Abstract
Aggregatibacter actinomycetemcomitans strains that express cytolethal distending toxin (Cdt) are associated with localized aggressive periodontitis. However, the in vivo targets of Cdt in the human oral cavity have not been firmly established. Here, we demonstrate that A. actinomycetemcomitans Cdt kills proliferating and nonproliferating U937 monocytic cells at a comparable specific activity, approximately 1.5-fold lower than that against the Cdt-hypersensitive Jurkat T-cell line. Cdt functioned both as a DNase and a phosphatidylinositol 3-phosphate (PIP3) phosphatase, and these activities were distinguished by site-specific mutagenesis of the active site residues of CdtB. Using these mutants, we determined that the DNase activity of CdtB is required for cell cycle arrest and caspase-dependent induction of apoptosis in proliferating U937 cells. In contrast, Cdt holotoxin induced apoptosis by a mechanism independent of caspase- and apoptosis-inducing factor in nonproliferating U937 cells. Furthermore, apoptosis of nonproliferating U937 cells was unaffected by the Cdt mutant possessing reduced phosphatase activity or by the addition of a specific PIP3 phosphatase inhibitor, suggesting that the induction of apoptosis is independent of phosphatase activity. These results indicate that Cdt intoxication of proliferating and nonproliferating U937 cells occurs by distinct mechanisms and suggest that macrophages may also be potential in vivo targets of Cdt.
Aggregatibacter (Actinobacillus) actinomycetemcomitans is associated with localized aggressive periodontitis (LAP) (40, 47, 48), a severe form of periodontal disease that results in the rapid destruction of the periodontal ligament and resorption of alveolar bone. Furthermore, growing evidence suggests that the oral cavity is a microbial reservoir for various systemic infections. In this regard, A. actinomycetemcomitans has also been associated with a variety of nonoral infections, including endocarditis, bacteremia, pericarditis, septicemia, pneumonia, infectious arthritis, osteomyelitis, synovitis, skin infections, urinary tract infections, and abscesses (45).
Although A. actinomycetemcomitans expresses a variety of potential virulence factors, including epithelial cell adhesins (12, 13), an RTX leukotoxin (21, 23), and a cytolethal distending toxin (Cdt) (39, 41), their contributions to disease are not well understood. However, several studies suggest that Cdt may be important in the pathogenesis of LAP. For example, A. actinomycetemcomitans strains that possess the cdt operon are strongly associated with patients diagnosed with LAP (43). In addition, 97% of clinical A. actinomycetemcomitans isolates obtained from periodontitis patients in Sweden, Japan, Kenya, and Brazil possessed the cdt operon and were cytotoxic to CHO cells (10). In a separate study, Ahmed et al. (1) found that 86% of clinical A. actinomycetemcomitans isolates expressed Cdt and were toxic to HEp-2 cells.
Cdt holotoxin is a tripartite complex comprised of subunits CdtA, CdtB, and CdtC (25, 33, 34). The CdtB protein is the active subunit and functions as a type I DNase (9, 24). However, a recent study shows that CdtB also functions as a phosphatidylinositol 3-phosphate (PIP3) phosphatase and that many of the catalytic residues required for DNase activity are also necessary for phosphatase activity (36). CdtB is internalized by target cells, and internalization is inhibited by monensin, suggesting that entry occurs via the endocytic pathway (2). CdtA and CdtC are thought to interact with the target cell surface and may facilitate internalization of CdtB (2, 25, 27, 37). However, Mao and DiRienzo (27) suggest that both CdtB and CdtC are internalized by CHO cells and that CdtC may also possess toxic activity. CdtA is a putative lipoprotein that localizes to the bacterial outer membrane and is processed during secretion of the holotoxin (44).
The in vivo cellular targets of the Cdt toxins are not well defined. Cdt holotoxin induces arrest in the G2 phase of the cell cycle in a variety of proliferating cells, including epithelial cells, fibroblasts, human periodontal ligament cells, and lymphocytes (2, 3, 5, 8, 19, 20, 24, 25, 27, 28, 38, 41). Interestingly, Shenker et al. reported that the specific activity of Cdt against stimulated primary T lymphocytes was five- to 10-fold greater than that against HeLa cells (39) and subsequently showed that the Jurkat T-cell line is hypersensitive to Cdt intoxication (36). These results suggest that lymphocytes may be a primary physiologic target of the A. actinomycetemcomitans Cdt. Additional evidence that cells of the host immune response may be targeted by Cdt came from studies showing that purified Haemophilus ducreyi or Campylobacter jejuni Cdt induced apoptosis in nonproliferating dendritic cells (DCs) and macrophages (16, 26, 42, 46), although the specific activities against these cell types were not determined. Together, these studies suggest that many different cell types are potential targets of Cdt and that active proliferation may not be strictly required for Cdt intoxication.
In this report, we show that A. actinomycetemcomitans Cdt induces apoptosis in both proliferating and nonproliferating U937 monocytic cells at a similar specific activity. Reconstituted Cdt holotoxin was shown to possess both DNase and PIP3 phosphatase activities. Site-specific mutagenesis of CdtB active site residues generated one mutant with reduced DNase but significant phosphatase activity and a second mutant that was reduced in both activities. Cell cycle arrest and caspase 3-dependent induction of apoptosis in proliferating, nondifferentiated U937 cells were dependent on the DNase activity of CdtB. In contrast, Cdt-induced apoptosis in nonproliferating, differentiated U937 cells occurred by a mechanism independent of caspase- and apoptosis-inducing factor (AIF) and did not require a functional PIP3 phosphatase activity. These results suggest that Cdt intoxication of proliferating and nonproliferating U937 cells occurs by distinct mechanisms and that macrophages may be potential in vivo targets of A. actinomycetemcomitans Cdt.
MATERIALS AND METHODS
Strains and culture conditions.
Escherichia coli strains Top10 and BL21 (Invitrogen, Carlsbad, CA) were grown in Luria-Bertani (LB) broth. When necessary, the medium was supplemented with ampicillin at 100 μg/ml. A. actinomycetemcomitans strains 652 and JP2 were cultured under microaerophilic conditions at 37°C in brain heart infusion (Difco) medium supplemented with 40 mg of NaHCO3 per liter. Due to the similarities between Legionella pneumophila-induced and Mycobacterium tuberculosis-induced apoptosis in cultured U937 cells and primary human monocyte-derived macrophages (4, 30), U937 cells were chosen for these studies. The Jurkat T-lymphocyte cell line and the U937 human monocytic cell line were grown in RPMI 1640 medium (Mediatech Cellgro, Herndon, VA) supplemented with 10% bovine growth serum (HyClone, Logan, UT) and 5% penicillin/streptomycin (Sigma, St. Louis, MO) in 5% CO2 at 37°C. When noted, U937 cells were differentiated into an adherent macrophage-like cell with the addition of 100 pg/ml phorbol myristic acid followed by a 48-h to 72-h incubation in 5% CO2 at 37°C. Flow cytometric analysis of differentiated U937 cells exhibited no population in G2, indicating that very few undifferentiated, proliferating U937 cells were present in these wells.
Cdt-expressing plasmids.
CdtA-, CdtB-, and CdtC-expressing plasmids were constructed as follows. An NcoI restriction endonuclease site was introduced at the 5′ ends and an XbaI site was introduced at the 3′ ends of the cdtA, cdtB, and cdtC genes by PCR amplification of 652 chromosomal DNA using CdtA-NcoI-5 and CdtA-XbaI-3 for cdtA, CdtB-F and CdtB-R for cdtB, and CdtC-F and CdtC-R for cdtC (see Table 1 for sequences). DNA was amplified using the following parameters: initially 95°C for 5 min and then 95°C for 1 min, 55°C for 1 min, and 72°C for 3 min for 30 cycles, followed by 72°C for 10 min. The amplified products were ligated into pGEM-T Easy (Promega, Madison, WI) and transformed into competent E. coli BL21 cells. The resulting plasmids and pBAD/gIII A (Invitrogen) were digested with NcoI and XbaI (New England Biolabs, Ipswich, MA); fragments were purified by electrophoresis through a 1% agarose gel and ligated using T4 DNA ligase. After transformation into competent E. coli BL21, recombinants were checked for correct insertion by restriction endonuclease digestion with NcoI and XbaI. The appropriate plasmids were transferred into E. coli Top10 in preparation for protein expression. The resulting plasmids were named pBAD/gIIIA-cdtA, pBAD/gIIIA-cdtB, and pBAD/gIII-cdtC.
TABLE 1.
Primers used in this study
| Primer | Sequencea |
|---|---|
| CdtA-NcoI-5 | GGCACCATGGGGAAGAAGTTTTTACCTGG |
| CdtA-XbaI-3 | GGCATCTAGAGGATTAACCGCTGTTGCTTC |
| CdtB-F | GGCACCATGGAGAACTTGAGTGATTTCAAAGTAGC |
| CdtB-R | GGCATCTAGACGATCACGAACAAAACTAACAGG |
| CdtC-F | GGCACCATGGAGTCAAATCCTGATCCGAC |
| CdtC-R | GGCATCTAGACTACCCTGATTTCTCCCCAC |
| H160G-F | CTGATGTATTTTTTACAGTGGGTGCTTTGGCCACAGGTGG |
| H160G-R | CCACCTGTGGCCAAAGCACCCACTGTAAAAAATACATCAG |
| D199G-F | GGATGGTTGTTGGTGGTTTCAATCGTGCGCCG |
| D199G-R | CGGCGCACGATTGAAACCACCAACAACCATCC |
Restriction endonuclease sequences are in italics.
Two constructs expressing variants of CdtB containing a glycine substitution for the catalytic residues histidine-160 and aspartate-199 (referred to as pBAD/gIIIA-cdtB-H160G and pBAD/gIIIA-cdtB-D199G) were constructed using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). Briefly, 50 ng of pBAD/gIIIA-cdtB DNA was combined with 1.5 μl of forward primer (H160G-F or D199G-F) (10 μM), 1.5 μl of reverse primer (H160G-R or D199G-R) (see Table 1 for sequences) (10 μM), 1 μl of deoxynucleoside triphosphates (supplied by manufacturer), and 1 μl of 2.5 U of PfuUltra polymerase/ml. Amplification was performed with the following parameters: 95°C for 3 min and then 16 cycles of 95°C for 30 s, 55°C for 1 min, and 72°C for 8.5 min, followed by 72°C for 10 min. The amplified products were digested with DpnI to degrade the parental DNA and transformed into Top10 cells. Mutated cdtB genes were verified by nucleotide sequencing.
Cdt protein expression and purification.
Cultures of Top10 containing each cdt construct were grown to early exponential phase in 100 ml LB medium supplemented with ampicillin, and expression of the Cdt subunit was induced by the addition of 0.2% arabinose. Growth was continued for an additional 3 to 4 h, after which the cells were sonicated three times on ice in 1× Tris-EDTA buffer containing 0.2 mg/ml lysozyme and centrifuged at 10,000 × g for 15 min to remove cellular debris. The pellet was then resuspended in 1× Tris-EDTA buffer containing 0.2 mg/ml lysozyme, sonicated three more times on ice, and centrifuged at 10,000 × g for 15 min. The pooled supernatants were collected in Tween 20-coated tubes. The individual Cdt subunits were purified from the resulting supernatants by affinity chromatography over a copper resin (HisTrap resin; Amersham Pharmacia Biotech). The column was first washed with 10 ml distilled H2O, followed by 24 ml of loading buffer (20 mM sodium phosphate, pH 7.5, 0.5 M NaCl, 100 mM imidazole). The protein extract was loaded, and the column was subsequently washed with 24 ml of loading buffer, 24 ml of loading buffer supplemented with 2.5 mg/ml polymyxin B, and then 24 ml of loading buffer. Bound Cdt subunits were eluted with two 8-ml aliquots of elution buffer (20 mM sodium phosphate, pH 7.5, 0.5 M NaCl, 500 mM imidazole). Eluted proteins were dialyzed overnight against 0.1× phosphate-buffered saline (PBS) and lyophilized. Prior to use, each subunit was suspended in distilled H2O and the protein concentration was determined using the Bradford assay (Bio-Rad, Hercules, CA) (6) according to the manufacturer's instructions. Purity was assessed by Coomassie blue staining after polyacrylamide gel electrophoresis of each subunit on 4 to 15% NuPAGE gradient gels (Bio-Rad). To reconstitute Cdt holotoxin, equal amounts of CdtA, CdtB, and CdtC were added to a Tween 20-coated tube and incubated at 37°C for at least 30 min. For some experiments, samples were then boiled at 100°C for 20 min and pulsed briefly prior to use.
Cell cycle arrest.
A total of 1 × 106 U937 cells were suspended in fresh medium containing the desired amount of Cdt holotoxin and incubated for 48 h in low-adherence tissue culture wells. Cells were then washed with PBS, placed on ice, and suspended in the dark in 500 μl PBS-0.1% Triton X-100 supplemented with 2.5 μg propidium iodide and 500 μg RNase. Cells were incubated for 10 to 60 min on ice and analyzed using a FACSCalibur flow cytometer (BD, San Jose, CA) using the FL2 (PE) channel. A line was fit to the data and the maximum G2 activity was calculated. From this curve, the 50% inhibitory concentration (IC50) was identified as the holotoxin concentration which resulted in 50% of the maximum.
Determination of necrosis.
Cdt-mediated cell killing of 1 × 106 differentiated U937 cells was quantified by measuring lactate dehydrogenase (LDH) release using the CytoTox 96 nonradioactive cytotoxicity method (Promega, Madison, WI). Briefly, cells were suspended in fresh medium containing the desired amount of Cdt holotoxin and incubated for 48 h. Following lysis and centrifugation at 180 × g for 5 min, 50 μl of the overlying medium was added to 50 μl of substrate (provided by the manufacturer) and incubated at room temperature in the dark for 30 min. Fifty microliters of stop solution (provided by the manufacturer) was added to each well, and the A490 was measured. Triton X-100 (0.1%) was added to control wells to achieve 100% lysis of U937 cells. Data are presented as percent LDH compared to Triton X-100-treated control wells.
Determination of apoptosis.
A total of 1 × 106 differentiated and undifferentiated U937 cells were suspended in 1 ml of fresh medium containing 10 to 1,000 ng Cdt holotoxin and incubated for 48 h in 5% CO2 at 37°C in low-adherence tissue culture wells. Cells suspended in medium containing 1 μM staurosporine (Ready-Set-Go; Sigma) for 18 to 24 h were used as a positive control. For some experiments, cells were pretreated with 30 to 120 nM of the phosphatase inhibitor dipotassium bisperoxo(picolinato)oxo-vanadate [bpV(pic)] for 2 h followed by incubation with Cdt holotoxin and inhibitor (EMD Calbiochem, San Diego, CA).
For annexin V experiments, cells were washed twice in cold PBS and suspended in 500 μl ice-cold 1× binding buffer supplemented with 5 μl of fluorescein isothiocyanate (FITC)-annexin V and 2.5 μl of 7-amino-actinomycin D (7-AAD; BD Biosciences, San Jose, CA). After a 15-min incubation on ice, 20,000 cells were analyzed using a FACSCalibur flow cytometer using the FL1 (fluorescein isothiocyanate [FITC]) and FL3 (PerCP) channels. Data were plotted as a percentage of annexin-V single-positive cells, which represent apoptotic cells that are not permeable to the 7-AAD dead cell marker.
For terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) experiments, cells were washed in PBS and fixed with 3% paraformaldehyde (Accustain formalin solution, 10%; Sigma) for 1 h while shaking gently. Cells were washed again in PBS and incubated with TUNEL reaction mixture in the dark for 1 h at 37°C as per the manufacturer's instructions (in situ cell death detection kit, fluorescein; Roche Applied Science). Cells were suspended in 500 μl PBS, and 20,000 cells were analyzed by flow cytometry as described above using the FL1 (FITC) channel. Data were plotted as percent TUNEL-positive cells.
For DNA fragmentation enzyme-linked immunosorbent assay (ELISA) experiments, cells were lysed, centrifuged to remove cellular debris, and mixed with anti-histone-biotin and peroxidase-conjugated DNA antibody (anti-DNA-POD) in streptavidin-coated wells according to the manufacturer's directions (cell death detection ELISAPLUS; Roche Applied Science, Indianapolis, IN). After shaking gently for 2 h, the wells were washed and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate solution was added until color development was sufficient for photometric analysis at an optical density at 405 nm. Data were plotted as percent DNA-histone complexes compared to the negative control.
For caspase 3 experiments, cells were washed in blocking buffer (0.5% bovine serum albumin, 0.1% saponin in PBS), fixed in 3% paraformaldehyde for 10 min, and blocked for 1 h. Anti-active caspase 3 (1:1,000; Biovision, Mountain View, CA) was added to the wells in blocking buffer for 45 min, followed by an Alexa Fluor 488-conjugated anti-rabbit antibody (Molecular Probes/Invitrogen) for 45 min in the dark. Twenty thousand cells were analyzed by flow cytometry as explained above using the FL1 (FITC) channel. Data were plotted as percent anti-active caspase 3-positive cells.
For poly-caspase experiments, cells were resuspended for 1 h at 37°C at 5% CO2 in PBS and 6-carboxyfluorescein (FAM)-fluorochrome inhibitor of caspase reagent (provided by the manufacturer; FAM-FLICA kit; Immunochemistry Technologies, Bloomington, MN), washed in the provided buffer, and read on the flow cytometer using the FL1 (FITC) filter.
For AIF experiments, cells were fixed with 3% paraformaldehyde for 30 min and autofluorescence was quenched with NH4Cl2 for 10 min. Following permeabilization and blocking with 0.1% saponin-10% bovine growth serum in PBS (blocking buffer), the cells were incubated with anti-AIF (1:100; Abcam) followed by Alexa Fluor 647-conjugated anti-rabbit (1:200; Molecular Probes Invitrogen) in blocking buffer for 1 h each. Sytox green was incubated in 10 mM Tris, pH 7 (1:5,000), for 10 min and the cells were covered with Mowiol (generously donated by Rey Carabeo) and sealed onto a slide with nail polish. Slides were viewed using an Olympus FluoView confocal laser scanning microscope (Olympus, Pittsburgh, PA) under ×60 magnification.
Analysis of DNase activity.
DNase activity of CdtB was determined using a modification of the protocol by Elwell and Dreyfus (9). Briefly, 0.3 μg CdtB was incubated for 30 min at 37°C with 0.5 μg pUC19 DNA in 25 mM HEPES, pH 7.4, 4 mM MgCl2, and 4 mM CaCl2. The DNA was visualized by electrophoresis through a 1% (wt/vol) agarose gel stained with ethidium bromide.
Analysis of PIP3 phosphatase activity.
The phosphatidylinositol 3-kinase assay (Echelon, Salt Lake City, UT) was adapted to monitor Cdt-mediated degradation of PI(3,4,5,)P3 by using the PIP3 supplied as a positive control in the kit as a substrate for Cdt. Briefly, 3 μg CdtB was incubated for 2 h at 37°C in a Tween 20-coated tube with Tris-buffered saline plus 0.5% Tween 20 and 50 pmol PIP3. For some experiments, cells were incubated with CdtB in the presence of 15 nM or 30 nM of the phosphatase inhibitor bpV(pic) (EMD Calbiochem, San Diego, CA). PIP3 detection antibody was diluted 1:200 in blocking buffer (Tris-buffered saline plus 0.5% Tween 20) and added to each tube. Following a 1-h incubation at room temperature, the mixture was added to a PIP3-coated 96-well strip plate where free antibody was allowed to bind to the plate. The wells were then washed with blocking buffer and incubated with a horseradish peroxidase-conjugated secondary antibody for an additional hour before developing with ABTS substrate solution. The development of color was stopped with the addition of 0.5 M H2SO4, and samples were analyzed spectrophotometrically at an optical density at 405 nm. Data are represented as PIP3 remaining in the tube after a 2-h incubation with toxin, as determined by using an experimentally derived standard curve of PIP3.
RESULTS
Cdt induces cell cycle arrest in proliferating U937 macrophages.
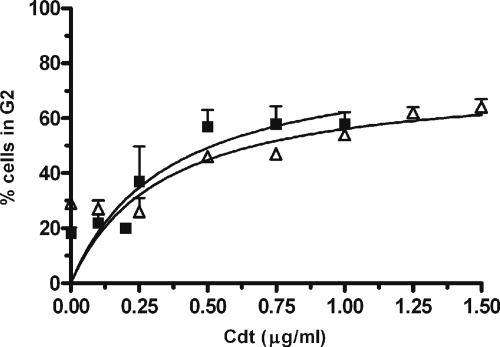
Mise et al. (29) previously showed that A. actinomycetemcomitans Cdt induced cell cycle arrest in proliferating U937 cells. To compare the sensitivity of U937 cells to Cdt-hypersensitive Jurkat cells, cultures were incubated with Cdt holotoxin and cell cycle arrest was determined by flow cytometry. As shown in Fig. 1, both cell lines exhibited a dose-dependent increase of cells arrested in G2. Jurkat cells showed a 20% increase in G2-arrested cells at 0.25 μg Cdt and an increase of 35% at 0.5 μg Cdt. U937 cells were essentially unaffected by 0.25 μg Cdt and exhibited a 17% and 25% increase in G2-arrested cells at 0.5 μg Cdt and 1.0 μg Cdt, respectively. A line was fit to the data, and the maximum G2 activity was calculated. From this curve, the IC50 was identified as the holotoxin concentration which resulted in 50% of the maximum. The IC50 is approximately 0.3 μg/ml for Jurkat cells and 0.4 μg/ml for U937 cells; thus, the specific activity of Cdt holotoxin against proliferating U937 cells is less than 1.5-fold lower than that against Jurkat T cells. Differentiated, nonproliferating U937 cells were used as a control. These cells exhibited no population in G2 either in the absence or presence of Cdt (data not shown).
FIG. 1.
Cdt induces cell cycle arrest in proliferating U937 macrophage cells. Jurkat cells (black squares) and undifferentiated proliferating U937 cells (white triangles) were incubated with 0- to 1.5-μg Cdt holotoxin for 48 h and cell cycle arrest was determined by flow cytometry as described in Materials and Methods. Cells incubated with 1 μM staurosporine for 18 to 24 h were used as a positive control. All experiments were carried out in triplicate, and data are represented as the percentage of cells in the G2 phase of the cell cycle.
Cdt induces apoptosis in both proliferating and nonproliferating U937 cells.
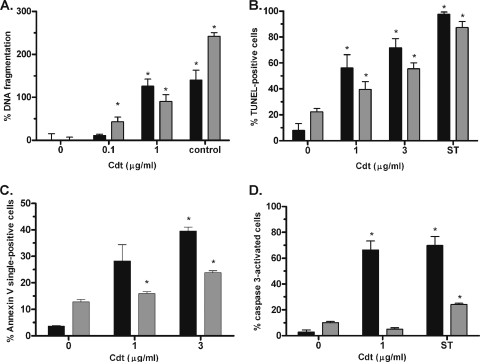
Cdt-dependent induction of G2 arrest leads to apoptosis and extensive DNA fragmentation in Jurkat cells, but Mise et al. (29) did not examine apoptosis in U937 cells. As shown in Fig. 2, treatment of proliferating U937 cells with 1 μg/ml Cdt induced apoptosis, resulting in a 126% increase of DNA-histone complexes (Fig. 2A), a ninefold increase of TUNEL-positive cells (Fig. 2B), an 11-fold increase in annexin V single-positive cells (Fig. 2C), and a 22-fold increase in caspase 3 activation (Fig. 2D) relative to that of untreated cells. Surprisingly, Cdt treatment of differentiated, nonproliferating U937 cells also resulted in a significant increase in DNA-histone complexes (Fig. 2A), DNA fragmentation (Fig. 2B), and a modest but significant increase in annexin V-positive cells (Fig. 2C) when treated with 1 μg/ml holotoxin. Annexin V-positive cells increased further upon exposure to 3 μg holotoxin (Fig. 2C), indicating that the response to Cdt was dose dependent. There are a higher number of TUNEL-positive cells than G2-arrested cells shown in Fig. 1 and 2B because Fig. 1 shows only those cells with a 4N amount of DNA. Since DNA in apoptotic cells is rapidly degraded, the number of apoptotic cells shown in Fig. 1 is underestimated. Treatment of nonproliferating U937 cells for 24 h with 1 μg/ml of Cdt resulted in no significant increase in TUNEL-positive or annexin V-positive cells compared to untreated cells. These results suggest that Cdt is also active against and induces apoptosis in nonproliferating U937 cells. However, Cdt-induced apoptosis of nonproliferating U937 cells was independent of caspase 3 activation (Fig. 2D), suggesting that the mechanism of action of Cdt may differ in proliferating and nonproliferating cells.
FIG. 2.
Cdt induces apoptosis in both proliferating and nonproliferating U937 macrophages. Induction of apoptosis in undifferentiated proliferating U937 cells (black bars) and differentiated nonproliferating U937 cells (gray bars) was determined by measuring DNA-histone complexes (A), TUNEL-positive cells (B), annexin V single-positive cells (C), and activation of caspase 3 (D). A DNA-histone mixture provided by the manufacturer (control) was used a positive control in panel A, and cells incubated with 1 μM staurosporine for 18 to 24 h were used as a positive control in panels B to D. The formation of DNA-histone complexes is plotted as the percent increase relative to the negative control (no Cdt). Data from TUNEL and caspase 3 experiments are plotted as percent positive cells. The results of the annexin V experiment are expressed as percent annexin-V single-positive cells, which represent apoptotic cells that are not permeable to the 7-AAD dead cell stain. Data represent an average of at least three separate experiments. Asterisks indicate a P value of <0.05 and refer to a comparison with the negative control.
Since Cdt-induced apoptosis in nonproliferating U937 cells was unexpected, the mechanism of cell death in these cells was investigated further. To test for necrosis, differentiated U937 cells were treated with holotoxin and culture supernatants were tested for the release of LDH. Although 60% of toxin-treated cells were TUNEL positive after 48 h (Fig. 2B), LDH release was only 10 to 20% of the Triton X-100-treated control cells (Fig. 3A), suggesting that apoptosis is the primary mechanism leading to cell death in nonproliferating cells. Next, the activation of poly-caspases was assessed by monitoring the binding of active caspases to a caspase inhibitor sequence linked to a fluorescent probe. As shown in Fig. 3B, 67% of staurosporine-treated cells were positive for caspase activation after 48 h compared to 30 to 35% of untreated cells. There was no significant increase in apoptotic cells with wild-type or mutant Cdt treatment compared to the untreated control. These results suggest that Cdt-mediated apoptosis of nonproliferating U937 cells occurs independently of caspase activation. Finally, the redistribution of AIF from the mitochondria into the nucleus was monitored to determine the involvement of AIF in apoptosis. Although colocalization of AIF with the nuclear stain Sytox green was observed in staurosporine-treated cells, there was no colocalization in untreated cells or cells treated with Cdt (Fig. 3C).
FIG. 3.
Apoptosis of nonproliferating U937 cells is caspase and AIF independent. LDH release (A), poly-caspase activation (B), and AIF localization (C) were assessed in nonproliferating U937 cells after incubation with wild-type (WT) or mutant forms of Cdt. Cells incubated with 1 μM staurosporine (ST) for 18 to 24 h were used as a positive control. LDH release is plotted as a percentage of total cell lysis (Triton X-100-treated cells), and caspase activation is represented as a percentage of fluorescent (FAM) cells. Asterisks indicate a P value of <0.05 and refer to a comparison with the untreated cells. The microscopy pictures in panel C depict AIF (red) and the Sytox green/nucleus (green). Control represents untreated nonproliferating U937 cells. Colocalization of the two markers is also shown (yellow). Data represent an average of at least three separate experiments.
Role of DNase and phosphatase activities of Cdt in induction of apoptosis.
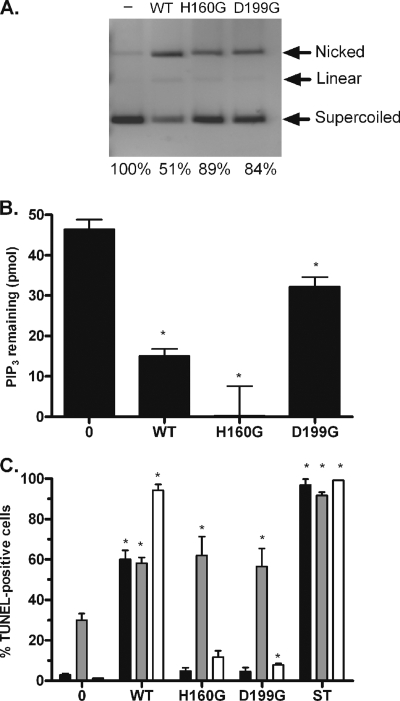
Although Cdt was originally identified as a DNase, Shenker et al. (36) recently showed that Cdt can also function as a PIP3 phosphatase. As shown in Fig. 4, the CdtB enzymatic subunit from A. actinomycetemcomitans possesses both DNase (compare lanes 1 and 2 in Fig. 4A) and PIP3 phosphatase (Fig. 4B) activities, consistent with this report. Site-specific mutagenesis of two putative active site amino acids of CdtB, His160 to Gly (H160G) or Asp199 to Gly (D199G), each resulted in a partial loss of DNase activity (Fig. 4A). However, consistent with previous reports (9, 31, 36), the DNase activity of Cdt was approximately six orders of magnitude lower than that of DNase I (data not shown). Interestingly, H160G retained phosphatase activity, whereas D199G was significantly reduced (Fig. 4B). A similar result was obtained with a dose response of wild-type and mutant forms of CdtB (data not shown). Thus, the two reported functions of Cdt were distinguished by the active site-specific mutations.
FIG. 4.
Role of DNase and PIP3 phosphatase activities in the induction of U937 cell apoptosis. His160 and Asp199, two catalytic residues of A. actinomycetemcomitans CdtB, were altered to glycine by site-directed mutagenesis, and CdtB proteins containing each mutation were purified. These polypeptides were designated H160G and D199G. (A) The DNase activity of the purified CdtB proteins was determined by agarose gel electrophoresis after incubating 0.3 μg CdtB, CdtB-H160G, and CdtB-D199G with 0.5 μg supercoiled pUC19 plasmid DNA for 2 h. Lane 1 contains untreated pUC19 DNA. Nicked, linear, and supercoiled DNA and the percentage of supercoiled DNA compared to the negative control are noted on the image. (B) The PIP3 phosphatase activity of 3 μg CdtB, CdtB-H160G, and CdtB-D199G was determined using the phosphatidylinositol 3-kinase assay as described in Materials and Methods. The data are represented as PIP3 remaining in the tube after a 2-h incubation with the toxin, as determined by using an experimentally derived standard curve of PIP3. (C) Apoptosis of undifferentiated proliferating U937 cells (black), differentiated nonproliferating U937 cells (gray), and Jurkat cells (white) was determined by TUNEL using 1 μg/ml reconstituted Cdt holotoxin comprised of wild type (WT), CdtB-H160G, or CdtB-D199G polypeptides. TUNEL-positive cells were identified by flow cytometry, and staurosporine-treated cells (ST) were used as a positive control. For each experiment, at least 20,000 cells were analyzed, and the data shown represent an average of at least three separate experiments. The data were plotted as the percentage of TUNEL-positive cells. Asterisks indicate a P value of <0.05 and refer to a comparison with the corresponding untreated cells.
To determine how the phosphatase and DNase activities of CdtB contribute to the toxicity of Cdt holotoxin against Jurkat cells and proliferating and nonproliferating U937 cells, cultures were exposed to reconstituted holotoxin containing either wild-type CdtB or the H160G or D199G variant. As shown in Fig. 4C, treatment of proliferating Jurkat and U937 cells with wild-type holotoxin resulted in 94% and 60% TUNEL-positive cells, respectively, whereas treatment with reconstituted holotoxin comprised of either site-specific mutant resulted in little increase in TUNEL-positive cells. The mutant and wild-type holotoxins induced equivalent levels of TUNEL-positive cells when incubated with nonproliferating U937 cells, suggesting that the mutated forms of CdtB form holotoxin complexes as efficiently as wild-type CdtB. Since both CdtB variants retain some PIP3 phosphatase activity, the induction of apoptosis in proliferating cells appears to be dependent primarily on the DNase activity of CdtB. In contrast, apoptosis of nonproliferating U937 cells after treatment with wild-type or mutant holotoxin comprising the CdtB-H160G or CdtB-D199G subunits was similar, suggesting that the mechanism of Cdt action differs in proliferating and nonproliferating U937 cells.
Inhibition of CdtB phosphatase activity does not affect apoptosis of nonproliferating U937 cells.
To determine if the PIP3 phosphatase activity contributes to apoptosis in nonproliferating U937 cells, bpV(pic), a specific PIP3 phosphatase inhibitor (22), was incubated with cells in the presence of wild-type holotoxin. As shown in Fig. 5A, bpV(pic) inhibited CdtB phosphatase activity in a dose-dependent manner, with complete inhibition of CdtB at a concentration of 30 nM. However, treatment of U937 cells with Cdt holotoxin in the presence of up to four times this inhibitory dose had no effect on the induction of TUNEL-positive cells (Fig. 5B), suggesting that Cdt-induced apoptosis of nonproliferating U937 cells is independent of the PIP3 phosphatase activity of CdtB. Inhibitors of DNase activity, e.g., divalent metal ion chelators, also inhibited the phosphatase activity of Cdt (data not shown), and thus it was not possible to directly assess the role of DNase activity in nonproliferating U937 cells.
FIG. 5.
Inhibition of Cdt PIP3 phosphatase activity does not influence Cdt-induced apoptosis of nonproliferating U937 cells. (A) Three micrograms of wild-type CdtB was incubated with 0 nM, 15 nM, or 30 nM of the phosphatase inhibitor bpV(pic) for 15 min prior to the addition of PIP3, and phosphatase activity was determined as described in Materials and Methods. The data are represented as PIP3 remaining in the tube after a 2-h incubation with toxin, as determined by using an experimentally derived standard curve of PIP3. To control for the interference of bpV(pic) on the detection system, reaction mixtures containing the inhibitor were normalized by subtracting the difference between no bpV(pic) and those containing bpV(pic). (B) Apoptosis of differentiated nonproliferating U937 cells was determined by TUNEL using 3 μg of reconstituted Cdt holotoxin comprised of wild-type CdtB in the presence or absence of bpV(pic). TUNEL-positive cells were identified by flow cytometry. For each experiment, at least 20,000 cells were analyzed, and the data are plotted as the percentage of TUNEL-positive cells. Staurosporine-treated cells (ST) were used as the positive control. Data represent an average of at least three separate experiments. Asterisks indicate a P value of <0.05 and refer to a comparison with the negative control.
DISCUSSION
A. actinomycetemcomitans strains expressing Cdt are strongly associated with LAP, but the role of Cdt in disease and the target(s) for cytotoxic activity in vivo have not been clearly defined. Shenker et al. (39) showed that the specific activity of Cdt was five- to 10-fold higher against human lymphocytes than against HeLa cells and suggested that lymphocytes may be a primary target for A. actinomycetemcomitans Cdt. However, although Jotwani et al. suggest that some lymphocyte proliferation may occur locally in the oral cavity (17, 18), it is likely that the majority of lymphocytes infiltrating the gingival pocket are not actively proliferating. Furthermore, nonproliferating macrophages and DCs comprise the majority of the leukocytes and are some of the first immune cells present at the site of A. actinomycetemcomitans infection (15, 32). Thus, it is not clear how Cdt-dependent targeting of proliferating lymphocytes may contribute to A. actinomycetemcomitans infection and persistence. Studies to investigate the effect of Cdt on both proliferating and nonproliferating immune cells may better reflect the environment faced by A. actinomycetemcomitans.
Our results show that A. actinomycetemcomitans Cdt efficiently kills both proliferating and nonproliferating U937 monocytic cells. These results suggest that monocytic cells may also be targeted by A. actinomycetemcomitans, consistent with studies demonstrating that H. ducreyi Cdt is capable of intoxicating monocytic cells (16, 26, 42, 46). However, since these previous studies did not determine the specific activity of CdtB to monocytes, it was difficult to compare the toxin's activity against other known cell types. Here, we show that the specific activity of Cdt against proliferating and nonproliferating U937 cells was similar and was approximately 1.5-fold lower than the Cdt activity against the hypersensitive Jurkat T-cell line (36). These data suggest that A. actinomycetemcomitans may target monocytic cells as a strategy to downregulate the host immune system, although we cannot exclude the possibility that cultured and primary macrophages may not respond identically to holotoxin. It is of interest that the A. actinomycetemcomitans Cdt sequence is most similar to that of H. ducreyi Cdt, since H. ducreyi Cdt has been reported to induce apoptosis in nonproliferating DCs and macrophages and prevents cytokine release and T-cell activation by DCs (46). Additionally, a recent report demonstrated that A. actinomycetemcomitans Cdt may induce localized immunosuppression by inhibiting nitric oxide production in murine peritoneal macrophages (11). Thus, A. actinomycetemcomitans Cdt may contribute to infection by inhibiting nonproliferating antigen-presenting cells and preventing or delaying activation of the adaptive immune response.
Cdt has previously been reported to exhibit DNase activity in vitro, and several lines of evidence suggest that this activity may contribute to the induction of apoptosis in target cells. Cdt intoxication results in the arrest of proliferating cells in the G2 phase of the cell cycle (9, 14, 24), and mutation of CdtB residues that are homologous to active site amino acids of DNase I inhibits cell cycle arrest (9, 24, 36). In addition, H. ducreyi Cdt induces phosphorylation of the histone H2AX and relocation of the DNA repair complex Mre11 in HeLa cells (26), and both of these processes were significantly delayed in ataxia telangiectasia mutated-deficient lymphoblastoid cell lines compared to wild-type cells (7). However, Shenker et al. (35) showed that overexpression of Bcl-2 or treatment with the caspase 3 inhibitor zVAD inhibited Cdt-induced apoptosis and DNA fragmentation in Jurkat cells but did not prevent cell cycle arrest. Subsequently, CdtB was shown to function as a PIP3 phosphatase, and interestingly, many of the catalytic residues required for DNase activity are also required for PIP3 phosphatase activity (36). Our results show that A. actinomycetemcomitans CdtB possesses both DNase and PIP3 phosphatase activities and that these activities can be distinguished by site-specific mutagenesis of the active site residues of CdtB. The CdtB variant H160G possessed reduced DNase activity but maintained PIP3 phosphatase activity, whereas the D199G variant was reduced in both activities. These properties allowed us to investigate the role of the DNase and PIP3 phosphatase activities in the intoxication of proliferating and nonproliferating cells. It should be noted that although our CdtB-D199G variant and the CdtB-D199S mutant reported by Shenker et al. (36) lost both activities, our CdtB-H160G variant retained phosphatase activity, unlike the corresponding CdtB-H160Q mutant reported by Shenker et al. This suggests that some flexibility exists in the amino acid requirement at position 160 with respect to the PIP3 phosphatase activity of CdtB. Furthermore, it is of interest that the H160G variant may have increased activity over the wild-type form. There is no structural information regarding a conformational change, but we can speculate that the replacement of glycine for histidine at position 160 may increase substrate binding and/or catalytic activity.
The CdtB-H160G and CdtB-D199G variants were ineffective in causing cell cycle arrest and apoptosis in proliferating cells, consistent with results reported for site-specific mutants of the E. coli Cdt (9) and C. jejuni Cdt (24). Since CdtB-H160G retained its PIP3 phosphatase activity, this result suggests that the phosphatase activity of CdtB may not be required to induce cell cycle arrest in proliferating U937 cells. However, we cannot exclude the possibility that induction of apoptosis in proliferating U937 cells requires both DNase and phosphatase activities of Cdt and that reduction of either activity is sufficient to prevent cell death.
In contrast to proliferating cells, Cdt-mediated apoptosis in nonproliferating U937 cells is unusual since it does not require caspase activation or mobilization of AIF. We are further investigating how apoptosis occurs in these cells by examining the contribution of other proteases such as cathepsins, calpains, serine proteases, or the proteasome complex. In addition, apoptosis in nonproliferating cells still occurred when cells were exposed to holotoxin comprising the CdtB-D199G variant or to holotoxin containing the wild-type CdtB in the presence of the specific PIP3 phosphatase inhibitor bpV(pic). This suggests that Cdt-mediated apoptosis in nonproliferating cells is independent of the PIP3 phosphatase activity. The contribution of the DNase activity of CdtB in the induction of apoptosis of nonproliferating U937 cells could not be directly assessed since inhibitors of DNase also blocked phosphatase activity. However, both site-specific CdtB mutants were inactive against proliferating U937 cells but remained fully active against nonproliferating cells. Thus, nonproliferating cells are either more sensitive to Cdt DNase, since residual activity remained in both CdtB variants, or apoptosis in nonproliferating cells occurs independently of the DNase or PIP3 phosphatase activity of Cdt.
In summary, the A. actinomycetemcomitans Cdt induces apoptosis in both proliferating and nonproliferating U937 monocytic cells at a similar specific activity. The induction of apoptosis in proliferating U937 cells was caspase dependent and required the DNase activity of CdtB. In contrast, apoptosis in nonproliferating cells was AIF and caspase independent and was not affected by CdtB active site mutations. These results suggest that Cdt intoxication of proliferating and nonproliferating U937 cells occurs by distinct mechanisms and that macrophages may be potential in vivo targets of A. actinomycetemcomitans Cdt.
Acknowledgments
We thank DeAnna James for construction of the wild-type Cdt expression plasmids; Deanne Pierce, Carlo Daep, and George Hajishengallis for critical reading of the manuscript; Rey Carabeo for the generous donation of Mowiol; and George Hajishengallis for helpful technical suggestions and assistance with the flow cytometer.
This work was supported by Public Health Service grants RO1DE10729 (D.R.D.) and F32 DE018068 (S.D.P.R.) from the National Institutes of Health.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 26 May 2009.
REFERENCES
- 1.Ahmed, H. J., L. A. Svensson, L. D. Cope, J. L. Latimer, E. J. Hansen, K. Ahlman, J. Bayat-Turk, D. Klamer, and T. Lagergard. 2001. Prevalence of cdtABC genes encoding cytolethal distending toxin among Haemophilus ducreyi and Actinobacillus actinomycetemcomitans strains. J. Med. Microbiol. 50860-864. [DOI] [PubMed] [Google Scholar]
- 2.Akifusa, S., W. Heywood, S. P. Nair, G. Stenbeck, and B. Henderson. 2005. Mechanism of internalization of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiology 1511395-1402. [DOI] [PubMed] [Google Scholar]
- 3.Akifusa, S., S. Poole, J. Lewthwaite, B. Henderson, and S. P. Nair. 2001. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect. Immun. 695925-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arcila, M. L., M. D. Sanchez, B. Ortiz, L. F. Barrera, L. F. Garcia, and M. Rojas. 2007. Activation of apoptosis, but not necrosis, during Mycobacterium tuberculosis infection correlated with decreased bacterial growth: role of TNF-α, IL-10, caspases and phospholipase A2. Cell. Immunol. 24980-93. [DOI] [PubMed] [Google Scholar]
- 5.Belibasakis, G., A. Johansson, Y. Wang, R. Claesson, C. Chen, S. Asikainen, and S. Kalfas. 2002. Inhibited proliferation of human periodontal ligament cells and gingival fibroblasts by Actinobacillus actinomycetemcomitans: involvement of the cytolethal distending toxin. Eur. J. Oral Sci. 110366-373. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 7.Cortes-Bratti, X., C. Karlsson, T. Lagergard, M. Thelestam, and T. Frisan. 2001. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J. Biol. Chem. 2765296-5302. [DOI] [PubMed] [Google Scholar]
- 8.DiRienzo, J. M., M. Song, L. S. Wan, and R. P. Ellen. 2002. Kinetics of KB and HEp-2 cell responses to an invasive, cytolethal distending toxin-producing strain of Actinobacillus actinomycetemcomitans. Oral Microbiol. Immunol. 17245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elwell, C. A., and L. A. Dreyfus. 2000. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 37952-963. [DOI] [PubMed] [Google Scholar]
- 10.Fabris, A. S., J. M. DiRienzo, M. Wikstrom, and M. P. Mayer. 2002. Detection of cytolethal distending toxin activity and cdt genes in Actinobacillus actinomycetemcomitans isolates from geographically diverse populations. Oral Microbiol. Immunol. 17231-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes, K. P., M. P. Mayer, E. S. Ando, A. G. Ulbrich, J. G. Amarente-Mendes, and M. Russo. 2008. Inhibition of interferon-γ-induced nitric oxide production in endotoxin-activated macrophages by cytolethal distending toxin. Oral Microbiol. Immunol. 23360-366. [DOI] [PubMed] [Google Scholar]
- 12.Fine, D. H., D. Furgang, H. C. Schreiner, P. Goncharoff, J. Charlesworth, G. Ghazwan, P. Fitzgerald-Bocarsly, and D. H. Figurski. 1999. Phenotypic variation in Actinobacillus actinomycetemcomitans during laboratory growth: implications for virulence. Microbiology 1451335-1347. [DOI] [PubMed] [Google Scholar]
- 13.Fine, D. H., P. Goncharoff, H. Schreiner, K. M. Chang, D. Furgang, and D. Figurski. 2001. Colonization and persistence of rough and smooth colony variants of Actinobacillus actinomycetemcomitans in the mouths of rats. Arch. Oral Biol. 461065-1078. [DOI] [PubMed] [Google Scholar]
- 14.Frisk, A., M. Lebens, C. Johansson, H. Ahmed, L. Svensson, K. Ahlman, and T. Lagergard. 2001. The role of different protein components from the Haemophilus ducreyi cytolethal distending toxin in the generation of cell toxicity. Microb. Pathog. 30313-324. [DOI] [PubMed] [Google Scholar]
- 15.Gemmell, E., K. Yamazaki, and G. J. Seymour. 2007. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol. 2000 4314-40. [DOI] [PubMed] [Google Scholar]
- 16.Hickey, T. E., G. Majam, and P. Guerry. 2005. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytolethal distending toxin. Infect. Immun. 735194-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jotwani, R., and C. W. Cutler. 2003. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. J. Dent. Res. 82736-741. [DOI] [PubMed] [Google Scholar]
- 18.Jotwani, R., A. K. Palucka, M. Al-Quotub, M. Nouri-Shirazi, J. Kim, D. Bell, J. Banchereau, and C. W. Cutler. 2001. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J. Immunol. 1674693-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamen, P. R. 1983. Inhibition of keratinocyte proliferation by extracts of Actinobacillus actinomycetemcomitans. Infect. Immun. 421191-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang, P., J. Korostoff, A. Volgina, W. Grzesik, and J. M. DiRienzo. 2005. Differential effect of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans on co-cultures of human oral cells. J. Med. Microbiol. 54785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolodrubetz, D., T. Dailey, J. Ebersole, and E. Kraig. 1989. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect. Immun. 571465-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai, J. P., J. T. Dalton, and D. L. Knoell. 2007. Phosphatase and tensin homologue deleted on chromosome ten (PTEN) as a molecular target in lung epithelial wound repair. Br. J. Pharmacol. 1521172-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lally, E. T., I. R. Kieba, D. R. Demuth, J. Rosenbloom, E. E. Golub, N. S. Taichman, and C. W. Gibson. 1989. Identification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem. Biophys. Res. Commun. 159256-262. [DOI] [PubMed] [Google Scholar]
- 24.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290354-357. [DOI] [PubMed] [Google Scholar]
- 25.Lara-Tejero, M., and J. E. Galan. 2001. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 694358-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, L., A. Sharipo, E. Chaves-Olarte, M. G. Masucci, V. Levitsky, M. Thelestam, and T. Frisan. 2002. The Haemophilus ducreyi cytolethal distending toxin activates sensors of DNA damage and repair complexes in proliferating and non-proliferating cells. Cell. Microbiol. 487-99. [DOI] [PubMed] [Google Scholar]
- 27.Mao, X., and J. M. DiRienzo. 2002. Functional studies of the recombinant subunits of a cytolethal distending holotoxin. Cell. Microbiol. 4245-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer, M. P., L. C. Bueno, E. J. Hansen, and J. M. DiRienzo. 1999. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect. Immun. 671227-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mise, K., S. Akifusa, S. Watarai, T. Ansai, T. Nishihara, and T. Takehara. 2005. Involvement of ganglioside GM3 in G2/M cell cycle arrest of human monocytic cells induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Infect. Immun. 734846-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molmeret, M., S. D. Zink, L. Han, A. Abu-Zant, R. Asari, D. M. Bitar, and Y. Abu Kwaik. 2004. Activation of caspase-3 by the Dot/Icm virulence system is essential for arrested biogenesis of the Legionella-containing phagosome. Cell. Microbiol. 633-48. [DOI] [PubMed] [Google Scholar]
- 31.Nesić, D., Y. Hsu, and C. E. Stebbins. 2004. Assembly and function of a bacterial genotoxin. Nature 429429-433. [DOI] [PubMed] [Google Scholar]
- 32.Page, R. C., and H. E. Schroeder. 1976. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab. Investig. 34235-249. [PubMed] [Google Scholar]
- 33.Saiki, K., T. Gomi, and K. Konishi. 2004. Deletion and purification studies to elucidate the structure of the Actinobacillus actinomycetemcomitans cytolethal distending toxin. J. Biochem. (Tokyo) 136335-342. [DOI] [PubMed] [Google Scholar]
- 34.Saiki, K., K. Konishi, T. Gomi, T. Nishihara, and M. Yoshikawa. 2001. Reconstitution and purification of cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiol. Immunol. 45497-506. [DOI] [PubMed] [Google Scholar]
- 35.Shenker, B. J., D. R. Demuth, and A. Zekavat. 2006. Exposure of lymphocytes to high doses of Actinobacillus actinomycetemcomitans cytolethal distending toxin induces rapid onset of apoptosis-mediated DNA fragmentation. Infect. Immun. 742080-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shenker, B. J., M. Dlakic, L. P. Walker, D. Besack, E. Jaffe, E. LaBelle, and K. Boesze-Battaglia. 2007. A novel mode of action for a microbial-derived immunotoxin: the cytolethal distending toxin subunit B exhibits phosphatidylinositol 3,4,5-triphosphate phosphatase activity. J. Immunol. 1785099-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shenker, B. J., R. H. Hoffmaster, A. Zekavat, N. Yamaguchi, E. T. Lally, and D. R. Demuth. 2001. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J. Immunol. 167435-441. [DOI] [PubMed] [Google Scholar]
- 38.Shenker, B. J., M. E. Kushner, and C. C. Tsai. 1982. Inhibition of fibroblast proliferation by Actinobacillus actinomycetemcomitans. Infect. Immun. 38986-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shenker, B. J., T. McKay, S. Datar, M. Miller, R. Chowhan, and D. Demuth. 1999. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J. Immunol. 1624773-4780. [PubMed] [Google Scholar]
- 40.Slots, J., H. S. Reynolds, and R. J. Genco. 1980. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 291013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugai, M., T. Kawamoto, S. Y. Peres, Y. Ueno, H. Komatsuzawa, T. Fujiwara, H. Kurihara, H. Suginaka, and E. Oswald. 1998. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect. Immun. 665008-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svensson, L. A., A. Tarkowski, M. Thelestam, and T. Lagergard. 2001. The impact of Haemophilus ducreyi cytolethal distending toxin on cells involved in immune response. Microb. Pathog. 30157-166. [DOI] [PubMed] [Google Scholar]
- 43.Tan, K. S., K. P. Song, and G. Ong. 2002. Cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Occurrence and association with periodontal disease. J. Periodontal Res. 37268-272. [DOI] [PubMed] [Google Scholar]
- 44.Ueno, Y., M. Ohara, T. Kawamoto, T. Fujiwara, H. Komatsuzawa, E. Oswald, and M. Sugai. 2006. Biogenesis of the Actinobacillus actinomycetemcomitans cytolethal distending toxin holotoxin. Infect. Immun. 743480-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Winkelhoff, A. J., and J. Slots. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontol. 2000 20122-135. [DOI] [PubMed] [Google Scholar]
- 46.Xu, T., A. Lundqvist, H. J. Ahmed, K. Eriksson, Y. Yang, and T. Lagergard. 2004. Interactions of Haemophilus ducreyi and purified cytolethal distending toxin with human monocyte-derived dendritic cells, macrophages and CD4+ T cells. Microbes Infect. 61171-1181. [DOI] [PubMed] [Google Scholar]
- 47.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 121-20. [DOI] [PubMed] [Google Scholar]
- 48.Zambon, J. J., J. Slots, and R. J. Genco. 1983. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect. Immun. 4119-27. [DOI] [PMC free article] [PubMed] [Google Scholar]