Abstract
Granulocyte-macrophage colony-stimulating factor enhanced the efficacy of liposomal amphotericin B (LAMB) in a murine model of disseminated infection by Rhizopus oryzae, significantly prolonging survival and reducing tissue burden. The use of gamma interferon (IFN-γ) alone was ineffective, and IFN-γ combined with LAMB did not improve the results obtained with LAMB alone.
Zygomycosis is a frequently lethal invasive infection (2). The standard therapy for zygomycosis has not yet been resolved (2). Historically, conventional amphotericin B (AMB) was the drug of choice for invasive zygomycosis, but its use is limited by its potential toxicity. The lipid formulation of AMB (LAMB) allows higher doses to be administered on account of its low toxicity and represents first-line therapy (17). In murine zygomycosis, LAMB has showed efficacy and has been even better than AMB deoxycholate (11, 12). The efficacy of posaconazole is controversial, as some clinical data show good results (9, 20) but some authors have demonstrated that this drug is poorly active against Rhizopus oryzae, the most common species causing zygomycosis (13, 16). Since the mortality rate is often high in disseminated zygomycosis, despite aggressive therapy, new strategies for the treatment of this infection are urgently needed (5).
Cytokines are critical components of the functional host defenses promoting activation and recruitment of granulocyte and mononuclear phagocyte effector cells (18). Over the last decade, the usefulness of these compounds as adjunctive agents in antifungal therapy in the treatment of severe fungal infections has been evaluated (2, 10). In particular, gamma interferon (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF) have showed efficacy as adjunctive agents in the treatment of experimental cryptococcosis and histoplasmosis in mice (3, 4) and also in several clinical cases of zygomycosis (1, 5, 8, 14). Moreover, these cytokines have induced an increase in in vitro polymorphonuclear leukocyte-induced hyphal damage to R. oryzae (6).
In this study, we have evaluated the effects of IFN-γ and GM-CSF, alone and combined with LAMB, in a murine model of infection by R. oryzae.
Two clinical isolates of Rhizopus oryzae, FMR 6485 and FMR 8542, were used in this study. The isolates were cultured on potato dextrose agar at 35°C. The AMB MICs were identical for both strains (0.5 μg/ml) (16).
Male OF1 mice were used in this study. All animal care procedures were supervised and approved by the Universitat Rovira i Virgili Animal Welfare Committee. Animals were immunodepressed 1 day prior to infection by administering a single dose of 200 mg of cyclophosphamide per kg of body weight intraperitoneally plus a single dose of 150 mg of 5-fluorouracil per kg intravenously (i.v.). Mice were challenged with 0.2 × 105 CFU/animal. Ten mice were used for survival studies and 10 for tissue burden studies, with the latter group being identified before the study started. All mice received ceftazidime (5 mg/day subcutaneously [s.c.]) from day 1 to day 7 after infection.
LAMB was administered i.v. at doses of 5 or 10 mg/kg of body weight/dose once daily. Human recombinant IFN-γ (GenScript Corporation) was administered i.v. at a dose of 105 U once daily (3); human recombinant GM-CSF (GenScript Corporation) was administered s.c. at a dose of 5 μg/kg/day once daily (19). The different groups were treated as follows: LAMB at 5 or 10 mg/kg of body weight i.v., IFN-γ at 105 U i.v., GM-CSF at 5 μg/kg/day s.c., LAMB at 10 mg/kg i.v. plus IFN-γ at 105 U i.v., and LAMB at 10 mg/kg i.v. plus GM-CSF at 5 μg/kg/day s.c.
All treatments began 24 h after challenge, and the therapies lasted for 7 days (16). Survival of mice was evaluated daily for 30 days. For tissue burden studies, mice were sacrificed on day 4 postinfection. Kidneys and brains were removed aseptically and were homogenized in 1 ml of sterile saline; care was taken to minimize tissue trauma. Serial 10-fold dilutions of the homogenates were plated on potato dextrose agar and incubated for 18 to 24 h at 35°C. Mean survival times were estimated by the Kaplan-Meier method and compared among groups by using the log rank test. Colony counts in tissue burden studies were analyzed by the Kruskal-Wallis test. When the results of this test were significant, we used the Mann-Whitney U test to compare treatment pairs. The Bonferroni correction method was used to avoid an increase in type I errors due to multiple comparisons. When P was <0.05, the observed differences were declared to be statistically significant.
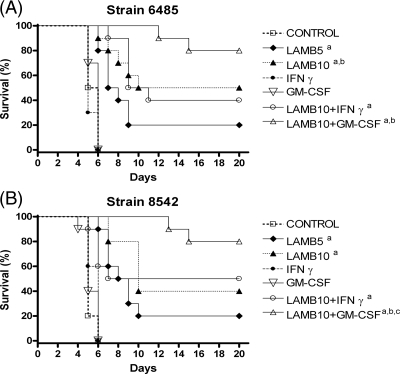
For both strains, the two doses of LAMB and the combination of LAMB with IFN-γ or GM-CSF significantly prolonged survival with respect to the rates for the control and the groups treated only with IFN-γ or GM-CSF (Fig. 1). LAMB combined with GM-CSF was able to prolong survival with respect to the rate for the group treated with LAMB at 10 mg/kg for strain FMR 8542. The combination of LAMB with IFN-γ showed efficacy similar to that of monotherapy with LAMB at 10 mg/kg, with no differences between them. Neither of the two cytokines prolonged survival.
FIG. 1.
Cumulative mortality of mice infected with Rhizopus oryzae FMR 6485 (A) or R. oryzae FMR 8542 (B) and treated with LAMB, GM-CSF, and IFN-γ. a, P values of <0.05 for comparison with the control, IFN-γ (100,000 U), and GM-CSF (5 μg/kg); b, P values of <0.05 for comparison with LAMB (5 mg/kg); c, P values of <0.05 for comparison with LAMB (10 mg/kg).
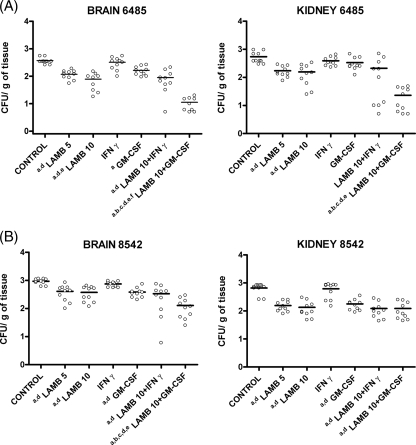
All the treatments, with the exception of IFN-γ, significantly reduced the fungal load in brain and kidney in comparison to that for the control group for both strains. In addition, GM-CSF and IFN-γ plus LAMB failed to reduce tissue burden in kidney for strain FMR 6485 (Fig. 2). The combination of LAMB at 10 mg/kg with GM-CSF was the most effective treatment, reducing the fungal load in brain and kidney tissues, although only in brain tissues for strain FMR 8542.
FIG. 2.
Effects of antifungal treatment on colony counts of Rhizopus oryzae strains FMR 6485 (A) and FMR 8542 (B) in brain and kidney tissues of mice. a, P values of <0.002 for comparison with the control; b, P values of <0.002 for comparison with LAMB (5 mg/kg); c, P values of <0.002 for comparison with LAMB (10 mg/kg); d, P values of <0.002 for comparison with IFN-γ (100,000 U); e, P values of <0.002 for comparison with GM-CSF (5 μg/kg); f, P values of <0.002 for comparison with LAMB (10 mg/kg) plus IFN-γ. CFU/g of tissue are expressed as log10 scale. Horizontal lines indicate mean values.
AMB showed efficacy similar to that in a previous study (16), which proved the reproducibility of this murine model.
LAMB in general shows efficacy in zygomycosis treatment, but in many cases, the patients die. In a recent review of 120 cases of zygomycosis in patients with hematological malignances, LAMB was associated with a 67% survival rate, compared to a 39% survival rate for AMB deoxycholate (7). In severe zygomycosis, correction of metabolic disturbances and reversal of immunosuppression are as essential as the other therapeutic measures for successful management (2). For this reason, we have assessed the efficacy of cytokines as adjuvant agents for returning the host immune response and tried to restore the host's defenses. In our model, only the combination of LAMB with GM-CSF was significantly better than the treatment with LAMB alone. These results agree with those observed in a few clinical cases of zygomycosis in which GM-CSF was administered successfully as an adjunctive therapy with AMB or its lipid formulation (5, 8, 14).
These studies suggest a potential use for GM-CSF as an immunomodulator for improving the benefits of therapy with LAMB against zygomycosis.
Acknowledgments
This work was supported by a grant from Fondo de Investigaciones Sanitarias from the Ministerio de Sanidad y Consumo of Spain (PI 050031).
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Abzug, M. J., and T. J. Walsh. 2004. Interferon-gamma and colony-stimulating factors as adjuvant therapy for refractory fungal infections in children. Pediatr. Infect. Dis. J. 23:769-773. [DOI] [PubMed] [Google Scholar]
- 2.Chayakulkeeree, M., M. A. Ghannoum, and J. R. Perfect. 2006. Zygomycosis: the re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 25:215-229. [DOI] [PubMed] [Google Scholar]
- 3.Clemons, K. V., J. E. Lutz, and D. A. Stevens. 2001. Efficacy of recombinant gamma interferon for treatment of systemic cryptococcosis in SCID mice. Antimicrob. Agents Chemother. 45:686-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deepe, G. S., Jr., and R. Gibbons. 2000. Recombinant murine granulocyte-macrophage colony-stimulating factor modulates the course of pulmonary histoplasmosis in immunocompetent and immunodeficient mice. Antimicrob. Agents Chemother. 44:3328-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Diaz, J. B., L. Palau, and G. A. Pankey. 2001. Resolution of rhinocerebral zygomycosis associated with adjuvant administration of granulocyte-macrophage colony-stimulating factor. Clin. Infect. Dis. 32:166-170. [DOI] [PubMed] [Google Scholar]
- 6.Gil-Lamaignere, C., M. Simitsopoulou, E. Roilides, A. Maloukou, R. M. Winn, and T. J. Walsh. 2005. Interferon-γ and granulocyte-macrophage colony-stimulating factor augment the activity of polymorphonuclear leukocytes against medically important zygomycetes. J. Infect. Dis. 191:1180-1187. [DOI] [PubMed] [Google Scholar]
- 7.Gleissner, B., A. Schilling, I. Anagnostopolous, I. Siehl, and E. Thiel. 2004. Improved outcome of zygomycosis in patients with hematological diseases? Leuk. Lymphoma 45:1351-1360. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez, C. E., D. R. Couriel, and T. J. Walsh. 1997. Disseminated zygomycosis in a neutropenic patient: succesful treatment with amphotericin B lipid complex and granulocyte colony-stimulating factor. Clin. Infect. Dis. 24:192-196. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg, R. N., K. Mullane, J. A. van Burik, I. Raad, M. J. Abzug, G. Anstead, R. Herbrecht, A. Langston, K. A. Marr, G. Schiller, M. Schuster, J. R. Wingard, C. E. Gonzalez, S. G. Revankar, G. Corcoran, R. J. Kryscio, and R. Hare. 2006. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 50:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hübel, K., D. C. Dale, and W. C. Liles. 2002. Therapeutic use of cytokines to modulate phagocyte function for the treatment of infectious diseases: current status of granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, macrophage colony-stimulating factor, and interferon-γ. J. Infect. Dis. 185:1490-1501. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim, A. S., V. Avanessian, B. Spellberg, and J. E. Edwards, Jr. 2003. Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob. Agents Chemother. 47:3343-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim, A. S., T. Gebremariam, M. I. Husseiny, D. A. Stevens, Y. Fu, J. E. Edwards, Jr., and B. Spellberg. 2008. Comparison of lipid amphotericin B preparations in treating murine zygomycosis. Antimicrob. Agents Chemother. 52:1573-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim, A. S., T. Gebremariam, J. A. Schwartz, J. E. Edwards, Jr., and B. Spellberg. 2009. Posaconazole mono- or combination therapy for treatment of murine zygomycosis. Antimicrob. Agents Chemother. 53:772-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mastroianni, A. 2004. Paranasal sinus mucormycosis in an immunocompetent host: efficacy and safety of combination therapy with liposomal amphotericin B and adjuvant rHuGM-CSF. Infez. Med. 12:278-283. [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Rodríguez, M. M., C. Serena, M. Mariné, F. J. Pastor, and J. Guarro. 2008. Posaconazole combined with amphotericin B, an effective therapy for a murine disseminated infection caused by Rhizopus oryzae. Antimicrob. Agents Chemother. 52:3786-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers, T. R. 2008. Treatment of zygomycosis: current and new options. J. Antimicrob. Chemother. 61:35-39. [DOI] [PubMed] [Google Scholar]
- 18.Safdar, A. 2007. Difficulties with fungal infections in acute myelogenous leukemia patients: immune enhancement strategies. Oncologist 12:2-6. [DOI] [PubMed] [Google Scholar]
- 19.Simitsopoulou, M., C. Gil-Lamaignere, N. Avramidis, A. Maloukou, S. Lekkas, E. Havlova, L. Kaurounaki, D. Loebenberg, and E. Roilides. 2004. Antifungal activities of posaconazole and granulocyte-macrophage colony-stimulating factor ex vivo and in mice with disseminated infection due to Scedosporium prolificans. Antimicrob. Agents Chemother. 48:3801-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobón, A. M., M. Arango, D. Fernández, and A. Restrepo. 2003. Mucormycosis (zygomycosis) in a heart-kidney transplant recipient: recovery after posaconazole therapy. Clin. Infect. Dis. 36:1488-1491. [DOI] [PubMed] [Google Scholar]