Abstract
Telavancin is an investigational lipoglycopeptide antibiotic currently being developed for the treatment of serious infections caused by gram-positive bacteria. The bactericidal action of telavancin results from a mechanism that combines the inhibition of cell wall synthesis and the disruption of membrane barrier function. The purpose of the present study was to further elucidate the mechanism by which telavancin interacts with the bacterial membrane. A flow cytometry assay with the diethyloxacarbocyanine dye DiOC2(3) was used to probe the membrane potential of actively growing Staphylococcus aureus cultures. Telavancin caused pronounced membrane depolarization that was both time and concentration dependent. Membrane depolarization was demonstrated against a reference S. aureus strain as well as phenotypically diverse isolates expressing clinically important methicillin-resistant (MRSA), vancomycin-intermediate (VISA), and heterogeneous VISA (hVISA) phenotypes. The cell wall precursor lipid II was shown to play an essential role in telavancin-induced depolarization. This was demonstrated both in competition binding experiments with exogenous d-Ala-d-Ala-containing ligand and in experiments with cells expressing altered levels of lipid II. Finally, monitoring of the optical density of S. aureus cultures exposed to telavancin showed that cell lysis does not occur during the time course in which membrane depolarization and bactericidal activity are observed. Taken together, these data indicate that telavancin's membrane mechanism requires interaction with lipid II, a high-affinity target that mediates binding to the bacterial membrane. The targeted interaction with lipid II and the consequent disruption of both peptidoglycan synthesis and membrane barrier function provide a mechanistic basis for the improved antibacterial properties of telavancin relative to those of vancomycin.
The increasing prevalence of serious infections caused by gram-positive bacteria, including those caused by methicillin (meticillin)-resistant Staphylococcus aureus (MRSA), highlights the need for new agents with enhanced antimicrobial properties (2, 10, 21, 26, 35). One promising approach has been the development of lipoglycopeptide antibiotics, semisynthetic derivatives of glycopeptides that contain hydrophobic substituents and that possess improved antimicrobial properties (1, 4, 13, 32, 39). Telavancin, a lipoglycopeptide derivative of vancomycin, exhibits enhanced potency in vitro, concentration-dependent bactericidal activity, and activity both in vitro and in vivo against organisms that display reduced susceptibility to vancomycin (17, 18, 23, 24, 28, 31, 33, 36, 42). Telavancin has been evaluated in phase III clinical trials for the treatment of complicated skin and skin structure infections and hospital-acquired pneumonia (46, 53).
The bactericidal action of telavancin results from a mechanism that includes the inhibition of cell wall synthesis and the disruption of essential membrane barrier functions (25). Telavancin possesses the glycopeptide core of vancomycin, which binds with a high affinity to the acyl-d-alanyl-d-alanine (d-Ala-d-Ala) terminus of cell wall precursors through a network of hydrogen bonds and hydrophobic packing interactions (3, 45). Inhibition of cell wall synthesis by telavancin therefore involves binding to late-stage peptidoglycan precursors, including membrane-embedded lipid II. These interactions prevent both the polymerization of the precursor into peptidoglycan and subsequent cross-linking events. Telavancin also binds to bacterial membranes and causes membrane depolarization and increased membrane permeability. The mechanism by which telavancin binds to and disrupts the function of the bacterial membrane has not been determined.
The present study was undertaken to further explore the interaction of telavancin with the bacterial membrane. Using a flow cytometry assay optimized for the accurate measurement of membrane potential in bacteria, we demonstrate that telavancin causes pronounced, concentration-dependent depolarization in S. aureus cells. Isolates of S. aureus expressing important and emerging resistance phenotypes, such as MRSA, heterogeneous vancomycin-intermediate S. aureus (hVISA), vancomycin-intermediate S. aureus (VISA), and daptomycin-nonsusceptibile MRSA, are equally susceptible to depolarization by telavancin. We provide evidence, through multiple lines of investigation, that membrane disruption by telavancin requires binding to the bacterial specific target, lipid II. Finally, we demonstrate that telavancin does not lyse bacteria during the time course that membrane effects are assayed. Importantly, the latter observation indicates that telavancin-induced membrane depolarization is not a consequence of a weakened cell wall. The findings of the studies reported here enhance our understanding of telavancin's mechanism of action and, in assays designed to be representative of physiological conditions, demonstrate that therapeutically relevant concentrations of telavancin inhibit essential functions of the bacterial membrane.
MATERIALS AND METHODS
Bacterial strains.
The S. aureus strains used in this study included ATCC 33591 (MRSA), ATCC 29213 (methicillin-susceptible S. aureus), ATCC 700698 (hVISA Mu3), and ATCC 700699 (VISA Mu50); all strains were obtained from the American Type Culture Collection (ATCC; Manassas, VA). A daptomycin-nonsusceptible MRSA strain (strain MED 2034) was obtained from the telavancin clinical program.
Antibacterials, media, and reagents.
Telavancin and THRX-881620 were manufactured by Theravance, Inc. (South San Francisco, CA). [14C]telavancin was prepared by ViTrax (Placentia, CA) with the radiolabel on the aminomethyl carbon substitution of the resorcinol position. Vancomycin, penicillin G, nisin, carbonyl cyanide m-chlorophenylhydrazone (CCCP), N,N′-diacetyl-l-Lys-d-Ala-d-Ala (dKAA), acetyl-l-Lys-d-Ala-d-Ala, fosfomycin, d-cycloserine, bacitracin, 1-decanol, lysostaphin, and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical Co. (St. Louis, MO); and 3,3′-diethyloxacarbocyanine iodide [DiOC2(3)] was obtained from Invitrogen (Carlsbad, CA). Telavancin stock solutions (4 mg/ml) were prepared by solubilizing telavancin powder in a 1:1 (vol/vol) mixture of DMSO and water that was acidified by adding 28 μl of 1 N HCl per 10 ml of solution. Serial dilutions of telavancin were also performed in acidified 50% DMSO. Polypropylene laboratory ware was used for the preparation of stock solutions and the subsequent dilution procedures. Nisin stock solutions were prepared in deionized water, which was warmed, vortexed, and briefly bath sonicated, prior to filtration through a 0.2-μm-pore-size syringe (polyethersulfone). Cation-adjusted Mueller Hinton II broth (MHB; Difco, Detroit, MI) was used for bacterial growth. Viable counts were determined by plating on tryptic soy agar plates (Hardy Diagnostics, Santa Monica, CA). Cultures subjected to the nongrowing condition were incubated in Dulbecco's phosphate-buffered saline with cations (PBS; Invitrogen, Carlsbad, CA).
Flow cytometry.
The membrane potential was assayed by flow cytometry with the diethyloxacarbocyanine dye DiOC2(3), which allows the variation in cell size to be normalized by analysis of the ratio of red fluorescence to green fluorescence (40, 48). Metabolically active S. aureus generates a membrane potential of approximately −120 mV; DiOC2(3) fluorescence can report changes across the entire range of −30 to −130 mV. Depolarization experiments were conducted with S. aureus ATCC 29213, unless otherwise noted. Cultures were grown to early exponential phase (A625 = 0.3) in MHB at 37°C. Cell concentrations were adjusted to 106 CFU/ml by dilution in MHB, compounds were added to 5-ml aliquots (where indicated, dKAA was added before the addition of telavancin), and the samples were incubated at 37°C in a shaking water bath. At selected intervals, 250-μl samples were aspirated and mixed 1:1 with DiOC2(3) in MHB, with the final concentration of DiOC2(3) being 30 μM. Samples were stained for 5 min prior to evaluation.
An Epics XL flow cytometer (Beckman Coulter Inc., Fullerton, CA) was used to collect 5,000 events for each sample at the medium flow rate, and the signal was acquired with logarithmic amplification. The collection of fluorescence from a fixed number of individual cells ensures that the culture is uniformly sampled, irrespective of cell density. Green fluorescence emission was detected through a 525-nm (40-nm band-pass) emission filter (FL1), and red fluorescence was detected through a 620-nm (30-nm band-pass) emission filter (FL3). Analysis was performed with FCS Express software (De Novo Software, Los Angeles, CA). In order to distinguish normal and depolarized populations of cells, gates were applied to plots of red versus green fluorescence, with the percent depolarization representing the cell count within the depolarized region divided by the total cell count. The depolarized region was defined by gating a region only slightly exceeding the boundary of a fully depolarized population induced by CCCP. The 50% inhibitory concentrations IC50s were determined from the percent depolarization by fitting the concentration-response curve by nonlinear regression to a sigmoidal four-parameter equation by the use of SigmaPlot software (Systat, San Jose, CA).
In our analyses of membrane potential, we sought to ensure that only perturbations caused by the direct interaction of compound with the membrane were recorded as depolarization. Accordingly, our assay was optimized to exclude secondary effects, such as those resulting from a weakened cell wall, that can induce stress to the membrane and cause depolarization. Experiments with penicillin G to inhibit cell wall synthesis either partially (sub-MIC) or completely (supra-MIC) showed that the membrane potential could be accurately measured in cells treated for up to 90 min. Therefore, 90 min was used as the endpoint for the depolarization assay, unless otherwise noted.
Three different inhibitors of cell wall synthesis, fosfomycin, d-cycloserine, and bacitracin, were used to modulate the lipid II levels in S. aureus cells. Cultures were pretreated with these compounds at 37°C with shaking for 10 min to suppress lipid II production. After pretreatment with fosfomycin (250 μg/ml), d-cycloserine (64 μg/ml), or bacitracin (250 μg/ml), cells were exposed to 32 μg/ml telavancin for 15 min or 2 μg/ml nisin for 5 min. The percent depolarization was calculated relative to the level of depolarization of control samples without pretreatment. Modified culture conditions were also used to modulate the lipid II levels. Cells were incubated under each condition (PBS at 37°C or MHB at 23°C) for 10 min before exposure to 32 μg/ml telavancin.
Binding of [14C]telavancin to bacteria.
S. aureus cultures (108 CFU/ml) were incubated at 37°C in either MHB or PBS in a deep-well polypropylene microplate. Cultures were exposed to [14C]telavancin (8 μg/ml), and at each time point, samples were transferred to a 0.2-μm-pore-size filter plate (MultiScreen-HTS-GV; Millipore). The filter plate was prewashed with PBS containing 0.002% Tween 20 to reduce nonspecific binding. The samples were filtered and then washed three times with PBS-0.002% Tween 20, a process that was verified to effectively wash unbound [14C]telavancin through the filter so that only bacteria remained. The filter plate was dried overnight at 37°C. Scintillant (MicroScint-20; Perkin-Elmer) was applied to the dried samples, which were then read on a Wallac MicroBeta scintillation counter (Perkin-Elmer).
Antimicrobial activity.
MICs were determined by the use of dry-form panels manufactured by Trek Diagnostic Systems (Cleveland, OH). Time-kill assays were performed according to methods defined by the CLSI (12).
Cell lysis.
Turbidity was evaluated with a PharmaSpec UV-1700 spectrophotometer (Shimadzu, Columbia, MD). S. aureus (ATCC 33591) cultures were grown to early exponential phase (A625 = 0.3) in MHB, 5 ml was aliquoted into 50-ml conical tubes, compounds were added to the desired final concentration, and the samples were incubated in a shaking 37°C water bath. At each time point, a 100-μl sample was aspirated and transferred to a quartz microcuvette, and the optical density at 625 nm was recorded. Lysostaphin, which cleaves the pentaglycine cross-link in peptidoglycan, was used for assay validation and reduced the optical density to background levels, consistent with complete cell lysis.
Microscopy.
Phase-contrast images of the bacteria were captured on a Zeiss Axioskop equipped with a Plan-Neofluar ×100/1.3 objective and with a Photometrics CoolsnapFx charge-couple-device camera. Cells were deposited on a slide, a coverslip was applied, and the edges were sealed. The bacteria were analyzed for lysis by the degree of cellular contrast; intact cells appeared dark, whereas lysed cells were transparent. Bacteria were imaged at 0, 2, and 6 h posttreatment. Populations of at least 30 cells were evaluated for each experimental condition.
RESULTS
Effect of telavancin on bacterial membrane potential.
To further investigate telavancin's mechanism of action, we employed a flow cytometry-based depolarization assay with the fluorescent dye DiOC2(3), which has been demonstrated to accurately measure the membrane potential in bacteria (40, 41, 48). Monitoring changes to membrane potential is a useful method for assessment of the significance of a membrane effect, as it reports the functional integrity of the bacterial membrane. The membrane potential is sensitive to modest disruptions of barrier function, like those that would allow small solutes such as protons to cross the membrane, and requires a sustained effect to maintain the depolarized state. Dissipation of the membrane potential (i.e., depolarization) leads directly to the loss of function of essential aspects of cell physiology, such as ATP generation and nutrient uptake (22).
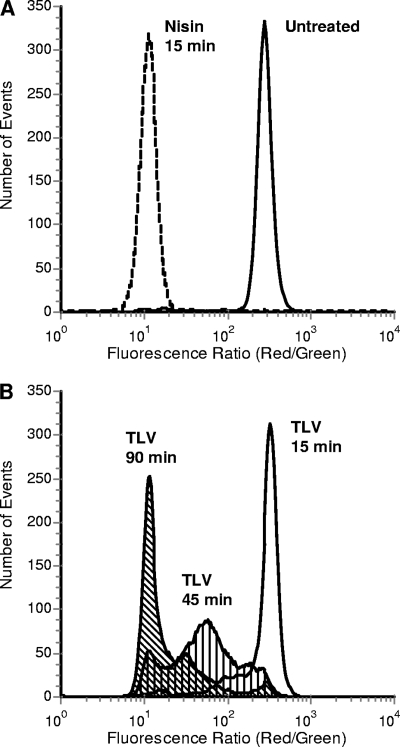
The effect of telavancin on bacterial membrane potential was assessed under the test conditions (cation-adjusted MHB, physiological temperature, and low cell density) that are routinely employed for antibiotic susceptibility testing. Histogram displays of cellular red fluorescence/green fluorescence ratios show distinctions in the magnitude of depolarization induced in antibiotic-treated cultures. In Fig. 1, the results for an untreated, fully polarized (normal metabolic state) population of S. aureus (ATCC 29213) cells are depicted in the uniform distribution on the farthest right in the graph. Upon depolarization, the populations were observed to move leftward. Nisin, a pore-forming lantibiotic used as a control (8), fully depolarized S. aureus cells at 15 min (Fig. 1A). When the S. aureus cells were treated with the proton ionophore CCCP, they were similarly completely depolarized (data not shown). Telavancin demonstrated time-dependent depolarization activity against S. aureus cells. Cultures exposed to 8 μg/ml telavancin became increasingly depolarized as the duration of exposure increased; a broad depolarization response observed at 45 min changed to a nearly uniform distribution of maximally depolarized cells by 90 min (Fig. 1B).
FIG. 1.
Dissipation of S. aureus membrane potential measured by flow cytometry with DiOC2(3). Cell populations exhibiting depolarization appear toward the left on the x axis. (A) Fully polarized (untreated) and depolarized (nisin, 8 μg/ml) cells; (B) depolarization of S. aureus by telavancin (TLV). The cultures were incubated for 15, 45, and 90 min in the presence of 8 μg/ml telavancin.
In order to analyze a wider variety of conditions, cell population histograms were converted to percent depolarization. A definition of depolarization was applied by gating a region only slightly exceeding the boundaries of a fully depolarized population induced by CCCP or nisin. Percent depolarization was the cell count within the depolarized region divided by the total cell count.
Concentration-response curves were obtained for telavancin and control compounds, and nonlinear regression was applied to establish IC50s. Telavancin (MIC, 0.25 μg/ml) demonstrated concentration-dependent depolarization activity against S. aureus, with an IC50 of 3.6 μg/ml. The IC50 determined for nisin (MIC, 4 μg/ml) was 2.9 μg/ml. In contrast, no IC50 could be calculated for vancomycin (MIC, 1 μg/ml), even at concentrations up to 96 μg/ml.
Membrane depolarization in phenotypically diverse S. aureus strains.
Telavancin depolarization activity was investigated in strains that exhibit important and emerging resistance phenotypes. Since telavancin shares the core structure of vancomycin, it was important to test isolates with reduced susceptibility to vancomycin in order to determine whether these phenotypes might affect the activity of telavancin. Telavancin maintained potent activity against and dissipated the membrane potential of S. aureus cells expressing clinically important phenotypes such as methicillin resistance, vancomycin intermediate, heterogeneous vancomycin intermediate and daptomycin nonsusceptibility (Table 1). In all strains, the percent depolarization increased over time, resulting in S. aureus populations in which a majority of the cells were fully depolarized.
TABLE 1.
Telavancin depolarization activity against phenotypically diverse S. aureus isolates
| Strain | Relevant phenotype | % Depolarizationa |
|---|---|---|
| ATCC 33591 | Methicillin resistant | 77 ± 7 |
| ATCC 700699 | Vancomycin intermediate (Mu50) | 61 ± 3 |
| ATCC 700698 | Heterogeneous vancomycin intermediate (Mu3) | 78 ± 4 |
| MED 2034 | Daptomycin nonsusceptible | 77 ± 5 |
Percent depolarization is reported for treatment with 32 μg/ml telavancin, and the values represent the means ± standard deviations from at least three independent experiments.
The strain exhibiting the lowest level of sensitivity to vancomycin, Mu50 (MIC, 8 μg/ml), was susceptible to telavancin with an MIC of 0.5 μg/ml, and against this strain, telavancin induced 61% depolarization. Against the other isolates tested, telavancin maintained comparable antimicrobial potency (MIC, ≤0.5 μg/ml) and depolarization activity (≥77%). A daptomycin-nonsusceptible S. aureus isolate (MIC, 4 μg/ml) was among the isolates evaluated. Considering that the antimicrobial mode of action of daptomycin occurs via membrane depolarization, it was important to determine whether telavancin retained its membrane-related mechanism of action against a strain with this important phenotype. Telavancin exerted a strong depolarization effect (77%) against the daptomycin-nonsusceptible S. aureus isolate.
Antagonism of telavancin-induced membrane depolarization by d-Ala-d-Ala ligand.
The mechanism by which telavancin binds to and disrupts the function of the bacterial membrane has not yet been determined. Telavancin may bind to the membrane through an interaction with membrane-embedded lipid II or, alternatively, by direct association with the membrane independent of lipid II. Telavancin has been shown to bind with a high affinity to the d-Ala-d-Ala residues of cell wall precursors (25). Previous binding studies with radiolabeled telavancin showed reduced binding to the membrane in the presence of the tripeptide dKAA, which mimics the structure of cell wall precursors, including lipid II. We conducted depolarization studies to determine whether lipid II, which is embedded in the bacterial membrane, plays a role in the membrane-related mechanism of action of telavancin.
When depolarization assays were conducted in the presence of dKAA, significant antagonism of telavancin activity at the S. aureus membrane was observed (Table 2). Upon pretreatment with 0.05, 0.5, and 5 mM dKAA, a pattern of graded antagonism was observed, with nearly maximal antagonism being achieved by 5 mM dKAA at 60 min with both 8 and 32 μg/ml telavancin. Control experiments demonstrated that dKAA had no effect on the assay. As expected, a molar excess of dKAA also antagonized the antibacterial activity. The telavancin MIC shifted 16-fold (from 0.25 to 4 μg/ml) in the presence of 5 mM dKAA, which links depolarization activity to susceptibility.
TABLE 2.
Antagonism of telavancin-induced depolarization by d-Ala-d-Ala ligand
| dKAA concn (mM)a | MIC (μg/ml) | % Depolarization with telavancin atb:
|
|
|---|---|---|---|
| 8 μg/ml | 32 μg/ml | ||
| 0 | 0.25 | 49 ± 3 | 94 ± 8 |
| 0.05 | 0.5 | 33 ± 14 | 68 ± 13 |
| 0.5 | 1 | 17 ± 4 | 19 ± 1 |
| 5 | 4 | 7 ± 6 | 14 ± 2 |
S. aureus cells were pretreated with dKAA prior to exposure to telavancin for 60 min.
Values represent the means ± standard deviations from at least three independent experiments.
Telavancin-induced depolarization is lipid II dependent.
The antagonism data presented above suggest that telavancin-induced depolarization may require binding to lipid II. To test this hypothesis directly, depolarization assays were conducted with cells expressing altered levels of lipid II. Three different inhibitors of cell wall synthesis, fosfomycin, d-cycloserine, and bacitracin, were used to reduce the lipid II levels in S. aureus cells. Fosfomycin is an inhibitor of MurA, the enzyme responsible for the first step in peptidoglycan synthesis, and thus blocks the formation of UDP-MurNAc. d-Cycloserine inhibits both alanine racemase and d-Ala-d-Ala ligase, two enzymes required for the synthesis of the d-Ala-d-Ala dipeptide of lipid II. Bacitracin binds directly to undecaprenyl pyrophosphate, the portion of lipid II that remains in the membrane once GlcNAc-MurNAc is polymerized, and prevents its use in subsequent cycles of lipid II synthesis. All three inhibitors thus block the synthesis of lipid II.
S. aureus cells were preincubated with each of the lipid II synthesis inhibitors and were then exposed to telavancin or nisin. The time and concentration of exposure to telavancin and nisin were selected to achieve 50 to 70% depolarization for each compound alone (for telavancin, 32 μg/ml and 15 min; for nisin, 2 μg/ml and 5 min). As has been described by other investigators (8), we observed that the membrane depolarization activity of nisin was lipid II dependent (Table 3). Fosfomycin and d-cycloserine reduced the activity of nisin to 26% and 36% of the levels observed in control cultures, respectively. Telavancin-induced depolarization was suppressed to a similar extent (29% to 38%) by these agents and by bacitracin (27%) (Table 3). Interestingly, bacitracin treatment suppressed the activity of nisin to 2% of the control levels. This observation suggests that the target shared by nisin and bacitracin, the pyrophosphate moiety of undecaprenyl pyrophosphate, may play a role in nisin-mediated depolarization. All three inhibitors of lipid II synthesis suppressed telavancin-induced depolarization by approximately two-thirds compared to that for cells that were not pretreated.
TABLE 3.
Effect of lipid II synthesis inhibitors on depolarization of S. aureus by telavancin
| Antibiotica | % Depolarizationb
|
|
|---|---|---|
| Telavancin | Nisin | |
| None | 100 | 100 |
| Fosfomycin | 29 ± 6 | 26 ± 4 |
| d-Cycloserine | 38 ± 8 | 36 ± 1 |
| Bacitracin | 27 ± 4 | 2 ± 1 |
S. aureus cells were pretreated for 10 min prior to the addition of telavancin or nisin.
Depolarization was calculated relative to that for the control sample without pretreatment. Values represent the means ± standard deviations from at least two independent experiments.
The cellular levels of lipid II were also manipulated by shifting the cultures to either nutrient-free medium or a reduced temperature. The amount of lipid II available to deliver new MurNAc-(pentapeptide)-GlcNAc precursors for peptidoglycan synthesis is dependent on cellular metabolism (56). A slowly growing or nongrowing cell will have a reduced rate of lipid II production, which can be induced by lower temperatures or minimal medium (9, 11).
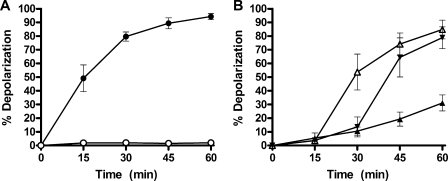
Cultures of S. aureus were grown in MHB and then washed and resuspended in PBS and allowed to equilibrate for 10 min at 37°C, prior to treatment with telavancin. At all concentrations across the dose range of 2 to 32 μg/ml telavancin, depolarization was completely suppressed. Figure 2A shows the time course of depolarization for 32 μg/ml telavancin in PBS versus that for telavancin in MHB. The membrane potential of untreated cells observed under the conditions and times explored in these assays was not different from that observed under the standard condition of MHB at 37°C.
FIG. 2.
Effect of reduced cellular levels of lipid II on the depolarization of S. aureus by telavancin. (A) Bacteria treated at 37°C in MHB (closed circles) or PBS (open circles); (B) bacteria treated at 23°C in MHB (closed triangles), followed by a temperature shift to 37°C at 15 min (open triangles) and 30 min (upside-down triangles). All samples were treated with 32 μg/ml telavancin. Values represent the means ± standard deviations from three independent experiments.
In another experiment, cells were grown normally and shifted to 23°C for 10 min prior to treatment with 32 μg/ml telavancin (Fig. 2B). Depolarization was suppressed but was seen to slowly rise to 30% by 60 min, whereas there was >90% depolarization at 37°C, a culture temperature that supports optimal cell growth and division. Additionally, samples treated with 32 μg/ml telavancin were shifted from 23°C to 37°C at time points of 15 min and 30 min (Fig. 2B). The shift from a suboptimal growth temperature to the optimal growth temperature resulted in a dramatic increase in the level of depolarization seen at the next time point that a sample was obtained. In fact, irrespective of when the temperature is shifted for the sample, 15 min at 37°C results in a similar percentage of depolarized cells (approximately 50%), which also matches the percentage at the 15-min time point under the standard assay conditions.
We also explored a telavancin analog with substantially reduced binding affinity to d-Ala-d-Ala residues. THRX-881620 is the hexapeptide (des-N-methyl-leucyl) derivative of telavancin that lacks the N-terminal amino acid of the carboxylate binding pocket (25). THRX-881620 (MIC, 8 μg/ml) induced only 35% depolarization relative to the level induced by telavancin. Like telavancin, however, THRX-881620 did not depolarize cells incubated in PBS.
Control experiments were performed to confirm that modified culture conditions, such as temperature and nutrient content, did not alter the overall properties of the membrane. 1-Decanol, a model surfactant, partitions into the membrane due to its amphipathic properties (20, 54) and induces depolarization independently of lipid II. We reasoned that if the modified culture conditions did not influence membrane properties, then 1-decanol would maintain its depolarization activity across all conditions. 1-Decanol caused the immediate and maximal depolarization of S. aureus at concentrations similar to its MIC (128 μg/ml), and this activity was consistent when it was compared between 37°C and 23°C as well as between MHB and PBS.
Binding of [14C]telavancin to bacteria.
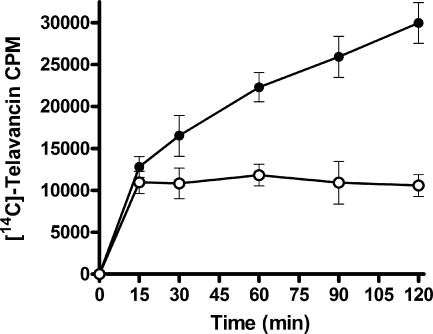
A radiolabeled binding assay was used to quantitate the amount of telavancin bound to S. aureus. Cells were treated with [14C]telavancin and monitored over a 2-h time period (Fig. 3). The association of [14C]telavancin with metabolically active cells (MHB) increased over time. In contrast, the association of [14C]telavancin with cells incubated in nutrient-free medium (PBS) occurred only during the initial binding period (15 min), after which no further binding was observed. After 2 h, 2.8-fold more telavancin was bound to cells in MHB than to cells in PBS.
FIG. 3.
Binding of [14C]telavancin to S. aureus cells. Bacteria were cultured at 37°C in MHB (closed circles) or PBS (open circles). Values represent the means ± standard deviations of three independent experiments.
Bactericidal activity in the absence of cell lysis.
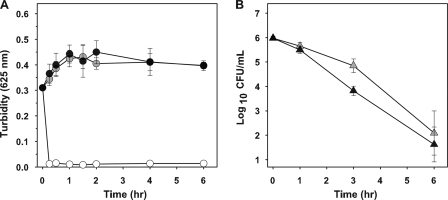
A series of experiments was conducted to determine whether the direct action of telavancin on the membrane results in cell lysis. The degree of cellular lysis was determined spectroscopically by measuring the turbidity of the culture at 625 nm. S. aureus cultures exposed to 8 and 32 μg/ml telavancin for 6 h showed no significant change in optical density, indicating a lack of cell lysis (Fig. 4). Similarly, the turbidity of cultures exposed to vancomycin did not change significantly (data not shown). In contrast, lysostaphin reduced the optical density of control cultures to background levels, consistent with complete cell lysis. When viable counts were monitored in parallel with turbidity, the viable counts showed that telavancin effectively reduced the inoculum by more than 3 log10 CFU/ml at each concentration (Fig. 4).
FIG. 4.
Comparison of culture turbidity to viable counts in S. aureus exposed to telavancin. Curves represent turbidity (A, circles) and viable counts (B, triangles) for cultures exposed to 8 μg/ml (gray symbols) and 32 μg/ml (black symbols) telavancin. Lysostaphin (open circles) was used as a positive control for cell lysis. Values represent the means ± standard deviations from three independent experiments.
Lysis can also be considered to occur through irreversible membrane dissolution, which may be independent of whether the cell maintains an intact wall. Since turbidity may not fully detect this type of lysis, phase-contrast microscopy was used to allow the direct observation of cellular integrity. No change in cellular integrity was observed in cells following exposure to telavancin (up to 32 μg/ml) that were monitored for up to 6 h.
DISCUSSION
The essential function of a cell membrane is to separate the cell from its environment. The membrane permits a cell to maintain a unique intracellular composition as well as ionic gradients across the membrane, both of which are essential for cellular viability. In bacteria, the proton gradient is required for both ATP synthesis and nutrient import and is the primary contributor to membrane potential (22). Collapse of the proton gradient (i.e., depolarization) inhibits these functions and thus results in the loss of bacterial viability (16, 30).
Telavancin effectively depolarized S. aureus, as seen in cell population histograms (Fig. 1). Concentration-response curves were used to establish an IC50 of 3.6 μg/ml for telavancin-induced membrane depolarization. This activity was observed at concentrations that are approximately 10-fold higher than the telavancin MIC but well below clinically achievable levels in plasma (maximum concentration in plasma, 82 μg/ml) (52). In contrast, no IC50 could be calculated for vancomycin, even at concentrations as high as 96 μg/ml, indicating that vancomycin has no direct activity against the bacterial membrane. At all concentrations tested, the degree of depolarization increased with time. S. aureus cells exposed to telavancin at 8 μg/ml exhibited a broad depolarization response at 45 min and were fully depolarized by 90 min (Fig. 1). The time dependence and broad depolarization response may be explained by the heterogeneity of lipid II present in bacterial populations; cells undergoing active division should produce more lipid II than those that have just completed a division cycle or that have yet to begin another division cycle. Furthermore, the path to reach lipid II will be shorter at stages of growth where the septum is beginning to form than at stages toward the end of septum formation (43). The time at which a majority of the population was completely depolarized correlated to the loss of bacterial viability (Fig. 4) and is best explained by the need to accumulate a sufficient concentration of telavancin in the membrane.
Binding studies with [14C]telavancin were conducted to determine the amount of antibiotic that binds to the cell. The concentration of telavancin bound to the cell continued to increase over time and correlated to the onset of depolarization, resulting in 2.8-fold more telavancin bound to cells cultured in MHB than those cultured in PBS. Since cells cannot grow or divide when they are suspended in PBS, binding under this condition represents the static number of d-Ala-d-Ala binding sites in the cell, consisting of uncrosslinked cell wall, nascent peptidoglycan, and lipid II. The differential between MHB and PBS likely represents new lipid II synthesis. While no further cell division can occur once the cell is exposed to telavancin, the cell wall machinery can generate new lipid II molecules to which telavancin can bind. This result is similar to that for another lipid II-dependent antibiotic, mersacidin (9).
Telavancin specifically binds to terminal d-Ala-d-Ala residues in cell wall precursors. Cell fractionation studies with radiolabeled telavancin showed that the majority of the telavancin bound to the cell was localized to the membrane (25). These observations prompted us to investigate the role of lipid II in telavancin-induced membrane depolarization. In our depolarization assay, the activity of telavancin was suppressed by the substrate-mimicking peptide dKAA (Table 2). The results of the competitive binding assay suggest that membrane disruption is caused when telavancin binds to lipid II and that telavancin lacks disruptive activity when binding is blocked. Antagonism by dKAA elevated the telavancin MIC by 16-fold, which is in agreement with the depolarization assay results. This concept is further supported by the results obtained with THRX-881620, the hexapeptide analog of telavancin with an impaired binding affinity for d-Ala-d-Ala. Relative to telavancin, THRX-881620 induced only one-third the depolarization and also exhibited weaker antimicrobial activity.
In order to more rigorously test the dependence of telavancin on lipid II, depolarization assays were conducted with cells expressing altered levels of lipid II. Depolarization was suppressed under all conditions that reduced lipid II levels in S. aureus, including pretreatment with lipid II synthesis inhibitors, suspension in nutrient-free medium, and incubation at low temperature, demonstrating the requirement of telavancin to bind to lipid II in order to disrupt membrane function.
We demonstrate the dependence of telavancin on lipid II by blocking the synthesis of lipid II with specific cell wall inhibitors. S. aureus cells were pretreated with either fosfomycin, d-cycloserine, or bacitracin and were then exposed to telavancin or nisin. All three inhibitors of lipid II synthesis suppressed telavancin-induced depolarization by approximately two-thirds compared to the level of depolarization of cells that were not pretreated (Table 3). Nisin-induced depolarization was similarly suppressed by the three inhibitors, except that bacitracin pretreatment caused even stronger suppression (to 2% of the control levels). This observation suggests that the target shared by nisin and bacitracin, the pyrophosphate moiety of undecaprenyl pyrophosphate, may play a role in nisin-mediated depolarization.
Depolarization by telavancin was completely suppressed when cells were suspended in nutrient-free medium (Fig. 2A). Thus, an orthogonal approach to the modulation of lipid II levels gives a result similar to that achieved with the antibiotic pretreatment described above. The suspension of bacteria in isotonic PBS with cations permits physiological gradients across the membrane but deprives the cell of the nutrients required for cell wall synthesis and growth. In the absence of cell wall synthesis and lipid II generation, cells lack the specific cellular target so that telavancin is unable to bind to the membrane and cause depolarization. Further support for this concept is derived from the results of [14C]telavancin binding experiments with bacteria. The concentration of telavancin that bound to bacteria increased over time when the bacteria were grown in MHB but remained constant when the bacteria were suspended in PBS. Similarly, the binding of another lipid II-dependent antibiotic, mersacidin, to bacteria was substantially reduced when the bacteria were in PBS than when they were in growth medium (9). Under conditions that support metabolic activity and thus the continuous synthesis of lipid II, telavancin is presented with additional target molecules, thereby increasing the concentration of telavancin in the membrane to levels that result in the disruption of normal function.
An intermediate growth condition was induced by incubation of the bacteria at a reduced temperature in broth. Bacteria cultured at 23°C in MHB sustained a degree of cell wall synthesis, with corresponding lipid II generation, which permitted telavancin to depolarize approximately one-third of the cell population by 60 min (Fig. 2B). Cultures that were shifted from 23°C to 37°C after telavancin treatment showed a dramatic increase in depolarization at the next time point that matched the magnitude of depolarization seen under standard conditions. This slowly growing culture at 23°C might be considered representative of S. aureus cells at an infection site that are near their low limit of growth. Therefore, even very slowly growing S. aureus cells produce sufficient levels of lipid II to be targeted by telavancin. Any increase in the bacterial growth rate is effectively countered by increased telavancin activity. By varying the cellular lipid II content with defined conditions, we observed the dynamic nature of telavancin-induced depolarization as a function of the presence or absence of its target, lipid II.
A number of antibiotics target lipid II, but they all act differently both in the binding contacts required and in the ability to affect cell wall synthesis or membrane function (5, 56). For example, the antibacterial action of nisin results from its high affinity for lipid II combined with its ability to assemble into nisin-lipid II complexes that form pores in the membrane (7, 8, 57). In contrast, telavancin lacks the structural characteristics required to form discrete pores. It must therefore exert its membrane-disruptive activity through other mechanisms, with one such possibility being the induction of positive membrane curvature (34) via the insertion of the decylaminoethyl side chain into the membrane. In a series of experiments with model membranes, telavancin-induced proton permeation was shown to be anionic phospholipid dependent but lipid II independent (6), suggesting that lipid II is not involved in the biophysical mechanism of membrane disruption. Therefore, in bacteria, membrane-embedded lipid II appears to act exclusively as a high-affinity target that mediates the binding of telavancin to the membrane.
As described in a recent surveillance of clinical isolates (17), telavancin retains potency against MRSA, VISA, hVISA, and daptomycin-nonsusceptible staphylococci. In good agreement, isolates of S. aureus expressing these resistance phenotypes are equally susceptible to depolarization by telavancin (Table 1). Exposure to telavancin therefore results in the significant disruption of membrane function, regardless of the phenotype. The strong depolarization activity against VISA and daptomycin-nonsusceptible strains highlights the fact that telavancin retains its membrane-related mechanism of action against these emerging, clinically important phenotypes. VISA and hVISA strains are increasingly identified as possible causes of vancomycin treatment failure in the clinic (27, 37, 38, 47).
In VISA isolates, resistance to glycopeptides is mediated by alterations in the structural organization of the cell wall, most notably, by thickened cell walls containing an increased number of cell wall d-Ala-d-Ala residues (14, 15, 19, 29, 49, 50). These binding sites act as decoy targets for vancomycin. The overproduction of uncrosslinked d-Ala-d-Ala residues creates a reservoir in the mature cell wall that effectively sequesters vancomycin and prevents it from reaching the lethal target sites of membrane-embedded lipid II and nascent peptidoglycan. Binding experiments show that the affinity of vancomycin for soluble d-Ala-d-Ala residues is four- to sixfold higher than that of telavancin (25). Affinities for soluble d-Ala-d-Ala can be considered a surrogate measure for uncrosslinked cell wall residues. In contrast, the binding affinity of telavancin for the d-Ala-d-Ala moiety of lipid II is 160-fold higher than that for soluble d-Ala-d-Ala (6). The strong affinity of telavancin for lipid II, combined with a weaker cell wall affinity, enables it to more readily pass through the wall to sites of peptidoglycan biosynthesis.
Cell lysis is a common terminal event for bacteria killed by cell wall-active antibiotics, particularly those of the beta-lactam class (55). However, the substrate-dependent mechanism of glycopeptides results in a different impact on the cell wall, and it has been demonstrated that vancomycin suppresses autolysis, presumably by blocking access to murein hydrolases (51). On the basis of the results of the turbidity assay and microscopic analysis presented above and as further corroborated by transmission electron microscopy (44), telavancin does not trigger autolysis, nor does it cause lysis by direct action on the membrane. Therefore, membrane disruption does not result from a weakened cell wall. In addition, these data support the fact that telavancin does not act as a nonspecific surfactant. Furthermore, when turbidity was evaluated in parallel with viable counts, telavancin is bactericidal without causing cell lysis. We conclude from these findings that telavancin disrupts the functional integrity of the bacterial membrane as a primary component of its mode of action.
Telavancin exerts its antibacterial effects through a mechanism of action that combines the inhibition of cell wall synthesis and the disruption of essential membrane barrier functions. In this report, we have further elucidated the membrane-related mechanism of action of telavancin. Telavancin selectively associates with the bacterial membrane by binding to lipid II, conferred by the intramolecular cooperativity of d-Ala-d-Ala binding and membrane anchoring. The accumulation of telavancin in the membrane leads to impaired barrier function. We propose that lipid II binding positions the lipophilic side chain of telavancin into the membrane, which enables it to perturb the lipid bilayer. The exact mechanism of biophysical disruption remains under investigation. In summary, through interaction with the membrane-embedded target lipid II, telavancin both inhibits peptidoglycan synthesis and disrupts membrane barrier function. Our findings provide a rational mechanistic basis for the potent antibacterial activity of telavancin.
Acknowledgments
We thank S. Hegde, K. Krause, and E. Breukink for critical review of the manuscript and D. Novo, H. Shapiro, and M. Renelli for helpful discussion of flow cytometry.
Footnotes
Published ahead of print on 26 May 2009.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Allen, N. E., D. L. LeTourneau, and J. N. Hobbs, Jr. 1997. The role of hydrophobic side chains as determinants of antibacterial activity of semisynthetic glycopeptide antibiotics. J. Antibiot. (Tokyo) 50:677-684. [DOI] [PubMed] [Google Scholar]
- 2.Baquero, F. 1997. Gram-positive resistance: challenge for the development of new antibiotics. J. Antimicrob. Chemother. 39(Suppl. A):1-6. [DOI] [PubMed] [Google Scholar]
- 3.Barna, J. C., and D. H. Williams. 1984. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu. Rev. Microbiol. 38:339-357. [DOI] [PubMed] [Google Scholar]
- 4.Beauregard, D. A., D. H. Williams, M. N. Gwynn, and D. J. Knowles. 1995. Dimerization and membrane anchors in extracellular targeting of vancomycin group antibiotics. Antimicrob. Agents Chemother. 39:781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breukink, E., and B. de Kruijff. 2006. Lipid II as a target for antibiotics. Nat. Rev. Drug Discov. 5:321-332. [DOI] [PubMed] [Google Scholar]
- 6.Breukink, E., P. P. A. Humphrey, B. M. Benton, and I. Visscher. 2006. Evidence for a multivalent interaction between telavancin and membrane-bound lipid II, abstr. C1-678. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 7.Breukink, E., H. E. van Heusden, P. J. Vollmerhaus, E. Swiezewska, L. Brunner, S. Walker, A. J. Heck, and B. de Kruijff. 2003. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J. Biol. Chem. 278:19898-19903. [DOI] [PubMed] [Google Scholar]
- 8.Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. [DOI] [PubMed] [Google Scholar]
- 9.Brotz, H., G. Bierbaum, K. Leopold, P. E. Reynolds, and H. G. Sahl. 1998. The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob. Agents Chemother. 42:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers, H. F. 1997. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 10:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung, H. Y., L. Vitkovic, and E. Freese. 1983. Rates of peptidoglycan turnover and cell growth of Bacillus subtilis are correlated. J. Bacteriol. 156:1099-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical Laboratory Standards Institute. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline M26-A. Clinical Laboratory Standards Institute, Wayne, PA.
- 13.Cooper, R. D., N. J. Snyder, M. J. Zweifel, M. A. Staszak, S. C. Wilkie, T. I. Nicas, D. L. Mullen, T. F. Butler, M. J. Rodriguez, B. E. Huff, and R. C. Thompson. 1996. Reductive alkylation of glycopeptide antibiotics: synthesis and antibacterial activity. J. Antibiot. (Tokyo) 49:575-581. [DOI] [PubMed] [Google Scholar]
- 14.Cui, L., A. Iwamoto, J. Q. Lian, H. M. Neoh, T. Maruyama, Y. Horikawa, and K. Hiramatsu. 2006. Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui, L., H. Murakami, K. Kuwahara-Arai, H. Hanaki, and K. Hiramatsu. 2000. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 44:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinning, A. J., I. S. Al-Adham, I. M. Eastwood, P. Austin, and P. J. Collier. 1998. Pyrithione biocides as inhibitors of bacterial ATP synthesis. J. Appl. Microbiol. 85:141-146. [DOI] [PubMed] [Google Scholar]
- 17.Draghi, D. C., B. M. Benton, K. M. Krause, C. Thornsberry, C. Pillar, and D. F. Sahm. 2008. Comparative surveillance study of telavancin activity against recently collected gram-positive clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:2383-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draghi, D. C., B. M. Benton, K. M. Krause, C. Thornsberry, C. Pillar, and D. F. Sahm. 2008. In vitro activity of telavancin against recent gram-positive clinical isolates: results of the 2004-05 Prospective European Surveillance Initiative. J. Antimicrob. Chemother. 62:116-121. [DOI] [PubMed] [Google Scholar]
- 19.Finan, J. E., G. L. Archer, M. J. Pucci, and M. W. Climo. 2001. Role of penicillin-binding protein 4 in expression of vancomycin resistance among clinical isolates of oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:3070-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franklin, T. J., and G. A. Snow. 2005. Biochemistry and molecular biology of antimicrobial drug action. Springer, New York, NY.
- 21.Grundmann, H., M. Aires-de-Sousa, J. Boyce, and E. Tiemersma. 2006. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368:874-885. [DOI] [PubMed] [Google Scholar]
- 22.Harold, F. M. 1986. The vital force: a study of bioenergetics. W. H. Freeman Co., New York, NY.
- 23.Hegde, S. S., N. Reyes, R. Skinner, and S. Difuntorum. 2008. Efficacy of telavancin in a murine model of pneumonia induced by methicillin-susceptible Staphylococcus aureus. J. Antimicrob. Chemother. 61:169-172. [DOI] [PubMed] [Google Scholar]
- 24.Hegde, S. S., N. Reyes, T. Wiens, N. Vanasse, R. Skinner, J. McCullough, K. Kaniga, J. Pace, R. Thomas, J. P. Shaw, G. Obedencio, and J. K. Judice. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 48:3043-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiramatsu, K., L. Cui, M. Kuroda, and T. Ito. 2001. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 9:486-493. [DOI] [PubMed] [Google Scholar]
- 27.Howden, B. P., P. D. Johnson, P. B. Ward, T. P. Stinear, and J. K. Davies. 2006. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 50:3039-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Judice, J. K., and J. L. Pace. 2003. Semi-synthetic glycopeptide antibacterials. Bioorg. Med. Chem. Lett. 13:4165-4168. [DOI] [PubMed] [Google Scholar]
- 29.Komatsuzawa, H., K. Ohta, S. Yamada, K. Ehlert, H. Labischinski, J. Kajimura, T. Fujiwara, and M. Sugai. 2002. Increased glycan chain length distribution and decreased susceptibility to moenomycin in a vancomycin-resistant Staphylococcus aureus mutant. Antimicrob. Agents Chemother. 46:75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koul, A., N. Dendouga, K. Vergauwen, B. Molenberghs, L. Vranckx, R. Willebrords, Z. Ristic, H. Lill, I. Dorange, J. Guillemont, D. Bald, and K. Andries. 2007. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 3:323-324. [DOI] [PubMed] [Google Scholar]
- 31.Krause, K. M., M. Renelli, S. Difuntorum, T. X. Wu, D. V. Debabov, and B. M. Benton. 2008. In vitro activity of telavancin against resistant gram-positive bacteria. Antimicrob. Agents Chemother. 52:2647-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leadbetter, M. R., S. M. Adams, B. Bazzini, P. R. Fatheree, D. E. Karr, K. M. Krause, B. M. Lam, M. S. Linsell, M. B. Nodwell, J. L. Pace, K. Quast, J. P. Shaw, E. Soriano, S. G. Trapp, J. D. Villena, T. X. Wu, B. G. Christensen, and J. K. Judice. 2004. Hydrophobic vancomycin derivatives with improved ADME properties: discovery of telavancin (TD-6424). J. Antibiot. (Tokyo) 57:326-336. [DOI] [PubMed] [Google Scholar]
- 33.Leuthner, K. D., C. M. Cheung, and M. J. Rybak. 2006. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:338-343. [DOI] [PubMed] [Google Scholar]
- 34.Lipowsky, R. 1991. The conformation of membranes. Nature 349:475-481. [DOI] [PubMed] [Google Scholar]
- 35.Lowy, F. D. 2003. Antimicrobial resistance: the example of Staphylococcus aureus. J. Clin. Investig. 111:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madrigal, A. G., L. Basuino, and H. F. Chambers. 2005. Efficacy of telavancin in a rabbit model of aortic valve endocarditis due to methicillin-resistant Staphylococcus aureus or vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3163-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moise, P. A., G. Sakoulas, A. Forrest, and J. J. Schentag. 2007. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 51:2582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neoh, H. M., S. Hori, M. Komatsu, T. Oguri, F. Takeuchi, L. Cui, and K. Hiramatsu. 2007. Impact of reduced vancomycin susceptibility on the therapeutic outcome of MRSA bloodstream infections. Ann. Clin. Microbiol. Antimicrob. 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicas, T. I., D. L. Mullen, J. E. Flokowitsch, D. A. Preston, N. J. Snyder, R. E. Stratford, and R. D. Cooper. 1995. Activities of the semisynthetic glycopeptide LY191145 against vancomycin-resistant enterococci and other gram-positive bacteria. Antimicrob. Agents Chemother. 39:2585-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novo, D., N. G. Perlmutter, R. H. Hunt, and H. M. Shapiro. 1999. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry 35:55-63. [DOI] [PubMed] [Google Scholar]
- 41.Novo, D. J., N. G. Perlmutter, R. H. Hunt, and H. M. Shapiro. 2000. Multiparameter flow cytometric analysis of antibiotic effects on membrane potential, membrane permeability, and bacterial counts of Staphylococcus aureus and Micrococcus luteus. Antimicrob. Agents Chemother. 44:827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pace, J. L., K. Krause, D. Johnston, D. Debabov, T. Wu, L. Farrington, C. Lane, D. L. Higgins, B. Christensen, J. K. Judice, and K. Kaniga. 2003. In vitro activity of TD-6424 against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3602-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira, P. M., S. R. Filipe, A. Tomasz, and M. G. Pinho. 2007. Fluorescence ratio imaging microscopy shows decreased access of vancomycin to cell wall synthetic sites in vancomycin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 51:3627-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renelli, M., B. Harris, T. J. Beveridge, and B. M. Benton. 2007. Transmission electron microscopy (TEM) study of the ultrastructural effects of telavancin, a novel lipoglycopeptide, on methicillin-resistant Staphylococcus aureus, abstr. C1-1470. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 45.Reynolds, P. E. 1989. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 8:943-950. [DOI] [PubMed] [Google Scholar]
- 46.Rubinstein, E., G. R. Corey, M. E. Stryjewski, H. W. Boucher, F. C. Genter, S. L. Barriere, M. M. Kitt, and H. D. Friedland. 2008. Telavancin for hospital-acquired pneumonia, including ventilator-associated pneumonia: the ATTAIN studies. Clin. Microbiol. Infect. 14(Suppl. 7):S14-S15. [Google Scholar]
- 47.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro, H. M. 2000. Membrane potential estimation by flow cytometry. Methods 21:271-279. [DOI] [PubMed] [Google Scholar]
- 49.Sieradzki, K., M. G. Pinho, and A. Tomasz. 1999. Inactivated pbp4 in highly glycopeptide-resistant laboratory mutants of Staphylococcus aureus. J. Biol. Chem. 274:18942-18946. [DOI] [PubMed] [Google Scholar]
- 50.Sieradzki, K., and A. Tomasz. 2003. Alterations of cell wall structure and metabolism accompany reduced susceptibility to vancomycin in an isogenic series of clinical isolates of Staphylococcus aureus. J. Bacteriol. 185:7103-7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sieradzki, K., and A. Tomasz. 2006. Inhibition of the autolytic system by vancomycin causes mimicry of vancomycin-intermediate Staphylococcus aureus-type resistance, cell concentration dependence of the MIC, and antibiotic tolerance in vancomycin-susceptible S. aureus. Antimicrob. Agents Chemother. 50:527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stryjewski, M. E., V. H. Chu, W. D. O'Riordan, B. L. Warren, L. M. Dunbar, D. M. Young, M. Vallee, V. G. Fowler, Jr., J. Morganroth, S. L. Barriere, M. M. Kitt, and G. R. Corey. 2006. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob. Agents Chemother. 50:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stryjewski, M. E., D. R. Graham, S. E. Wilson, W. O'Riordan, D. Young, A. Lentnek, D. P. Ross, V. G. Fowler, A. Hopkins, H. D. Friedland, S. L. Barriere, M. M. Kitt, and G. R. Corey. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46:1683-1693. [DOI] [PubMed] [Google Scholar]
- 54.Togashi, N., A. Shiraishi, M. Nishizaka, K. Matsuoka, K. Endo, H. Hamashima, and Y. Inoue. 2007. Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules 12:139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomasz, A. 1979. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu. Rev. Microbiol. 33:113-137. [DOI] [PubMed] [Google Scholar]
- 56.van Heijenoort, J. 2007. Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol. Mol. Biol. Rev. 71:620-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiedemann, I., E. Breukink, C. van Kraaij, O. P. Kuipers, G. Bierbaum, B. de Kruijff, and H. G. Sahl. 2001. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276:1772-1779. [DOI] [PubMed] [Google Scholar]