Abstract
Fifty-two percent of 1,288 poultry isolates of campylobacters were ampicillin resistant, and resistance was more common among Campylobacter coli isolates (67.4%) than among Campylobacter jejuni isolates (47.5%). Production of β-lactamase was typically associated with resistance to ampicillin, amoxicillin (amoxicilline), penicillin, and ticarcillin. Regardless of β-lactamase production, all isolates were resistant to piperacillin (MICs ≥ 256 μg/ml), and most were resistant to carbenicillin, cloxacillin, and cephalosporins. Of all ampicillin-resistant campylobacters tested, 91% (347/380) carried the blaOXA-61 gene, and 77% (136/175) of those tested with nitrocefin produced a β-lactamase, presumably OXA-61. The isoelectric point (pI) of OXA-61 was 8.7, and the molecular mass was 31.0 kDa. Insertional inactivation of blaOXA-61 in C. jejuni NCTC 11168 and two ampicillin-resistant isolates resulted in increased susceptibility to ampicillin, co-amoxiclav (amoxicillin and clavulanic acid), penicillin, carbenicillin, oxacillin, and piperacillin, but the effects on MICs of cephalosporins and imipenem were negligible. Some C. jejuni isolates that lacked blaOXA-61 produced a β-lactamase, CjBla2, with a pI of 9.2 and molecular mass of 32.4 kDa. Mass spectrometry confirmed that the most prevalent β-lactamase was the product of blaOXA-61, but CjBla2 was not identified. OXA-61 is prevalent among Campylobacter spp. of veterinary origin and is similar to the β-lactamase previously reported in human isolates. Production of OXA-61 was associated with resistance to penams but not cephalosporins. Co-amoxiclav remained active against all isolates tested.
Resistance to ampicillin and other β-lactam agents has been widely reported among Campylobacter spp. isolated from humans and poultry. In 1997, 34% of Campylobacter jejuni isolates from humans in England and Wales were ampicillin resistant (24). Data submitted to the International Surveillance Network for Enteric Infections (25) showed that of the 1,889 human isolates of Campylobacter spp. tested by participating laboratories worldwide during 2005 to 2006, 26.5% were resistant to ampicillin. Corcoran et al. (4) reported that ampicillin resistance was prevalent (48.5%) among Irish isolates of Campylobacter spp. and that the incidence of antimicrobial resistance in poultry isolates was higher than in those from humans. Similarly, McGill et al. (16) found that 25% of C. jejuni isolates from retail food samples in Ireland were ampicillin resistant compared to 17% of human isolates.
β-Lactamase-producing human isolates of C. jejuni have been shown to be significantly less susceptible to ampicillin, amoxicillin (amoxicilline), and ticarcillin than β-lactamase-negative strains (11). Other workers have shown that β-lactamase production in C. jejuni is associated with resistance to ampicillin and amoxicillin (5). However, the roles of β-lactamases in the mechanism of resistance to ampicillin in campylobacters are not yet clear (11, 21, 22), and the production of β-lactamase is not always associated with resistance to β-lactams. Lariviere et al. (13) demonstrated that 89.3% human clinical isolates of C. jejuni from Montreal, Canada, produced a β-lactamase detectable by nitrocefin, although only 14.6% of isolates were ampicillin resistant. Similarly, Fliegelman et al. (6) detected β-lactamase activity in 25/27 C. jejuni isolates and 29/31 C. coli isolates, although the MIC90 of ampicillin for the strains was only 8 μg/ml.
A gene encoding β-lactamase located on a chromosome in campylobacter was postulated by Taylor et al. (23), as resistance to ampicillin was not cotransferred with tetracycline resistance by conjugation. A 774-bp gene encoding a 257-amino-acid putative periplasmic class D β-lactamase, Cj0299, is located on the C. jejuni NCTC 11168 genome (18). The corresponding gene, from a human clinical isolate, C. jejuni GC015, was cloned and characterized and shown to confer resistance to ampicillin, penicillin, and carbenicillin in C. jejuni (1). This β-lactamase, designated OXA-61, showed identity to other OXA-type enzymes in Pseudomonas aeruginosa and Acinetobacter baumannii. Orthologues of OXA-61 are present in C. jejuni RM1221 and Campylobacter lari RM2100 and have 99% and 52% protein identity, respectively, to Cj0299 (http://campy.bham.ac.uk/).
However, C. jejuni can produce more than one type of β-lactamase. In a conference abstract, Lucain et al. (14) described four enzymes based on their differing activity against eight β-lactams, relative rates of hydrolysis, molecular weight, immunological specificity, and isoelectric point (pI). The predominant β-lactamase found was termed type A, a 30-kDa protein with a pI of 8.3, which was active against penicillin, ampicillin, oxacillin, and carbenicillin and weakly active against cephalothin (cefalotin) but inactive against cephaloridine, cefuroxime, and cefotaxime. Lachance et al. (12) showed that a β-lactamase from C. jejuni hydrolyzed ampicillin, amoxicillin, penicillin, and cloxacillin and partially hydrolyzed cephalothin. The profile of β-lactamase was similar to the profile of the type A enzyme reported by Lucain et al. (14), had a pI of 8.8, and was inhibited by tazobactam, clavulanic acid, sulbactam, and cefoxitin but not EDTA or p-chloromercuribenzoate. The 10 sequenced Campylobacter genomes suggest that putative metallo-β-lactamases are produced. However, two putative zinc hydrolases, structurally similar to the metallo-β-lactamase superfamily from C. jejuni, conferred no resistance to β-lactams. Furthermore, no β-lactamase activity was observed in Escherichia coli or C. coli transformed with plasmids carrying the cloned zinc hydrolase genes, suggesting that these enzymes do not function as metallo-β-lactamases (2).
Most cases of human campylobacteriosis do not require antimicrobial treatment, but in severe cases where therapy is required, the drugs of choice are erythromycin and fluoroquinolones. Ampicillin is not recommended for the treatment of campylobacter infections (3). However, campylobacters resistant to both erythromycin and ciprofloxacin occur. Gillespie et al. (7) showed that in travelers returning to the United Kingdom with infection by Campylobacter coli, 13.1% were resistant to both agents. In a recent study of veterinary isolates, 11/841 isolates (1.3%) were resistant to both agents (19). Should such isolates cause an infection in humans that requires treatment, then an oral ß-lactam, such as co-amoxiclav (amoxicillin and clavulanic acid), could be an appropriate agent, especially as resistance to this combination in Campylobacter spp. is rare; only 0.6% of isolates were recorded be resistant by the Enter-net project in 2005 to 2006 (25). When treatment of erythromycin- and ciprofloxacin-resistant Campylobacter infections has been necessary, anecdotal evidence indicates that imipenem or co-amoxiclav has been used.
The aim of this study was to characterize β-lactamase-mediated β-lactam resistance among well characterized and typed veterinary isolates of Campylobacter spp, isolated from chicken flocks in the United Kingdom from 2000 to 2006. In addition, we determined the effect of β-lactamase production upon the susceptibility of campylobacters to β-lactam agents.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains were isolated between 2000 and 2006 from chicken flocks in the United Kingdom, during studies OZO501 and VMO2200 funded by the Department for Environment, Food and Rural Affairs (9, 10, 19; N. C. Elviss, L. K. Williams, F. Jorgensen, S. A. Chisholm, A. J. Lawson, C. Swift, R. J. Owen, D. J. Griggs, M. M. Johnson, T. J. Humphrey, and L. J. V. Piddock, submitted for publication). In total, 1,288 Campylobacter isolates were studied; 967 of the isolates were C. jejuni, 319 were C. coli, and 2 were C. lari. Of these, 1,115 isolates were from feces, 64 from ceca, and 109 from the poultry house or farm environment. In addition, C. jejuni NCTC 11168, 81-176, 81116, C. coli NCTC 11366, and C. lari NCTC 11352 were used.
Determination of antimicrobial resistance.
The agar doubling dilution procedure recommended by the NCCLS (now CLSI) Campylobacter Working Group (15) was used throughout the study as described previously (9) to determine the MICs of a range of β-lactam agents, including penams, cephalosporins, and carbapenems for ampicillin- and amoxicillin-resistant isolates. C. jejuni NCTC 11168 and C. coli NCTC 11366 were used as control strains. Designation of strains as antibiotic susceptible or resistant was made using a cutoff concentration for ampicillin of 8 μg/ml. At the time of the study, there were no specific guidelines for Campylobacter and the other agents, so reference to the guidelines for Enterobacteriaceae by the British Society for Antimicrobial Chemotherapy and CLSI were used. For Cj0299-inactivated mutants, MICs of β-lactam agents were also determined in the presence of the efflux pump inhibitor Phe-Arg-β-naphthylamide (PAβN; 20 μg/ml).
PCR detection of Cj0299 gene encoding a putative periplasmic β-lactamase.
Ampicillin-resistant strains were tested for the presence of Cj0299 encoding a putative periplasmic β-lactamase in C. jejuni NCTC 11168 (18). Bacteria were grown on Mueller-Hinton agar plus 5% horse blood at 37°C in 7.5% CO2 for 48 h. Bacterial colonies were harvested from the agar plate, and a suspension of the colonies was prepared in sterile distilled water. The cells were lysed by boiling for 5 min, and the lysate was centrifuged to remove cell debris. PCR of Cj0299 was performed with a reaction mixture volume of 25 μl using PCR Mastermix (Abgene, Epsom, United Kingdom), 0.5 μl boiled cell lysate, and 250 nM primers each of 469 (5′-GAGTATAATACAAGCGGCAC-3′) and 470 (5′-CCAATTCTTCTTGCCACTTC-3′) covering nucleotides (nt) 79 to 359 of Cj0299 and encompassing the predicted active site of the β-lactamase (Fig. 1). An initial denaturation step of 5 min at 94°C was carried out. The initial denaturation step was followed by 30 cycles, with 1 cycle consisting of 30 s at 94°C, 45 s at 56°C, and 30 s at 72°C. A final extension step of 10 min at 72°C was then performed. A DNA amplimer of the correct size (281 bp) observed on a 1% agarose gel indicated that the strain was positive for Cj0299. Primers were synthesized commercially (Invitrogen Ltd., Paisley, United Kingdom). DNA sequencing of a 1,218-bp fragment comprising Cj0299 and flanking regions (nt −291 to +153) (Fig. 2) was performed with primers 467 (5′-ATACGCACTTACCATGG-3′) and 468 (5′-ATCGGCGATACGCTTTT-3′) commercially by the Functional Genomics and Proteomics Laboratory, University of Birmingham.
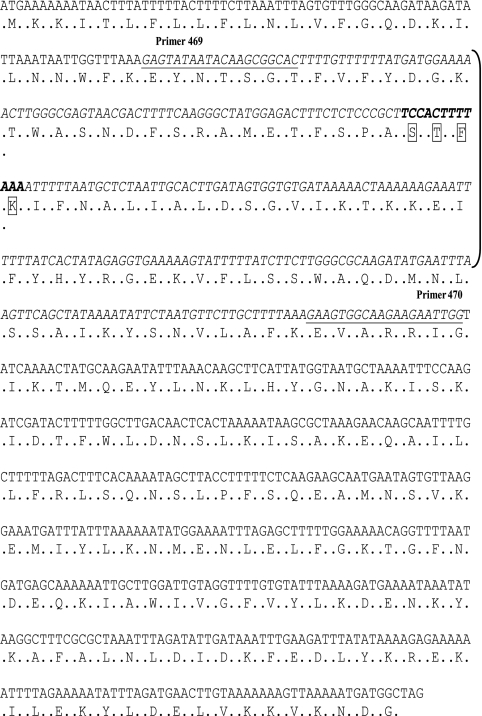
FIG. 1.
DNA sequence of Campylobacter jejuni subsp. jejuni NCTC 11168 Cj0299. The location where primers 469 and 470 anneal is underlined. The DNA sequence from positions 273321 to 274094 amplified and used as a probe in Southern blotting is shown in italic type. The region amplified is indicated by brackets. The amino acid sequence encoded by the DNA sequence is shown. The DNA sequence encoding the active site tetrad, STFK (indicated in boxes), is shown in bold type.
FIG. 2.
Diagrammatic representation of the genes in the region surrounding Cj0299 (positions 263708 to 283707) exported from Xbase (http://xbase.bham.ac.uk).
Detection of β-lactamase activity.
Ampicillin-resistant isolates representing each strain type of Campylobacter spp. from each sampling time and farm (9, 10, 19; Elviss et al., submitted) were tested for production of β-lactamase. Bacteria were grown on Mueller-Hinton agar plus 5% horse blood at 37°C in 7.5% CO2 for 48 h. Bacterial colonies were harvested from the agar plate, and a turbid suspension was prepared in 1 ml sterile distilled water. The cell suspension was lysed using a MSE Soniprep 150 microprobe (Sanyo Biomedical, Loughborough, United Kingdom) for 2 min, pausing for 30 s after each 30 s of sonication. Fifty microliters of sonicate was added to 10 μl of the rehydrated nitrocefin solution (500 mg/ml) (Oxoid Ltd., Basingstoke, United Kingdom) in the well of a microtiter tray, and a color change from yellow to red within 5 min indicated a β-lactamase-positive strain. For those isolates that gave a negative reaction with nitrocefin, the reaction was repeated, and the reaction mixture was incubated for 30 min.
Isoelectric focusing.
Crude β-lactamase preparations were electrophoresed on a broad-range isoelectric focusing gel (Ampholine PAG plate pH 3.5 to 9.5; GE Healthcare Life Sciences, United Kingdom) using the Multiphor II electrophoresis system (GE Healthcare Life Sciences, United Kingdom). β-Lactamase bands were visualized by overlaying the gel with 500 mg/ml nitrocefin (Oxoid Ltd., Basingstoke, United Kingdom). The pIs of the β-lactamases were determined by comparison to a broad-range pI marker (pH 3 to 10, GE Healthcare Life Sciences, United Kingdom).
Extraction and purification of β-lactamases for mass spectrometry (MS) analysis.
Campylobacters were cultured in 200 ml Mueller-Hinton broth at 37°C in 7.5% CO2 with shaking. Bacterial cells were harvested by centrifugation, resuspended in sterile distilled water, and lysed by sonication as described above. The gross cell debris was removed by centrifugation. The protein concentration of the supernatant was estimated using the Bradford reagent assay kit (Sigma-Aldrich, Gillingham, United Kingdom). The supernatant was passed through a 0.45-μm sterile filter to remove any remaining cell particles. The filtrate was diluted two- to threefold with 25 mM sodium acetate buffer (pH 5.5) to adjust the pH to approximately 5.5. The β-lactamase was purified and separated from proteins present at high levels using Vivapure mini H type S ion-exchange minispin columns (Vivascience; Sartorius Ltd., Epsom, United Kingdom) following the manufacturer's protocol. Positively charged proteins, including the β-lactamase, bound to the column, and were eluted using a stepwise NaCl gradient (0.1 M to 1.0 M NaCl) in 25 mM sodium acetate buffer (pH 5.5). Each 400-μl NaCl fraction was collected and added to the upper reservoir of a Vivaspin centrifugal concentrator (molecular mass cutoff, 10 kDa; Vivascience, Sartorius Ltd., Epsom, United Kingdom) and then centrifuged at 10,000 × g for 10 to 15 min at 4°C to reduce the volume to ca. 50 μl. Four hundred microliters of 50 mM cold phosphate buffer (pH 7.0) was added to the upper reservoir of the centrifugal concentrator, centrifuged as described above and then repeated to desalt and neutralize the sample. Five microliters of each fraction was tested with nitrocefin to identify which fraction contained the β-lactamase. The eluted fractions were electrophoresed on a NuPAGE Novex Bis-Tris sodium dodecyl sulfate (SDS)-polyacrylamide gel (Invitrogen Ltd., Paisley, United Kingdom), and protein bands were visualized by silver staining (17). Samples containing β-lactamase were electrophoresed in duplicate on a NuPAGE gel; one-half was stained as described above, and the other was shaken in renaturation buffer (100 mM Tris HCl, 2% [wt/vol] Triton X-100 [pH 7.0]) for 4 h at 37°C to allow the proteins to renature. The β-lactamase was then visualized by overlaying the gel with 500 mg/ml nitrocefin (Oxoid).
Identification of β-lactamases by MS.
β-Lactamase bands were excised from SDS-polyacrylamide minigels using a sterile scalpel and sent for protein identification by QTOF (quadrupole time-of-flight), or high-resolution FTICR (Fourier transform ion cyclotron resonance) MS (Functional Genomics and Proteomics, University of Birmingham). QTOF data were analyzed using the Mascot software (Matrix Science Ltd., Boston, MA), and FTICR data were analyzed using TurboSEQUEST (Bioworks 3.2; Thermo Scientific, East Grinstead, United Kingdom).
Insertional inactivation of Cj0299.
A construct, pC1, was made to allow the chloramphenicol acetyltransferase (cat) gene to be inserted into Cj0299, essentially by the method of Pumbwe et al. (20), with minor modifications. Chromosomal DNA was isolated from C. jejuni NCTC 11168 using the DNAce spin kit (Bioline, London, United Kingdom). Primers 467 and 468 were used to amplify the 1.2-kb fragment flanking the 774-bp coding region of Cj0299. The PCR product was cloned into pGEM-T Easy (Promega) following the manufacturer's protocol to give construct pI. A BglII recognition site and a 280-bp deletion was introduced into Cj0299 in construct pI by inverse PCR using primers 503 blaF-inv (5′-GCG[AGATCT]GTGCCGCTTGTATTATACTC-3′) and 504 blaR-inv (5′-GCG[AGATCT]GAAGTGGCAAGAAGAATTGG-3′). The cat gene of pRY109 was cloned into the BglII site of the Cj0299 fragment in pI to generate construct pCI. The β-lactamase active site tetrad STFK (1) is located close to the center of the 280-bp fragment deleted from Cj0299 of C. jejuni NCTC 11168 by inverse PCR; thus, the Cj0299 fragment in construct pCI lacks the active site.
To ensure that the cat gene had inserted in the correct position, pCI was analyzed by restriction digestion with EcoRI and by standard PCR with combinations of the primers for Cj0299 (primers 469 [5′-GAGTATAATACAAGCGGCAC-3′] and 470 [5′-CCAATTCTTCTTGCCACTTC-3′]) and cat genes (primer 541 [5′-AGAGTTCAGGACCGCATTAG-3′] and primer 536 [5′-GGCATGATGCACTTGAATCG-3′]). C. jejuni strain NCTC 11168 and isolates P1093 and P338 (both isolates of C. jejuni, Cj0299 and nitrocefin positive) were transformed with pCI by the method of Van Vliet et al. (26). Transformants were cultured on Muller-Hinton agar plates supplemented with 10 μg/ml chloramphenicol for 3 to 5 days. To ensure that the observed chloramphenicol resistance was on the chromosome, the transformants were checked for plasmid, and chromosomal DNA was isolated and analyzed by PCR using combinations of Cj0299 and cat primers as described above.
Southern blot hybridization of Cj0299.
Bacteria were grown on Mueller-Hinton agar plus 5% horse blood at 37°C in 7.5% CO2 for 48 h. Bacterial colonies were harvested from the agar plate, and a turbid suspension was prepared in 1 ml sterile distilled water. Genomic DNA was extracted using a Wizard Genomic DNA purification kit (Promega) following the manufacturer's protocol and was quantified using a Nanodrop spectrophotometer (Thermo Scientific). DNA (5 μg) was digested with 30 U/μg of HindIII (Promega) for 4 h and then electrophoresed through a 0.8% agarose gel at 45 V for 8 h. DNA was vacuum transferred to a Hybond-N+ membrane (GE Healthcare) and fixed to the membrane using a UV cross-linker (Stratagene). Blots were prehybridized in AlkPhos Direct hybridization buffer (GE Healthcare) at 60°C for 15 min. The probe for Cj0299 was the 281-bp Cj0299 PCR amplimer (generated from C. jejuni NCTC 11168 using primers 469 and 470). The probe was labeled with alkaline phosphatase following the AlkPhos Direct labeling kit guidelines (GE Healthcare). The blot was incubated overnight at a high-stringency temperature of 60°C with the alkaline phosphatase-labeled probe. Low-salt-stringency primary wash (2 M area, 3.5 M SDS, 50 mM NaPO4, 150 mM NaCl, 1 mM MgCl2) was carried out following the AlkPhos Direct labeling detection system guidelines CDP-Star (RPN 3690; Amersham). The secondary wash buffer was 2 M NaCl-1 M Tris. The combination of high-stringency temperature and low-stringency salt in the wash buffer gave rise to the medium stringency of the Southern blotting (http://www.patentlens.net/daisy/RiceGenome/3663/3612.html). Blots were flooded with CDP-Star detection reagent (Amersham) for 5 min before being wrapped in Saran Wrap and placed in a lightproof cassette with a sheet of Hyperfilm (GE Healthcare) for various times ranging from 20 to 90 min. After this time, the background signal became too dark to ascertain any bands on the film. In addition, no further bands appeared. Film was developed manually using photographic developer and fixer (Kodak).
RESULTS
Phenotype of β -lactam resistance in Campylobacter spp.
Of the 1,288 isolates, 52.4% (675) were resistant to ampicillin (MIC ≥ 8 μg/ml), with ampicillin resistance being more common among C. coli isolates (67.4%) than among C. jejuni isolates (47.5%) (Table 1). One of the two C. lari strains was ampicillin resistant (MIC = 8 μg/ml). C. jejuni isolates (n = 92) and C. coli isolates (n = 19) representing every strain type isolated at each time point in each study (9, 10, 19) were tested for susceptibility to a further 16 β-lactam agents (Table 1). All isolates, regardless of their susceptibility to ampicillin, were highly resistant to piperacillin (MICs ≥ 256 μg/ml). The majority were also resistant to amoxicillin, penicillin, ticarcillin, carbenicillin, piperacillin, cloxacillin, cefazolin, cefuroxime, cefamandole, cefoxitin, and aztreonam. The exceptions were seven isolates of C. jejuni, which were susceptible to one or more of this group of β-lactams. All isolates were susceptible to imipenem and meropenem (MICs ≤ 0.12 μg/ml) and to ertapenem (MICs ≤ 0.5 μg/ml) and moderately susceptible to cefotaxime (MIC50 of 4 μg/ml; MIC90 of 8 μg/ml).
TABLE 1.
MIC50s, MIC90s, and MIC ranges of β-lactam agents for C. jejuni and C. coli
| β-Lactam agenta | MIC(μg/ml) of β-lactam agent for C. jejuni (n = 967)
|
MIC(μg/ml) of β-lactam agent for C. coli (n = 319)
|
||||
|---|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | |
| AMP | 64 | 128 | 0.5-256 | 64 | 128 | 4-256 |
| AMX | 64 | 128 | 0.5->128 | 128 | >128 | 8->128 |
| AMC | 4 | 8 | 0.25-32 | 16 | 32 | 2-32 |
| PEN | 64 | 256 | <8-256 | 128 | 128 | <8-256 |
| TIC | 64 | 128 | <4-256 | 64 | 128 | 8-256 |
| CAR | 128 | 256 | 1->256 | 128 | 256 | 32->256 |
| PIP | ≥512 | >512 | 128->512 | 512 | 512 | 512 |
| TZP | 16 | 32 | 1-128 | 16 | 32 | 16-32 |
| CLX | 256 | >256 | <8->256 | 256 | >256 | 256->256 |
| CEF | >256 | >256 | 8->256 | 256 | >256 | 128->256 |
| CXM | 128 | 128 | 1->256 | 128 | 256 | 64-256 |
| FAM | >256 | >256 | <8->256 | >256 | >256 | 256->256 |
| FOX | 128 | 256 | 2->256 | 128 | >256 | 64->256 |
| CTX | 4 | 8 | 0.25-16 | 4 | 8 | 2-16 |
| MEM | 0.015 | 0.12 | 0.008-0.5 | 0.12 | 0.12 | 0.015-0.25 |
| IPM | 0.03 | 0.06 | <0.015-0.25 | 0.06 | 0.12 | 0.015-0.25 |
| ATM | 64 | >128 | 0.12->128 | 64 | >128 | 8->128 |
Abbreviations: AMP, ampicillin; AMX, amoxicillin; AMC, co-amoxiclav (ampicillin-clavulanic acid); PEN, benzylpenicillin; TIC, ticarcillin; CAR, carbenicillin; PIP, piperacillin; TZP, tazocin (piperacillin-tazobactam); CLX, cloxacillin; CEF, cefalothin; CXM, cefuroxime; FAM, cefamandole; FOX, cefoxitin; CTX, cefotaxime; MEM, meropenem; IPM, imipenem; ATM, aztreonam.
All isolates were more susceptible to co-amoxiclav (amoxicillin and clavulanic acid) and tazocin (piperacillin and tazobactam) than to amoxicillin and piperacillin alone (Table 1). The MICs of co-amoxiclav were ≤32 μg/ml and were 4- to 64-fold lower than those of amoxicillin. The MICs of tazocin were 16- to 32-fold lower than piperacillin alone for all isolates tested, although the MICs were still high (typically 32 μg/ml; Table 1).
Presence of Cj0299 and β-lactamase activity.
Forty-seven ampicillin-sensitive Campylobacter isolates (MIC = 4 μg/ml) were tested for the presence of Cj0299 (blaOXA-61); 59% (28/47) carried this gene. Nineteen of the ampicillin-sensitive isolates did not give an amplimer by PCR. Thirteen randomly chosen nonreplicate ampicillin-sensitive isolates representing each strain type (as determined by phage, serotype, flaA, and/or pulsed-field gel electrophoresis type) were also tested for β-lactamase production (Table 2). Of the 13 isolates tested, eight strains hydrolyzed nitrocefin, of which four gave an amplimer for Cj0299. Five isolates did not produce a ß-lactamase by nitrocefin testing and did not give an amplimer for Cj0299.
TABLE 2.
Association between the presence of Cj0299 (blaOXA-61) and β-lactamase production in ampicillin-susceptible and -resistant Campylobacter spp.
| β-Lactamase activitya | No. of ampicillin-susceptible campylobacters (n = 13)b
|
No. of ampicillin-resistant campylobacters (n = 175)c
|
||
|---|---|---|---|---|
| blaOXA-61-positived | blaOXA-61-negativee | blaOXA-61-positive | blaOXA-61-negative | |
| Detected | 4 | 4 | 136 | 22 |
| Not detected | 0 | 5 | 11 | 6 |
β-Lactamase activity detected by hydrolysis of nitrocefin.
Number of ampicillin-susceptible (MIC ≤ 4μg/ml) campylobacters.
Number of ampicillin-resistant (MIC ≥ 8 μg/ml) campylobacters.
blaOXA-61-positive, blaOXA-61 was detected by PCR.
blaOXA-61-negative, no amplimer was obtained for blaOXA-61.
Of the 380 ampicillin-resistant Campylobacter isolates (MIC ≥ 8 μg/ml) tested by PCR for the presence of Cj0299, 91% (347) carried the gene. The 33 ampicillin-resistant Campylobacter isolates that did not give an amplimer for Cj0299 were reexamined on two or more occasions to confirm the result and under a range of PCR conditions (e.g., different annealing temperatures [from 45 to 56°C], different combinations of PCR and sequencing primers, and magnesium concentrations ranging from 0.5 to 5 mM). These isolates consistently give no amplimer for Cj0299. One hundred seventy-five ampicillin-resistant isolates representing each strain type were also tested with nitrocefin for the production of β-lactamase (Table 2). Of the isolates that produced an amplimer for Cj0299, most (77%; 136) hydrolyzed nitrocefin, indicating that a β-lactamase was produced. Interestingly, 22 ampicillin-resistant isolates produced a β-lactamase, although no PCR amplimer for Cj0299 was obtained. No β-lactamase was detected for 17 isolates, of which 11 gave an amplimer for Cj0299. Of note, the remaining six isolates were multiply drug resistant (resistant to ampicillin, nalidixic acid, tetracycline; 5/6 isolates were resistant to erythromycin, chloramphenicol and ciprofloxacin).
The presence of Cj0299 and the production of β-lactamase was typically associated with resistance to ampicillin, amoxicillin, penicillin, and ticarcillin.
Insertional inactivation of Cj0299 and characterization of mutants.
The Cj0299 gene was successfully inactivated by insertional mutagenesis in C. jejuni NCTC 11168 (isolate P270), and two ampicillin-resistant isolates of C. jejuni, P338 and P1093. No transformants were obtained from C. jejuni P316 and P1156 or C. coli P321. The MICs of a range of β-lactams were determined for the Cj0299-disrupted mutants from each isolate and compared to those of the parental isolate (Table 3). Inactivation of Cj0299 in C. jejuni NCTC 11168 resulted in two- to eightfold-increased susceptibility to ampicillin, co-amoxiclav, penicillin, carbenicillin, oxacillin, and tazocin and 64-fold increase in susceptibility to piperacillin. Susceptibility to cephalosporins, carbapenems, and aztreonam was unaffected by inactivation of Cj0299 in C. jejuni NCTC 11168. Inactivation of Cj0299 in C. jejuni isolates P338 and P1093 resulted in 4- to 64-fold-increased susceptibility to ampicillin, co-amoxiclav, penicillin, ticarcillin, carbenicillin, piperacillin, oxacillin, and meropenem. There was little or no effect on the MICs of cephalosporins and imipenem by inactivation of Cj0299 in isolates P338 and P1093. The presence of 20 μg/ml of the efflux pump inhibitor PAβN had no effect on the MICs of ampicillin, co-amoxiclav, penicillin, piperacillin, ticarcillin, oxacillin, and cefoxitin for the isolates or their respective Cj029-inactivated mutants. All isolates were resistant to 20 μg/ml PAβN alone (data not shown).
TABLE 3.
Presence of Cj0299 (blaOXA-61), β-lactamase production, and susceptibility to β-lactams in representative isolates of C. jejuni and C. coli
| Campylobacter isolatea | blab | BLAc | pId | MIC(μg/ml) of β-lactame
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | AMX | AMC | PEN | TIC | CAR | PIP | TZP | CLX | CXM | FOX | CTX | MEM | IPM | ATM | ||||
| C. jejuni isolates | ||||||||||||||||||
| P270f | + | − | 4 | 4 | 1 | 8 | 2 | 16 | 512 | 16 | 128 | 32 | 32 | 2 | 0.08 | 0.03 | 64 | |
| P270 blaOXA-61::cat | 0.5 | 0.25 | 2 | 2 | 4 | 8 | 4 | 128 | 64 | 32 | 2 | 0.008 | 0.03 | |||||
| P835 | − | − | 2 | 0.25 | 0.5 | 4 | 4 | 8 | >256 | >16 | 256 | 64 | 256 | 4 | <0.015 | 0.06 | 128 | |
| P850 | − | − | 32 | 1 | 4 | 64 | 128 | 256 | >256 | >8 | 256 | 128 | 256 | 8 | 0.03 | 0.06 | >32 | |
| P1962 | − | − | 2 | 2 | 0.5 | 8 | <4 | <16 | >256 | >16 | 256 | 64 | 256 | 1 | 0.015 | 0.015 | 4 | |
| P285 | + | + | 64 | 64 | 8 | 128 | 64 | 256 | >256 | >16 | 256 | 128 | 256 | 8 | 0.015 | 0.015 | >128 | |
| P286 | + | + | 64 | 64 | 8 | 128 | 64 | 256 | >256 | >16 | 256 | 128 | 256 | 8 | 0.25 | 0.25 | >128 | |
| P305 | + | + | 8.9 | 64 | 64 | 8 | 128 | 128 | >256 | >256 | - | 256 | 64 | 128 | 4 | 0.06 | 0.06 | >128 |
| P316 | + | + | 8.7 | 64 | 32 | 8 | 128 | 64 | 128 | >256 | >16 | 256 | 64 | 128 | 4 | 0.12 | 0.06 | >128 |
| P338 | + | + | 8.9 | 64 | 64 | 8 | 128 | 64 | 128 | >256 | >16 | 128 | 128 | 512 | 8 | 0.12 | 0.06 | 128 |
| P338 blaOXA-61::cat | 2 | 1 | 16 | 4 | 8 | 8 | 8 | 128 | 128 | 512 | 8 | 0.015 | 0.03 | |||||
| P790 | + | + | 8.7 | 64 | 64 | 16 | 128 | 128 | 256 | 128 | >16 | >256 | 64 | 64 | 4 | 0.12 | 0.12 | >128 |
| P1093 | + | + | 128 | 64 | 32 | 256 | 32 | 256 | 128 | 256 | 512 | 128 | 128 | 8 | 0.5 | 0.12 | ||
| P1093 blaOXA-61::cat | 2 | 0.5 | 8 | 8 | 16 | 16 | 32 | 256 | 128 | 128 | 4 | 0.03 | 0.12 | |||||
| P1618 | + | + | 64 | 64 | 2 | 64 | 64 | 128 | >512 | 32 | 128 | 256 | 256 | 16 | 0.008 | 0.015 | 64 | |
| P1650 | + | + | 64 | 64 | 4 | 64 | 64 | 64 | >512 | 32 | 256 | 128 | 128 | 4 | 0.008 | 0.06 | 128 | |
| P1730 | + | + | 64 | 64 | 8 | 64 | 32 | 128 | >512 | 16 | 256 | 128 | 256 | 4 | 0.015 | 0.015 | 32 | |
| P1698 | + | − | 128 | 128 | 1 | <8 | 64 | 32 | >512 | 16 | 128 | 64 | 128 | 4 | 0.015 | 0.06 | 128 | |
| P1699 | + | − | 64 | 64 | 4 | <8 | 128 | 64 | 256 | <8 | 256 | 64 | 128 | 4 | 0.015 | 0.03 | <1 | |
| P843 | − | + | 9.2 | 32 | 64 | 4 | 64 | 128 | <16 | >256 | >16 | >256 | 64 | 256 | 4 | 0.03 | 0.06 | >128 |
| P854 | − | + | 9.2 | 128 | 128 | 4 | 256 | 128 | >256 | >256 | >8 | 256 | 128 | 128 | 4 | 0.03 | 0.06 | 4 |
| C. coli isolates | ||||||||||||||||||
| P321 | + | + | 8.6 | 128 | 64 | 8 | 64 | 64 | 256 | >256 | >16 | 256 | 64 | 64 | 4 | 0.12 | 0.12 | >128 |
| P1627 | + | wk + | 32 | 8 | 4 | 8 | 64 | 32 | 512 | 16 | 256 | 64 | 64 | 4 | 0.03 | 0.12 | >128 | |
| P1826 | + | + | 128 | 128 | 8 | 128 | 32 | 128 | 512 | 16 | 256 | 64 | 128 | 2 | 0.06 | 0.03 | 32 | |
The isolates for which the DNA sequence of Cj0299 (blaOXA-61) was determined are shown in boldface type.
+, bla gene detected by PCR; −, bla gene not detected by PCR.
+, β-lactamase (BLA) positive reaction with nitrocefin; −, β-lactamase negative reaction with nitrocefin; wk +, weakly positive reaction.
The pI or isoelectric point was determined by isoelectric focusing.
Abbreviations: AMP, ampicillin; AMX, amoxicillin; AMC, co-amoxiclav (amoxicillin-clavulanic acid); PEN, benzylpenicillin; TIC, ticarcillin; CAR, carbenicillin; PIP, piperacillin; TZP, tazocin (piperacillin-tazobactam); CLX, cloxacillin; CXM, cefuroxime; FOX, cefoxitin; CTX, cefotaxime; MEM, meropenem; IPM, imipenem; ATM, aztreonam. The boldface MICs indicate the agents for which lower MICs were obtained for blaOXA-61-inactivated mutants.
P270, strain NCTC 11168.
DNA sequence of Cj0299 in ampicillin-susceptible and -resistant isolates.
The DNA sequence of Cj0299 in C. jejuni NCTC 11168 was 99.6% identical to that of OXA-61. However, the upstream sequence of NCTC 11168 had a substitution at nt 66 (T→G) in the 122-bp conserved region, prior to the initiation codon of Cj0299 compared to the sequence in the same regions upstream of OXA-61 described by Alfredson and Korolik (1; GenBank accession number AY587956). The DNA sequence of a 1,218-bp PCR product flanking and including Cj0299 was determined for 10 β-lactamase-producing (nitrocefin-positive) isolates. Cj0299 was not sequenced for those isolates that did not produce β-lactamase. The DNA sequence of the open reading frame of Cj0299 in the 11 Campylobacter isolates (C. jejuni isolates P285, P286, P305, P316, P338, P790, P1093, P1156, and P1618 and C. coli isolates P321 and P1826) was identical to that of Cj0299 C. jejuni NCTC 11168 with three exceptions. Isolate P316 had a substitution at nt 22 (T→C) in the 122-bp conserved region, upstream of the initiation codon for Cj0299. Isolate P1156 had a substitution of threonine for alanine at codon 116. Isolates P305 and P338 had substitutions of asparagine for aspartic acid at codon 228. None of the isolates studied had the Glu→Gly substitution at position 202 of OXA-61 described by Alfredson and Korolik (1; GenBank accession number AY587956). These data support those of Alfredson and Korolik (1) that Cj0299 encodes the ß-lactamase OXA-61. The additional mutations described in blaOXA-61 suggest that these are variations in the same enzyme, as all of the conserved motifs in OXA are retained. Until enzyme kinetics are carried out, it is premature to request alternative OXA numbers, as there is no indication to suggest that the sequence variations alter the substrate specificity or kinetics of the β-lactamase. The sequence variation may just be natural polymorphisms in the DNA sequence. Hereafter, Cj0299 is referred to as blaOXA-61.
Isoelectric focusing and SDS-PAGE of β-lactamases.
β-Lactamases were isolated from seven isolates, and the isolectric point was determined by isoelectric focusing. The pI of the single β-lactamase from the five isolates that gave an amplimer for blaOXA-61 and were nitrocefin positive (four C. jejuni isolates [P305, P316, P338, and P790] and one C. coli isolate [P321]) was 8.69 ± 0.14 (n = 11 values). SDS-polyacrylamide gel electrophoresis (SDS-PAGE) indicated that the molecular mass was 31.0 ± 1.5 kDa (n = 3). The pI of the β-lactamases from the two isolates that did not give an amplimer for blaOXA-61 but were nitrocefin positive (C. jejuni P843 and P854) was 9.21 ± 0.10 (n = 8 values). SDS-PAGE showed that the molecular mass was 32.4 ± 0.98 kDa (n = 4). These data suggest that the two groups of isolates produce different β-lactamases. The β-lactamase from C. jejuni P843 and P854 is hereafter referred to as CjBla2.
Identification of β-lactamases by MS.
The β-lactamases from three of the isolates (one C. coli isolate, P321, representing the blaOXA-61/nitrocefin-positive isolates) (pI 8.6) and two C. jejuni isolates that produced CjBla2 (isolates P843 and P854) (pI 9.2) were analyzed by MS to confirm the identity of the enzymes. The β-lactamase from C. jejuni NCTC 11168 was not extracted, as it gave a low yield. Analysis of the QTOF-MS data using the Mascot software showed that the β-lactamase produced by isolate P321 significantly matched two C. jejuni proteins with a probability-based Mowse algorithm score of 167 (P < 0.05), a class D β-lactamase from C. jejuni (mass of 29,956; GenBank AY587956.1 accession number QPPZ0) and a probable periplasmic β-lactamase Cj0299 C. jejuni (mass of 30,028; Protein Information Resource accession number G81448). These proteins correspond to OXA-61 (1) and Cj0299 (18), respectively, and confirm that the β-lactamase of P321 is the product of the Cj0299 gene.
QTOF-MS analysis of CjBla2 produced by isolates P843 and P854 (both of which lacked the blaOXA-61 gene) did not reveal any homology to OXA-61 or Cj0299 or to β-lactamases of other bacterial species. Thus, the more sensitive FTICR-MS, using a liquid chromatography injection, was used in order to identify the protein from these two strains. These data were analyzed using BLAST searches against the campylobacter, helicobacter, and pseudomonas protein databases but did not yield any matches to known β-lactamases or penicillin binding proteins. These data indicate that CjBla2 is not the product of a mutated blaOXA-61 (Cj0299) gene. The most frequent hit for proteins of the correct molecular mass in the C. jejuni database was with formyltetrahydrofolate deformylase, PurU (accession number CAB73055), an enzyme involved in nucleotide transport and metabolism. However, inactivation of the purU gene in isolates P843 and P854 had no effect upon the MIC of β-lactams (data not shown). These data suggest that CjBla2 had coeluted with PurU, which was the more abundant protein. Further experiments are under way to identify CjBla2.
CjBla2 is unlikely to be a variant of blaOXA-61.
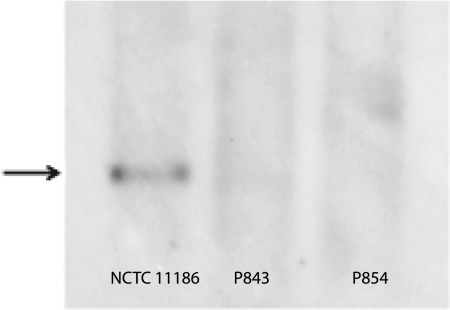
P843 and P854, the two isolates that produce CjBla2, were subject to Southern blot hybridization and probed for blaOXA-61. Hybridization was obtained with DNA from C. jejuni NCTC 11168 but not with DNA from isolate P843 or P854 (Fig. 3) even under conditions of low stringency (data not shown). These data suggest that if the gene encoding CjBla2 is a variant of blaOXA-61, then the sequences differ by at least 50%.
FIG. 3.
Southern blot hybridization of HindIII-digested genomic DNA from C. jejuni NCTC 11168, P843, and P854 probed with the blaOXA-61 gene under conditions of low-stringency washes, 60°C annealing temperature, and 20-min exposure. Both P843 and P854 isolates lack the blaOXA-61 gene (indicated by the black arrow to the left of the gel).
DISCUSSION
In this study, we showed that ampicillin resistance and β-lactamase production in campylobacters isolated from poultry was strongly associated with the presence of the blaOXA-61 gene carried on the chromosome. However, all ampicillin- and amoxicillin-resistant isolates were susceptible to co-amoxiclav, imipenem, meropenem, and ertapenem, and most of these isolates were susceptible to cefotaxime. Susceptibility to amoxicillin and piperacillin was increased in the presence of β-lactamase inhibitors, similar to findings with human clinical isolates (10). The β-lactamase-producing strains were more resistant to penicillins, such as ampicillin, amoxicillin, and ticarcillin, but as found by others (5, 11), susceptibility to cephalosporins was the same in both β-lactamase-producing or non-β-lactamase-producing strains. The β-lactamase described by Lachance et al. (11) had an isoelectric point of 8.8, which agrees with our data from ampicillin-resistant veterinary isolates (pI 8.7). In addition, we found that the molecular size and the MS analysis confirmed that this β-lactamase was the product of the blaOXA-61 gene. Thus, our data suggest that the characteristics and spectrum of activity of β-lactamase produced by veterinary C. jejuni isolates were similar to those reported for human clinical isolates.
Inactivation of the blaOXA-61 gene increased susceptibility to β-lactams, including ampicillin, penicillin, carbenicillin, oxacillin, and piperacillin, in both ampicillin-sensitive and -resistant strains. Susceptibility to cephalosporins and imipenem was unaffected by inactivation of blaOXA-61. Alfredson and Korolik (1) showed that by cloning OXA-61 into a β-lactam-susceptible clinical C. jejuni strain they were able to confer resistance to ampicillin, piperacillin, and carbenicillin, but not to cefotaxime or imipenem. However, they observed a fivefold increase in the MIC of meropenem. In the present study, susceptibility to meropenem was increased slightly in mutants in which blaOXA-61 was inactivated. It is interesting that increased susceptibility to β-lactams was observed in C. jejuni NCTC 11168 blaOXA-61::cat, when no β-lactamase activity could be detected in this strain with nitrocefin. These data indicate that the β-lactamase encoded by blaOXA-61 is involved in resistance to penams in Campylobacter spp. and confers some innate resistance in β-lactam-susceptible strains of Campylobacter.
Production of β-lactamase was not always associated with resistance to β-lactams, as some ampicillin-sensitive isolates hydrolyzed nitrocefin. Lariviere et al. (13) found 89% human clinical isolates of C. jejuni produced β-lactamase, although only 14.6% were ampicillin resistant. β-Lactamase production was not quantified in nitrocefin-positive isolates in this early or earlier studies, but we postulate that ampicillin-resistant isolates produce more enzyme than susceptible isolates do. However, these data may suggest that β-lactamases have a function other than mediating β-lactam resistance in campylobacters. Comparative growth kinetics of parental strains and blaOXA-61::cat mutants showed that inactivation of blaOXA-61 had no deleterious effect upon growth in liquid media (unpublished data), which suggests that this function is not essential for survival.
Our data suggest that a novel β-lactamase, CjBla2, which has a more basic pI and higher molecular mass than the product of the blaOXA-61 gene, is produced by some ampicillin-resistant isolates that lack the blaOXA-61 gene. Although MS confirmed that the β-lactamase produced by blaOXA-61-positive strains was the product of the gene, the identity of the CjBla2 β-lactamase could not be determined, despite use of a more sensitive MS technique. Earlier work by Lucain et al. (14) described four β-lactamases in C. jejuni, which were distinguished by their spectrum of activity, molecular mass, and pI. Although the type B enzyme described by Lucain et al. (14) had a higher pI than the more common type A β-lactamase (which most probably correlates to OXA-61), the molecular mass was lower and the activity profile differed from that of type A. In the present study, irrespective of production of OXA-61 or CjBla2, there was little difference in susceptibility of the isolates to any of the β-lactams tested.
To our knowledge, this is the first study to determine the incidence of the blaOXA-61 gene in campylobacters of veterinary origin. It is also the first study of Campylobacter spp. from any source to correlate the presence of the gene with β-lactamase production and susceptibility to a wide range of β-lactam agents. In addition, we have shown that inactivation of blaOXA-61 in C. jejuni results in increased susceptibility to penams and meropenem, but not to cephalosporins. Our data also suggests that in addition to blaOXA-61, there is at least one further β-lactamase, CjBla2, in C. jejuni.
Acknowledgments
This work was supported by Department for Environment, Food and Rural Affairs project grants OZO501 and VMO2200.
pRY109 was kindly supplied by C. W. Penn and J. M. Ketley. C. jejuni NCTC 11168 purU::cat was supplied by Andy Grant (8). We thank Vito Ricci for technical support for this project and Laine Wallace of the Functional Genomics and Proteomics laboratory for assistance with proteomics analysis.
Footnotes
Published ahead of print on 8 June 2009.
REFERENCES
- 1.Alfredson, D. A., and V. Korolik. 2005. Isolation and expression of a novel molecular class D β-lactamase, OXA-61, from Campylobacter jejuni. Antimicrob. Agents Chemother. 49:2515-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfredson, D. A., and V. Korolik. 2007. Identification of putative zinc hydrolase genes of the metallo-β-lactamase superfamily from Campylobacter jejuni. FEMS Immunol. Med. Microbiol. 49:159-164. [DOI] [PubMed] [Google Scholar]
- 3.Blaser, M. J. 1995. Campylobacter and related species, p. 1948-1956. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, New York, NY.
- 4.Corcoran, D., T. Quinn, L. Cotter, P. Whyte, and S. Fanning. 2006. Antimicrobial resistance profiling and fla-typing of Irish thermophilic Campylobacter spp. of human and poultry origin. Lett. Appl. Microbiol. 43:560-565. [DOI] [PubMed] [Google Scholar]
- 5.Fleming, P. C., A. D'Amico, S. De Grandis, and M. A. Karmali. 1982. The detection and frequency of beta-lactamase production in Campylobacter jejuni, p. 217. In D. G. Newell (ed.), Campylobacter. Epidemiology, pathogenesis and biochemistry. MTP Press, Lancaster, United Kingdom.
- 6.Fliegelman, R. M., R. M. Petrak, L. J. Goodman, J. Segreti, G. M. Trenholme, and R. L. Kaplan. 1985. Comparative in vitro activities of twelve antimicrobial agents against Campylobacter species. Antimicrob. Agents Chemother. 27:429-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillespie, I. A., S. J. O'Brien, J. A. Frost, G. K. Adak, P. Horby, A. V. Swan, M. J. Painter, K. R. Neal, and Campylobacter Sentinel Surveillance Scheme Collaborators. 2002. A case-case comparison comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg. Infect. Dis. 9:937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant, A. J., C. Coward, M. A. Jones, C. A. Woodall, P. A. Barrow, and D. J. Maskell. 2005. Signature-tagged transposon mutagenesis studies demonstrate the dynamic nature of cecal colonization of 2-week-old chickens by Campylobacter jejuni. Appl. Environ. Microbiol. 71:8031-8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griggs, D. J., M. M. Johnson, J. A. Frost, T. Humphrey, F. Jørgensen, and L. J. V. Piddock. 2005. Incidence and mechanism of ciprofloxacin resistance in Campylobacter spp. isolated from commercial poultry flocks in the United Kingdom before, during, and after fluoroquinolone treatment. Antimicrob. Agents Chemother. 49:699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphrey, T. J., F. Jørgensen, J. A. Frost, H. Wadda, G. Domingue, N. C. Elviss, D. J. Griggs, and L. J. V. Piddock. 2005. Prevalence and subtypes of ciprofloxacin-resistant Campylobacter spp. in commercial poultry flocks before, during, and after treatment with fluoroquinolones. Antimicrob. Agents Chemother. 49:690-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lachance, N., C. Gaudreau, F. Lamothe, and L. A. Lariviere. 1991. Role of the β-lactamase of Campylobacter jejuni in resistance to β-lactam agents. Antimicrob. Agents Chemother. 35:813-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lachance, N., C. Gaudreau, F. Lamothe, and F. Turgeon. 1993. Susceptibilities of β-lactamase-positive and -negative strains of Campylobacter coli to β-lactam agents. Antimicrob. Agents Chemother. 37:1174-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lariviere, L. A., C. L. Gaudreau, and F. F. Turgeon. 1986. Susceptibility of clinical isolates of Campylobacter jejuni to twenty-five antimicrobial agents. J. Antimicrob. Chemother. 18:681-685. [DOI] [PubMed] [Google Scholar]
- 14.Lucain, C., H. Goossens, and J. C. Pechere. 1985. Beta-lactamases in Campylobacter jejuni, abstr. 005, p. 36-37. In A. D. Pearson, M. B. Skirrow, H. Lior, and B. Rowe (ed.), Campylobacter III. Public Health Laboratory Service, London, United Kingdom.
- 15.McDermott, P. F., S. M. Bodeis, F. M. Aarestrup, S. Brown, M. Traczewski, P. Fedorka-Cray, M. Wallace, I. A. Critchley, C. Thornsberry, S. Graff, R. Flamm, J. Beyer, D. Shortridge, L. J. Piddock, V. Ricci, M. M. Johnson, R. N. Jones, B. Reller, S. Mirrett, J. Aldrobi, R. Rennie, C. Brosnikoff, L. Turnbull, G. Stein, S. Schooley, R. A. Hanson, and R. D. Walker. 2004. Development of a standardized susceptibility test for campylobacter with quality-control ranges for ciprofloxacin, doxycycline, erythromycin, gentamicin, and meropenem. Microb. Drug Resist. 10:124-131. [DOI] [PubMed] [Google Scholar]
- 16.McGill, K., D. Cowley, L. Moran, P. Scates, A. O'Leary, R. H. Madden, C. Carroll, E. McNamara, J. E. Moore, S. Fanning, J. D. Collins, and P. Whyte. 2006. Antibiotic resistance of retail food and human Campylobacter isolates on the island of Ireland from 2001-2002. Epidemiol. Infect. 134:1282-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortz, E., T. N. Krogh, H. Vorum, and A. Gorg. 2001. Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionisation-time of flight analysis. Proteomics 1:1359-1363. [DOI] [PubMed] [Google Scholar]
- 18.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 19.Piddock, L. J. V., D. Griggs, M. M. Johnson, V. Ricci, N. C. Elviss, L. K. Williams, F. Jorgensen, S. A. Chisholm, A. J. Lawson, C. Swift, T. J. Humphrey, and R. J. Owen. 2008. Persistence of Campylobacter species, strain types, antibiotic resistance and mechanisms of tetracycline resistance in poultry flocks treated with chlortetracycline. J. Antimicrob. Chemother. 62:303-315. [DOI] [PubMed] [Google Scholar]
- 20.Pumbwe, L., and L. J. V. Piddock. 2002. Identification and molecular characterisation of CmeB, a Campylobacter jejuni multidrug efflux pump. FEMS Microbiol. Lett. 206:185-189. [DOI] [PubMed] [Google Scholar]
- 21.Reina, J., M. J. Ros, and A. Serra. 1994. Susceptibilities to 10 antimicrobial agents of 1,220 Campylobacter strains isolated from 1987 to 1993 from feces of pediatric patients. Antimicrob. Agents Chemother. 38:2917-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tajada, P., J. L. Gomez-Garces, J. I. Alos, D. Balas, and R. Cogollos. 1996. Antimicrobial susceptibilities of Campylobacter jejuni and Campylobacter coli to 12 β-lactam agents and combinations with β-lactamase inhibitors. Antimicrob. Agents Chemother. 40:1924-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor, D. E., S. A. de Grandis, M. A. Karmali, and P. C. Fleming. 1981. Transmissible plasmids from Campylobacter jejuni. Antimicrob. Agents Chemother. 19:831-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thwaites, R. T., and J. A. Frost. 1999. Drug resistance in Campylobacter jejuni, C. coli, and C. lari isolated from humans in north west England and Wales, 1997. J. Clin. Pathol. 52:812-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United Kingdom Health Protection Agency. 2006. International surveillance network for the enteric infections Salmonella and VTEC O157: Enter-net Quarterly Campylobacter Reports. 2005-2006. United Kingdom Health Protection Agency, London, United Kingdom. http://www.hpa.org.uk/hpa/inter/enter-net_reports.htm.
- 26.Van Vliet, A. H. M., A. C. Wood, J. Henderson, K. Wooldridge, and J. M. Ketley. 1998. Genetic manipulation of enteric Campylobacter spp. Methods Microbiol. 27:407-419. [Google Scholar]