Abstract
SMP-601 (also known as PTZ601, PZ-601, or SM-216601) is a novel parenteral carbapenem with potent activity against multidrug-resistant gram-positive pathogens, including vancomycin-resistant Enterococcus faecium (VREF) and methicillin-resistant Staphylococcus aureus (MRSA). The pharmacodynamics of SMP-601 against VREF and MRSA were investigated in neutropenic murine thigh infection models. The percentage of the dosing interval that the unbound SMP-601 concentration exceeded the MIC (f%T>MIC) was the pharmacokinetic-pharmacodynamic parameter that correlated most closely with efficacy with R2 values of 0.81 to 0.84 for two strains of VREF and 0.92 to 0.93 for two strains of MRSA, whereas the R2 values for the area under the concentration-time curve from 0 to 24 h divided by the MIC were 0.12 to 0.89, and the R2 values for the peak level divided by the MIC were 0 to 0.22. The f%T>MIC levels required for static or killing efficacy against two strains of VREF (9 to 19%) apparently were lower than those against two strains of MRSA (23 to 37%). These results suggested that SMP-601 showed time-dependent in vivo efficacy against VREF and MRSA, and SMP-601 had a sufficient therapeutic effect against VREF infections at lower exposure conditions compared to those for with MRSA infections.
The increasing rates of resistance among both hospital- and community-acquired bacterial pathogens, such as Staphylococcus aureus, coagulase-negative staphylococci, and enterococci, are serious problems in antibacterial chemotherapy (2, 11, 15). Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci are of particular concern. The proportion of enterococci resistant to vancomycin continues to rise in the hospital setting, with the overwhelming majority of infections being due to Enterococcus faecium (16). This situation has prompted attempts to discover new antimicrobials with activities against multidrug-resistant gram-positive pathogens. SMP-601 (also known as PTZ601, PZ-601, or SM-216601) is a novel parenteral carbapenem possessing a novel dihydropyrrolylthiazole moiety at the C-2 side chain (Fig. 1), and it has potent activity against multidrug-resistant gram-positive pathogens, including MRSA and vancomycin-resistant Enterococcus faecium (VREF) (23).
FIG. 1.
Chemical structure of SMP-601.
Pharmacokinetic-pharmacodynamic (PK-PD) studies of animal infection models are useful for elucidating the targeting exposures associated with optimal activity, and PK-PD relationships determined in animal infection models can be used to establish dosage regimens for clinical development (5, 6). The objective of this study was to examine the in vivo pharmacodynamic profile of SMP-601 against clinical isolates of VREF and MRSA using a neutropenic murine thigh infection model. However, in regard to VREF, it was reported that the 24-h growth of VREF in the murine thigh was negligible and that the anti-VREF activity of the drug may have been overestimated in the model (7, 17). Therefore, a new VREF thigh infection model was established, and the pharmacodynamic profile of SMP-601 against VREF was investigated.
(This work was presented in part at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, 17 to 20 September 2007, Chicago, IL [9].)
MATERIALS AND METHODS
Organisms.
Four clinically isolated strains of MRSA (SP-12249 and TL-3677) and VREF (TL-3273 and TL-3621) were used.
Antimicrobial agents.
SMP-601 was synthesized at Dainippon Sumitomo Pharma Co., Ltd., Osaka, Japan.
Susceptibility testing.
MICs were determined by the broth microdilution method using cation-adjusted Mueller-Hinton broth (MHB; Nippon BD Company Ltd., Tokyo, Japan) according to CLSI guidelines (4). The final inocula were approximately 105 CFU/well.
Time-kill study.
The bactericidal activity of SMP-601 against VREF was assessed using a time-kill assay with brain heart infusion broth (BHIB; Nippon BD Company Ltd., Tokyo, Japan) with or without 1% (wt/vol) carrageenan (Sigma-Aldrich, Inc., St. Louis, MO) (3). The final inocula were approximately 105 CFU/ml. Viable cell counts were determined by a general plating method at 1, 2, 4, 6, and 8 h after SMP-601 addition (0.5 [1/4× MIC], 2 [1× MIC], and 8 μg/ml [4× MIC]).
Murine thigh infection model.
Four-week-old male slc:ICR mice were purchased from Japan SLC, Inc. (Shizuoka, Japan), and adapted to standardized environmental conditions (temperature, 23 ± 2°C; humidity, 55% ± 10%) for 1 week before the experiments. All animal procedures were performed in accordance with the institutional animal guidelines. Mice were rendered neutropenic by injecting cyclophosphamide (Shionogi & Co., Ltd., Osaka, Japan) subcutaneously 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before the thigh infection study. Overnight cultures of each strain in MHB were diluted 500-fold for MRSA or 10-fold for VREF with fresh MHB and then cultured at 37°C to an absorbance at 580 nm of about 0.3 for MRSA and of about 0.2 for VREF. For MRSA, the culture was diluted 10-fold with fresh MHB and used as the bacterial inoculum (approximately 107 CFU/ml). For VREF, the culture was diluted 100- or 1,000-fold with fresh BHIB with or without 1% (wt/vol) carrageenan, which induces local acute inflammation, and used as the bacterial inoculum (approximately 105 to 106 CFU/ml). Of these bacterial inoculum suspensions, 0.1 ml of each was inoculated into both thighs of groups of two mice each 2 h before the start of chemotherapy. The mice then were treated subcutaneously for 24 h with multiple SMP-601 regimens divided into 1, 2, 3, 4, 6, 8, or 12 doses. The total doses of SMP-601 ranged from 2.5 to 640 mg/kg. At each administration, 100 mg/kg of cilastatin (Ranbaxy Laboratories, Ltd., India), a dehydropeptidase-I (DHP-I) inhibitor, was coadministered to prevent SMP-601 degradation by this rodent enzyme. SMP-601 dosage forms were prepared by dissolving it with a 10-mg/ml cilastatin solution. Mice were sacrificed 24 h after the start of chemotherapy, and both thighs were harvested aseptically and homogenized in 5 ml of sterilized saline. Untreated controls were sacrificed at the start of chemotherapy and after 24 h. Aliquots of 10-fold dilutions of the homogenized tissue were plated on Mueller-Hinton agar (MHA) for CFU determinations.
Impact of 1% (wt/vol) carrageenan on in vivo activity.
Additional studies were designed to determine the impact of 1% (wt/vol) carrageenan on the activity of SMP-601 in the murine thigh infection models using MRSA SP-12249 and VREF TL-3273. Approximately 107-CFU/ml bacterial inoculum suspensions were prepared in MHB with or without 1% (wt/vol) carrageenan for MRSA and approximately 105-CFU/ml bacterial inoculum suspensions were prepared in BHIB with 1% (wt/vol) carrageenan for VREF. SMP-601 at a level of 5, 20, and 80 mg/kg with 100 mg/kg of cilastatin was administrated by subcutaneous injection once 2 h after the infection.
Pharmacokinetics.
Single-dose pharmacokinetics of SMP-601 were examined in neutropenic ICR mice infected in the thigh with MRSA SP-12249 or VREF TL-3273. SMP-601 at a level of 10, 20, 40, or 80 mg/kg with 100 mg/kg cilastatin was administrated by subcutaneous injection into three mice in each group. A sample of heart blood was obtained at 5, 15, 30, 60, and 90 min for doses of 10 and 20 mg/kg or at 5, 15, 30, 60, 90, and 120 min for doses of 40 and 80 mg/kg after drug administration. SMP-601 serum concentrations were determined by the bioassay method with Bacillus subtilis ATCC 6633 as the indicator organism (23). The lower and upper limits of the quantitation of the assay were 0.1 and 100 μg/ml, respectively. The intra- and interday variation was less than 15%. The pharmacokinetic parameters were calculated using a one-compartment model analysis.
Protein binding.
Protein binding in the murine serum was analyzed using ultrafiltration methods (19). The degree of binding was measured using SMP-601 concentrations of 10, 30, and 100 μg/ml.
Data analysis.
Three PK-PD parameters (the percentage of the dosing interval that the unbound drug concentration exceeded the MIC [f%T>MIC], the area under the concentration-time curve from 0 to 24 h divided by the MIC [fAUC/MIC], and the peak level divided by the MIC [fCmax/MIC]) for each dosing regimen were calculated using pharmacokinetic parameters, murine serum protein binding (76%), and MICs. The correlation between efficacy and the three PK-PD parameters was evaluated using the sigmoid dose-effect model. This model is derived from the Hill equation: E = (Emax × DN)/(ED50N + DN), where E is the effect or, in this case, the log change in CFU per thigh between treated mice and untreated controls after the 24 h period of the study, Emax is the maximum effect, D is the 24-h total dose, ED50 is the dose required to achieve 50% of Emax, and N is the slope of the dose-effect curve. The indices Emax, ED50, and N were calculated using nonlinear least-squares regression. The magnitude of f%T>MIC and fAUC/MIC required to achieve a static effect (no change in the bacterial density compared to the numbers at the start of chemotherapy) or 1- and 2-log kills (1- and 2-log10 reductions, respectively, in the bacterial density compared to the numbers at the start of chemotherapy) also were calculated. This analysis was performed using the Statistical Analysis System for Windows. (SAS Institute Inc., Cary, NC).
RESULTS
VREF thigh infection model.
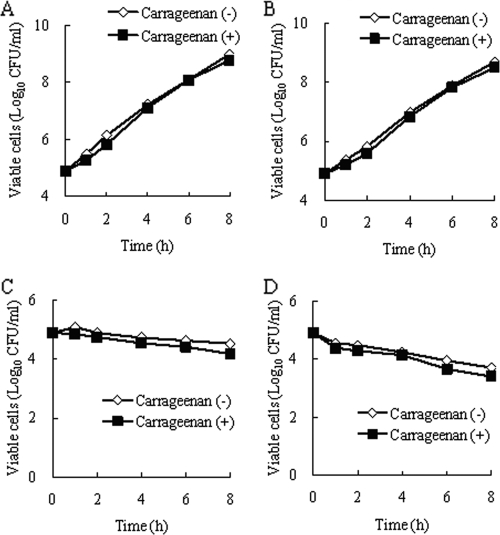
The growth of VREF TL-3273 in a neutropenic murine thigh with or without 1% (wt/vol) carrageenan is shown in Table 1. Bacterial growth was not observed at all without 1% (wt/vol) carrageenan in the bacterial inoculum suspension (−0.02 log10 CFU/thigh for 24 h). On the contrary, sufficient bacterial growth was observed with 1% (wt/vol) carrageenan in the bacterial inoculum suspension (1.41 log10 CFU/thigh for 24 h). The impact of 1% (wt/vol) carrageenan on the growth of VREF and the anti-VREF activity of SMP-601 was determined in an in vitro time-kill study (Fig. 2). For the growth control, bacterial growth with 1% (wt/vol) carrageenan was not different from that without 1% (wt/vol) carrageenan. The antibacterial activity of SMP-601 at 0.5 μg/ml (1/4× MIC), 2 μg/ml (1× MIC), and 8 μg/ml (4× MIC) was not affected by adding 1% (wt/vol) carrageenan to the bacterial growth medium. Therefore, it was concluded that 1% (wt/vol) carrageenan did not affect the growth of VREF or the anti-VREF activity of SMP-601, and this new murine VREF thigh infection model was established with sufficient in vivo bacterial growth.
TABLE 1.
Impact of 1% (wt/vol) carrageenan on the growth of VREF TL-3273 in a neutropenic murine thigh
| Presence of 1% (wt/vol) carrageenan | Inoculum (log10 CFU/thigh) | Viable cell count (log10 CFU/thigh)a at:
|
Change in viable cell counts during 24 h (log10 CFU/thigh) | |
|---|---|---|---|---|
| 2 hb | 26 hb | |||
| Without | 3.88 | 4.01 ± 0.21 | 3.99 ± 0.53 | −0.02 |
| With | 3.88 | 4.97 ± 0.11 | 6.38 ± 1.02 | 1.41 |
The means ± standard deviations data are for two mice (four thighs).
Time after infection.
FIG. 2.
Impact of 1% (wt/vol) carrageenan on the growth of VREF and the anti-VREF activity of SMP-601. (A) Growth control of VREF TL-3273 in BHIB with or without 1% (wt/vol) carrageenan. Shown is the in vitro activity of SMP-601 against VREF TL-3273 in BHIB with or without 1% (wt/vol) carrageenan at 0.5 μg/ml (1/4×MIC) (B), 2 μg/ml (1×MIC) (C), and 8 μg/ml (4×MIC) (D).
Impact of 1% (wt/vol) carrageenan on in vivo activity.
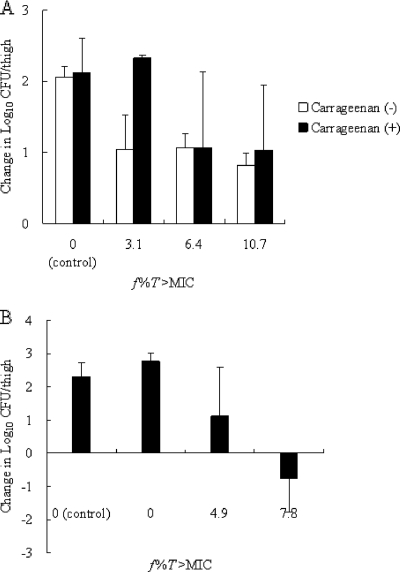
For VREF, because bacterial growth was not observed at all without 1% (wt/vol) carrageenan in the murine thigh infection model, the activity of SMP-601 without 1% (wt/vol) carrageenan was not able to be evaluated. Therefore, the activity of SMP-601 with 1% (wt/vol) carrageenan was compared to that without 1% (wt/vol) carrageenan against MRSA SP-12249 to determine the impact of 1% (wt/vol) carrageenan on the in vivo activity of SMP-601 (Fig. 3A). The change in the log10 CFU/thigh value compared to the numbers at the start of therapy with 1% (wt/vol) carrageenan was similar to that without 1% (wt/vol) carrageenan. Therefore, it was suggested that 1% (wt/vol) carrageenan did not affect the in vivo activity of SMP-601. In contrast to MRSA, organ burden reduction was observed at a low f%T>MIC level (7.8%) for VREF (Fig. 3B).
FIG. 3.
Change in the number of log10 CFU/thigh compared to numbers at the start of SMP-601 therapy against MRSA SP-12249 with or without 1% (wt/vol) carrageenan (A) and VREF TL-3273 with 1% (wt/vol) carrageenan (B) in the murine thigh infection model.
Pharmacokinetics.
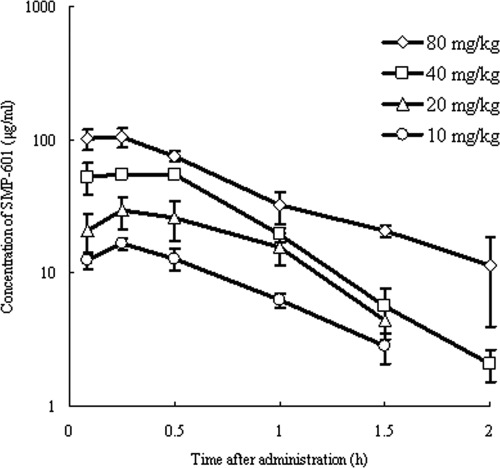
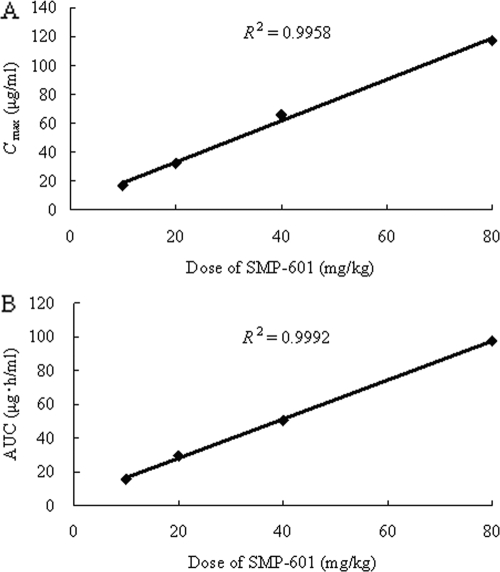
The time-concentration profiles of SMP-601 in neutropenic mice infected in the thigh with MRSA SP-12249 after single subcutaneous doses of 10, 20, 40, and 80 mg/kg are shown in Fig. 4. The relationship between dose and Cmax and the relationship between the dose and AUC is shown in Fig. 5. Both the Cmax and AUC increased in proportion to the dose (R2>0.99). The pharmacokinetics of SMP-601 demonstrated linearity in thigh-infected neutropenic mice. The protein binding of SMP-601 in murine serum at concentrations of 10, 30, and 100 μg/ml is shown in Table 2.
FIG. 4.
Serum concentrations of SMP-601 after the administration of single subcutaneous doses of 10, 20, 40, and 80 mg/kg to neutropenic mice infected in the thigh with MRSA SP-12249. Each symbol represents the means ± standard deviations for three mice.
FIG. 5.
Relationship between dose versus Cmax (A) and dose versus AUC (B) for various doses of SMP-601.
TABLE 2.
Protein binding of SMP-601 in murine serum
| Concn of SMP-601 (μg/ml) | Protein binding (%)
|
||
|---|---|---|---|
| Assay 1 | Assay 2 | Mean | |
| 100 | 78 | 78 | 78 |
| 30 | 75 | 76 | 76 |
| 10 | 74 | 75 | 75 |
Impact of 1% (wt/vol) carrageenan on pharmacokinetics.
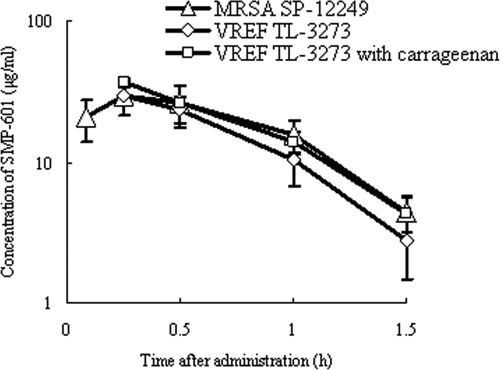
The time-concentration profiles of SMP-601 in neutropenic mice infected in the thigh with MRSA SP-12249 or VREF TL-3273 with or without 1% (wt/vol) carrageenan after single subcutaneous doses of 20 mg/kg were quite similar (Fig. 6). It was suggested that 1% (wt/vol) carrageenan or a bacterial strain did not influence the pharmacokinetics of SMP-601 in mice.
FIG. 6.
Serum concentrations of SMP-601 after the administration of single subcutaneous doses of 20 mg/kg to neutropenic mice infected in the thigh with MRSA SP-12249 or VREF TL-3273 with or without 1% (wt/vol) carrageenan. Each symbol represents the means ± standard deviations for three mice.
PK-PD index determination and magnitude of the PK-PD index associated with efficacy.
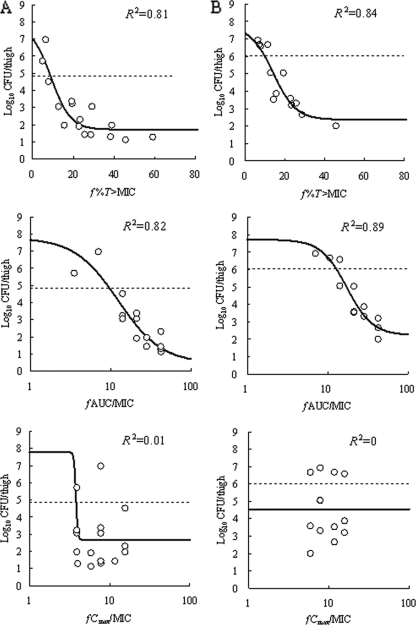
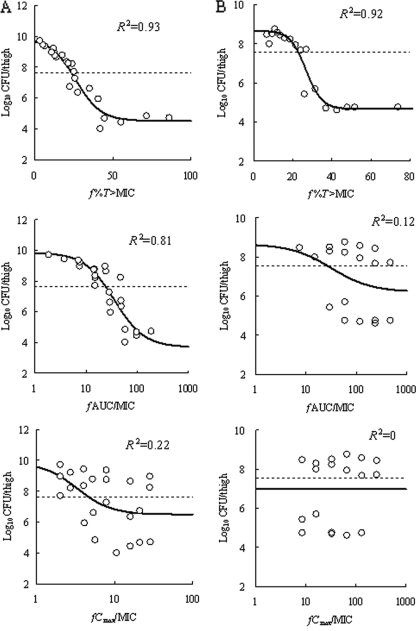
At the start of chemotherapy, mice had 4.85 ± 0.11, 6.03 ± 0.12, 7.65 ± 0.34, and 7.57 ± 0.10 log10 CFU/thigh of VREF TL-3273, VREF TL-3621, MRSA SP-12249, and MRSA TL-3677, respectively. The increase in bacterial density during 24 h in untreated control mice was 2.93, 1.70, 2.21, and 1.10 log10 CFU/thigh, respectively. The relationship between the PK-PD parameters (based on free drug concentrations) and the efficacy of SMP-601 is shown in Fig. 7 for VREF and Fig. 8 for MRSA. f%T>MIC and fAUC/MIC were the parameters that best correlated with efficacy, with R2 values for an f%T>MIC of 0.81 for VREF TL-3273, 0.84 for VREF TL-3621, and 0.93 for MRSA SP-12249 and with R2 values for an fAUC/MIC of 0.82 for VREF TL-3273, 0.89 for VREF TL-3621, and 0.81 for MRSA SP-12249, whereas the R2 values for the fCmax/MIC were 0.01 for VREF TL-3273, 0 for VREF TL-3621, and 0.22 for MRSA SP-12249. For MRSA TL-3677, f%T>MIC was the parameter that best correlated with efficacy, with an R2 value of 0.92, whereas the R2 values for fAUC/MIC and fCmax/MIC were 0.12 and 0, respectively.
FIG. 7.
Relationship between PK-PD parameters (f%T>MIC, fAUC/MIC, and fCmax/MIC) and the efficacy of SMP-601 against VREF TL-3273 (A) and TL-3621 (B) in the neutropenic murine thigh infection model. Each parameter is calculated from the free (unbound) serum concentration of SMP-601. The fCmax/MIC values at each dosing regimen were set to more than three against TL-3273 and more than four against TL-3621. Each symbol represents the mean data from two mice (four thighs). The dashed lines represent the organism burden at the start of chemotherapy. R2 is the coefficient of determination.
FIG. 8.
Relationship between PK-PD parameters (f%T>MIC, fAUC/MIC, and fCmax/MIC) and efficacy of SMP-601 against MRSA SP-12249 (A) and TL-3677 (B) in the neutropenic murine thigh infection model. Each parameter is calculated from the free (unbound) serum concentration of SMP-601. Each symbol represents the mean data from two mice (four thighs). The dashed lines represent the organism burden at the start of chemotherapy. R2 is the coefficient of determination.
The magnitudes of f%T>MIC required to achieve a static effect or 1- and 2-log kill during 24 h are listed in Table 3. The f%T>MIC levels required for efficacy against the two strains of VREF apparently were lower than those against the two strains of MRSA.
TABLE 3.
Magnitudes of f%T>MIC required for static effect, 1-log kill, or 2-log kill in the present neutropenic murine thigh infection model
| Organism | MIC (μg/ml) |
f%T>MIC
|
||
|---|---|---|---|---|
| Static effect | 1-Log kill | 2-Log kill | ||
| VREF TL-3273 | 2 | 9 | 12 | 16 |
| VREF TL-3621 | 2 | 10 | 14 | 19 |
| MRSA SP-12249 | 1 | 23 | 30 | 37 |
| MRSA TL-3677 | 0.12 | 23 | 27 | 32 |
Impact of fCmax on activity of SMP-601.
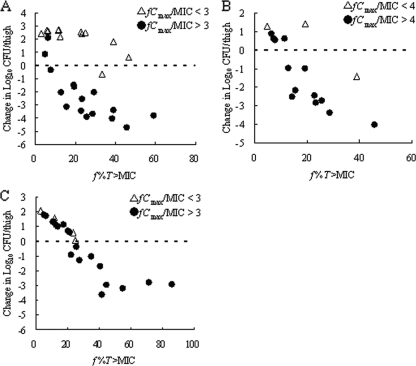
The impact of the fCmax/MIC on the activity of SMP-601 against VREF TL-3273, VREF TL-3621, and MRSA SP-12249 was analyzed using the neutropenic murine thigh infection model (Fig. 9). Under low-Cmax conditions (fCmax/MIC < 3), the f%T>MIC levels required for a static effect against VREF TL-3273 apparently were higher than those under high-Cmax conditions (fCmax/MIC > 3). Similarly, under low-Cmax conditions (fCmax/MIC < 4), the f%T>MIC levels required for a static effect against VREF TL-3621 apparently were higher than those under high-Cmax conditions (fCmax/MIC > 4). In contrast, the f%T>MIC levels required for a static effect against MRSA SP-12249 were invariable irrespective of the fCmax/MIC.
FIG. 9.
Relationship between f%T>MIC and the efficacy of SMP-601 against VREF TL-3273 (A), VREF TL-3621 (B), and MRSA SP-12249 (C) in the neutropenic murine thigh infection model under low-Cmax conditions (fCmax/MIC < 3 or 4) and high-Cmax conditions (fCmax/MIC > 3 or 4). Each symbol represents the mean data from two mice (four thighs). The dashed lines represent the organism burden at the start of chemotherapy.
DISCUSSION
The increasing resistance of gram-positive bacteria to many classes of antibacterial agents is a cause for great clinical concern. SMP-601 is a novel parenteral carbapenem that has potent activity against multidrug-resistant gram-positive pathogens, including VREF and MRSA. The current studies demonstrated the in vivo pharmacodynamic activity of SMP-601 against VREF and MRSA in a neutropenic murine thigh infection model.
It was reported previously that lower doses of daptomycin might be applicable for the treatment of infections caused by VREF in the murine thigh infection model; however, the 24-h growth of VREF in the untreated murine thigh was negligible and the anti-VREF activity of daptomycin might have been overestimated in the thigh infection model, so additional studies with a model that showed better growth in the murine thigh were needed (17). Therefore, a new VREF thigh infection model was established with sufficient bacterial growth by adding 1% (wt/vol) carrageenan, which itself did not affect the anti-VREF activity of SMP-601, to the bacterial inoculum suspension. The reason why sufficient bacterial growth was observed in the new murine thigh model is not clear, but the induction of acute inflammation by 1% (wt/vol) carrageenan might enhance bacterial growth in the muscle tissue. On the other hand, it was reported that the depletion of macrophages could be achieved by intraperitoneal treatment with carrageenan (18, 22, 24), and that the depletion of macrophages enhanced the susceptibility of mice to Streptococcus pyogenes infection (10). Despite the differences in the injection route of carrageenan and bacterial species, the possibility that the depletion of macrophages by treatment with carrageenan contributed to the growth of VREF in the murine thigh was considered in this study.
The induction of inflammation at the site of infection by 1% (wt/vol) carrageenan might affect the distribution of SMP-601 to the site of infection. However, it is difficult to determine the SMP-601 concentration at the strict site of infection, namely extracellular fluid; therefore, the impact of 1% (wt/vol) carrageenan on the activity of relatively low doses of SMP-601 (f%T>MIC = 3.1 to 10.7) against MRSA SP-12249 in the murine thigh infection model was examined. Carrageenan at 1% (wt/vol) did not affect the in vivo activity of SMP-601 against MRSA, so it was suggested that the inflammation caused by 1% (wt/vol) carrageenan did not affect the distribution of SMP-601 to the extracellular fluid, which was the major infection site of MRSA and VREF. Additionally, the impact of 1% (wt/vol) carrageenan on the pharmacokinetics of SMP-601 in infected mice was examined, and 1% (wt/vol) carrageenan did not affect them. β-Lactams, including carbapenems, mainly distribute to the extracellular fluid. The concentration of β-lactams in the extracellular fluid generally is equivalent to the blood concentration. Therefore, the increase in blood flow through muscle tissue caused by inflammation has no effect on the antibiotic exposure against these extracellular pathogens.
Like other β-lactams, including carbapenems (14, 20), it was predicted that f%T>MIC would be the PK-PD parameter most closely correlated with the efficacy of SMP-601, but this study demonstrated that both f%T>MIC and fAUC/MIC correlated with the in vivo activity of SMP-601 against VREF TL-3273, VREF TL-3621, and MRSA SP-12249. The efficacy of ME-1036, a novel parenteral carbapenem, also correlated with both f%T>MIC and fAUC/MIC against MRSA strains (12). However, in this study this might be because the interrelationships between these two PK-PD parameters (f%T>MIC and fAUC/MIC) were relatively high (f%T>MIC versus fAUC/MIC had an R2 of 0.63 in the VREF TL-3273 model, an R2 of 0.72 in the VREF TL-3621 model, and an R2 of 0.74 in the MRSA SP-12249 model). In fact, only f%T>MIC correlated with the in vivo activity of SMP-601 against MRSA TL-3677, because the interrelationships between f%T>MIC and fAUC/MIC were low, with an R2 of 0.19. Conversely, the correlation of the fCmax/MIC and efficacy resulted in a very low R2 value; therefore, increasing concentrations did not increase the antimicrobial effect, as is the case for other β-lactams.
It was reported previously that β-lactams displayed some concentration-dependent activity (6, 21). Under low-Cmax conditions (fCmax/MIC < 3 to 4), the f%T>MIC levels required for a static effect against VREF apparently were higher than those under high-Cmax conditions (fCmax/MIC > 3 to 4). In contrast, the f%T>MIC levels required for a static effect against MRSA were invariable irrespective of the fCmax/MIC. Therefore, it was indicated that SMP-601 showed time-dependent in vivo efficacy against VREF under more than one specific Cmax condition. This finding is very important for the design of dosing regimens of SMP-601 against VREF infections.
Previous studies of carbapenems have identified that an f%T>MIC of 20 to 30% was required to achieve a bacteriostatic response, and an f%T>MIC of 40 to 50% was required to achieve a bactericidal response (8, 13). The results of this study against MRSA were consistent with these values; however, f%T>MIC levels required for efficacy against VREF apparently were lower than those against MRSA. The reason for this finding may be related to differences in the virulence in mammalian tissues between VREF and MRSA, not the usage of 1% (wt/vol) carrageenan in this model.
The murine serum concentration time profile, murine serum protein binding, and f%T>MIC targets against S. aureus shown in this study are considerably different from the data presented by Craig and Andes (1). First, their murine serum concentrations of SMP-601 were lower. The discrepancy between these data apparently are due to the difference in cilastatin usage. Namely, Craig and Andes conducted the pharmacokinetic study of SMP-601 without the coadministration of cilastatin. Second, the present results of murine serum protein binding were 75 to 78%, whereas their protein binding was less than 5%, and the reason for this discrepancy is unexplained. The reproducibility of the present results was confirmed; however, this discrepancy is controversial. Finally, the f%T>MIC targets required for efficacy against S. aureus are different between these two studies. However, in their study, f%T>MIC targets differed between five strains (static effect, 2 to 13%; 1-log kill, 6 to 25%; and 2-log kill, 16 to 25%), so the distinction between the two sets of data are thought to be due to differences in the strains. Alternatively, the distinction may be due to differences in protein binding as described above.
Although a limitation of this study was the use of just two VREF and two MRSA isolates, the better-than-expected low value of the required f%T>MIC for in vivo efficacy against VREF suggested a potent therapeutic effect of SMP-601 against VREF infections, and these pharmacodynamic targets would be beneficial in the design of dosing regimens for clinical trials with this compound.
Acknowledgments
We thank Luigi Xerri (Protez Pharmaceuticals, Malvem, PA) and Neil S. Ryder (Novartis Institutes for BioMedical Research, Inc., Cambridge, MA) for their helpful commenting on the manuscript. We also are very grateful to Koichi Fujimoto, Takayuki Uda, Kokichi Urasaki, Yasuko Hirai, and Tatsuo Nakayama for excellent technical assistance.
Footnotes
Published ahead of print on 1 June 2009.
REFERENCES
- 1.Andes, D., and W. A. Craig. 2006. In vivo pharmacodynamic activity of a new carbapenem, PZ601, against multiple bacteria in murine thigh and lung infection models, abstr. F1-232, p. 199. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., American Society for Microbiology, Washington, DC.
- 2.Chambers, H. F. 2005. Community-associated MRSA resistance and virulence converge. N. Engl. J. Med. 352:1485-1487. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 1999. Methods for determining bactericidal activity of antimicrobial agents. Approved standard M26-A. CLSI, Wayne, PA.
- 4.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7. CLSI, Wayne, PA.
- 5.Craig, W. A. 1993. Relevance of animal models for clinical treatment. Eur. J. Clin. Microbiol. Infect. Dis. 12(Suppl. 1):S55-S57. [DOI] [PubMed] [Google Scholar]
- 6.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 7.Dandekar, P. K., P. R. Tessier, P. Williams, C. H. Nightingale, and D. P. Nicolau. 2003. Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. J. Antimicrob. Chemother. 52:405-411. [DOI] [PubMed] [Google Scholar]
- 8.Drusano, G. L. 2003. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin. Infect. Dis. 36:S42-S50. [DOI] [PubMed] [Google Scholar]
- 9.Eguchi, K., and K. Kanazawa. 2007. In vivo pharmacodynamic activity of SMP-601 (PZ-601) in a new VRE thigh infection model using carrageenan-treated mice, abstr. F1-349. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 10.Goldmann, O., M. Rohde, G. S. Chhatwal, and E. Medina. 2004. Role of macrophages in host resistance to group A streptococci. Infect. Immun. 72:2956-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones, R. N. 2001. Resistance patterns among nosocomial pathogens: trends over the past few years. Chest 119:397S-404S. [DOI] [PubMed] [Google Scholar]
- 12.Kijima, K., Y. Takayama, J. Nagura, Y. Tanaka, M. Kurazono, K. Yamada, T. Sugano, H. Takata, E. Shitara, and M. Yonezawa. 2004. ME1036 (CP5609), a novel parenteral carbapenem: pharmacokinetics and in vivo pharmacodynamic activities against methicillin-resistant Staphylococcus aureus in a murine thigh infection model, abstr. F-332, p. 197. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 13.Kuti, J. L., P. K. Dandekar, C. H. Nightingale, and D. P. Nicolau. 2003. Use of Monte Carlo simulation to design an optimized pharmacodynamic dosing strategy for meropenem. J. Clin. Pharmacol. 43:1116-1123. [DOI] [PubMed] [Google Scholar]
- 14.Maglio, D., M. A. Banevicius, C. Sutherland, C. Babalola, C. H. Nightingale, and D. P. Nicolau. 2005. Pharmacodynamic profile of ertapenem against Klebsiella pneumoniae and Escherichia coli in a murine thigh model. Antimicrob. Agents Chemother. 49:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moellering, R. C., Jr. 2006. The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann. Intern. Med. 144:368-370. [DOI] [PubMed] [Google Scholar]
- 16.Rice, L. B. 2006. Antimicrobial resistance in gram-positive bacteria. Am. J. Infect. Control 34:S11-S73. [DOI] [PubMed] [Google Scholar]
- 17.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosroseno, W., and E. Herminajeng. 2002. The role of macrophages in the induction of murine immune response to Actinobacillus actinomycetemcomitans. J. Med. Microbiol. 51:581-588. [DOI] [PubMed] [Google Scholar]
- 19.Sunagawa, M., M. Itoh, K. Kubota, A. Sasaki, Y. Ueda, P. Angehrn, A. Bourson, E. Goetschi, P. Hebeisen, and R. L. Then. 2002. New anti-MRSA and anti-VRE carbapenems; synthesis and structure-activity relationships of 1β-methyl-2-(thiazol-2-ylthio)carbapenems. J. Antibiot. (Tokyo) 55:722-757. [DOI] [PubMed] [Google Scholar]
- 20.Takata, T., K. Aizawa, A. Shimizu, S. Sakakibara, H. Watabe, and K. Totsuka. 2004. Optimization of dose and dose regimen of biapenem based on pharmacokinetic and pharmacodynamic analysis. J. Infect. Chemother. 10:76-85. [DOI] [PubMed] [Google Scholar]
- 21.Turnidge, J. D. 1998. The pharmacodynamics of β-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]
- 22.Udono, H., D. L. Levey, and P. K. Srivastava. 1994. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc. Natl. Acad. Sci. USA 91:3077-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda, Y., K. Kanazawa, K. Eguchi, K. Takemoto, Y. Eriguchi, and M. Sunagawa. 2005. In vitro and in vivo antibacterial activities of SM-216601, a new broad-spectrum parenteral carbapenem. Antimicrob. Agents Chemother. 49:4185-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki, K., T. Nguyen, and E. R. Podack. 1999. Cutting edge: tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J. Immunol. 163:5178-5182. [PubMed] [Google Scholar]