Abstract
Elderly individuals have increased morbidity and mortality associated with infectious diseases due in part to the progressive age-associated decline in immune function. Despite this, the old mouse model of Mycobacterium tuberculosis infection has revealed a CD8- and gamma interferon (IFN-γ)-dependent early resistance to infection. In this study, we investigated the mechanism by which CD8 T cells from old mice contributed to the early immune response to M. tuberculosis. Following a low-dose aerosol infection with M. tuberculosis, CD8 T cells were identified as being a dominant source of IFN-γ expression in the lungs of old mice early after infection, before the typical onset of antigen-specific immunity. In addition, M. tuberculosis-induced IFN-γ production by CD8 T cells isolated from naïve old mice was major histocompatibility complex class I independent but was dependent on interleukin-12p70, confirming an innate role of CD8 T cells during M. tuberculosis infection. Moreover, the ability of CD8 T cells from old mice to produce increased innate IFN-γ levels in response to M. tuberculosis infection was defined as a unique function of CD8 T cells from old mice and not the aged lung environment. Finally, we have identified increased expression of SET as being one possible mechanism by which CD8 T cells from old mice produce enhanced levels of IFN-γ. Additional characterizations of the signaling events that lead to enhanced innate IFN-γ production by CD8 T cells in old mice may lead to novel strategies to further enhance or perpetuate beneficial immune responses in the elderly.
The world's elderly population is rapidly expanding and is predicted to reach 1.5 billion by the year 2050, a 28% increase since 2000 (http://esa.un.org/unpp/). The largest concentration of elderly individuals (projected to be 78% by the year 2050) is in developing countries (http://esa.un.org/unpp/), where many infectious diseases, including tuberculosis (35), are endemic. Given the increased susceptibility of the elderly to infectious diseases, this rapid rise in the elderly population poses a significant threat to global health care. Research focused on characterizing the immune response of the elderly to pathogens that cause substantial morbidity and mortality in elderly individuals is an area that has been significantly neglected; however, such studies would have a considerable impact on the prevention and treatment of infectious diseases in the elderly.
As an individual ages, significant immunological changes occur, which contribute to the enhanced morbidity and mortality associated with infectious diseases in the elderly. After puberty, thymic atrophy leads to a progressive decrease in the output of naïve T cells and decreased diversity in the T-cell repertoire (7). Consequently, the periphery of an elderly individual is dominated by antigen-experienced or memory T cells, leading to a significant impairment of immune responses to new antigenic challenges (36). In addition to the immune defects in the T-cell compartment, significant deficiencies in B cells (27), NK cells (21, 22), macrophages (15, 20), and dendritic cells (19, 28) have been reported. Despite the abundant evidence that the immune system of the aged is significantly altered, there is mounting data suggesting that some components of the immune system, particularly CD8 T cells, remain functionally intact or are enhanced in old age (11, 26, 34). The characterization of immune responses in the elderly, specifically to pathogens, may lead to more effective vaccination and therapeutic strategies in this highly vulnerable and expanding population.
We previously demonstrated that although old mice fail to contain a chronic infection with Mycobacterium tuberculosis (23, 32), during the first 2 weeks following infection, old mice display an enhanced resistance to infection that is gamma interferon (IFN-γ) and CD8 dependent (32, 33). In addition, characterization of in vitro responses of naïve CD8 T cells from old mice to Th1 cytokines identified an interleukin-12 (IL-12)-driven mechanism of enhanced IFN-γ production by these cells (34). Since IL-12 is detected early in the lungs of M. tuberculosis-infected old mice (34), we hypothesized that this same mechanism of innate IFN-γ production was also biologically relevant during M. tuberculosis infection.
The aim of the present study was to define and characterize the mechanism by which CD8 T cells contribute to innate immune responses following M. tuberculosis infection. Using both in vivo and in vitro models of M. tuberculosis infection of old mice, we demonstrated that CD8 T cells were a major source of innate IFN-γ. In addition, in vitro assays using purified pulmonary CD8 and CD11c cells from naïve mice conclusively demonstrated that M. tuberculosis-driven IFN-γ production by CD8 T cells from old mice was dependent on IL-12p70 and independent of major histocompatibility complex (MHC)-T-cell receptor interactions. Finally, we provide evidence that suggests that one mechanism by which CD8 T cells from old mice are capable of enhanced innate IFN-γ production is through the regulation of IL-12 signaling.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) or through a contract with the National Institute on Aging (Supplied by Charles River Laboratories). Young mice were 2 to 3 months of age, and old mice were 16 to 18 months of age. Two- to six-month-old IL-12p35−/−, β2m−/−, and C57BL/6 wild-type control female mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Repeat experiments were performed using young and old BALB/c mice in some studies, with similar results.
Cell isolation.
Lungs were perfused through the pulmonary artery with phosphate-buffered saline containing 50 U/ml of heparin (Sigma, St. Louis, MO) and placed into Dulbecco's modified Eagle's medium (DMEM) (500 ml; Mediatech, Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum (Atlas Biologicals, Ft. Collins, CO), 1% HEPES buffer (1 M; Sigma), 1% l-glutamine (200 nM; Sigma), 10 ml of a 100× minimal essential medium-nonessential amino acid solution (Sigma), 5 ml of a penicillin-streptomycin solution (50,000 U penicillin and 50 mg streptomycin; Sigma), and 0.1% β-mercaptoethanol 2-hydroxyethylmercaptan (50 mM; Sigma) (complete DMEM). A single-cell suspension was obtained using enzymatic digestion as described previously (32). Viable cells were counted using trypan blue exclusion and resuspended at working concentrations in complete DMEM. Single-cell suspensions of splenocytes were obtained by pressing individual spleens through a sterile 70-μm nylon mesh screen, followed by erythrocyte lysis with ACK lysis buffer (0.15 M NH4Cl, 1 mM KHCO3) for 3 min. Cells were washed in complete DMEM, counted using trypan blue exclusion, and resuspended at working concentrations in complete DMEM.
Cell purifications.
For CD8+, NK, and CD11c+ cell purifications, single-cell suspensions from individual mice were resuspended in complete DMEM and cultured for 1 h in 100-mm tissue culture dishes (BD Biosciences) at 37°C with 5% CO2 to allow cell adherence. For CD8+ and NK cell purification, nonadherent cells were collected by vigorous pipetting, counted using trypan blue exclusion, and centrifuged. Cell pellets were incubated with 50 μl of CD8 DM beads (BD Biosciences) per 107 cells for 30 min at 4°C. For NK cells, cells were incubated with 50 μl of anti-DX5 antibody per 107 cells for 15 min at 4°C, washed, and incubated with 50 μl of anti-phycoerythrin (PE) beads per 107 cells for 30 min at 4°C. Cells were resuspended in 1 ml of complete DMEM and purified using a BD IMagnet apparatus according to the manufacturer's instructions (BD Biosciences). Purified cells were counted using trypan blue exclusion and adjusted to working concentrations in complete DMEM. CD8+ and NK cells were assessed for purity by flow cytometry and typically exceeded 95% purity. For CD11c+ cell purification, adherent cells were washed with warm phosphate-buffered saline twice and incubated with trypsin-EDTA (Sigma) for 10 min at 37°C. Adherent cells were collected, washed in complete DMEM, and counted using trypan blue exclusion. Cells were incubated with 2.5 μg of CD11c+ biotin (BD Biosciences) for 15 min at 4°C and washed in complete DMEM. Fifty microliters of streptavidin beads (BD Biosciences) per 107 cells was incubated with the cells for 30 min at 4°C and resuspended in 1 ml of complete DMEM. CD11c+ cells were purified on a BD IMagnet apparatus according to the manufacturer's instructions. Purified cells were counted using trypan blue exclusion and adjusted to working concentrations in complete DMEM. Purity was assessed by flow cytometry and was typically greater than 90%.
In vitro cell culture.
Single-cell suspensions obtained from whole lungs were cultured in complete DMEM without antibiotics either alone or with M. tuberculosis Erdman (multiplicity of infection [MOI] of 0.5) for 12 to 24 h (reverse transcription [RT]-PCR) or 48 h (IFN-γ enzyme-linked immunosorbent assay [ELISA]). For overlay cultures, CD11c+ cells were cultured along with M. tuberculosis Erdman and CD8+ cells (1:1:1 ratio of CD8 to CD11c to M. tuberculosis cells) for 48 h. For IL-12 neutralization, anti-IL-12 (clone C17.8; BioXCell, West Lebanon, NH) or rat immunoglobulin G2A isotype control was added at 1 μg/ml. Antibody concentrations were optimized earlier.
For antigen-independent assays, CD11c+ cells were cultured either alone or with M. tuberculosis Erdman (MOI of 1) for 72 h at 37°C with 5% CO2. Culture supernatants were frozen at −80°C. Prior to use, cell supernatants were thawed and filtered with a 0.2-μm microcentrifuge filter (Corning, Lowell, MA). CD8+ cells were cultured with 150 μl of filtered CD11c+ supernatant for 48 h at 37°C with 5% CO2.
To assess the induction of IFN-γ production by p35- and p40-containing cytokines, CD8+ cells purified from the spleens of naïve old mice were cultured with medium, IL-12p70 (5 ng/ml; PeproTech, Rocky Hill, NJ), IL-23 (20 ng/ml; R&D Systems, Minneapolis, MN), IL-12p40 (20 ng/ml; PeproTech), or IL-12p40p40 (20 ng/ml; R&D Systems) for 48 h. All cultures also contained IL-18 (20 ng/ml; R&D Systems) to amplify the amount of detectable IFN-γ. IL-18 alone does not drive IFN-γ production in old CD8 T cells.
To assess SET expression levels in vitro, CD8+ cells purified from the spleen were cultured either alone or with a combination of IL-2 (100 ng/ml; R&D Systems), IL-12p70 (5 ng/ml; PeproTech), and IL-18 (10 ng/ml, R&D Systems) for 1, 4, and 7 h.
For flow cytometric analysis, CD8+ cells that were purified from the lungs of individual mice were cultured with 40 μM 1,9-dideoxyforskolin (Calbiochem, Gibbstown, NJ) or a dimethyl sulfoxide (DMSO) control for 30 min and then cultured with a combination of IL-2 (100 ng/ml), IL-12p70 (5 ng/ml), and IL-18 (10 ng/ml) or complete DMEM for 4 h.
In vivo bacterial infections.
M. tuberculosis Erdman (ATCC 35801) was obtained from the American Type Culture Collection (Manassas, VA) and grown in Proskauer-Beck liquid medium containing 0.05% Tween 80 (Sigma) to mid-log phase. Bacterial suspensions were frozen in aliquots at −80°C. Mice were aerogenically infected with a low dose of M. tuberculosis Erdman (50 to 100 CFU). Lungs were harvested for CD8+ and NK cell purification at 0, 8, and 12 days postinfection.
Real-time PCR.
Purified CD8+ and NK cells were homogenized in 1 ml Trizol (Invitrogen, Carlsbad, CA) and frozen at −80°C. RNA was isolated by chloroform extraction and isopropanol precipitation. RNA was further purified using an RNeasy minikit (Qiagen, Valencia, CA). RNA was then reverse transcribed with Omniscript RT (Qiagen), and cDNA was amplified by real-time PCR using a Bio-Rad (Hercules, CA) iCycler. TaqMan gene expression assays for IFN-γ and SET were used for all real-time PCR assays (Applied Biosystems, Foster City, CA). The 18S rRNA gene was used as an endogenous normalizer, and the delta delta cycle threshold method was used for relative quantification of mRNA expression.
Flow cytometry.
BD Phosflow Protocol III for mouse splenocytes or thymocytes was used to measure intracellular pSTAT4 and IFN-γ in CD8 T cells from the lungs of individual mice. Lung cells were concomitantly stained with 0.3 to 0.6 μg IFN-γ PE-Cy7 (clone XMG1.2), pSTAT4Tyr693 Alexa Fluor 488 (clone 38/pSTAT4), CD3ɛ PE (clone 145-2C11), and CD8 Alexa Fluor 647 (clone 53-6.7) or isotype control antibodies (BD Biosciences). Samples were run on a LSRII flow cytometer, and data were analyzed using FACSDiva software (BD Biosciences). Lymphocytes were gated based on their characteristic forward- and side-scatter profiles. CD8 T cells were subsequently identified by the presence of specific fluorescently labeled antibodies against CD8 in combination with CD3ɛ. IFN-γ and pSTAT4 were measured within this population of CD8 T cells, and quadrant gates were set according to the staining of isotype control antibodies.
ELISA.
Cell culture supernatants cultured for 48 h were thawed, and the concentration of IFN-γ protein was measured by ELISA. Purified anti-IFN-γ (clone R4-6A2) and biotin anti-IFN-γ (clone XMG1.2) were used for capture and detection antibodies, respectively (BD Biosciences).
Statistical analysis.
Statistical significance was determined using Prism 4 software (GraphPad Software, San Diego, CA). The unpaired two-tailed Student t test was used for comparisons of young and old mice, and a one-way analysis of variance (ANOVA) with Tukey's posttest was used for multigroup comparisons.
RESULTS
M. tuberculosis infection drives IFN-γ expression in pulmonary CD8 T cells in old mice.
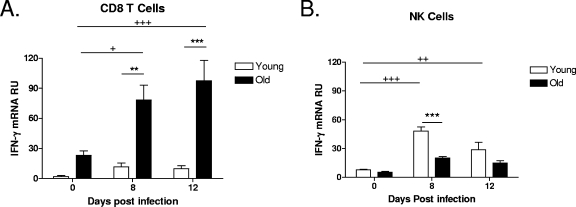
During M. tuberculosis infection, old mice express an elevated level of IFN-γ mRNA throughout the first 8 to 12 days (34), and we have hypothesized that resident CD8 T cells within the aged lung are the source of IFN-γ. To determine the in vivo cellular source of IFN-γ within the lungs of old mice during M. tuberculosis infection, young and old mice were infected with a low-dose aerosol of M. tuberculosis, and at various time points postinfection, CD8 T cells were purified from the lungs, and IFN-γ mRNA was quantified using RT-PCR directly ex vivo without further stimulation. Baseline IFN-γ mRNA levels within purified CD8 T cells were increased in naïve old mice relative to those in young mice (day 0), suggesting that these cells may be primed to rapidly synthesize IFN-γ (Fig. 1A). In response to M. tuberculosis infection, old mice had an additional and significant increase in IFN-γ mRNA levels within CD8 T cells as infection progressed (Fig. 1A). In contrast, pulmonary CD8 T cells from young mice did not generate IFN-γ mRNA in response to M. tuberculosis infection throughout the 12-day period studied. Therefore, CD8 T cells within the lungs of old mice have the capacity to synthesize IFN-γ in response to infection with M. tuberculosis.
FIG. 1.
CD8 T cells isolated from the lungs of old mice are a major source of IFN-γ following M. tuberculosis infection. Young and old mice were infected aerogenically with M. tuberculosis Erdman, and lungs were harvested at 0, 8, and 12 days postinfection (n = 3 to 5). CD8 cells (A) and NK cells (B) were purified from the lungs of young and old mice using magnetic beads. Purified cells were homogenized in Trizol and frozen at −80°C. RNA was isolated and reverse transcribed, and cDNA was amplified for IFN-γ by RT-PCR. The relative expression levels of IFN-γ mRNA are shown as means ± standard errors of the means (SEM) and are representative of data from at least two individual experiments. Statistical significance was determined using a one-way ANOVA with Tukey's posttest (**, P value of <0.01; ***, P value of <0.001 [significance between young and old mice]; +, P value of <0.05; ++, P value of <0.01; +++, P value of <0.001 [significance between time points for old mice, as there were no statistically significant increases for young mice]). RU, relative units.
NK cells, like CD8 T cells from old mice, can also be an early source of IFN-γ in response to infection (10, 13), and therefore, we purified NK cells from the lungs of old and young mice during early M. tuberculosis infection and determined IFN-γ mRNA levels within this specific cell subset. In contrast to CD8 T cells, there was no significant increase in the levels of IFN-γ mRNA within NK cells from the lungs of old mice in response to M. tuberculosis infection (Fig. 1B). NK cells purified from the lungs of M. tuberculosis-infected young mice expressed a significant increase in IFN-γ mRNA levels after 8 days of infection, which dissipated by day 12, indicating an early functional role for NK cells in the lungs of young mice. We therefore formally demonstrate that CD8 T cells are an early source of IFN-γ mRNA in the lungs of old mice during M. tuberculosis infection and also show that the dominant cellular sources of innate IFN-γ during M. tuberculosis infection in vivo may differ between young and old mice.
Pulmonary CD8 T cells isolated from naïve old mice produce innate IFN-γ following in vitro stimulation with M. tuberculosis.
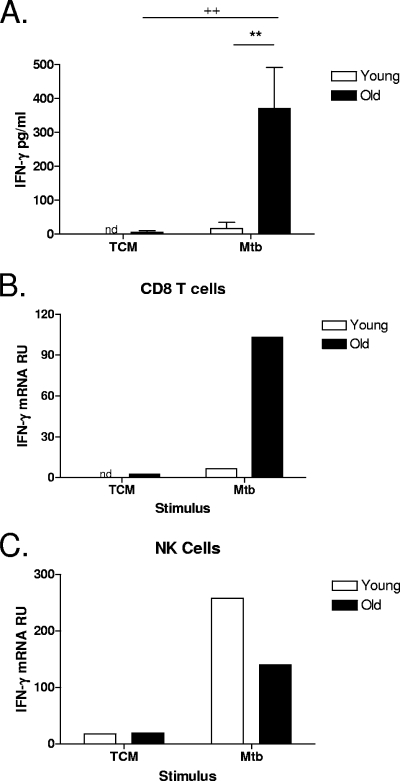
To determine the mechanism by which CD8 T-cell-derived IFN-γ from old mice contributed to early immune responses to M. tuberculosis infection, we established an in vitro infection model. Initially, to demonstrate that lung cells from old mice produced innate IFN-γ when cultured in vitro with M. tuberculosis, lung cells were isolated from naïve young and old mice and stimulated in vitro with M. tuberculosis for 48 h. Following in vitro M. tuberculosis stimulation, lung cells from old mice produced significantly more IFN-γ protein than did lung cells from their young counterparts (Fig. 2A). To then demonstrate that CD8 T cells were the source of innate IFN-γ in the whole-lung cultures, lung cells were isolated from naïve young and old mice and cultured with or without M. tuberculosis for 12 to 24 h. In order to obtain sufficient numbers of cells for RT-PCR, lung cells were pooled by age. CD8 T cells were purified, and IFN-γ mRNA was quantified using RT-PCR. After 24 h of stimulation with M. tuberculosis, the level of expression of IFN-γ mRNA in CD8 T cells isolated from lung cell cultures of old mice was 100-fold higher than that in nonstimulated CD8 T cells and, as predicted from our in vivo infections, substantially higher than that in young CD8 T cells (Fig. 2B). As in our in vivo model, NK cells purified from lung cell cultures of young mice had higher expression levels of IFN-γ mRNA in response to in vitro stimulation with M. tuberculosis than did NK cells isolated from the lungs of old mice (Fig. 2C). Taken together, these data confirm that in old mice, CD8 T cells are an early and significant source of innate IFN-γ. Furthermore, we have validated an in vitro model that reliably reproduces our in vivo findings, allowing for the elucidation of the mechanism leading to CD8 T-cell-derived IFN-γ production in old mice.
FIG. 2.
CD8 T cells isolated from the lungs of naïve old mice produce innate IFN-γ in response to in vitro stimulation with M. tuberculosis. Lung cells were isolated from naïve young and old mice and stimulated with M. tuberculosis Erdman (Mtb) (MOI of 0.5) for 48 h (A) (n = 4 to 5) or 12 to 24 h (B and C) (n = 4 to 5, pooled). (A) IFN-γ was measured in culture supernatants of individual mice by ELISA. Data are represented as means ± SEM and are representative of data from two individual experiments. Statistical significance was determined using a one-way ANOVA with Tukey's posttest (**, P value of <0.01 [significance between young and old mice]; ++, P value of <0.01 [significance between treatment groups]). (B and C) CD8 (B) and NK (C) cells were purified from lung cultures using magnetic beads. Purified cells were homogenized in Trizol and frozen at −80°C. RNA was isolated and reverse transcribed, and cDNA was amplified for IFN-γ by RT-PCR. The relative expression levels of IFN-γ mRNA are shown and are representative of data from at least two individual experiments. nd, not detected; TCM, tissue culture media; RU, relative units.
Increased IFN-γ production is an intrinsic property of CD8 T cells from old mice.
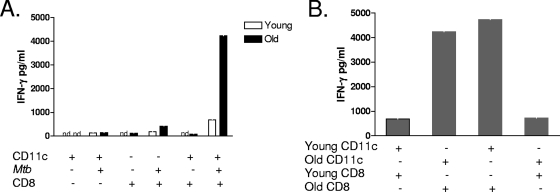
To determine the role of the aged lung microenvironment in driving CD8 T-cell IFN-γ secretion, we developed an in vitro overlay assay using CD11c cells isolated from the lungs of naïve young and old mice infected with M. tuberculosis in vitro. CD8 and CD11c cells were sequentially purified from the lungs of naïve young and old mice. Due to the limited number of both CD8 and CD11c cells obtained from the lungs of naïve mice, cells from individual mice were pooled by age. CD11c cells were infected with M. tuberculosis and incubated with purified CD8 cells for 48 h. Initial experiments involved the culturing of young CD8 T cells with young CD11c cells and old CD8 T cells with old CD11c cells (Fig. 3A). IFN-γ was measured in the cell culture supernatant by ELISA. Figure 3A shows that CD8 T cells purified from the lungs of naïve young mice produced very little IFN-γ in response to M. tuberculosis-infected CD11c cells from young mice. In contrast, IFN-γ production was abundant from CD8 T cells isolated from the lungs of naïve old mice following stimulation with M. tuberculosis-infected CD11c cells from old mice. These data provide evidence that stimulation with M. tuberculosis-infected CD11c cells is sufficient to drive IFN-γ production by CD8 T cells from old mice.
FIG. 3.
Increased IFN-γ production is an intrinsic property of aged CD8 T cells. CD8 and CD11c cells were purified from individual lung cell suspensions using magnetic beads. CD8 and CD11c cells were pooled by age group (n = 8). Purified CD11c cells were infected with M. tuberculosis (Mtb) (MOI of 1) and cultured with purified CD8 T cells for 48 h. (A) CD8 T cells from young and old mice were cultured with CD11c cells from the same age group. (B) CD8 T cells from young and old mice were cultured separately with M. tuberculosis-infected CD11c cells from both young and old mice. IFN-γ in culture supernatants was quantified by ELISA. Data are represented as pg/ml of IFN-γ and are representative of data from two individual experiments. nd, not detected.
To elucidate whether the capacity of CD8 T cells to secrete IFN-γ was a specific property of CD8 T cells from old mice, we established culture conditions in which the source of CD11c cells was young or old mice. As anticipated, CD8 T cells from young mice failed to produce substantial quantities of IFN-γ in response to M. tuberculosis-infected CD11c cells from young or old mice (Fig. 3B). In contrast, CD8 T cells from old mice were fully capable of secreting IFN-γ in response to M. tuberculosis-infected CD11c cells from either old or young mice (Fig. 3B). Therefore, the increased capacity of CD8 T cells from old mice to secrete IFN-γ in response to M. tuberculosis infection is a consequence of altered CD8 T-cell function in old age and not altered macrophage function.
M. tuberculosis-induced IFN-γ production by CD8 T cells is antigen independent.
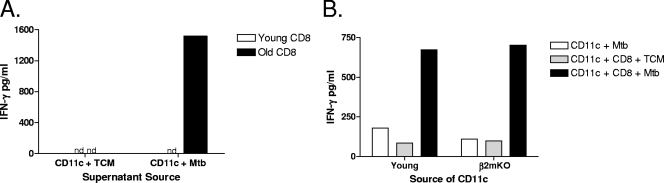
The capacity of CD8 T cells isolated from the lungs of noninfected old mice to produce IFN-γ in response to M. tuberculosis stimulation (Fig. 3) suggests that CD8 T cells from old mice produced IFN-γ in an antigen-independent manner. To verify this, supernatants were collected from M. tuberculosis-infected CD11c cells, filtered through a 0.2-μm filter to remove any cell-associated particulates, and cultured with purified CD8 T cells from young or old mice. CD8 T cells that were purified from the lungs of naïve old mice produced IFN-γ when stimulated with filtered supernatant derived from M. tuberculosis-infected CD11c cells from old mice (Fig. 4A), indicating that a soluble product was sufficient to stimulate CD8 T cells from old mice to secrete IFN-γ. As expected, CD8 T cells purified from the lungs of naïve young mice did not produce IFN-γ in response to filtered supernatants from M. tuberculosis-infected CD11c cells (Fig. 4A).
FIG. 4.
CD8-derived IFN-γ production is antigen independent. Lung cells were isolated from young and old naïve mice using gentle enzymatic digestion. (A) CD11c cells were purified from young and old mice, pooled (n = 3 to 10), and cultured for 72 h with M. tuberculosis (Mtb) (MOI of 1). Cell supernatants were frozen, thawed, and filtered through a 0.2-μm filter. Supernatants were cultured with purified CD8 T cells (n = 3 to 7, pooled) from young and old mice for 48 h. (B) CD11c cells were purified from young wild-type (n = 5) and young β2m knockout (β2mKO) (n = 5) mice and pooled by group. CD11c cells were infected with M. tuberculosis (MOI of 1) and cultured with CD8 T cells that were purified and pooled from old mice (n = 10) for 48 h. IFN-γ was quantified in culture supernatants by ELISA. Data are represented as pg/ml of IFN-γ and are representative of data from at least two individual experiments. nd, not detected; TCM, tissue culture media.
To conclusively demonstrate that antigen presentation was not necessary for M. tuberculosis-induced IFN-γ production by CD8 T cells from the lungs of naïve old mice, β2m knockout mice were used as the source of CD11c cells. β2m is an essential component of MHC class I molecules, and therefore, mice with homozygous disruptions in the β2m gene lack functional MHC class I on the cell surface (14). Figure 4B shows that pulmonary CD8 T cells isolated from naïve old mice cultured with M. tuberculosis-infected CD11c cells from young β2m knockout mice were fully capable of producing IFN-γ. Taken together, these data demonstrate that M. tuberculosis-induced IFN-γ production by CD8 T cells from old mice is an antigen-independent event mediated by a soluble factor produced by M. tuberculosis-infected CD11c cells.
IL-12 is essential for M. tuberculosis-induced IFN-γ production by CD8 T cells.
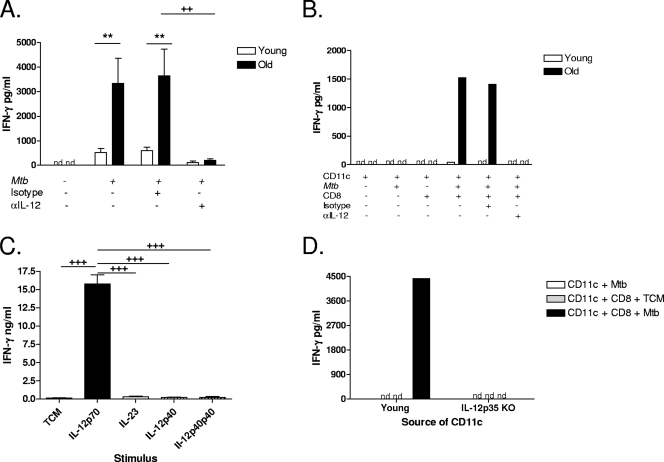
CD8 T cells can be stimulated to secrete IFN-γ in the presence of IL-12, IL-18, and IL-2 (2) and, as we have shown, for old mice by IL-12 alone (25, 34). We therefore anticipated that the stimulatory soluble factor produced by M. tuberculosis-infected CD11c cells would be IL-12. Lung cell suspensions were isolated from naïve young and old mice and cultured with M. tuberculosis in the presence of a neutralizing antibody to IL-12p40. Lung cells from naïve old mice produced significantly more IFN-γ than their young counterparts, and this was ablated with the inclusion of a neutralizing antibody to IL-12p40 (Fig. 5A). Furthermore, IFN-γ production by CD8 T cells from old mice in the presence of M. tuberculosis-infected CD11c cells was also ablated by a neutralizing antibody to IL-12p40 (Fig. 5B). These data indicate that M. tuberculosis-driven IL-12p40 production is necessary for the stimulation of CD8 T cells from old mice to secrete IFN-γ.
FIG. 5.
CD8-derived IFN-γ is IL-12 dependent. (A, B, and D) Lung cells were isolated from young and old mice using gentle enzymatic digestion. (A) Lung cells from individual mice (n = 3 to 5) were cultured with M. tuberculosis (Mtb) (MOI of 0.5) for 48 h in the presence of M. tuberculosis either alone or in combination with 1 μg/ml anti-IL-12 (αIL-12) or isotype control antibody. (B) CD8 and CD11c cells were purified from individual lung cell suspensions using magnetic beads. CD8 and CD11c cells were pooled by age group (n = 5 to 7). Purified CD11c cells were infected with M. tuberculosis (MOI of 1) and cultured with purified CD8 T cells and 1 μg/ml anti-IL-12 or isotype control for 48 h. (C) CD8 cells were purified from the spleens of old mice (n = 1 to 2) and pooled. Purified CD8 cells were cultured with IL-12p70 (5 ng/ml), IL-23 (20 ng/ml), IL-12p40 (20 ng/ml), or IL-12p40p40 (20 ng/ml) for 48 h. All cultures contained IL-18 (20 ng/ml). (D) CD11c cells were purified from young wild-type (n = 5) and young IL-12p35 knockout (KO) (n = 5) mice and pooled by group. CD11c cells were infected with M. tuberculosis (MOI of 1) and cultured with CD8 cells that were purified and pooled from old mice (n = 10) for 48 h. IFN-γ in culture supernatants was quantified by ELISA. Data are represented as means ± SEM (A and C) or pg/ml (B and D) and are representative of data from at least two individual experiments. Statistical significance was determined using a one-way ANOVA with Tukey's posttest (**, P value of <0.01 [significance between young and old mice]; ++, P value of <0.01; +++, P value of <0.001 [significance between treatment groups]). nd, not detected; TCM, tissue culture media.
Biologically active IL-12p70 is composed of two subunits, p40 and p35. While the p35 subunit is found only in biologically active IL-12, the p40 subunit is found as monomers, as homodimers, and in biologically active IL-12 and IL-23. Therefore, in addition to IL-12p70, the neutralizing antibody to IL-12p40 neutralizes IL-23 and also any biological activity of p40. To formally demonstrate that IL-12p70, and not any other known p35- or p40-containing cytokine, was sufficient to directly stimulate CD8 T cells from old mice to secrete IFN-γ, CD8 T cells were purified from the spleens of naïve old mice and cultured with commercially available p35- and p40-containing cytokines. We previously demonstrated that the stimulation of CD8 T cells from old mice with IL-12p70 alone induces low but detectable quantities of IFN-γ (25, 34). For these studies, IL-18 was included in all wells to amplify the quantity of IFN-γ produced by old CD8 T cells as measured by IFN-γ ELISA. IL-12p70 but not IL-23, IL-12p40, or IL-12p40p40 was sufficient to directly stimulate CD8 T cells from old mice to secrete IFN-γ (Fig. 5C).
We confirmed that bioactive IL-12p70 was required for the stimulation of CD8 T cells from old mice to secrete IFN-γ in response to M. tuberculosis infection by using IL-12p35 knockout mice as the source of CD11c cells in our assay. CD8 T cells isolated from the lungs of naïve old mice failed to produce IFN-γ when cultured with M. tuberculosis-infected CD11c cells purified from the lungs of naïve IL-12p35 knockout mice (Fig. 5D). When expressed as percent inhibition, IFN-γ production by CD8 T cells isolated from old mice stimulated with M. tuberculosis-infected IL-12p35 knockout CD11c cells was inhibited 82 and 100% relative to that by CD11c cells from young wild-type controls (in two separate experiments, respectively). These data conclusively demonstrate that M. tuberculosis-induced innate IFN-γ production by CD8 T cells from naïve old mice is dependent on biologically active IL-12p70.
Hyperresponsiveness of CD8 T cells from old mice to IL-12p70 is associated with elevated levels of SET, a phosphatase inhibitor.
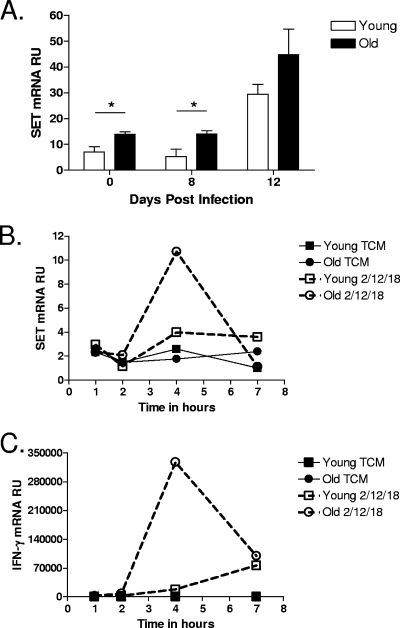
We have shown here that the capacity of CD8 T cells to secrete IFN-γ in response to M. tuberculosis infection is due to distinct functional properties of CD8 T cells; however, the molecular pathways that lead to IL-12p70 hyperreactivity in this unique subset have not been fully elucidated. Recently, IFN-γ production by human NK cells was shown to be regulated by SET, an inhibitor of the phosphatase PP2A (31). We therefore hypothesized that elevated SET expression levels are one mechanism by which CD8 T cells from old mice produce increased levels of IFN-γ. To test this hypothesis, CD8 T cells were purified from M. tuberculosis-infected young and old mice at various time points postinfection. RNA was isolated, and SET mRNA expression was measured using RT-PCR. The level of SET expression in CD8 T cells from naïve old mice, and day 8-infected old mice, was elevated compared to levels in CD8 T cells from young mice (Fig. 6A). To address the role of SET in more detail, we measured SET expression in CD8 T cells purified from naïve young and old mice that had been stimulated with a Th1 cytokine cocktail of IL-12, IL-18, and IL-2 by RT-PCR. SET mRNA levels in CD8 T cells from old mice stimulated with the Th1 cytokine cocktail peaked at 4 h, and SET mRNA levels were nearly threefold higher than those of stimulated young CD8 T cells at this time point (Fig. 6B). This peak in SET expression correlated with peak IFN-γ mRNA expression levels (Fig. 6C).
FIG. 6.
The level of SET expression is elevated in CD8 T cells isolated from the lungs of old mice. (A) Young and old mice were infected aerogenically with M. tuberculosis, and lungs were harvested at 0, 8, and 12 days postinfection (n = 4 to 5). Single-cell suspensions were obtained by gentle enzymatic digestion, and CD8 T cells were purified by use of magnetic beads. (B and C) Single-cell suspensions were obtained from the spleens of naïve young and old mice (n = 5), and CD8 T cells were purified by use of magnetic beads and pooled by age. CD8 T cells were cultured with a cytokine cocktail of IL-2 (100 ng/ml), IL-12 (5 ng/ml), and IL-18 (10 ng/ml) for 1, 4, and 7 h. Cells were homogenized in Trizol and frozen at −80°C. RNA was isolated and reverse transcribed, and cDNA was amplified for SET and IFN-γ by RT-PCR. The relative level of expression of SET (A and B) or IFN-γ (C) message is shown as means ± SEM (A) or as relative units (RU) (B and C). Statistical significance between young and old mice at each time point was determined using the Student t test (A) (*, P value of <0.05). TCM, tissue culture media.
Chemically overriding SET inhibition of PP2A in CD8 T cells from old mice leads to decreased IL-12p70 signaling and IFN-γ production.
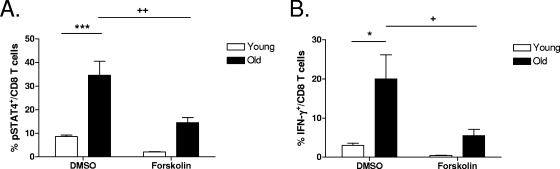
To demonstrate that increased levels of SET in CD8 T cells from old mice, acting via its suppression of PP2A activity, could influence downstream IL-12-induced IFN-γ production, CD8 T cells were purified from the lungs of young and old mice and treated with 1,9-dideoxyforskolin (an activator of PP2A which overcomes SET inhibition). Cells were subsequently cultured with a Th1 cytokine cocktail, and STAT4 phosphorylation, which is indicative of IL-12p70 signaling, in addition to IFN-γ production, was determined. Treatment with 1,9-dideoxyforskolin significantly decreased STAT4 phosphorylation (Fig. 7A) and IFN-γ production (Fig. 7B) levels by CD8 T cells from old mice. Taken together, these data suggest that elevated levels of SET may contribute to the elevated levels of IFN-γ produced by CD8 T cells from old mice in response to M. tuberculosis infection.
FIG. 7.
Overcoming SET inhibition of PP2A activity results in decreased levels of STAT4 phosphorylation and subsequent IFN-γ production. Single-cell suspensions of lung cells from young and old mice (n = 4 to 5) were obtained by gentle enzymatic digestion. CD8 T cells were purified from individual mice using magnetic beads and cultured for 30 min with 40 μM 1,9-dideoxyforskolin or a DMSO control. IL-2 (100 ng/ml), IL-12 (5 ng/ml), and IL-18 (10 ng/ml) were added to the cultures for 4 h. Following incubation, cells were fixed, permeabilized, and stained with anti-CD3, anti-CD8, anti-IFN-γ, and anti-pSTAT4 for flow cytometric analysis. Data are shown as means ± SEM and are representative of data from at least two independent experiments. Statistical significance was determined using a one-way ANOVA with Tukey's posttest (*, P value of <0.05; ***, P value of <0.001 [for comparisons between young and old mice]; +, P value of <0.05; ++, P value of <0.01 [for comparisons between DMSO and 1,9-dideoxyforskolin treatments]).
DISCUSSION
An understanding of the immune response of the elderly to relevant pathogens is becoming an urgent health care priority with the projected rapid expansion of this population. It is well known that numerous immunological deficiencies emerge with age, resulting in an increased susceptibility of the elderly to infection. One strategy to combat the increased morbidity and mortality associated with infectious agents in the elderly is to identify ways to prevent or reverse established immunological deficiencies (1, 9). An alternative strategy is to identify components of the aged immune system that respond positively to such infectious agents and determine mechanisms by which successful effector functions can be targeted or manipulated to improve immunological responses in the elderly. By focusing on this alternative strategy, we have defined a mechanism by which a population of pulmonary CD8 T cells in the lungs of old mice mediates early resistance to M. tuberculosis infection. Our results indicate that this population of CD8 T cells contributes to the innate immune response of old mice to M. tuberculosis infection via the antigen-independent and IL-12p70-dependent production of IFN-γ. We have therefore identified a population of CD8 T cells present in old mice that makes a unique and effective, albeit transient, contribution to the immune response to a relevant pathogen.
Old mice and humans are indeed more susceptible to M. tuberculosis infection than their young counterparts; however, our laboratory and others have demonstrated that during the first 2 weeks of an aerogenic infection with M. tuberculosis, old mice are more resistant to infection (6, 32, 34). IFN-γ and CD8 T cells were later identified as being essential components of the early resistance phenotype in old mice (32, 33), and we now demonstrate that CD8 T cells are a major source of IFN-γ expressed during the innate phase of M. tuberculosis infection. The conventional response of CD8 T cells to M. tuberculosis is mediated by M. tuberculosis-specific peptides presented in the context of MHC class I. Although conventional immune responses may dominate, there is mounting evidence that CD8 T cells are capable of contributing to innate immunity (3-5, 8, 16, 34) as well as evidence suggesting that NK cells contribute to specific acquired immune responses (29). By using M. tuberculosis-infected CD11c cells isolated from the lungs of naïve β2m knockout mice, this study demonstrates that IFN-γ production by CD8 T cells from the lungs of naïve old mice in response to M. tuberculosis infection was not dependent on classical MHC class I antigen presentation. To the best of our knowledge, this is the first direct demonstration of CD8 T cells from naïve mice responding to M. tuberculosis infection in an antigen-independent manner.
Our previous studies characterizing the phenotype and function of pulmonary CD8 T cells in old mice revealed the ability of this population of CD8 T cells to respond to Th1 cytokines by producing IFN-γ (34), and here we extend those studies by demonstrating the biological relevance to a respiratory pathogen. M. tuberculosis-induced IFN-γ production by CD8 T cells isolated from the lungs of naïve old mice was ablated in the presence of IL-12p40 neutralizing antibody or when naïve IL-12p35 knockout mice were used as a source of CD11c cells, conclusively demonstrating that IL-12p70 was essential. Taken together, these data suggest that CD8 T cells from old mice are capable of innate IFN-γ production in response to all pathogens that induce IL-12p70. In the case of M. tuberculosis infection, this response seems to be beneficial, at least in the short term, but could be detrimental during infections with other infectious agents. In fact, recent data suggest that IFN-γ produced in the lung in response to influenza virus infection inhibits initial bacterial clearance of secondary bacterial infections (30), which are common and a significant cause of morbidity and mortality in the elderly. This highlights the necessity of an understanding of the immune responses of the elderly to pathogens.
Given that increased IFN-γ production in response to M. tuberculosis is mediated by IL-12p70 production, two non-mutually-exclusive mechanisms to explain the hyperresponsive phenotype of old CD8 T cells were evaluated. First, the aged lung environment during M. tuberculosis infection could be significantly different from that of a young mouse. We have in fact shown that in vivo, the lungs of M. tuberculosis-infected old mice contain significantly more IL-12 than do the lungs of their young counterparts (34). Data presented in this study demonstrated that the amount of IL-12 produced by M. tuberculosis-infected CD11c cells isolated from either young or old mice was sufficient to drive equivalent amounts of IFN-γ production by CD8 T cells from old mice. These data suggest that while M. tuberculosis-infected lung cells from old mice may make more IL-12 than those from young mice, the difference is not biologically relevant, and therefore, the aged lung environment does not drive the hyperresponsive function of these cells. It is important to highlight that although the unique features of the aged lung environment are not biologically relevant to the increased level of innate IFN-γ production by CD8 T cells, it is likely that the environment in an aged mouse directly contributes to the development of such cells (12, 18).
Since the aged lung environment does not contribute to the increased IFN-γ production by CD8 T cells from naïve old mice in response to M. tuberculosis, the hyperresponsive effector function of these cells must therefore be a consequence of some unique feature or features of old CD8 T cells. Given that CD8 T cells from naïve old mice are functionally similar to NK cells in that both cell types produce innate IFN-γ in response to cytokines (24), we investigated the mechanism by which NK cells produce enhanced levels of IFN-γ in our model. The increased level of expression of SET was shown to mediate enhanced IFN-γ production by human CD56bright NK cells (31) via an inhibition of PP2A activity through the binding of the C subunit of the phosphatase (17). Our laboratory has recently demonstrated that CD8 T cells from old mice have enhanced STAT4 phosphorylation levels in response to IL-12 (25), highlighting a role of altered IL-12 signaling in old CD8 T cells. In this study, we demonstrated that the level of SET expression is elevated in CD8 T cells purified from the lungs of M. tuberculosis-infected old mice and in CD8 T cells stimulated with Th1 cytokines. Furthermore, the in vitro expression of SET correlated with IFN-γ production in old CD8 T cells. We also provided evidence that overcoming the SET inhibition of PP2A resulted in decreased levels of IL-12 signaling and IFN-γ production in Th1 cytokine-stimulated CD8 T cells from old mice. These data suggest that one possible mechanism by which CD8 T cells from old mice produce increased levels of IFN-γ is via increased levels of expression of SET.
Taken together, our data presented here and in previous studies demonstrate that during the innate phase of an aerogenic M. tuberculosis infection, resident pulmonary CD11c cells produce IL-12p70 in response to infection. Resident CD8 T cells have increased quantities of the IL-12β2 receptor mRNA (25) and increased levels of SET expression and are therefore uniquely able to respond to IL-12p70 by producing increased levels IFN-γ. Increased levels of IFN-γ production contribute to mycobacterial control in the lung and result in a decreased mycobacterial burden in the lungs throughout the first 2 weeks of infection. Additional studies to understand the transient nature of resistance and to characterize the signaling events leading to enhanced IFN-γ production are currently ongoing and will contribute to the development of more effective strategies to combat infectious diseases in the elderly.
Acknowledgments
This work was supported by National Institutes of Health grant AG21097 to J.T. E.K.R. was supported by a fellowship from PHPID. osu.edu and the Targeted Investment in Excellence of the OAA, President and Research Offices of Ohio State.
We thank Rossana Trotta for insightful discussions regarding SET.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 26 May 2009.
REFERENCES
- 1.Aspinall, R., and W. Mitchell. 2008. Reversal of age-associated thymic atrophy: treatments, delivery, and side effects. Exp. Gerontol. 43700-705. [DOI] [PubMed] [Google Scholar]
- 2.Berg, R. E., C. J. Cordes, and J. Forman. 2002. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur. J. Immunol. 322807-2816. [DOI] [PubMed] [Google Scholar]
- 3.Berg, R. E., E. Crossley, S. Murray, and J. Forman. 2003. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 1981583-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, R. E., E. Crossley, S. Murray, and J. Forman. 2005. Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes. J. Immunol. 1751751-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg, R. E., and J. Forman. 2006. The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr. Opin. Immunol. 18338-343. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, A. M., J. E. Callahan, J. P. Griffin, A. D. Roberts, and I. M. Orme. 1995. Old mice are able to control low-dose aerogenic infections with Mycobacterium tuberculosis. Infect. Immun. 633259-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez-Gerpe, L., and M. Rey-Mendez. 2003. Evolution of the thymus size in response to physiological and random events throughout life. Microsc. Res. Tech. 62464-476. [DOI] [PubMed] [Google Scholar]
- 8.D'Orazio, S. E., M. J. Troese, and M. N. Starnbach. 2006. Cytosolic localization of Listeria monocytogenes triggers an early IFN-gamma response by CD8+ T cells that correlates with innate resistance to infection. J. Immunol. 1777146-7154. [DOI] [PubMed] [Google Scholar]
- 9.Dorshkind, K., E. Montecino-Rodriguez, and R. A. Signer. 2009. The ageing immune system: is it ever too old to become young again? Nat. Rev. Immunol. 957-62. [DOI] [PubMed] [Google Scholar]
- 10.Feng, C. G., M. Kaviratne, A. G. Rothfuchs, A. Cheever, S. Hieny, H. A. Young, T. A. Wynn, and A. Sher. 2006. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J. Immunol. 1777086-7093. [DOI] [PubMed] [Google Scholar]
- 11.Herndler-Brandstetter, D., E. Veel, G. T. Laschober, G. Pfister, S. Brunner, S. Walcher, W. Parson, G. Lepperdinger, and B. Grubeck-Loebenstein. 2008. Non-regulatory CD8+CD45RO+CD25+ T-lymphocytes may compensate for the loss of antigen-inexperienced CD8+CD45RA+ T-cells in old age. Biol. Chem. 389561-568. [DOI] [PubMed] [Google Scholar]
- 12.Jones, S. C., K. Clise-Dwyer, G. Huston, J. Dibble, S. Eaton, L. Haynes, and S. L. Swain. 2008. Impact of post-thymic cellular longevity on the development of age-associated CD4+ T cell defects. J. Immunol. 1804465-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junqueira-Kipnis, A. P., A. Kipnis, A. Jamieson, M. G. Juarrero, A. Diefenbach, D. H. Raulet, J. Turner, and I. M. Orme. 2003. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J. Immunol. 1716039-6045. [DOI] [PubMed] [Google Scholar]
- 14.Koller, B. H., P. Marrack, J. W. Kappler, and O. Smithies. 1990. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 2481227-1230. [DOI] [PubMed] [Google Scholar]
- 15.Kong, K. F., K. Delroux, X. Wang, F. Qian, A. Arjona, S. E. Malawista, E. Fikrig, and R. R. Montgomery. 2008. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J. Virol. 827613-7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lertmemongkolchai, G., G. Cai, C. A. Hunter, and G. J. Bancroft. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 1661097-1105. [DOI] [PubMed] [Google Scholar]
- 17.Li, M., A. Makkinje, and Z. Damuni. 1996. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J. Biol. Chem. 27111059-11062. [DOI] [PubMed] [Google Scholar]
- 18.Linton, P. J., S. P. Li, Y. Zhang, B. Bautista, Q. Huynh, and T. Trinh. 2005. Intrinsic versus environmental influences on T-cell responses in aging. Immunol. Rev. 205207-219. [DOI] [PubMed] [Google Scholar]
- 19.Moretto, M. M., E. M. Lawlor, and I. A. Khan. 2008. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J. Immunol. 1817977-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murciano, C., E. Villamon, A. Yanez, J. E. O'Connor, D. Gozalbo, and M. L. Gil. 2006. Impaired immune response to Candida albicans in aged mice. J. Med. Microbiol. 551649-1656. [DOI] [PubMed] [Google Scholar]
- 21.Nogusa, S., B. W. Ritz, S. H. Kassim, S. R. Jennings, and E. M. Gardner. 2008. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech. Ageing Dev. 129223-230. [DOI] [PubMed] [Google Scholar]
- 22.Ogata, K., E. An, Y. Shioi, K. Nakamura, S. Luo, N. Yokose, S. Minami, and K. Dan. 2001. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin. Exp. Immunol. 124392-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orme, I. M. 1987. Aging and immunity to tuberculosis: increased susceptibility of old mice reflects a decreased capacity to generate mediator T lymphocytes. J. Immunol. 1384414-4418. [PubMed] [Google Scholar]
- 24.Papamichail, M., S. A. Perez, A. D. Gritzapis, and C. N. Baxevanis. 2004. Natural killer lymphocytes: biology, development, and function. Cancer Immunol. Immunother. 53176-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rottinghaus, E. K., B. Vesosky, and J. Turner. Interleukin-12 is sufficient to promote antigen-independent interferon-γ production by CD8 T cells in old mice. Immunology, in press. [DOI] [PMC free article] [PubMed]
- 26.Schwanninger, A., B. Weinberger, D. Weiskopf, D. Herndler-Brandstetter, S. Reitinger, C. Gassner, H. Schennach, W. Parson, R. Wurzner, and B. Grubeck-Loebenstein. 2008. Age-related appearance of a CMV-specific high-avidity CD8+ T cell clonotype which does not occur in young adults. Immun. Ageing 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi, Y., T. Yamazaki, Y. Okubo, Y. Uehara, K. Sugane, and K. Agematsu. 2005. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J. Immunol. 1753262-3267. [DOI] [PubMed] [Google Scholar]
- 28.Stout-Delgado, H. W., X. Yang, W. E. Walker, B. M. Tesar, and D. R. Goldstein. 2008. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J. Immunol. 1816747-6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun, J. C., J. N. Beilke, and L. L. Lanier. 2009. Adaptive immune features of natural killer cells. Nature 457557-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun, K., and D. W. Metzger. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 14558-564. [DOI] [PubMed] [Google Scholar]
- 31.Trotta, R., D. Ciarlariello, J. Dal Col, J. Allard II, P. Neviani, R. Santhanam, H. Mao, B. Becknell, J. Yu, A. K. Ferketich, B. Thomas, A. Modi, B. W. Blaser, D. Perrotti, and M. A. Caligiuri. 2007. The PP2A inhibitor SET regulates natural killer cell IFN-gamma production. J. Exp. Med. 2042397-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner, J., A. A. Frank, and I. M. Orme. 2002. Old mice express a transient early resistance to pulmonary tuberculosis that is mediated by CD8 T cells. Infect. Immun. 704628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner, J., and I. M. Orme. 2004. The expression of early resistance to an infection with Mycobacterium tuberculosis by old mice is dependent on IFN type II (IFN-gamma) but not IFN type I. Mech. Ageing Dev. 1251-9. [DOI] [PubMed] [Google Scholar]
- 34.Vesosky, B., D. K. Flaherty, and J. Turner. 2006. Th1 cytokines facilitate CD8-T-cell-mediated early resistance to infection with Mycobacterium tuberculosis in old mice. Infect. Immun. 743314-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 2008. Global tuberculosis control—surveillance, planning, financing. World Health Organization, Geneva, Switzerland.
- 36.Yager, E. J., M. Ahmed, K. Lanzer, T. D. Randall, D. L. Woodland, and M. A. Blackman. 2008. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 205711-723. [DOI] [PMC free article] [PubMed] [Google Scholar]