Abstract
Gentamicin (GM) is a widely used antibiotic but shows renal toxicity. We produced a serum against GM (anti-GM) conjugated to bovine serum albumin with N-(gamma-maleimidobutyryloxy)succinimide. The antiserum was monospecific for GM and did not cross-react with the analog streptomycin, tobramycin, kanamycin, or amikacin. The antiserum also detected glutaraldehyde-fixed GM, and this enabled us to develop an immunocytochemical method for detecting the uptake of GM in rat kidney. Twelve hours after a single intravenous administration of GM, immunocytochemistry revealed that GM accumulated in the S1, S2, and S3 segments of the proximal tubules, as well as in the distal tubules and collecting ducts. By 12 h after injection, the drug was detected in cytoplasmic granules of the proximal tubule cells. However, early (1 h) after injection, drug accumulation was detected in the microvilli of these cells. The distal tubules and collecting ducts contained scattered swollen cells, reminiscent of necrotic cells, in which both the nuclei and the cytoplasm reacted strongly with GM. No staining occurred in the kidneys of saline-injected control rats. These results agree with previous studies showing that GM is endocytosed in the proximal tubules and accumulates in lysosomes. Additionally, our results show that GM also accumulates in the distal tubules and collecting ducts. This was achieved by systematically varying the pretreatment conditions—an approach necessary for detecting GM in different subcellular compartments. This approach should be useful for accurately detecting the uptake and toxicity of the antibiotic in different tissues.
Gentamicin (GM) is widely used as a bactericidal agent for the treatment of severe infections with gram-negative bacteria, such as Pseudomonas aeruginosa. Its antibacterial activity is due to an irreversible inhibition of bacterial protein synthesis. However, clinical use of aminoglycosides, such as GM, is limited by their ototoxicity and nephrotoxicity. The mechanisms behind the nephrotoxity have been investigated extensively, but how these drugs induce cellular malfunction and necrosis remains to be elucidated. Accurate localization of GM in cells and tissues can be expected to contribute to this effort. Previously, subcellular fractionation (27), micropuncture techniques (24), and autoradiography using radioactively labeled aminoglycosides (6, 26) have been used to study the mechanisms of nephrotoxity. Additionally, immunogold labeling methods have been used to study the subcellular distribution of aminoglycosides in proximal tubules of the nephron (1, 2, 3, 4, 20). However, these studies have been limited to ultrastructural analysis of proximal tubule cells. Recently, we have successfully developed immunocytochemical (ICC) procedures for detecting the cellular uptake of other water-diffusible small-molecule drugs, such as daunomycin (DM) (14, 15, 22, 23). These procedures make use of glutaraldehyde (GA)-fixed tissues, which undergo a series of pretreatments to unmask sites of accumulation of the drug.
We now report on the development of an ICC method for the uptake of GM in the kidneys of rats injected with the drug. Importantly, we found that systematic variation of the pretreatment conditions was necessary in order to fully detect the uptake of the drug in different cellular compartments. The new information obtained by this method is that GM accumulates not only in the cytoplasmic granules of the S1 and S2 segments but also in the S3 segment of the proximal tubules. Moreover, we also detected accumulation of GM in the nuclei and cytoplasm of cells in the distal tubules and collecting ducts. These results indicate the importance of varying tissue pretreatment conditions when one is studying the distribution of small-molecule drugs, which may be masked in different ways in different subcellular compartments, and they suggest that this approach is useful for detecting sites of uptake and potential toxicity of GM.
MATERIALS AND METHODS
Chemicals.
Gentamicin H2SO4 (GM) and daunomycin HCl (DM) were generously supplied by Shionogi Co. Ltd., Osaka, Japan, and Meiji Seika Co. Ltd., Tokyo, Japan, respectively. GA (25% in water) and Triton X-100 were obtained from Nacalai Tesque (Kyoto, Japan). Sodium borohydride (NaBH4), bovine serum albumin (BSA), human serum albumin (HSA), and protease (type XXIV [bacterial]) were from Sigma-Aldrich. Co. Inc. (St. Louis, MO). N-(Gamma-maleimidobutyryloxy)succinimide (GMBS) was from Dojin Chemical Co. (Kumamoto, Japan).
Preparation of immunogen (GM-GMBS-BSA conjugate).
The immunogen was prepared according to our previous method for anti-DM serum using the heterobifunctional agent GMBS (10, 11, 12, 13). Briefly, 5 mg (8.9 μmol) GM·H2SO4 in 0.1 M phosphate buffer (pH 6.8) (1.0 ml) and 0.8 mg (14.1 μmol) GMBS in 0.5 ml tetrahydrofuran were mixed, constantly stirred, and incubated at room temperature for 90 min, yielding a GMBS-acylated GM solution. Fifteen milligrams of acetylmercaptosuccinyl BSA (approximately 0.1 μmol) (10) was incubated in 200 μl of 0.1 M hydroxylamine, pH 6.8, at room temperature for 20 min to remove the acetyl group. The resulting mercaptosuccinyl BSA was diluted with 3 ml 0.1 M phosphate buffer (pH 7.0), added immediately to GMBS-acylated GM solution, and incubated for 30 min with slow stirring. The conjugate mixture was applied to a 2.5-cm by 45-cm Sephadex G-100 column equilibrated with 20 mM phosphate buffer (pH 7.0) and was eluted with the same buffer. The eluate, monitored at 280 nm, was collected in 3-ml fractions, and the peak fraction was used for immunization.
Antiserum.
The GM-GMBS-BSA conjugate described above was used for the production of anti-GM serum in three white female rabbits by the procedure of Hurn and Landon (18). The rabbits received 1.0 mg of the conjugate, emulsified in complete Freund's adjuvant, simultaneously by intramuscular and subcutaneous injection. This was followed by biweekly booster injections, in the same manner, of 0.5 mg of the conjugate, which were repeated three times. The rabbits were bled from an ear vein 2 weeks after the final injection. Sera were separated by centrifugation, heated at 55°C for 30 min, and stored at −30°C.
Synthesis of GM-GA-HSA conjugate.
The GM-GA-HSA conjugate was prepared according to our recent method using GA as a cross-linker (14). Briefly, HSA (10 mg) in 1.0 ml of 1 M sodium acetate and 1.0 ml of 3 mM GA were mixed and incubated at room temperature for 30 s with stirring; to this mixture was then added GM (1 mg) in 1.0 ml of 1 M sodium acetate. This was followed by incubation for 30 min before 10 mg NaBH4 was added to terminate the reaction. The reaction mixture was further incubated for 30 min and was dialyzed against 10 mM Tris-HCl buffer (pH 7.2) for 6 h at 4°C.
ELISA method.
An enzyme-linked immunosorbent assay (ELISA) was performed similarly to our previous method for monoclonal antibodies against spermine (13). Wells in microtiter plates were coated either with the GM-GMBS-BSA conjugate (10 μg/ml) or with the GM-GA-HSA conjugate (10 μg/ml), which were then incubated overnight at 4°C with dilutions of anti-GM serum, followed by goat anti-rabbit immunoglobulin G (IgG) labeled with horseradish peroxidase (HRP) (diluted 1:2,000) for 1 h at 25°C. The amount of enzyme conjugate bound to each well was measured using o-phenylenediamine as a substrate, and the absorbance at 492 nm was read with an automatic ELISA analyzer (ImmunoMini NJ-2300; Nalge Nunc International Co. Ltd., Tokyo, Japan).
Animals.
Normal adult male Wistar rats (Kyudo Experimental Animals, Kumamoto, Japan), weighing 200 to 250 g, were used in this study. The principles of laboratory animal care and specific national laws were observed. The animals were housed in temperature- and light-controlled rooms (21 ± 1°C; 12 h of light-12 h of darkness) and had free access to standard food and tap water. GM was given in a single intravenous dose (4 or 16 mg/kg of body weight) via the tail vein to 24 rats. For the next perfusion experiments, the rats were divided into four groups of three rats each and were fixed at 1 h, 12 h, 24 h, and 48 h after the intravenous drug administration. Three rats, receiving saline injections, were used for control purposes.
ICC.
Under sodium pentobarbital (60 mg/kg; Abbott Laboratories, North Chicago, IL) anesthesia, the rats were perfused intracardially with phosphate-buffered saline at 50 ml/min for 2 min at room temperature and then with a freshly prepared solution of 2% GA in 10 mM phosphate buffer at pH 7.2 for 6 min. Kidneys were quickly excised and postfixed in the same fixative (2% GA) overnight at 4°C and were subsequently embedded in paraffin in a routine way. The samples were cut into 5-μm-thick sections, treated with 6% H2O2 in TBS (50 mM Tris-HCl buffer, pH 7.4, containing 0.86% NaCl) for 30 min, reduced with 1.0% NaBH4 in TBS for 10 min, acidified with 1 N HCl for 30 min, and digested with 0.04% protease (type XXIV [bacterial]; Sigma-Aldrich, St. Louis, MO) in TBS for different periods (15 min to 2 h) at 30°C. During each process of the treatment, the specimens were washed three times with TBS. Next, the specimens were blocked with a protein solution containing 10% normal goat serum, 1.0% BSA, and 0.1% saponin in TBS for 1 h at room temperature; they were then directly incubated at 4°C overnight with anti-GM serum diluted 1:5,000 in TBS supplemented with 0.1% Triton X-100. The sections were washed three times with TBS supplemented with 0.1% Triton X-100, for 5 min each time, and were then incubated with HRP-labeled goat anti-rabbit IgG (whole IgG; Cappel, West Chester, PA), diluted 1:500, for 2 h at 4°C. After a rinse with TBS, the site of the antigen-antibody reaction was revealed for 10 min with diaminobenzidine and H2O2 (17).
ICC controls included the use of conventional staining controls (second-level controls) as well as antibleomycin serum. Absorption controls used conjugates of GM-GMBS-BSA, GM-GA-BSA, bleomycin-GMBS-BSA, mitomycin C-GMBA-BSA, and GMBA-BSA at concentrations ranging from 2 to 100 μg/ml and the free compounds GM, DM, bleomycin, and kanamycin at concentrations between 2 and 50 μg/ml.
RESULTS
Antibody dilution.
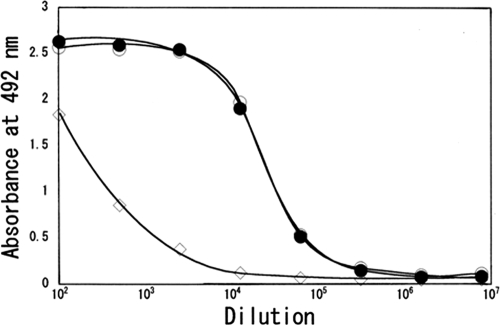
Anti-GM serum produced against GMBS-onjugated GM (GM-GMBS-BSA conjugate) was characterized by an ELISA system employing either GMBS-conjugated GM or GA-conjugated GM (GM-GA-HSA conjugate) as the solid-phase antigen. As shown in Fig. 1, the anti-GM serum bound almost equally well to both conjugates. This was promising, because GA can be used for fixing GM in cells and tissues prior to ICC. ELISA also showed that the antiserum reacted with unconjugated GM. However, it showed only low binding to the conjugating protein (BSA) (Fig. 1).
FIG. 1.
ELISA measurements of the binding of serially diluted anti-GM serum to GM-GA-HSA (filled circles), GM-GMBS-BSA (open circles), or BSA (diamonds).
Antibody specificity by inhibition ELISA.
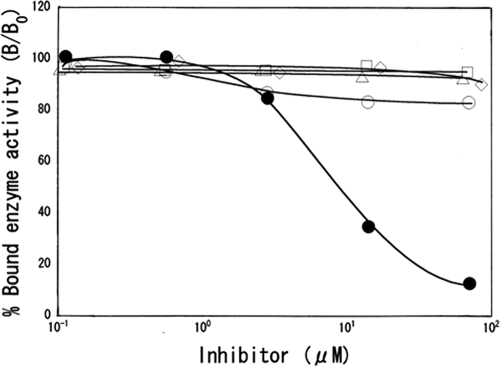
Antibody specificity was tested using an ELISA system with GM-GA-HSA conjugate as an antigen. The principle was competition between GM or analog antibiotics (streptomycin, kanamycin, amikacin, and tobramycin) (free in solution) and a fixed amount of GM applied to ELISA plates. Calibration curves were plotted showing the relationship between the concentrations of the analytes and the percentage of bound anti-GA antibody, giving dose-dependent inhibition curves with GM in the range of 1 μM to 70 μM (Fig. 2). The dose required for 50% inhibition of binding was 7 μM with GM. No inhibition occurred with any of the GM analogs (Fig. 2) or with the unrelated antibiotics peplomycin, mitomycin C, and actinomycin D, even at concentrations as high as 1 mM (data not shown).
FIG. 2.
ELISA measurements showing competition between different GM congeners and GM-GA-HSA for binding to the anti-GM serum. The curves show the ratio of bound enzyme activity (B) for various doses of GM (filled circles), kanamycin (open diamonds), amikacin (open triangles), tobramycin (open circles), or streptomycin (open squares) to the enzyme activity bound using the HRP-labeled secondary antibody alone (B0).
ICC.
We have recently developed an ICC procedure for the anticancer drug DM (14, 15, 22, 23), which requires a series of pretreatments prior to the ICC reaction. The same pretreatment conditions were tested and slightly modified for ICC detection of GM. Animals injected with GM were first perfusion-fixed with GA, followed by immersion fixation overnight at 4°C. Subsequently, sections were oxidized with 6% hydrogen peroxide for 30 min to enhance the intensity of immunostaining (22), reduced with 0.5% NaBH4 for 10 min to prevent nonspecific staining by reducing free aldehyde groups of GA bound in tissues, acidified with 1 N hydrochloric acid for 30 min in order to denature DNA, and finally digested with 0.04% protease at 30°C for 15 min to 2 h in order to facilitate the penetration of antibody. The protease digestion step, particularly, was critical and needed to be systematically varied (see below) in order to detect the uptake of GM in different cells and subcellular compartments. Anti-GM serum was then applied to the sections, and the site of antigen-antibody reaction was revealed with peroxidase-labeled goat anti-rabbit IgG.
GM uptake in rat kidney.
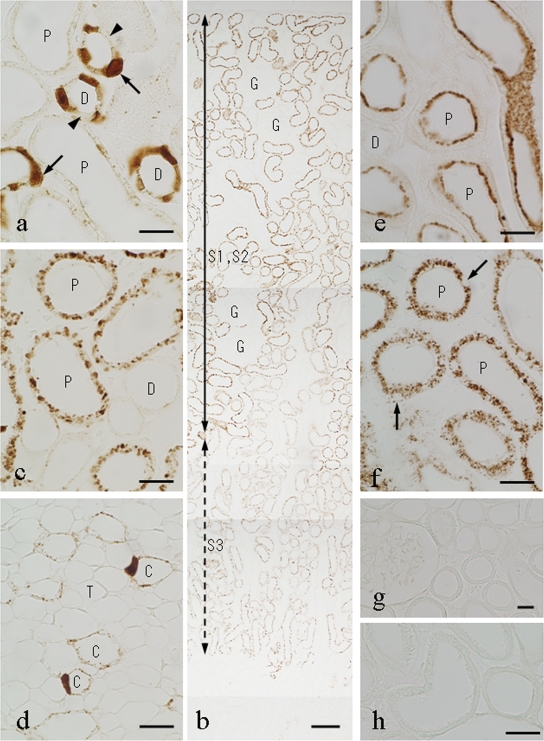
Virtually the same pattern of GM uptake in the kidney was observed at all time points studied for rats injected with either 4 or 16 mg/kg of GM. However, the intensity of staining was higher in rats injected with the higher dose. Kidney sections taken from rats 12 h postinjection that had been digested with 0.04% protease for 15 min at 30°C showed strong immunostaining for GM in the nuclei and cytoplasm of cells of the distal tubules (Fig. 3a), while weak staining occurred in cells of the collecting ducts and in cells of the thick portion of Henle's loop (Fig. 3d). Some of the distal tubule cells and collecting-duct cells were heavily stained for GM, while others were virtually unstained, indicating a very heterogenous uptake of GM (Fig. 3a). In both the distal tubules and the collecting ducts, some of the heavily stained cells were swollen and possessed large nuclei (Fig. 3a). Surprisingly, in contrast to previous results (1, 2, 3, 4, 20), only very weak or no staining was observed in cells of the proximal tubules (Fig. 3a). However, when the protease digestion was prolonged to 2 h, strong immunostaining appeared in the S1 and S2 portions of the proximal tubules (the straight proximal segments located in the cortex), whereas staining of the distal tubules and the collecting ducts was strongly diminished (Fig. 4). Staining of the proximal tubule cells was confined to cytoplasmic granules of irregular shape (Fig. 3b and c). Very weak staining was also present in the S3 portion of the proximal tubules (the medullary straight segment) (Fig. 3b and 4). In addition, the best immunostaining of the collecting-duct cells was obtained by ICC with a 30-min protease digestion of the kidney specimens (Fig. 3d) The glomeruli, thin portions of Henle's loop, and endothelial cells were virtually unstained under all protease digestion conditions tested (Fig. 3b). Similar results were observed for rats 24 and 48 h after GM injection. For control rats, receiving saline instead of GM, no staining was detected (Fig. 3g). Conventional ICC staining controls (second-level controls) were all negative (data not shown), and absorption controls using the GM-GA-BSA conjugate at 10 μg/ml abolished all staining (Fig. 3h).
FIG. 3.
Immunostaining for GM in the kidneys of rats 12 h (a to d and h) or 1 h (e and f) after GM injection or saline injection (g). GM ICC was carried out following digestion of the sections with 0.04% protease at 30°C for 15 min (a and e), 30 min (d), or 2 h (b, c, and f to h). (a) Twelve hours after GM injection (16 mg/kg), the nuclei as well as the cytoplasm of cells of the distal convoluted tubules (D) are immunostained for GM. Note that heavily stained cells are swollen (arrows) and that adjacent cells are virtually unstained (arrowheads). Only very slight immunostaining was observed in the proximal tubules (P). (b) Renal cortex. Immunostaining is strong in the S1 and S2 segments of the proximal tubules and weak in the S3 segment (medullary portions of the straight proximal tubule). Almost no immunoreaction was observed in the glomeruli (G) or the distal tubules. The S3 segment was identifiable by the presence of periodic acid-Schiff-positive brush borders. (c) Higher magnification of the renal cortex. Note strong immunostaining of cytoplasmic granules in proximal tubule cells and virtually no staining in the distal tubules. (d) Swollen cells that are heavily immunopositive for GM are present in the collecting ducts (C). T, thin limb of Henle's loop or blood capillary. (e and f) Strong immunostaining occurs on microvilli and apical regions of the cytoplasm of the proximal tubules 1 h after injection of GM, while there is almost no staining of the distal tubules. (f) Note strong immunostaining of cytoplasmic granules, but not of nuclei (arrows), in cells of the proximal tubules. (g) No immunoreaction is evident in the renal cortex cells of control rats injected with saline. (h) The staining was completely abolished by absorption of the anti-GM serum with GM-GMBS-BSA. Bars, 20 μm (a and c to h) and 100 μm (b).
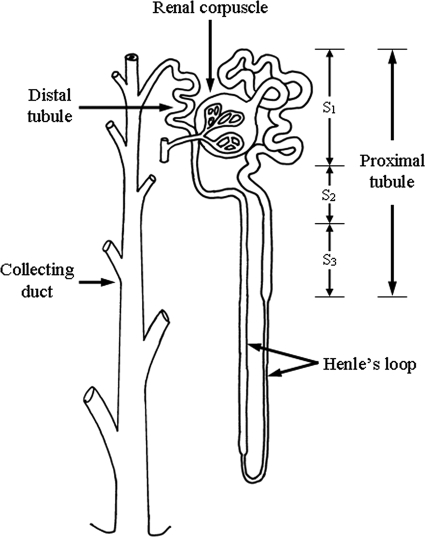
FIG. 4.
Schematic representation of a nephron. Three ultrastructurally distinct segments (S1, S2, and S3) are identifiable in the proximal tubule.
Additionally, we examined rats sacrificed 1 h after GM injection. The relationship between the protease digestion and the sites and intensity of immunoreaction for GM in the kidney was almost the same as that for the specimens taken 12 h after injection (Fig. 3f). However, at 1 h postinjection, the microvilli of the proximal tubule cells were strongly immunostained following a 15- to 60-min protease predigestion (Fig. 3e), but they were unstained when digestion was prolonged to 2 h (Fig. 3f).
DISCUSSION
Using immunoelectron microscopy and commercial antisera, Beauchamp et al. (1, 2, 3, 4) have previously localized sites of uptake of GM, tobramycin, daptomycin, and vancomycin in the rat kidney. These pioneering studies were, however, limited to studies of the proximal tubules. In this study, we have prepared anti-GM serum against GMBS-conjugated GM, characterized its specificity, and developed an ICC procedure useful for the study of whole-kidney sections at the light-microscopic level. Our results extend those of Beauchamp et al. (1, 2, 3, 4) by showing that drug uptake is not limited to the proximal tubules and that different pretreatment conditions are needed in order to detect the uptake of the drug in different cell types and compartments.
Anti-GM serum was produced against a GM-GMBS-BSA conjugate and tested for reactivity to both GMBS-conjugated GM and GA-conjugated GM. The antiserum bound almost equally well to both conjugates, indicating that it recognized the GM molecule rather than the conjugation site(s) of GMBS with GM. The anti-GM serum reacted with GM but not with a number of related and unrelated antibiotics. The fact that it recognized GM conjugated to HSA with GA suggested that it could be useful for ICC studies of GM uptake in GA-fixed tissues. In agreement with our previous experiences with small water-soluble antigens, animals injected with the drug were perfusion-fixed with GA in order to immobilize the antigen in the tissue as rapidly as possible.
Following systematic testing of several pretreatments aimed at demasking GM immunoreactivity in fixed tissues and reducing background staining, we succeeded in specifically localizing GM in the kidneys of rats injected with the drug. Interestingly, the optimal conditions for immunostaining with respect to protease digestion differed in the different segments of the nephron. These differences may reflect differences in the binding and masking of GM by proteins and other macromolecules in different cellular compartments, which may thus constitute different obstacles to the penetration of the antibody into such segments. Accordingly, to reveal the full complement of GM localization, results from different digestion procedures needed to be evaluated.
Our method localized GM uptake to cytoplasmic granules of proximal tubule cells—a result in excellent agreement with those of previous immunogold (1, 2, 3) and autoradiographic (6, 16, 26) studies. From the results of these studies, it appears that GM undergoes partial reabsorption by proximal tubule cells as a consequence of adsorptive endocytosis and then accumulates in lysosomes in the cells, leading to the development of lysosomal phospholipidosis. Accordingly, the irregular granules detected by us in the proximal tubule cells 12 to 48 h postinjection most probably represent lysosomes, while the accumulation of the drug in the brush border of the cells 1 h after injection most likely reflects ongoing endocytotic reabsorption of the drug. Our results also agree with the findings of Wedeen et al. (28), who detected GM accumulation in the S1 and S2 segments of the kidney by combined immunofluorescence and section freeze-dry autoradiography techniques. However, Wedeen et al. (28) did not detect uptake in other parts of the nephron—a fact that may reflect difficulties with the technique. An important difference, however, is that we used a strongly cross-linking fixative, which can be expected to immobilize GM efficiently in situ, while Wedeen et al. (28) used precipitating fixatives (acetone and ethanol), which may lead to dislocation or extraction of low-molecular-weight molecules such as GM. This may also have a bearing on the sensitivity of the method, since we, but not Wedeen et al. (28), could localize the uptake of GM to the S3 segments of the proximal tubules as well.
Furthermore, a new finding of the present study was that swollen, necrotic-like cells of the distal tubules and collecting ducts were heavily immunostained for GM. Previously, autoradiographic studies also demonstrated the transport of aminoglycosides in the distal tubules and collecting ducts as well as in the proximal tubules (6, 16, 19, 26). However, they did not describe such swollen, necrotic-like cells. These results suggest the possibility that GM nephrotoxicity is not restricted to the proximal tubules but extends to the distal tubules and collecting ducts. This agrees with the report by Parsons et al. (24) that acute GM-induced hypercalciuria is mediated by a decrease in calcium reabsorption in the early distal tubule. In the distal convoluted tubules, our ICC study detected striking differences between the abilities of neighboring cells to take up and/or retain GM. This suggests that physiological differences exist among these cells, perhaps in their expression of a surface receptor for GM. In fact, both the connecting segment and the collecting duct are composed of two distinct cell types: the principal cell and the intercalated cell (21). Hypercalciuria is the most frequently reported kidney malfunction following repeated administration of GM and is also seen following acute administration of the drug (5, 7, 8, 9, 24, 25). Previous studies localizing sites of action of GM in the kidney have focused on the proximal tubular and glomerular systems. Our present data reveal that GM also interacts with the distal tubular system—a finding that is consonant with GM-induced hypercalciuria and with the results reported by Parsons et al. (24) using micropuncture. Our data are the first to show the swollen, necrotic-like cells caused by the action of GM in the distal tubules.
In conclusion, our study clearly demonstrates that the anti-GM serum produced against GMBS-conjugated GM was useful for developing a GM ICC method. This method revealed that GM accumulated in the proximal and distal tubules as well as in the collecting ducts, pinpointing all of these sites as potential targets for GM toxicity.
Acknowledgments
We are grateful to K. Ohara, M. Eto, S. Hamada, M. Kiyama, and Y. Fujikubo for technical assistance throughout this study.
This study was supported in part by a grant (15590148) from the Japanese Society for the Promotion of Science.
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Beauchamp, D., P. Gourde, and M. G. Bergeron. 1991. Subcellular distribution of gentamicin in proximal tubular cells, determined by immunogold labeling. Antimicrob. Agents Chemother. 35:2173-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchamp, D., P. Gourde, M. Simard, and M. G. Bergeron. 1992. Subcellular localization of tobramycin and vancomycin given alone and in combination in proximal tubular cells, determined by immunogold labeling. Antimicrob. Agents Chemother. 36:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beauchamp, D., P. Gourde, M. Simard, and M. G. Bergeron. 1994. Subcellular distribution of daptomycin given alone or with tobramycin in renal proximal tubular cells. Antimicrob. Agents Chemother. 38:189-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchamp, D., G. Laurent, L. Grenier, P. Gourde, J. Zanen, J.-A. Heuson-Stiennon, and M. G. Bergeron. 1997. Attenuation of gentamicin-induced nephrotoxicity in rats by fleroxacin. Antimicrob. Agents Chemother. 41:1237-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett, W. M., J. P. Pulliam, G. A. Porter, and D. C. Houghton. 1985. Modification of experimental gentamicin nephrotoxicity by selective parathyroidectomy. Am. J. Physiol. 249:F832-F835. [DOI] [PubMed] [Google Scholar]
- 6.Bergeron, M. G., Y. Marois, C. Kuehn, and F. J. Silverblatt. 1987. Autoradiographic study of tobramycin uptake by proximal and distal tubules of normal and pyelonephritic rats. Antimicrob. Agents Chemother. 31:1359-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chahwala, S. B., and E. S. Harpur. 1983. Gentamicin-induced hypercalciuria in the rat. Acta Pharmacol. Toxicol. 53:358-362. [DOI] [PubMed] [Google Scholar]
- 8.Cronin, R. E., and J. A. Newman. 1985. Protective effect of thyroxine but not parathyroidectomy on gentamicin nephrotoxicity. Am. J. Physiol. 248:F332-F339. [DOI] [PubMed] [Google Scholar]
- 9.Elliott, W. C., D. S. Patchin, and D. B. Jones. 1987. Effect of parathyroid hormone activity on gentamicin nephrotoxicity. J. Lab. Clin. Med. 109:48-54. [PubMed] [Google Scholar]
- 10.Fujiwara, K., M. Yasuno, and T. Kitagawa. 1981. Novel preparation method of immunogen for hydrophobic hapten, enzyme immunoassay for daunomycin and adriamycin. J. Immunol. Methods 45:195-203. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara, K., H. Saikusa, M. Yasuno, and T. Kitagawa. 1982. Enzyme immunoassay for the quantification of mitomycin C using β-galactosidase as a label. Cancer Res. 42:1487-1491. [PubMed] [Google Scholar]
- 12.Fujiwara, K., T. Saita, N. Takenawa, N. Matsumoto, and T. Kitagawa. 1988. Enzyme-linked immunosorbent assay for the quantification of actinomycin D using β-d-galactosidase as a label. Cancer Res. 48:4843-4847. [PubMed] [Google Scholar]
- 13.Fujiwara, K., and Y. Masuyama. 1995. Monoclonal antibody against the glutaraldehyde-conjugated polyamine, spermine. Histochem. Cell Biol. 104:309-316. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara, K., H. Takatsu, and K. Tsukamoto. 2005. Immunocytochemistry for drugs containing an aliphatic primary amino group in the molecule, anticancer antibiotic daunomycin as a model. J. Histochem. Cytochem. 53:467-474. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara, K., M. Shin, D. M. Hougaard, and L.-I. Larsson. 2007. Distribution of anticancer antibiotic daunomycin in the rat heart and kidney revealed by immunocytochemistry using monoclonal antibodies. Histochem. Cell Biol. 127:69-77. [DOI] [PubMed] [Google Scholar]
- 16.Giurgea-Marion, L., G. Toubeau, G. Laurent, J. A. Heuson-Stiennon, and P. M. Tulkens. 1986. Impairment of lysosome-pinocytic vesicle fusion in rat kidney proximal tubules after treatment with gentamicin at low doses. Toxicol. Appl. Pharmacol. 86:271-285. [DOI] [PubMed] [Google Scholar]
- 17.Graham, R. C., Jr., and M. J. Karnovsky. 1966. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J. Histochem. Cytochem. 14:291-302. [DOI] [PubMed] [Google Scholar]
- 18.Hurn, B. A., and J. Landon. 1971. Antisera for radioimmunoassay, p. 121-142. In K. E. Kirkham and W. M Hunter (ed.), Radioimmunoassay methods. Churchill Livingstone Publishers, London, United Kingdom.
- 19.Kuhar, M. J., L. L. Mak, and P. S. Lietman. 1979. Autoradiographic localization of [3H]gentamicin in the proximal renal tubules of mice. Antimicrob. Agents Chemother. 15:131-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, L., L. Grenier, Y. Bergeron, M. Simard, M. G. Bergeron, G. Labrecque, and D. Beauchamp. 1994. Temporal changes of pharmacokinetics, nephrotoxicity, and subcellular distribution of tobramycin in rats. Antimicrob. Agents Chemother. 38:54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madsen, K. M. R., and C. C. Tisher. 1986. Structural-functional relationship along the distal nephron. Am. J. Physiol. 250:F1-F15. [DOI] [PubMed] [Google Scholar]
- 22.Ohara, K., M. Shin, L.-I. Larsson, and K. Fujiwara. 2007. Improved immunocytochemical detection of daunomycin. Histochem. Cell Biol. 127:603-608. [DOI] [PubMed] [Google Scholar]
- 23.Ohara, K., M. Shin, H. Nakamuta, L.-I. Larsson, D. M. Hougaard, and K. Fujiwara. 2007. Immunocytochemical studies on the distribution pattern of daunomycin in rat gastrointestinal tract. Histochem. Cell Biol. 128:285-290. [DOI] [PubMed] [Google Scholar]
- 24.Parsons, P. P., H. O. Garland, and E. S. Harpur. 2000. Localization of the nephron site of gentamicin-induced hypercalciuria in the rat: a micropuncture study. Br. J. Pharmacol. 130:441-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phipps, D. J., A. J. Spencer, and E. S. Harpur. 1990. Comparison of the renal response of male Fischer 344, Sprague Dawley and Wistar rats to administration of gentamicin. Toxicol. Lett. 53:203-205. [DOI] [PubMed] [Google Scholar]
- 26.Silverblatt, F., and C. Kuehn. 1979. Autoradiography of gentamicin uptake by the rat proximal tubule cell. Kidney Int. 15:335-345. [DOI] [PubMed] [Google Scholar]
- 27.Tulkens, P., and A. Trouet. 1978. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem. Pharmacol. 27:415-424. [DOI] [PubMed] [Google Scholar]
- 28.Wedeen, R. P., U. Batuman, C. Cheeks, E. Marquet, and H. Sobel. 1983. Transport of gentamicin in rat proximal tubule. Lab. Investig. 48:212-223. [PubMed] [Google Scholar]