Abstract
Ceftobiprole, a broad-spectrum cephalosporin with activity against methicillin (meticillin)-resistant staphylococci, was statistically noninferior to a combination of vancomycin plus ceftazidime in patients with complicated skin and skin structure infections (cSSSI). This analysis used data from this clinical trial to determine the relationship between therapeutic outcome and the percentage of time that the unbound ceftobiprole concentration exceeds the MIC (percent T>MIC). From the trial of ceftobiprole (500 mg every 8 h, 2-h infusion) for cSSSI due to gram-positive and/or gram-negative bacteria, data from 309 patients in the microbiological intent-to-treat analysis set with measured ceftobiprole concentrations and baseline MICs were used to assess the relationship between percent T>MIC and therapeutic outcome. Individual pharmacokinetic (PK) profiles were obtained from a three-compartment population PK model. The relationship between percent T>MIC and a clinical cure was determined. For the clinical trial dosing regimen, individual percent T>MICs were used to calculate fractional target attainment rates (TARs) for ≥30 and ≥50% T>MIC targets at various MICs. There was a statistically significant relationship between achieving a ≥30 or ≥50% T>MIC and a clinical cure (P = 0.003 and P = 0.007, respectively; Pearson's χ2 test). The fractional TAR was greater than 90% at a MIC of ≤4 mg/liter for patients with normal renal function. A relationship between percent T>MIC and a clinical cure with ceftobiprole was demonstrated. A ceftobiprole regimen of 500 mg every 8 h as a 2-h infusion has a high probability of achieving a target of ≥30 or ≥50% T>MIC for patients with cSSSI due to gram-positive and gram-negative pathogens.
Ceftobiprole is a broad-spectrum cephalosporin that binds to penicillin-binding protein 2a, the principal determinant of methicillin (meticillin) resistance in staphylococci, and to penicillin-binding proteins of many clinically important gram-positive and gram-negative pathogens (7, 9). It has been assessed in clinical trials involving patients with complicated skin and skin structure infections (cSSSI), including those caused by methicillin-resistant Staphylococcus aureus (MRSA) (15, 16). In one of these trials, which was conducted with patients with cSSSI due to gram-positive and/or gram-negative pathogens, ceftobiprole at 500 mg every 8 h administered as a 2-h infusion was statistically noninferior to a combination of vancomycin plus ceftazidime (15).
Pharmacokinetic-pharmacodynamic (PK-PD) measures correlate with therapeutic efficacy and are critical in choosing the optimal dosing regimen (1, 6, 8, 12, 17). For ceftobiprole, as for other β-lactams, the percentage of time that the unbound drug concentration exceeds the MIC (percent T>MIC) during the dosing interval best correlates with efficacy (1, 5). Combining this PK-PD measure with PK modeling allows the effects of variations in pathogen susceptibility and differences between hosts on the efficacy of ceftobiprole to be investigated. Mouton et al. (13) and Lodise et al. (11) used time-concentration data in Monte Carlo simulations to assess the probability of ceftobiprole succeeding as therapy. As these two analyses included the pharmacokinetics of ceftobiprole as defined in phase I studies and in a phase II study with subjects with cSSSI, they are estimates of what a dosing regimen might accomplish.
The objectives of the present analyses were to determine the relationship between the therapeutic outcome with ceftobiprole and percent T>MIC by using data from the phase III trial, which comprises a representative population of patients with cSSSI, and to investigate the probability of achieving a target percent T>MIC with the ceftobiprole regimen evaluated in this clinical study.
(This work was presented in part at the 16th Population Approach Group in Europe Meeting [H. Kimko, S. Xu, B. Murthy, M. Samtani, P. Nandy, R. Strauss, and G. Noel, 16th PAGE, abstr. 1222, 2007] and at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy [H. Kimko, B. Murthy, R. Strauss, S. Xu, P. Bagchi, P. Nandy, K. Bush, and G. J. Noel, abstr. A-590, Abstr. 47th ICAAC, 2007].)
MATERIALS AND METHODS
Relationship between a PK-PD measure (percent T>MIC) and therapeutic response.
The relationship between percent T>MIC and therapeutic outcome was analyzed for evaluable patients who had both PK and baseline MIC data. The therapeutic efficacy data used in the assessment of their relationship to ceftobiprole percent T>MIC were based on the clinical cure rates observed at a test-of-cure (TOC) visit that took place 7 to 14 days after the end of ceftobiprole therapy (500 mg was administered as a 2-h infusion every 8 h) in a trial with patients with cSSSI, including diabetic foot infection (ClinicalTrials.gov, no. NCT00210899 [http://clinicaltrials.gov/ct2/show/NCT00210899?term=NCT00210899&rank=1]) (15). The clinical cure rates at the TOC visit were similar between the clinically evaluable and intent-to-treat populations in the ceftobiprole and comparator treatment arms.
A three-compartment population PK model with first-order elimination provides a good fit for ceftobiprole plasma concentration-versus-time data. In the model, clearance was a function of creatinine clearance (CLCR) and health status. The volume of distribution (V) in the central compartment was a function of body weight and health status; V in the shallow peripheral compartment was a function of sex and health status; and V in the deep peripheral compartment was a function of sex. Renal function characterized by CLCR was the only factor that had a clinically relevant effect on the pharmacodynamics of ceftobiprole. The PK model and the demographics of the 309 subjects in the microbiological intent-to-treat population were used to generate individuals' full PK profiles every 0.1 h over the entire 8-h dosing interval. More-detailed information on the model-building procedure and its goodness of fit has been published previously (10).
Due to sparse sampling in many patients in this study, Bayesian-predicted PK profiles were used to calculate percent T>MIC. Protein binding of 16% was used to calculate percent T>MIC by using the time course of unbound ceftobiprole concentrations (14). With an individual's baseline pathogen MIC (the highest MIC was used for a patient with more than one pathogen), the PK profiles from the model were used to determine individual percent T>MICs. The individual percent T>MIC was used as a determinant to predict therapeutic outcome.
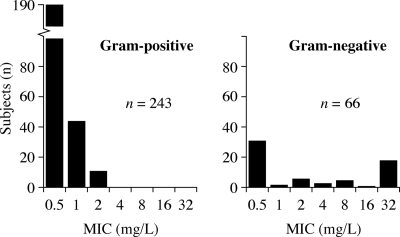
Given their high prevalence and importance as causes of serious skin infections, the analysis considered the following baseline pathogens and their ceftobiprole MICs: Enterobacteriaceae spp. (13.1%), Enterococcus faecalis (4.2%), Pseudomonas aeruginosa (8.0%), Staphylococcus spp.(65.4%), and Streptococcus spp. (9.3%) (Fig. 1). Organism identification had been confirmed by coagulase production and standard biochemical methods (API; bioMérieux, Durham, NC), and susceptibility testing was performed according to Clinical and Laboratory Standards Institute methodology (3, 4).
FIG. 1.
Distributions of ceftobiprole MICs of pathogens from patients with cSSSI.
The percent T>MIC targets assessed were 30 and 50%. These values were chosen on the basis of the results of animal model studies that identified a target of 30% to be appropriate for staphylococci and 50% to be appropriate for clinically important gram-negative bacteria (5). Pearson's χ2 test (P < 0.05) tested the independence of the percent T>MIC targets and therapeutic responses (i.e., clinical cure/failure at the TOC visit). In addition, the percent T>MIC cutoff point that divided patients into groups with lower and higher probabilities of a successful therapeutic outcome were estimated by using classification and regression tree (CART) analysis (2). For the CART analysis, the pathogen types of gram-positive and gram-negative bacteria could not be analyzed separately due to the limited range of percent T>MIC and the high clinical success rate of the patients with gram-positive infections. Therefore, the goal of the analysis was to understand any apparent relationship between percent T>MIC and clinical outcome.
The relationship between percent T>MIC as a continuous variable and the probability of a clinical cure was also investigated by logistic regression with a generalized linear model using S-PLUS 6.2 software (Insightful, Seattle, WA). In addition, the predictive abilities of two other PK-PD measures (i.e., peak concentration/MIC and area under the curve/MIC ratios) were assessed by using the same model.
Fractional TAR calculation.
Fractional target attainment rate (TAR) analysis was performed by using the 30 and 50% T>MIC targets. The three-compartment PK model was used to randomly generate 5,000 individual concentration-time profiles by using the body weight (median = 80 kg, first quarter = 70 kg, third quarter = 95 kg), sex (61% males), and CLCR value (median = 112 ml/min, first quarter = 86 ml/min, third quarter = 143 ml/min) distributions of the study population.
A series of Monte Carlo simulations using S-PLUS 6.2 software was performed to simulate plasma drug concentrations for the dosing regimen used in the clinical study (500 mg every 8 h, 2-h infusion) for simulated subjects with CLCR of ≥80 ml/min. The percent T>MIC was calculated for each simulated plasma concentration-time profile for MICs of 0.5, 1, 2, 4, 8, 16, and 32 mg/liter. The fractions of the simulated subjects who achieved the ≥30 and ≥50% T>MIC targets were then calculated for each MIC, which are equivalent to the probabilities of achieving the targets.
Overall TARs for the dosing regimen were also calculated. The TAR at each MIC was multiplied by the proportion of the population of isolates of the particular species with that MIC, and then the results for each MIC were added to yield the overall probability of achieving the target for each pathogen group. The proportion of pathogens with a particular MIC was based on the naturally occurring frequency in the study (Fig. 1).
RESULTS
Relationship between percent T>MIC thresholds and clinical cure.
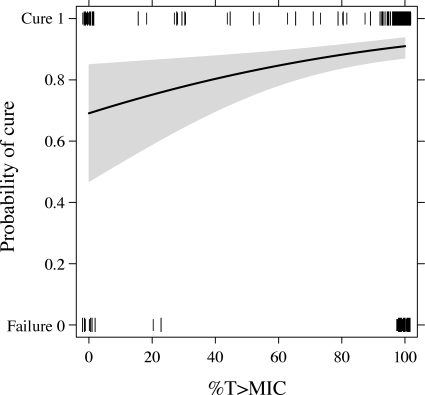
Achievement of ≥30 and ≥50% T≥MICs was significantly associated with the outcome of a clinical cure (P = 0.003 and 0.007, respectively; Table 1). For example, among subjects with a percent T>MIC of ≥50%, the clinical cure rate was 91% (259 of 285 subjects), whereas for those with a percent T>MIC below 50%, the clinical cure rate was 71% (17 of 24 subjects). The CART analysis showed that a 25% T>MIC divided patients into groups with lower and higher probabilities of a successful therapeutic outcome. The probability of therapeutic success increased with a higher percent T>MIC (P = 0.008; Fig. 2). The other two PK-PD measures tested, the peak concentration/MIC and area under the curve/MIC ratios, did not predict the likelihood of a clinical cure with ceftobiprole (P = 0.588 and P = 0.539, respectively).
TABLE 1.
Relationship between therapeutic outcome and percent T>MIC
| % T>MIC | No. (%) of clinical:
|
P valuea | |
|---|---|---|---|
| Cures | Failures | ||
| ≥30 | 261 (91) | 26 (9) | 0.003 |
| <30 | 15 (68) | 7 (32) | |
| ≥50 | 259 (91) | 26 (9) | 0.007 |
| <50 | 17 (71) | 7 (29) | |
Pearson's χ2 test.
FIG. 2.
Probability of therapeutic success by percent T>MIC (the shaded area indicates the 95% confidence interval) in patients receiving ceftobiprole at 500 mg every 8 h administered as a 2-h infusion. The tick marks aligned with the cure and failure categories represent the subjects' percent T>MICs and clinical outcomes.
TARs.
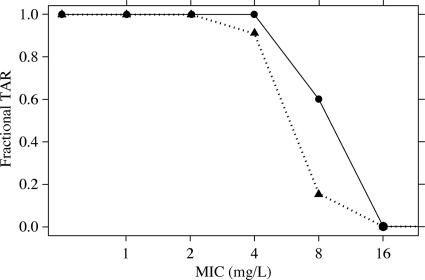
At an MIC of 4 mg/liter, fractional target attainment of a ≥30% T>MIC target was achieved in approximately 100% of the subjects with CLCR of ≥80 ml/min who were treated with ceftobiprole at 500 mg every 8 h administered as a 2-h infusion (Fig. 3). A rate of approximately 90% was apparent for the ≥50% T>MIC target. As would be expected with an increase in the MIC, the percent T>MIC decreased, resulting in a lower proportion of the simulated subjects achieving a target of 30 or 50% T>MIC.
FIG. 3.
Fractional TARs for 30 and 50% T>MICs by MIC in patients with cSSSI receiving ceftobiprole at 500 mg every 8 h administered as a 2-h infusion. Circles represent a 30% T>MIC; triangles represent a 50% T>MIC.
The overall TAR was also calculated, which reflects the actual distributions of each pathogen's MIC. The overall TARs for 30 and 50% T>MICs among subjects receiving ceftobiprole at 500 mg every 8 h administered as a 2-h infusion were 94.6 and 93.7%, respectively.
DISCUSSION
In the modeling work reported here, the relationship between the achievement of targets of 30 and 50% T>MICs and clinical cure in a phase III trial of ceftobiprole administered at 500 mg every 8 h as a 2-h infusion (15) aligned with that which was modeled before initiating this clinical trial (5). Using percent T>MICs as a continuous variable in a logistic regression model demonstrated that percent T>MIC was a predictor of clinical cure and confirms a preclinical study of ceftobiprole (5) and earlier modeling studies (11, 13).
The CART analysis showed that a 25% T>MIC separates subjects who have higher probabilities of a positive or negative therapeutic outcome and is comparable to a 30% T>MIC target for gram-positive pathogens. Initially, the relationship between percent T>MIC and clinical outcome was analyzed for each pathogen type separately. However, as the clinical cure rates were high (90.1% for gram-positive and 86.4% for gram-negative pathogens), there were too few subjects with negative clinical outcomes to allow meaningful logistic regression in the case of gram-positive infection. In addition, all of the subjects infected with gram-positive pathogens had at least a 65% T>MIC and all of those with clinical failure had a 100% T>MIC. Because of the narrow range of percent T>MIC, it was not meaningful to apply logistic regression to gram-positive infection subjects' data. Therefore, to understand the overall percent T>MIC and clinical outcome relationship, all of the available subjects' data had to be combined.
The PK-PD analysis indicates that ceftobiprole at 500 mg every 8 h as a 2-h infusion provided clinical antimicrobial activity at the higher end of the exposure-response relationship in subjects with normal renal function. For a target of 50% T>MIC, this regimen resulted in TARs of at least 90% for pathogens with an MIC of <4 mg/liter. In the murine thigh model, bacterial stasis is achieved when free-ceftobiprole concentrations exceed the MIC for 21% of the dosing interval for S. aureus and 41% of the dosing interval for Enterobacteriaceae (5). Likewise, the 2-log kill dose is achieved at free-drug concentrations exceeding the MIC for 29 and 65% of the dosing interval for these gram-positive and -negative bacteria, respectively (5). Thus, the regimen achieved targets that are predicted to be associated with efficacy for a broad spectrum of pathogens.
Using time-concentration data from 12 volunteers in a multiple-ascending-dose study, Mouton et al. (13) concluded that ceftobiprole at 750 mg every 12 h would have a high probability of therapeutic efficacy for gram-positive infections, including MRSA. Lodise et al. (11) completed additional simulations of the PD profile of ceftobiprole at 500 mg every 8 h as a 2-h infusion. The probabilities of achieving 40 and 60% T>MICs with this regimen exceeded 90% for MICs of ≤4 and ≤2 mg/liter, respectively. Based on the susceptibility distribution of methicillin-sensitive S. aureus and MRSA, the probability of achieving a 50% T>MIC exceeded 90%. For patients with non-AmpC-producing gram-negative pathogens, the probability of achieving a nearly maximal bactericidal effect with the regimen (i.e., a 60% T>MIC) was greater than 90%. The analysis by Lodise et al. was based on data from 150 subjects, among whom were 39 healthy volunteers and 22 intravenous drug users (who typically have higher drug clearances than otherwise healthy subjects). The PK model used in the present analysis was updated by including patients from eight phase I studies and from one phase II study and two phase III studies with subjects with cSSSI. Its use in this analysis further supports the conclusion that this dosage regimen would have a high probability of achieving the identified PD targets in patients with a broad range of pathogens.
The population used in our modeling represented a “typical” clinical trial population. As the distribution of pathogen species can differ according to infection type, the overall TAR was determined by correcting the pathogen-specific TAR with the natural frequency of occurrence of each species. The overall TAR was >90% in patients who had normal renal function (CLCR, ≥80 ml/min), which is consistent with the clinical cure rate reported for ceftobiprole in the cSSSI clinical study (15).
The probability of a cure in our analysis was approximately 70% when the subjects had ceftobiprole exposure that was less than the target 30% T>MIC (Fig. 2). A similar phenomenon has been reported elsewhere; there was a 50% cure rate with levofloxacin in patients with skin and skin structure infections when the exposure was less than its target PK-PD measure of the peak concentration/MIC ratio (17). The clinical cure in the subjects with less than 30% T>MIC may partly reflect a contribution of natural resolution of infection. Although patients are likely to have had measurable ceftobiprole concentrations that were lower than the target MIC, these sub-MICs may still have had some effect on the clinical course of the infection. Moreover, the clinical cure rates may also reflect prior surgical intervention and antibiotic therapy.
Ceftobiprole is primarily excreted by the kidneys, and exposure is higher in subjects with moderate and severe renal impairment (14). The modeling included data from patients with a spectrum of renal function, although the majority of patients (75%) had normal renal function (CLCR of ≥80 ml/min). Thus, experience with patients with impaired renal function is relatively limited and this should be considered when interpreting the findings from the model. The PK-PD calculations in the present study did not take into account drug accumulation. An assumption of no accumulation underestimates the exposure to ceftobiprole and the percent T>MIC and TARs. However, as it is desirable that the PD target be achieved after the first dose, assuming minimal accumulation should ensure that the target percent T>MIC is achieved with the first dosing event.
In summary, by using data from a phase III clinical trial, a strong relationship between percent T>MIC and therapeutic success with ceftobiprole has been demonstrated. There was a statistically significant relationship between the achievement of a ≥30 or ≥50% T>MIC and a clinical cure. The ceftobiprole regimen of 500 mg every 8 h as a 2-h infusion has a high probability of achieving the identified PD targets for patients with a broad range of gram-positive and gram-negative pathogens.
Acknowledgments
We thank Karen Bush for her review of the manuscript. We also thank Bindu Murthy for her project management. Medical writing support was provided by Chris Langford, PAREXEL MMS.
The analyses and studies described in this report were funded by Johnson & Johnson Pharmaceutical Research & Development.
Footnotes
Published ahead of print on 15 June 2009.
REFERENCES
- 1.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 2.Breiman, L., J. Friedman, R. Olshen, and C. Stone. 1998. Classification and regression trees. CRC Press LLC, Boca Raton, FL.
- 3.Clinical and Laboratory Standards Institute. 2005. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A6. Clinical and Laboratory Standards Institute, Wayne PA.
- 4.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial disk susceptibility tests, 15th ed. M2-A8. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Craig, W. A., and D. R. Andes. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob. Agents Chemother. 52:3492-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drusano, G. L., S. L. Preston, C. Fowler, M. Corrado, B. Weisinger, and J. Kahn. 2004. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J. Infect. Dis. 189:1590-1597. [DOI] [PubMed] [Google Scholar]
- 7.Entenza, J. M., P. Hohl, I. Heinze-Krauss, M. P. Glauser, and P. Moreillon. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 46:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, M. E. 2007. In-vitro profile of a new beta-lactam, ceftobiprole, with activity against methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 13(Suppl. 2):17-24. [DOI] [PubMed] [Google Scholar]
- 10.Kimko, H. H., B. Murthy, X. S. Xu, P. Nandy, R. Strauss, and G. J. Noel. 2009. Population pharmacokinetic analysis of ceftobiprole for the treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 53:1228-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lodise, T. P., Jr., R. Pypstra, J. B. Kahn, B. P. Murthy, H. C. Kimko, K. Bush, G. J. Noel, and G. L. Drusano. 2007. Probability of target attainment for ceftobiprole as derived from a population pharmacokinetic analysis of 150 subjects. Antimicrob. Agents Chemother. 51:2378-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodise, T. P., N. Patel, A. Renaud-Mutart, E. Gorodecky, T. R. Fritsche, and R. N. Jones. 2008. Pharmacokinetic and pharmacodynamic profile of ceftobiprole. Diagn. Microbiol. Infect. Dis. 61:96-102. [DOI] [PubMed] [Google Scholar]
- 13.Mouton, J. W., A. Schmitt-Hoffmann, S. Shapiro, N. Nashed, and N. C. Punt. 2004. Use of Monte Carlo simulations to select therapeutic doses and provisional breakpoints of BAL9141. Antimicrob. Agents Chemother. 48:1713-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy, B., and A. Schmitt-Hoffmann. 2008. Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity. Clin. Pharmacokinet. 47:21-33. [DOI] [PubMed] [Google Scholar]
- 15.Noel, G. J., K. Bush, P. Bagchi, J. Ianus, and R. S. Strauss. 2008. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin. Infect. Dis. 46:647-655. [DOI] [PubMed] [Google Scholar]
- 16.Noel, G. J., R. S. Strauss, K. Amsler, M. Heep, R. Pypstra, and J. S. Solomkin. 2008. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob. Agents Chemother. 52:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]