Abstract
Pyrazolopyrimidinediones are a novel series of compounds that inhibit growth of Helicobacter pylori specifically. Using a variety of methods, advanced analogues were shown to suppress the growth of H. pylori through the inhibition of glutamate racemase, an essential enzyme in peptidoglycan biosynthesis. The high degree of selectivity of the series for H. pylori makes these compounds attractive candidates for novel H. pylori-selective therapy.
Helicobacter pylori is a gram-negative pathogen whose colonization of the human gastric mucosa can cause gastritis that can lead to peptic ulceration (23). The organism is also implicated as a causative agent for certain types of gastric cancer (20, 23, 24, 27), and therefore, eradication of the organism is advised for people with ulcer disease (5). Recommended therapies consist of a proton pump inhibitor in combination with broad-spectrum antibacterials, but emerging resistance and poor patient compliance compromise the effectiveness of these treatments (23). Thus, alternative therapies without these issues are needed for continued successful eradication of H. pylori in patients.
The non-life-threatening nature and unique disease manifestation of H. pylori infections allow highly selective therapy directed only against the specific organism. The advantage of such a selective therapy would be to limit adverse effects caused by disturbances in the microbial gut flora, thereby improving patient compliance and reducing selection for resistance in other species.
A target-based research program that integrated genetic, biochemical, biophysical, and structural characterization of targets was undertaken to identify selective targets for therapy directed against H. pylori (17). Glutamate racemase (MurI), an essential enzyme in peptidoglycan biosynthesis (11, 12, 25) (Fig. 1), was identified through these efforts as a potentially selective target for therapy directed against H. pylori. A high-throughput screen was carried out with the enzyme, and pyrazolopyrimidinediones were identified as a class of selective H. pylori MurI inhibitors that also showed whole-cell activity (17). This study describes the microbiological characterization of this class of inhibitors.
FIG. 1.
Cytoplasmic steps of the peptidoglycan biosynthetic pathway. Amino acids are sequentially added to UDP-N-acetyl muramic acid (UDP-Mur) by MurC to -F to form the UDP-MurNac-pentapeptide, the final cytoplasmic peptidoglycan precursor (25). MurI provides d-glutamate. Inhibition of MurI results in the inhibition of peptidoglycan biosynthesis at the d-glutamate addition stage (MurD). As a result, inhibition of MurI will result in the depletion of MurD to -F products and accumulation of UDP-N-acetyl muramic acid-alanine (MurD substrate). Dap, diaminopimelic acid.
(These studies were presented in part at the 2005 Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC [10a].)
MATERIALS AND METHODS
Bacterial strains and susceptibility testing.
The bacterial strains, plasmids, and primers used in this study are listed in Table 1. Susceptibilities for H. pylori, Campylobacter coli, and Campylobacter jejuni were determined as described previously (16). MICs for anaerobic species were determined according to CLSI broth microdilution guidelines for Bacteroides fragilis (9). MICs for all other species were determined according to CLSI guidelines (10). Compounds were dissolved in dimethyl sulfoxide, and the final concentration of this solvent in all MIC assays was 2%, a concentration that was used as control.
TABLE 1.
Strains and vectors used in this study
| Strain, vector (clone), or primer | Relevant genotype, phenotype, or sequence | Source or reference |
|---|---|---|
| Strains | ||
| H. pylori | ||
| ARHp80 (AH244) | Wild type | 1 |
| Hp80.1 | FlaA KO | This study |
| Hp80.2 | FlaA KO; overexpressing Hp MurI | This study |
| J99 | Wild type | 2 |
| E. coli | ||
| TOP10 | F−mcrA Δ(mrr-hsdRMS mcrBC) φ80lacZΔM15 endA1 recA1 | 14; Invitrogen |
| Streptococcus pneumoniae | D39; NCTC7466 | This study |
| Enterococcus faecium | ATCC 19434 | ATCC |
| Haemophilus influenzae | ATCC 51907 | ATCC |
| Moraxella catarrhalis | ATCC 43617 | ATCC |
| B. fragilis | ATCC 25285 | ATCC |
| Clostridium difficile | Clinical isolate | This study |
| Lactobacillus jensenii | Clinical isolate | This study |
| Fusobacterium mortiferum | Clinical isolate | This study |
| Bacteroides thetaiotaomicron | Clinical isolate | This study |
| Fusobacterium necrophorum | Clinical isolate | This study |
| Prevotella melaninogenicus | Clinical isolate | This study |
| C. coli | ATCC 33559 | ATCC |
| C. jejuni | ATCC 33560 | ATCC |
| Vectors (clones) | ||
| pUC19 | Cloning vector; Apr | 29 |
| pRY109 | pUC18 containing cat cassette; Apr Cmr | 30 |
| PGEM-T | TA cloning vector; Apr | Promega |
| pET23b/J99murI | pET23b containing H. pylori strain J99 murI as an 810-bp NdeI-SalI fragment for overexpression | 17 |
| pSM103 | 468-bp J99 3′ flaA in pGEM-T | This study |
| pSM104 | 607-bp J99 5′ flaA in pGEM-T | This study |
| pSM105 | 607-bp AatII-NdeI (from pSM104) 5′ flaA fragment in pUC19 | This study |
| pSM107 | 468-bp PstI-SphI (from pSM103) 3′ flaA fragment in pSM105 | This study |
| pSM109 | 854-bp PstI-PstI (from pRY109) cat gene in pSM107 | This study |
| pSM116 | 810-bp NdeI-SalI (from pETb/J99 murI) murI-containing fragment in pSM109 | This study |
| Primers | This study | |
| FlaA-1 (AatII) | TACTGACGTCATTTAGGGGGCGGATGTGCAGTCCG | This study |
| FlaA-2 (NdeI) | AAGCCATATGTGTAACTCCTTGTTATAAAAAACCCAAAGG | This study |
| FlaA-3 (PstI) | GCGCCTGCAGGCAGCTATGCGATGAGTCAAGCC | This study |
| FlaA-4 (SphI) | ATAAAGCATGCTAGGGCGGACACACTCTGCAAGC | This study |
Frequency of spontaneous resistance development.
Spontaneous frequencies of resistance were determined in triplicate with H. pylori strains SS1 and ARHp80 by plating >109 cells (collected from blood agar plates) on blood agar plates containing serial twofold dilutions of compound D (Fig. 2) at 1, 2, 4, and 8× MIC. The plates were incubated for 7 days under microaerophilic conditions (5% O2, 10% CO2, and 85% N2) at 37°C. The frequency of resistance was expressed as the average number of mutants able to grow on compound-containing plates divided by the total cell inoculum. Colonies from different agar plates were randomly selected, and MICs were measured to confirm decreased susceptibility against compound D.
FIG. 2.
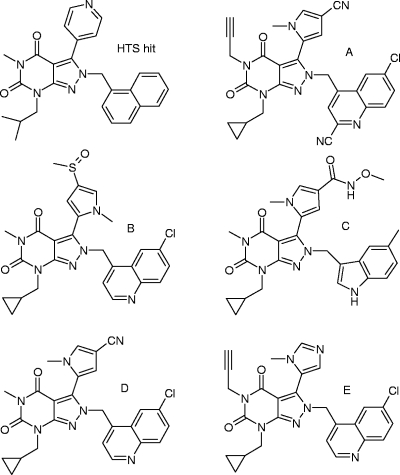
Pyrazolopyrimidinediones described in this study.
Peptidoglycan precursor analysis.
Peptidoglycan precursors were extracted and analyzed according to published procedures (13). H. pylori J99 was grown at 37°C in brucella broth (Difco) supplemented with 5% fetal calf serum (Biowhittaker) under microaerophilic conditions (5% O2, 10% CO2, and 85% N2). Exponentially grown cells were exposed to the compound at 2× MIC for about 3 h, which equals about one generation of uninhibited growth. The cells were rapidly cooled in an ice bath and harvested by centrifugation. The pellet was extracted with 5% cold trichloroacetic acid. After removal of the trichloroacetic acid through ether extraction, the supernatant was concentrated by lyophilization and salts were removed by size exclusion chromatography (Superdex peptide HR10/30 column; Pharmacia). UDP containing fractions were pooled and concentrated by lyophilization. The concentrates were analyzed using reversed-phase high-performance liquid chromatography on a 3-μm C18 ODS Hypersil column (Keystone Scientific). Chromatography was performed with 250 mM ammonium formate (pH 4.0) at 30°C for the first 25 min with a 0.5-ml/min flow rate and at 60°C with a flow rate of 1 ml/min for the remainder of the time. Peaks were identified based on the retention times of known standards, and the identity was confirmed by tandem mass spectrometry (28).
General DNA manipulations.
Standard molecular biology protocols were used for PCR, DNA cloning, agarose gel electrophoresis, and sequencing (21). The relevant oligodeoxynucleotide primers used in this study are listed in Table 1. Restriction endonucleases were purchased from New England Biolabs (Beverley, MA). The Rapid DNA Ligation Kit (Roche Diagnostics Corp., Indianapolis, IN) was used for DNA cloning. High Fidelity PCR Supermix (Invitrogen) was used to amplify DNA fragments for cloning and sequence analysis. All PCR-generated clones and selected PCR-generated DNA fragments were sequenced (both strands) using an ABI Prism 3100 Genetic Analyzer after preparing ABI Prism BigDye Terminator Cycle Sequencing v.2.0 Ready Reactions (PE Biosystems, Foster City, CA). The resulting DNA sequence chromatographs were assembled and analyzed using Sequencher software v.4.0.5 (Gene Codes Corporation, Ann Arbor, MI). The oligodeoxynucleotide primers used for PCR and sequencing were synthesized by Invitrogen (Table 1). DNA was extracted from agarose gels with the QIAEX II kit (Qiagen Inc., Valencia, CA).
Plasmid constructions.
A suicide protein expression vector was constructed to facilitate efficient site-specific integration of cloned DNA into the H. pylori chromosome, followed by overexpression. The flaA locus (jhp0548), encoding the flagellin A subunit, was used as the target site for integration into the H. pylori chromosome so that the flaA promoter would drive protein expression for selected genes, such as murI. This pUC19-based vector contains the ColE1 origin of replication and therefore does not replicate outside of the Enterobacteriaceae. The final vector was assembled as described below using cloning vectors, intermediate plasmids, oligonucleotide primers, and DNA templates listed in Table 1 and described below. Primers flaA1 and flaA2 (containing engineered AatII and NdeI sites, respectively) were used to amplify a 586-bp DNA fragment of the J99 chromosome corresponding to the flaA promoter region. This fragment was cloned into pGEM-T to make pSM104. Primers flaA3 and flaA4 (containing engineered PstI and SphI sites, respectively) were used to amplify a 438-bp DNA fragment corresponding to the 3′ region downstream of the flaA gene (3′ flaA). This fragment was cloned into pGEM-T to make pSM103. The 5′ flaA DNA fragment was excised from pSM104 (AatII-NdeI fragment) and cloned into pUC19 to make pSM105. The 3′ flaA DNA fragment was excised from pSM103 (PstI-SphI fragment) and cloned into pSM105 to make pSM107. Next, an 830-bp DNA fragment containing the chloramphenicol acetyltransferase (CAT) gene was excised from pRY109 using PstI and ligated into pSM107 to make pSM109. The J99 murI gene (jhp0496) was excised from pET23b/murI as an 810-bp NdeI-SalI DNA fragment and ligated into pSM109 to make pSM116.
Transformation of H. pylori.
H. pylori cells were transformed with DNA as previously described (26). Briefly, H. pylori was grown to an optical density at 600 nm (OD600) of 0.5 to 0.8. The cells were then concentrated by centrifugation and suspended in brucella broth to a concentration of 100 OD600 units, 20 μl of which (∼108 cells) was spotted onto blood agar plates (Remel) and incubated for 2 h at 37°C under microaerophilic conditions. Next, approximately 1 μg DNA was applied directly onto the spotted H. pylori cells (sterile water was used as a negative control) and incubated overnight. The spotted cells were suspended in 1 ml of brucella broth and spread directly onto blood agar plates containing 10 μg/ml chloramphenicol for the selection of transformants. Colonies generally appeared within 3 to 5 days. Transformants were characterized by PCR analysis and Western blot analysis.
Western blot analysis.
Exponentially growing H. pylori cells were concentrated and suspended in sodium dodecyl sulfate protein-loading buffer to a final concentration of 1 OD600 unit/100 μl and boiled for 5 min. Ten microliters of each sample was then run on Novex precast 4 to 20% Tris-glycine polyacrylamide gradient gels (Invitrogen; no. EC60252BOX), and the proteins were transferred onto a nitrocellulose membrane (Novex; no. LC2000) under the conditions described by the manufacturer (XCell SureLock Mini-Cell; Invitrogen). H. pylori FlaA-specific mouse monoclonal antibody 220a was obtained from T. J. Trust and used as described previously (15). Rabbit polyclonal anti-MurI antiserum was produced and characterized using standard protocols following the immunization of rabbits with purified H. pylori MurI (15, 17). Secondary alkaline phosphate (AP) conjugates (goat anti-mouse immunoglobulin G-AP and goat anti-rabbit immunoglobulin G-AP) were used according to the manufacturer's instructions (Bio-Rad; no. 170-6520 and 170-6518, respectively). The blots were developed using a premixed color development solution (Bio-Rad; no. 170-6432).
Morphology alterations.
Cells were grown at 37°C in brucella broth (Difco) supplemented with 5% fetal calf serum (Biowhittaker) under microaerophilic conditions (5% O2, 10% CO2, and 85% N2). Changes in morphology upon compound exposure were observed through phase-contrast microscopy using ×100 magnification.
RESULTS AND DISCUSSION
Activities of pyrazolopyrimidinediones.
Pyrazolopyrimidinediones were discovered through high-throughput screening (HTS) with H. pylori MurI (17). The original HTS hit (Fig. 2) showed selective growth inhibition of H. pylori (Table 2), and this selectivity was retained throughout the program, as shown for representative analogues A to E. The MIC90 of compound D against an additional 24 H. pylori clinical isolates that originated from different geographic regions and patients with various disease states and that exhibited different resistance profiles was 2 μg/ml. Compound D was also further profiled against a broader range of species, including commensals of the gut flora and species closely related to H. pylori, such as C. coli and C. jejuni. No activity was seen against any of these species (Table 2). The selective whole-cell activity of the pyrazolopyrimidinediones is in agreement with the observed selective inhibition of H. pylori MurI by these compounds, which can be explained by the unique biochemical and structural features of the enzyme (17).
TABLE 2.
Activities of pyrazolopyrimidinedionesa
| Compound | MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
|
H. pylori
|
E. coli | S. aureus | Other | ||||
| SS1 | ARHp80 | Hp80.2 | ARHp80 HefC−b | ||||
| HTS hit | 4 | 4 | 8 | 1 | >64 | >64 | >64c |
| A | 2 | 1 | >64 | 0.5 | >64 | >64 | ND |
| B | 16 | 8 | 16 | 0.25 | >64 | >64 | >64c |
| C | 16 | 16 | ND | 0.25 | >64 | >64 | >32c |
| D | 1 | 0.5 | 4 | 0.25 | >64 | >64 | >64d |
| E | 2 | 1 | 8 | 0.25 | >64 | >64 | NDe |
| Tetracycline | 0.25 | 0.13 | 0.13 | 0.015 | ND | ND | ND |
Structures are shown in Fig. 2.
Efflux mutant (16).
Panel included S. pneumoniae, E. faecium, H. influenzae, and M. catarrhalis.
Panel included S. pneumoniae, E. faecium, H. influenzae, and M. catarrhalis plus B. fragilis, C. difficile, L. jensenii, F. mortiferum, B. thetaiotaomicron, F. necrophorum, P. melaninogenicus, C. coli, and C. jejuni.
ND, not determined.
The activities of pyrazolopyrimidinediones against H. pylori could be compromised by efflux. Some compounds (e.g., B and C) showed much lower MICs against a mutant that lacked a component (hefC) (16) of a multidrug efflux pump system (Table 2). However, efflux appeared not to be a trait of the series in general, as other analogues were less severely impacted (e.g., compounds D and E). It is possible that reduced compound permeability and/or increased efflux in other species also plays a role in the high selectivity of the pyrazolopyrimidinediones for H. pylori, together with the reported selective enzymatic inhibition (17).
Spontaneous resistant mutants could be obtained only on plates containing 2× and 4× MIC with frequencies of 10−7 and 10−8, respectively. At 8× MIC, no mutants could be isolated (frequency < 10−9). Decreased susceptibility of isolated mutants to compound D was confirmed, and no cross-resistance to other, unrelated antibiotics was detected. These susceptibility data are consistent with the findings that the mutations causing this phenotype were mapped to murI (17).
Peptidoglycan precursor pool analyses.
Peptidoglycan precursor analyses were carried out to determine if growth suppression of H. pylori by the pyrazolopyrimidinediones was due to the inhibition of MurI. Inhibitors of MurI should inhibit the biosynthesis of peptidoglycan precursors at the stage where d-glutamate is linked to UDP-MurNAc-Ala (Fig. 1). Thus, it can be expected that MurI inhibition leads to a reduction in the UDP-MurNAc-dipeptide, -tripeptide, and -pentapeptide products and an accumulation of UDP-MurNAc-Ala substrate (Fig. 1).
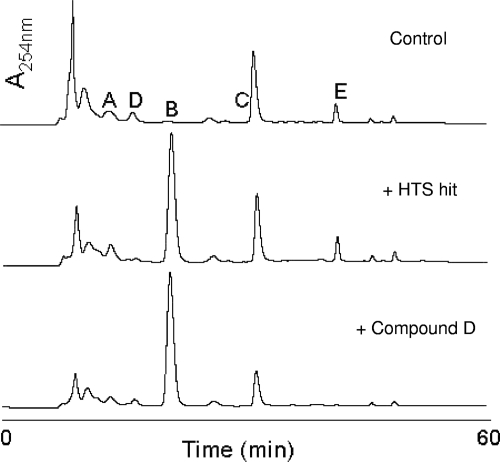
Analysis of the pool of cytoplasmic peptidoglycan precursors of untreated H. pylori showed a significant amount of UDP-MurNAc-pentapeptide (Fig. 3, top), similar to what was observed in Escherichia coli (18). When growth of H. pylori was suppressed by the original HTS hit, an accumulation of UDP-MurNAc-Ala (peak B) was observed (Fig. 3, middle), which was expected for inhibition of MurI within the cell. However UDP-MurNAc-pentapeptide (peak E), the final product of the cytoplasmic peptidoglycan precursor biosynthetic pathway, was still present in substantial amounts (Fig. 3, middle), calling into question the extent of inhibition of MurI and peptidoglycan biosynthesis by this compound. The presence of UDP-MurNAc-Ala-pentapeptide was not seen with a compound that showed improved activity (compound D) (Fig. 3, bottom), indicating complete inhibition of MurI in H. pylori by this compound.
FIG. 3.
High-performance liquid chromatography analysis of peptidoglycan precursors of H. pylori J99 in the absence (top) and presence of pyrazolopyrimidinediones (HTS hit [middle] and compound D [bottom]). Elution times of N-acetyl-muramic acid (A), N-acetyl-muramic acid-alanine (B), N-acetyl-muramic acid-alanine-glutamate (C), N-acetyl-muramic acid-alanine-glutamate diaminopimelic acid (D), and N-acetyl-muramic acid-alanine-glutamate diaminopimelic acid-alanine-alanine (E) peptidoglycan precursors (Fig. 1) are indicated.
Susceptibility of a MurI overexpression strain.
The peptidoglycan precursor results were confirmed in a mode-of-action study that measured MICs for a MurI-overexpressing strain. Recombinant strains Hp80.1 and Hp80.2 were constructed in the wild-type background of strain ARHp80 (Table 1) and confirmed by PCR and sequence analysis (data not shown). Hp80.1 is a flaA mutant, constructed using pSM109, in which the FlaA open reading frame is replaced by the CAT gene. Hp80.2 is a flaA mutant, constructed using pSM116, in which the FlaA open reading frame is displaced by H. pylori murI and the CAT gene. The overexpression of MurI in Hp80.2 was evaluated relative to ARHp80 and Hp80.1 by Western blot analysis (Fig. 4). ARHp80 produced a detectable band corresponding to FlaA (a high-abundance protein), but not to MurI (a low-abundance protein). The flaA knockout strains, Hp80.1 and Hp80.2, did not produce detectable FlaA, while the MurI overexpression strain, Hp80.2, produced a significant protein band corresponding to MurI (Fig. 4).
FIG. 4.
Overexpression of H. pylori MurI. An additional copy of H. pylori MurI was inserted in the genome of H. pylori behind the fla promoter (replacing the nonessential flaA). Overexpression of MurI and subsequent loss of FlaA expression were monitored by Western blotting using antibodies raised against H. pylori MurI (left blot) and FlaA (right blot). ARHp80 represents the wild-type strain, Hp80.1 represents ARHp80 transformed with the empty flaA allelic replacement construct pSM109, and Hp80.2 represents ARHp80 transformed with the murI expression construct pSM116.
The rationale for using a strain that overexpresses MurI is that it requires additional compound to inhibit the additional copies of the MurI target in the cell, thus resulting in an elevated MIC compared to the isogenic parental strain. The usefulness of such strains has been demonstrated for several targets (8, 19, 31). MIC determinations with the MurI-overexpressing strain HP80.2 indeed showed an elevated MIC for compound D, conclusively showing that growth suppression by this compound was due to the inhibition of MurI (Table 2). In contrast, the original HTS hit did not show elevated MICs for the MurI-overexpressing strain (Table 2), showing that although this compound inhibits MurI in the cell (Fig. 3, middle), growth suppression must be mediated via a different target. Identification of the off-target activity of the HTS hit was not pursued, as more optimized compounds all showed the desired mode of action.
These results illustrate the usefulness of different mode-of-action assays in the different stages of drug discovery. In the earlier stages, when weaker compounds are profiled, less definitive assays, such as the peptidoglycan precursor analysis assay, are very useful, as they can demonstrate that compounds enter the cell and interfere with the intended target (4), even though growth suppression may still occur through a different mechanism. As such, they provide confidence that growth suppression through the intended target can be achieved within the series upon compound optimization. This important information will be missed if only more definitive mode-of-action assays, such as the MurI-overexpressing strains, are used, with the risk that valuable compounds would be discarded outright as promiscuous growth inhibitors. However, as compounds are optimized, the more definitive higher-throughput assays gain value.
H. pylori morphology changes upon pyrazolopyrimidinedione exposure.
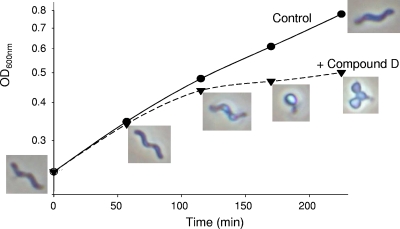
Inhibition of MurI leads to disturbance of peptidoglycan biosynthesis, and since peptidoglycan is a determinant of the cell shape (7), alterations in cell morphology are expected for compounds that suppress growth through MurI inhibition. When H. pylori was exposed to compound D, changes in its morphology were seen (Fig. 5). The cells lost their characteristic spiral shape and turned into transparent cocci. Such alterations are also seen when H. pylori enters stationary phase (22), except the change here was rapid upon compound exposure. The rapid response was typical of the pyrazolopyrimidinediones and was not seen when cell growth was inhibited with tetracycline for the same time (data not shown). When cells were exposed to amoxicillin, a β-lactam that inhibits peptidoglycan biosynthesis, similar rapid morphology changes were noted (6). Thus, the morphological changes seen with the pyrazolopyrimidinediones are in agreement with inhibition of peptidoglycan biosynthesis through MurI.
FIG. 5.
Morphology alterations of H. pylori following exposure at 2× MIC to pyrazolopyrimidinedione D starting at zero minutes.
In conclusion.
Pyrazolopyrimidinediones are newly discovered agents that suppress H. pylori growth through inhibition of MurI. The high selectivity of these compounds for H. pylori combined with their low propensity for resistance development make these analogues attractive candidates for therapy aimed specifically at H. pylori. Oral bioavailability is another important feature of therapy specifically directed against H. pylori, and improvements in that area could be achieved through prodrug approaches (3). However, the most potent compounds showed low solubility and high protein binding, and in order for this series to progress to compounds of clinical utility, improvements in these areas will be needed.
Acknowledgments
We thank Barbara Arsenault, Linda Otterson, and Jeanette Jones for susceptibility testing; Gejing Deng for mass spectrometric analysis of the cytoplasmic peptidoglycan precursors; and members of the MurI team for their support.
Footnotes
Published ahead of print on 11 May 2009.
REFERENCES
- 1.Alm, R. A., J. Bina, B. M. Andrews, P. Doig, R. E. W. Hancock, and T. J. Trust. 2000. Comparative genomics of Helicobacter pylori: analysis of the outer membrane protein families. Infect. Immun. 68:4155-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. de Jonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 3.Basarab, G. S., P. J. Hill, A. Rastagar, and P. J. H. Webborn. 2008. Design of Helicobacter pylori glutamate racemase inhibitors as selective antibacterial agents: a novel pro-drug approach to increase exposure. Bioorg. Med. Chem. Lett. 18:4716-4722. [DOI] [PubMed] [Google Scholar]
- 4.Baum, E. Z., S. M. Crespo-Carbone, A. Klinger, B. D. Foleno, I. Turchi, M. Macielag, and K. Bush. 2007. A MurF inhibitor that disrupts cell wall biosynthesis in Escherichia coli. Antimicrob. Agents Chemother. 51:4420-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazzoli, F. 2001. Key points from the revised Maastricht Consensus Report: the impact on general practice. Eur. J. Gastroenterol. Hepatol 13(Suppl. 2):S3-S7. [PubMed] [Google Scholar]
- 6.Berry, V., K. Jennings, and G. Woodnutt. 1995. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrob. Agents Chemother. 39:1859-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun, V., H. Gnirke, U. Henning, and K. Rehn. 1973. Model of the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J. Bacteriol. 114:1264-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, D. Z., D. V. Patel, C. J. Hackbarth, W. Wang, G. Dreyer, D. C. Young, P. S. Margolis, C. Wu, Z.-J. Ni, J. Trias, R. J. White, and Z. Yuan. 2000. Actinonin, a naturally occurring antibacterial agent, is a potent deformylase inhibitor. Biochemistry 39:1256-1262. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2001. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard. 5th ed. CLSI document M11-A5. CLSI, Wayne, PA.
- 10.Clinical and Laboratory Standards Institute. 2003. Performance standards for antimicrobial susceptibility testing; 13th informational supplement. CLSI document M100-S13. CLSI, Wayne, PA.
- 10a.de Jonge, B. L. M., A. Kutschke, M. Uria-Nickelsen, H. D. Kamp, and S. D. Mills. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1817. [DOI] [PMC free article] [PubMed]
- 11.Doublet, P., J. van Heijenoort, J.-P. Bohin, and D. Mengin-Lecreulx. 1993. The murI gene of Escherichia coli is an essential gene that encodes a glutamase racemase activity. J. Bacteriol. 175:2970-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher, S. L. 2008. Glutamate racemase as a target for drug discovery. Microb. Biotechnol. 1:345-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flouret, B., D. Mengin-Lecreulx, and J. van Heijenoort. 1981. Reverse-phase high-pressure liquid chromatography of uridine diphosphate N-acetylmuramyl peptide precursors of bacterial cell wall peptidoglycan. Anal. Biochem. 114:59-63. [DOI] [PubMed] [Google Scholar]
- 14.Grant, S. G., J. Jessee, F. R. Bloom, and D. Hanahan. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 87:4645-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kostrzynska, M., J. D. Betts, J. W. Austin, and T. J. Trust. 1991. Identification, characterization, and spatial localization of two flagellin species in Helicobacter pylori flagella. J. Bacteriol. 173:937-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutschke, A., and B. L. M. de Jonge. 2005. Compound efflux in Helicobacter pylori. Antimicrob. Agents Chemother. 49:3009-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundqvist, T., S. Fisher, G. Kern, R. H. A. Folmer, Y. Xue, D. T. Newton, T. A. Keating, R. A. Alm, and B. L. M. de Jonge. 2007. Exploitation of structural and regulatory diversity in glutamate racemases. Nature 447:817-822. [DOI] [PubMed] [Google Scholar]
- 18.Mengin-Lecreulx, D., B. Flouret, and J. van Heijenoort. 1982. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J. Bacteriol. 151:1109-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne, D. J., W. H. Miller, V. Berry, J. Brosky, W. J. Burgess, E. Chen, W. E. DeWolf, Jr., A. P. Fosberry, R. Greenwood, M. S. Head, D. A. Heerding, C. A. Janson, D. D. Jaworski, P. M. Keller, P. J. Manley, T. D. Moore, K. A. Newlander, S. Pearson, B. J. Polizzi, X. Qiu, S. F. Rittenhouse, C. Slater-Radosti, K. L. Salyers, M. A. Seefeld, M. G. Smyth, D. T. Takata, I. N. Uzinskas, K. Vaidya, N. G. Wallis, S. B. Winram, C. C. K. Yuan, and W. F. Huffman. 2002. Discovery of a novel and potent class of FabI-directed antibacterial agents. Antimicrob. Agents Chemother. 46:3118-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peek, R. M., and M. J. Blaser. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2:28-37. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis. (ed.). 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 22.Sorberg, M., M. Nilsson, H. Hanberger, and L. E. Nilsoson. 1996., Morphological conversion of Helicobacter pylori from bacillary to coccoid form. Eur. J. Clin. Microbiol. Infect. Dis. 15:216-219. [DOI] [PubMed] [Google Scholar]
- 23.Suerbaum, S., and P. Michetti. 2002. Medical progress: Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 24.Uemura, N., S. Okamoto, S. Yamamoto, N. Matsumura, S. Yamaguchi, M. Yamakido, K. Taniyama, N. Sasaki, and R. J. Schlemper. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784-789. [DOI] [PubMed] [Google Scholar]
- 25.van Heijenoort, J. 2001. Recent advances in the formation of the bacterial peptidoglycan monomer unit. Nat. Prod. Rep. 18:503-519. [DOI] [PubMed] [Google Scholar]
- 26.Wang, Y., K. P. Roos, and D. E. Taylor. 1993. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J. Gen. Microbiol. 139:2485-2493. [DOI] [PubMed] [Google Scholar]
- 27.Wong B. C-Y., S. K. Lam, W. M. Wong, J. S. Chen, T. T. Zheng, R. E. Feng, K. C. Lai, W. H. C. Hu, S. T. Yuen, S. Y. Leung, D. Y. T. Fong, J. Ho, C. K. Ching, and J. S. Chen. 2004. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291:187-194. [DOI] [PubMed] [Google Scholar]
- 28.Xu, N., Z. H. Huang, B. L. M. de Jonge, and D. Gage. 1997. Structural organization of peptidoglycan muropeptides by matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) and post-source decay analysis. Anal. Biochem. 248:7-14. [DOI] [PubMed] [Google Scholar]
- 29.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 30.Yao, R., R. A. Alm, T. J. Trust, and P. Guerry. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130:127-130. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, L., F. Fan, L. M. Palmer, M. A. Lonetto, C. Petit, L. L. Voelker, A. St. John, B. Bakosky, M. Rosenberg, and D. McDevitt. 2000. Regulated gene expression in Staphylococcus aureus for identifying conditional lethal phenotypes and antibiotic mode of action. Gene 255:297-305. [DOI] [PubMed] [Google Scholar]