. Diekema, M. A. Pfaller, R. A. Prince, K. Marchillo, J. Ashbeck, and J. Hou. 2008. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemons, K. V., M. Espiritu, R. Parmar, and D. A. Stevens. 2005. Comparative efficacies of conventional amphotericin B, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob. Agents Chemother. 49:4867-4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graybill, J. R., L. K. Najvar, G. M. Gonzalez, S. Hernandez, and R. Bocanegra. 2003. Improving the mouse model for studying the efficacy of voriconazole. J. Antimicrob. Chemother. 51:1373-1376. [DOI] [PubMed] [Google Scholar]
- 4.Gumbo, T., G. L. Drusano, W. Liu, L. Ma, M. R. Deziel, M. F. Drusano, and A. Louie. 2006. Anidulafungin pharmacokinetics and microbial response in neutropenic mice with disseminated candidiasis. Antimicrob. Agents Chemother. 50:3695-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hope, W. W., D. Mickiene, V. Petraitis, R. Petraitiene, A. M. Kelaher, J. E. Hughes, M. P. Cotton, J. Bacher, J. J. Keirns, D. Buell, G. Heresi, D. K. Benjamin, Jr., A. H. Groll, G. L. Drusano, and T. J. Walsh. 2008. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J. Infect. Dis. 197:163-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein, M., B. Obermaier, B. Angele, H. W. Pfister, H. Wagner, U. Koedel, and C. J. Kirschning. 2008. Innate immunity to pneumococcal infection of the central nervous system depends on toll-like receptor (TLR) 2 and TLR4. J. Infect. Dis. 198:1028-1036. [DOI] [PubMed] [Google Scholar]
- 7.Lachin, J. M. 1999. Worst-rank score analysis with informatively missing observations in clinical trials. Control. Clin. Trials 20:408-422. [DOI] [PubMed] [Google Scholar]
- 8.Mook-Kanamori, B. B., M. S. Rouse, C. I. Kang, D. van de Beek, J. M. Steckelberg, and R. Patel. 2009. Daptomycin in experimental murine pneumococcal meningitis. BMC Infect. Dis. 9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker, J. C., Jr., J. J. McCloskey, and R. S. Lee. 1981. Human cerebral candidosis—a postmortem evaluation of 19 patients. Hum. Pathol. 12:23-28. [DOI] [PubMed] [Google Scholar]
- 10.Reboli, A. C., C. Rotstein, P. G. Pappas, S. W. Chapman, D. H. Kett, D. Kumar, R. Betts, M. Wible, B. P. Goldstein, J. Schranz, D. S. Krause, and T. J. Walsh. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 356:2472-2482. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Portocarrero, J., E. Perez-Cecilia, O. Corral, J. Romero-Vivas, and J. J. Picazo. 2000. The central nervous system and infection by Candida species. Diagn. Microbiol. Infect. Dis. 37:169-179. [DOI] [PubMed] [Google Scholar]
- 12.Serena, C., F. J. Pastor, M. Marine, M. M. Rodriguez, and J. Guarro. 2007. Efficacy of voriconazole in a murine model of cryptococcal central nervous system infection. J. Antimicrob. Chemother. 60:162-165. [DOI] [PubMed] [Google Scholar]
- 13.Shih, W. 2002. Problems in dealing with missing data and informative censoring in clinical trials. Curr. Control. Trials Cardiovasc. Med. 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shuford, J. A., M. S. Rouse, K. E. Piper, J. M. Steckelberg, and R. Patel. 2006. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catheter infection model. J. Infect. Dis. 194:710-713. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez, J. A., and J. D. Sobel. 2006. Anidulafungin: a novel echinocandin. Clin. Infect. Dis. 43:215-222. [DOI] [PubMed] [Google Scholar]
- 16.Zornes, L. L., and R. E. Stratford. 1997. Development of a plasma high-performance liquid chromatographic assay for LY303366, a lipopeptide antifungal agent, and its application in a dog pharmacokinetic study. J. Chromatogr. B Biomed. Sci. Appl. 695:381-387. [DOI] [PubMed] [Google Scholar]