Abstract
N-acetylneuraminic acid (Neu5Ac, sialic acid) could provide a good substrate for enteropathogenic bacteria in the intestine, when the bacteria invade and colonize in human gut. In order to analyze the role of Neu5Ac catabolism in Vibrio vulnificus pathogenesis, a mutant with disruption of the nanA gene encoding Neu5Ac lyase was constructed by allelic exchanges. The nanA mutant was not able to utilize Neu5Ac as a sole carbon source and revealed an altered colony morphotype with reduced opacity in the presence of Neu5Ac. Compared to the wild type, the nanA mutant exhibited a low level of cytotoxicity toward INT-407 epithelial cells in vitro and reduced virulence in a mouse model. The disruption of nanA also resulted in a substantial decrease in histopathological damage in jejunum and colon tissues from the mouse intestine. These results indicated that NanA plays an important role in V. vulnificus pathogenesis. In addition, the nanA mutant was significantly diminished in growth with and adherence to INT-407 epithelial cells in vitro, and was defective for intestinal colonization, reflecting the impaired ability of the mutant to grow and survive with, persist in, and adhere to the intestine in vivo. Consequently, the combined results suggest that NanA and the capability of catabolic utilization of Neu5Ac contribute to V. vulnificus virulence by ensuring growth, adhesion, and survival during infection.
Microbial pathogenicity is a complex phenomenon that involves the products of many genes, called virulence factors, contributing not only to diseases but also to survival and multiplication on or within the host (20). For the development of diseases, survival and multiplication are clearly the priorities of the infecting microorganisms. It is likely that when enteropathogenic bacteria invade the human gut, many environmental changes, such as differences in types and concentrations of nutrients, would be encountered. In addition to the scarcity of specific nutrients, increased competition for the nutrient imposed by the host cells and endogenous bacterial flora would be encountered. Thus, the ability to acquire nutrients under these adverse environments is often crucial for enteropathogenic bacteria to survive and multiply in the intestine (5). For example, because the amount of free glucose would be quite small in the intestine, enteropathogenic bacteria must be able to use nutrients other than glucose to be a successful pathogen (11, 15).
Epithelial surfaces of the intestine that are exposed to bacteria are the major components of innate immunity. Therefore, bacteria have to penetrate the epithelial surfaces to invade the host. The mucus layer that overlays epithelial surfaces is the primary place where bacteria adhere and colonize. It has been observed that Escherichia coli mutants that have difficulty growing on or surviving in mucus are unable to colonize the mouse intestine (25). The mucus layer is composed of a variety of factors, but its main properties are attributable to the presence of mucins, which are complex linear polymorphic glycoproteins produced mainly by goblet cells (43). Mucins are highly glycosylated large glycoproteins (with molecular weights ranging from 5 × 103 to 4 × 106 Da), and up to 85% of their dry weight is carbohydrate (42). As such, the carbohydrate component of a mucin molecule is one of the most abundant mucosal polysaccharides (42), indicating that mucin sugars are important carbon sources to support the survival and growth of infecting enteropathogens.
Sialic acid is a generic term to represent a family of related nine-carbon sugar acids that are located prominently at the terminal end of the carbohydrate side chains of mucin (for recent reviews, see references 34 and 39). The most abundant sialic acid is N-acetylneuraminic acid (Neu5Ac); therefore, many enteropathogenic bacteria have evolved elaborate systems for the utilization of Neu5Ac as a potential carbon and nitrogen source. However, few definitive analyses of the genes encoding the proteins involved in the degradation of Neu5Ac have been reported. The nan (stands for N-acylneuraminate) systems of E. coli and Haemophilus influenzae, consisting of many genes, including nanA, nanK, and nanE (or homologues), are genetic systems of which the functions are well characterized at the molecular level (38, 39, 40). In E. coli, the Neu5Ac lyase (NanA) initiates the catabolism of Neu5Ac by cleaving it into pyruvate and N-acetylmannosamine (ManNAc), which is ultimately converted into an intermediate of central metabolism (fructose 6-phosphate) via the activities of many proteins, including NanK and NanE (39). Nevertheless, until now, very little has been known about the role of Neu5Ac degradation genes in the pathogenesis of bacteria (40).
The pathogenic marine bacterium Vibrio vulnificus is the causative agent of food-borne diseases, such as gastroenteritis and life-threatening septicemia in immunocompromised individuals (for recent reviews, see references 8, 18, and 37). A search of the GenBank database (http://www.ncbi.nlm.nih.gov) for homology to the amino acid sequence of E. coli NanA singled out the putative V. vulnificus NanA protein, suggesting that V. vulnificus is able to utilize Neu5Ac as a nutrient during infection. However, no studies have yet been reported on the effects of the capability of catabolic utilization of Neu5Ac on V. vulnificus virulence. Accordingly, in the present study, a V. vulnificus null mutant, in which the nanA gene was disrupted and thus is not able to utilize Neu5Ac as a nutrient, was constructed by allelic exchange. In order to demonstrate the possible roles of the catabolic utilization of Neu5Ac in V. vulnificus pathogenesis, the adhesion, survival, and multiplication as well as virulence of the nanA mutant were compared with those of the wild type by using tissue cultures and mice. It appeared from the results that NanA is essential for V. vulnificus virulence by ensuring adhesion, survival, and multiplication during infection.
MATERIALS AND METHODS
Strains, plasmids, and culture media.
The strains and plasmids used in this study are listed in Table 1. V. vulnifcus ATCC 29307 with spontaneous rifampin (rifampicin) resistance was named HG071 and used as a wild type. Unless noted otherwise, the V. vulnificus strains were grown in Luria-Bertani medium supplemented with 2.0% (wt/vol) NaCl (LBS) at 30°C. All the medium components were purchased from Difco (Detroit, MI), and the chemicals were purchased from Sigma (St. Louis, MO).
TABLE 1.
Plasmids and bacterial strains used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Bacterial strains | ||
| V. vulnificus | ||
| HG071 | ATCC 29307 with spontaneous Rifr mutation, virulent | Laboratory collection |
| HG072 | HG071 nanA::nptI, Kmr, Rifr | This study |
| E. coli SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λpir; Kmr; host for π-requiring plasmids; conjugal donor | 22 |
| Plasmids | ||
| pUC4K | pUC4 with the nptI gene; Apr, Kmr | Pharmacia |
| pDM4 | Suicide vector; oriR6K; Cmr | 23 |
| pGEM-T Easy | PCR product cloning vector; Apr | Promega |
| pJH0311 | 0.3-kb NruI fragment containing multicloning site of pUC19 cloned into pCOS5; Apr, Cmr | 7 |
| pHG0701 | pGEM-T Easy with the nanA gene; Apr | This study |
| pHG0703 | pDM4; nanA::nptI; Cmr, Kmr | This study |
| pHG0704 | pJH0311 with the nanA gene; Apr, Cmr | This study |
Apr, ampicillin resistant; Cmr, chloramphenicol resistant; Kmr, kanamycin resistant; Rifr, rifampin resistant.
Cloning of V. vulnificus nanA and generation of the nanA mutant.
A whole nanA open reading frame was amplified from the genomic DNA of V. vulnificus HG071 by PCR using a pair of oligonucleotide primers, NANA001 (5′-TCTAGAATGATGAACAAATTAAAAGG-3′) and NANA002 (5′-CTCGAGCTACAGATTAAGAAAATCC-3′). The primers were designed using the genomic sequence of V. vulnificus (GenBank accession number VV2_0730). The amplified 900-bp nanA was ligated into pGEM-T Easy (Promega, Madison, WI) to result in pHG0701 (Table 1).
To inactivate nanA in pHG0701 in vitro, a 1.2-kb nptI DNA conferring resistance to kanamycin (26) was inserted into a unique PvuI site present 314 bp away from the translational initiation codon of nanA, and the resulting 2.1-kb nanA::nptI mutant was ligated with XbaI-XhoI-digested pDM4 (23) to form pHG0703 (Table 1). E. coli SM10 λpir tra (containing pHG0703) (22) was used as a conjugal donor to V. vulnificus HG071 to generate the nanA mutant by homologous recombination. The conjugation and isolation of the transconjugants were conducted using methods previously described (13), and a resulting nanA::nptI mutant chosen for further analysis was named HG072 (Table 1).
Neu5Ac lyase activities.
Neu5Ac lyase activities were measured using the method described by Bulai et al. (4) with modifications. Briefly, V. vulnificus cells grown to an A600 of 0.5 with M9 broth (32) supplemented with 10 mM d-xylose, 10 mM l-proline, and 5 mM Neu5Ac were harvested, disrupted by sonication (48), and spun down by centrifugation to obtain clear cytosol. For the measurement of Neu5Ac lyase activity, the reaction was initiated by the addition of 500 μl of the cytosol (about 16 μg of total proteins) as an enzyme source to 25 μl of 32 mM Neu5Ac in aqueous solution and 75 μl of 1 M potassium phosphate buffer, pH 7.4. After incubation at 37°C for 2 h, the reaction was stopped by heating at 96°C for 3 min, and the liberated ManNAc was determined by a colorimetric method using p-dimethylamino-benzaldehyde as described elsewhere (4, 31). One unit of Neu5Ac lyase activity is defined as the amount that releases 1 μmol of ManNAc per min under the reaction conditions used. Protein concentrations were determined by the method used by Bradford (3), with bovine serum albumin as the standard.
Colony opacity.
The wild type and the nanA mutant were grown overnight, and subsequently, equal amounts of the strains were spotted onto the modified M9 medium used above and solidified with 1.5% agar (Bacto agar; Difco). The plates were incubated at 30°C for 24 h, and colony morphotypes were photographed by using a UMAX digital imaging system (UTA-1100; UMAX Technologies, Inc., Fremont, CA).
Utilization of Neu5Ac as a sole carbon source.
The ability of V. vulnificus strains to use Neu5Ac as a sole carbon source was assayed by measuring growth in the M9 broth supplemented with either 5 mM Neu5Ac or 5 mM glucose. Fifty-milliliter cultures of the modified M9 broth in 250-ml Erlenmeyer flasks were inoculated with an initial cell density (A600) of approximately 0.005 and incubated at 30°C with shaking. The inocula were from late-exponential-phase cultures in LBS.
Cytotoxicity assay.
Cytotoxicity was evaluated by the quantification of cytoplasmic lactate dehydrogenase (LDH) activity released by damage of plasma membranes (44). Preparation of INT-407 (ATCC CCL-6) human intestinal epithelial cells and infection with V. vulnificus strains were performed as described previously (28). Cytotoxicity was then determined by measuring the activity of LDH in the supernatant using a cytotoxicity detection kit (Roche, Mannheim, Germany) and expressed using the total LDH activity of the cells completely lysed by 1% Triton X-100 as 100%.
LD50 determination.
A group of six 7-week-old female mice (ICR specific pathogen-free; Seoul National University) was injected intraperitoneally with 100-μl serial dilutions of the V. vulnificus suspensions as described elsewhere (28). Mice were overloaded with iron immediately before injection of bacterial cells. The infected mice were observed for 24 h, and the 50% lethal doses (LD50s) were calculated using a method described by Reed and Muench (30). All manipulations of mice were approved by the Animal Care and Use Committee at Seoul National University.
Histopathological examination.
The female ICR mice were given drinking water containing rifampin (50 μg/ml) for 24 h to eliminate resident bacteria (21). After an 18-h starvation period without food and water, 100 μl of the bacterial inoculum, representing approximately 106 CFU of either the wild type or HG072, was given intragastrically to the mice. At 18 h following infection, the mice were killed, and segments taken from the jejuna and colons were sectioned transversely in their entirety and fixed overnight in 10% neutral buffered formalin. The fixed tissues were processed in paraffin, cut into five microsections, stained with hematoxylin-eosin, and examined by light microscopy.
Bacterial growth rates during infection.
The INT-407 cells were infected using the wild type or HG072 at a multiplicity of infection (MOI) of 10, and growth rates of the bacterial strains during the infection were monitored. For this purpose, the INT-407 cells were broken by treatment with 0.1% Triton X-100 for 5 min at regular intervals, and bacterial cells were recovered and enumerated as numbers of CFU on LBS agar plates.
Adhesion assay.
The INT-407 cell monolayer was seeded on glass coverslips placed at the bottom of the tissue culture plate and infected with the V. vulnificus strains at an MOI of 10 for 1 h. The monolayer was then washed two times with prewarmed phosphate-buffered saline (PBS) to remove nonadherent bacteria (17, 28). Following the last wash, the INT-407 cells were fixed in methanol, stained with 0.4% Giemsa, and examined under a light microscope (28). To quantify the adherent bacteria, the INT-407 cells were also broken after washing, and the adherent bacteria were recovered and enumerated as described above. The mean number of adherent bacteria per cell was used to represent the adhesion index of the strains.
Competition assay.
Colonization activities of the wild type and nanA mutant HG072 were determined with the mouse model by competition assays as described earlier (24, 41). Briefly, four female ICR mice (7 weeks old) were infected as described above for the histopathological examination, except that 100 μl of the bacterial inoculum, prepared by mixing the wild type and the nanA mutant at a 1:1 ratio, representing approximately 106 CFU of each strain, was given intragastrically to the mice. Their intestines were collected after a 1- to 48-h infection, washed, and homogenated. Equal amounts of the homogenates were spread on LBS agar containing either rifampin (100 μg/ml) alone to enumerate the sum of wild-type and nanA mutant cells or rifampin and kanamycin (100 μg/ml) to specifically count the nanA mutant cells. The ratio of the number of CFU recovered from the intestines to the number of CFU inoculated is defined as the colonization index.
Data analysis.
Averages and standard errors of the mean (SEM) were calculated from at least three independent experiments. The statistical significance of the difference among the V. vulnificus strains was evaluated using analysis of variance, and significance was accepted at P values of <0.005.
RESULTS
Identification of the nanA gene and construction of the nanA mutant of V. vulnificus.
The amino acid sequence deduced from the putative nanA nucleotide sequence revealed a protein, a putative NanA composed of 299 amino acids with a theoretical molecular mass of 32,199 Da and a pI of 5.51. The amino acid sequence of the V. vulnificus NanA was 26% identical to that of the E. coli and H. influenzae NanA, and this identity appeared evenly throughout the proteins (data not shown; http://www.ncbi.nlm.nih.gov). The predicted hydrophobicity profile (http://www.expasy.ch) was similar to that of the NanA of E. coli and H. influenzae and was consistent with the fact that the NanA protein is a cytosolic soluble protein (data not shown). To examine the role of NanA, the V. vulnificus nanA isogenic mutant HG072 (Table 1) was constructed by allelic exchanges, and the insertional disruption of the nanA gene in HG072 was confirmed by PCR as described previously (data not shown; 13).
Effect of the nanA mutation on Neu5Ac lyase activity and colony morphotypes.
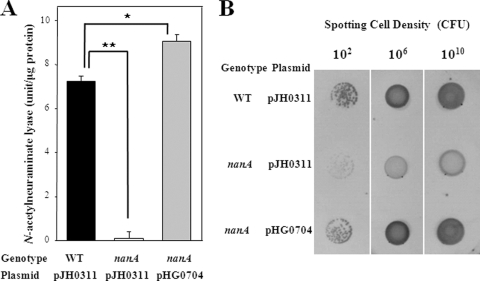
For the wild type, Neu5Ac lyase, initiating the catabolic degradation of Neu5Ac, was produced and reached a maximum at 7.2 units (Fig. 1A). The disruption of nanA in the mutant HG072 resulted in the complete loss of Neu5Ac lyase activity. These results demonstrated that the nanA gene encoded the V. vulnificus Neu5Ac lyase. The observation that the level of Neu5Ac lyase activity in the nanA mutant was almost undetectable revealed the existence of only one Neu5Ac lyase being produced by V. vulnificus.
FIG. 1.
Effect of the nanA mutation on the Neu5Ac lyase activity and colony morphotypes. Cultures of the wild type (WT) and the nanA mutant HG072 (nanA) were grown in modified M9 broth (A) or on a plate (B) containing 10 mM d-xylose, 10 mM l-proline, and 5 mM Neu5Ac. (A) Relative activities of Neu5Ac lyase were determined from samples removed at an A600 of 0.5 as described in the text. Error bars represent the SEM. *, P ≤ 0.01; **, P ≤ 0.005. (B) Colony morphotypes of the strains after 24 h of growth. Complementation of the mutant with a functional nanA (pHG0704) is also presented as indicated.
We examined whether the reintroduction of pHG0704 carrying a recombinant nanA could complement the decrease of Neu5Ac lyase activity of HG072. For this purpose, pHG0704 was constructed by subcloning the nanA amplified by a PCR using the primers NANA003 (5′-GAGCTCTGGCTACGGCCAGCCTTGG-3′) and NANA004 (5′-CCCGGGATCAAAAGCCACTGGTTTTTAATA-3′) into the broad host-range vector pJH0311 (7; Table 1). The Neu5Ac lyase activity of HG072 (pHG0704) was restored to a level comparable to the wild-type level of HG071 (Fig. 1A). Therefore, the decreased Neu5Ac lyase activity of HG072 was confirmed to result from the inactivation of functional nanA rather than any polar effects on genes downstream of nanA.
Morphotypes of V. vulnificus colonies are divided into opaque and translucent due to differences in the light transmission of the colonies. The wild-type HG071 was observed as an opaque colony type, and disruption of the nanA gene converted the wild type to a translucent colony type in the presence of Neu5Ac (Fig. 1B). The colony opacity of the complemented strain HG072 (pHG0704) was restored to the wild-type level (Fig. 1B), indicating that the nanA gene seems to contribute to the formation of the V. vulnificus opaque morphotype in the presence of Neu5Ac.
Effect of the nanA mutation on utilization of Neu5Ac as a sole carbon source.
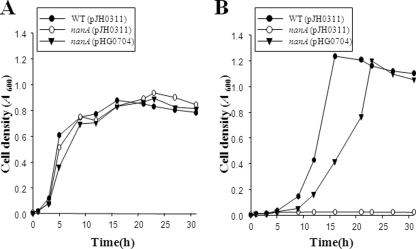
Growth was determined for the wild type, HG072, and HG072 (pHG0704) in M9 broth containing either 5 mM glucose or 5 mM Neu5Ac as a sole carbon source (Fig. 2). No significant differences were observed between the wild type, HG072, and HG072 (pHG0704) in their growth with M9 medium containing glucose (Fig. 2A). When Neu5Ac was used as a sole carbon source, 5 mM Neu5Ac supported the growth of the wild type and the complemented strain, and the stationary-phase yield of cells was even higher than that obtainable when glucose was used (Fig. 2B). In contrast to this result, HG072 that is deficient of functional NanA was not able to grow at all, indicating that the conversion of Neu5Ac into ManNAc is essential for the growth of V. vulnificus with Neu5Ac as a sole carbon source.
FIG. 2.
Growth kinetics of the V. vulnificus strains. Cultures of wild-type HG071, the nanA mutant HG072, or the complemented strain were grown in M9 medium supplemented with 5 mM glucose (A) or 5 mM Neu5Ac (B) as a sole carbon source. The data are the means from three independent experiments. WT, wild type; nanA (pJH0311), nanA mutant; nanA (pHG0704), complemented strain. Details are described in Materials and Methods.
NanA is important for cytotoxicity toward epithelial cells in vitro.
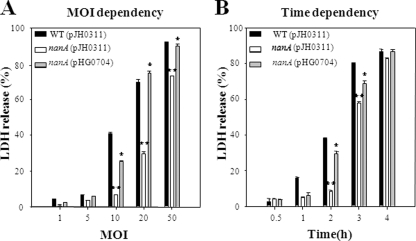
Monolayers of INT-407 cells infected with the wild type, HG072, and HG072 (pHG0704) strains at different MOIs were incubated for 2 h, after which the LDH-releasing activities were determined (Fig. 3A). The nanA mutant HG072 exhibited significantly less LDH-releasing activity when the MOIs were 10, 20, and 50. The level of LDH activity released from the INT-407 cells infected with HG072 was almost sixfold less than that from the cells infected with the wild type at an MOI of 10. Also, the LDH activities displayed from INT-407 cells infected at an MOI of 10 were compared at different incubation times as shown in Fig. 3B. When incubated for 2 h and 3 h, the cells infected with HG072 exhibited lower LDH activities than those of the cells infected with the wild type. The lower LDH activities were restored to the level obtained from the cells infected with the wild type when the cells were incubated with HG072 (pHG0704) (Fig. 3). These results suggest that NanA is important in regard to the ability of V. vulnificus to infect and injure host cells.
FIG. 3.
Effect of the nanA mutation on virulence of V. vulnificus to INT-407 cells. INT-407 cells were infected with wild-type HG071, the nanA mutant HG072, or the complemented strain at various MOIs for 2 h (A) or at an MOI of 10 for various incubation times (B). Error bars represent the SEM. *, P < 0.05; **, P < 0.005 relative to groups infected with the V. vulnificus wild type at each MOI or incubation time. WT, wild type; nanA (pJH0311), nanA mutant; nanA (pHG0704), complemented strain.
NanA is important for virulence in mice.
Predisposed individuals, i.e., those with underlying conditions related to their being immunocompromised, such as liver damage and excess levels of iron, are susceptible to infection with the pathogen V. vulnificus (18, 37). Therefore, mice were overloaded with iron to mimic V. vulnificus pathogenesis. The LD50s in the iron-overloaded mice after intraperitoneal infection with V. vulnificus strains were measured. For six iron-treated mice in each inoculation group and with inoculations ranging from 100 to 108 CFU in 10-fold increments, the intraperitoneal LD50 for the nanA mutant HG072 was 7.01 × 104 CFU, compared with an LD50 of 3.16 × 101 CFU for the wild type. Therefore, for the mouse model of intraperitoneal infection, in which the nanA mutant exhibited a >3-log increase in the LD50 over that of the isogenic parental strain, the nanA mutant appeared to be less virulent than its parental wild type. From this difference in LD50s, it is apparent that V. vulnificus NanA is important for V. vulnificus virulence in mice.
Histopathology of mice infected with V. vulnificus.
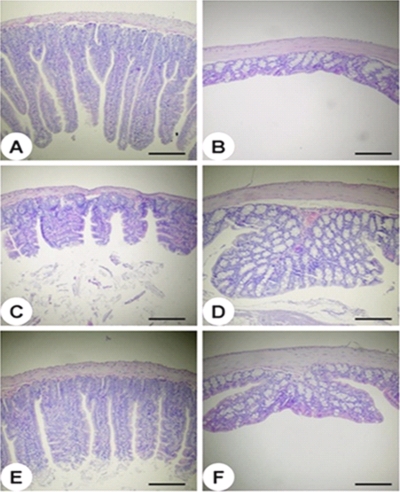
In order to understand the pathological changes occurring in the mouse intestine during infection with the wild type or HG072, histological examinations of intestinal sections of the jejuna (Fig. 4A, C, and E) and colons (Fig. 4B, D, and F) were performed. There were no histopathological changes in the jejuna (Fig. 4A) and colons (Fig. 4B) of mice that received PBS alone as a control. In comparison to the PBS-treated mice, the wild-type-treated mice revealed remarkably shorter villus lengths in the jejunum (Fig. 4C). In contrast, the HG072-treated mice had villi of lengths that were intermediate, between those of the control and wild-type-treated mice (Fig. 4E). Both wild-type and HG072-treated mice showed hypertrophy of the mucosal glands in their colons (Fig. 4D and 4F). However, mucosal glands in the colons of wild-type-treated mice (Fig. 4D) showed much greater hypertrophicity than those in the colons of HG072-treated mice (Fig. 4F). Thus, these results indicated that NanA is important for the virulence of the bacteria in the mouse intestine.
FIG. 4.
Histopathology of mouse intestine tissues after infection with the V. vulnificus strains. The jejunum (A, C, and E) and colon (B, D, and F) tissues of mice infected intragastrically with PBS (A and B), wild-type V. vulnificus (C and D), or the nanA mutant strain of V. vulnificus (E and F) were histopathologically examined. Hematoxylin and eosin stain. Scale bars = 200 μm.
Growth rates of the V. vulnificus strains during infection and toxic effects of Neu5Ac.
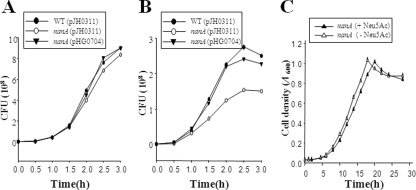
When taken together, the results presented above clearly demonstrated that the NanA protein required for V. vulnificus to utilize Neu5Ac as a carbon source is important for V. vulnificus pathogenesis. However, it is unlikely that NanA could directly injure the host (or host cells), as proposed for hemolysin, elastolytic protease, and RtxA (10, 16, 36). To examine whether reduced cytotoxicity of the nanA mutant (Fig. 3) resulted from defects in its growth, we compared the growth rate of the nanA mutant with that of the wild type. The growth rate of the nanA mutant in minimal essential medium (MEM) with 1% fetal bovine serum in the absence of INT-407 cells was not significantly different from that of the wild type (Fig. 5A). During the infection, however, the growth rate of the nanA mutant in the INT-407 tissue cultures was significantly lower than that of the wild type (Fig. 5B), indicating that the capability of using Neu5Ac from the INT-407 cells as a carbon source makes the growth rates different. Similar results revealing the difference between growth rates of the wild type and the nanA mutant during infection were also obtained when the bacteria were enumerated in the supernatant without using Triton (data not shown). Therefore, it is unlikely that growth of the nanA mutant is more strongly inhibited by (the factors released from) the host cells than is the growth of the wild type.
FIG. 5.
Growth rates of the V. vulnificus strains during infection and toxic effects of Neu5Ac. (A) Growth of the strains in MEM supplemented with 1% fetal bovine serum, in the absence of INT-407 cells, was determined by enumerating CFU on LBS agar plates at the indicated time intervals. (B) For growth of the strains during infection of INT-407 cells, the strains were used to infect the INT-407 cells at an MOI of 10 and then bacterial cells during infection were enumerated as described above. (C) Cultures were grown in modified M9 broth containing 10 mM d-xylose and 10 mM l-proline and supplemented either with or without 5 mM Neu5Ac as indicated. Growth was monitored by measuring the A600 of the cultures. WT, wild type; nanA (pJH0311), nanA mutant; nanA (pHG0704), complemented strain. The data are means from three independent experiments.
In E. coli, growth of a nanA mutant is inhibited by the toxic accumulation of intracellular Neu5Ac (39). Therefore, the toxic effect of Neu5Ac on growth of the V. vulnificus nanA mutant was measured in vitro, and the result demonstrated that growth of the V. vulnificus nanA mutant was not inhibited substantially by the presence of Neu5Ac in the growth medium (Fig. 5C). These results combined suggest that the decreased virulence of the nanA mutant likely resulted from its growth defect when it infected the INT-407 cells and that the growth defect might be caused by its inability to utilize Neu5Ac rather than by a toxic accumulation of Neu5Ac.
NanA is required for adhesion to epithelial cells in vitro.
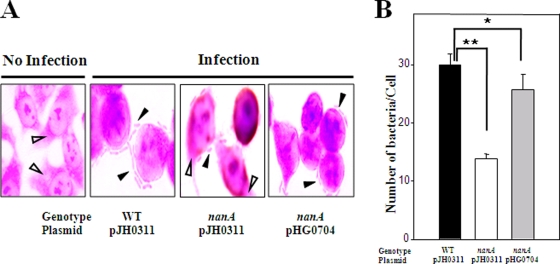
It has been reported that the change of colony morphotypes influences the surface hydrophobicity and surface charge of the bacterial cell, and altering the physiochemical characteristics of the cell surface has been postulated to modify the relative adhesive properties of bacteria (2, 19, 46). The adhesion abilities of the V. vulnificus strains were compared using INT-407 monolayers. The wild type and the complemented strain HG072 (pHG0704) exhibited formation of small clusters of aggregated bacteria on the surface of INT-407 cells (Fig. 6A). After 1 h of infection, the wild type and the complemented strain adhered to INT-407 cells and reached adhesion indexes of 30 and 26, respectively (Fig. 6B). In contrast, a much smaller area of the intestinal cell surface was found covered with the nanA mutant HG072, and no clusters of aggregated bacteria were observed (Fig. 6A and 6B). When infected for 1 h, the number of nanA mutants per cell on INT-407 monolayers was about twofold lower than that of the wild type (Fig. 6B), indicating that the nanA mutant was significantly impaired in its ability to attach to epithelial cells. These results suggest that NanA might contribute to V. vulnificus virulence by facilitating adhesion on host epithelial cells.
FIG. 6.
Adhesion of the V. vulnificus strains. (A) INT-407 cells were cultured on glass coverslips and infected at an MOI of 10. After incubation with the bacteria for 1 h, the INT-407 monolayers were rinsed to remove any nonadhering bacteria. Light micrographs (original magnification, ×1200) show the adhesion of the wild type, the nanA mutant, and the complemented strain to the INT-407 cells. The adhered V. vulnificus cells (closed arrowheads) and the cytoplasm of the INT-407 cells (open arrowheads) are indicated. (B) The adherent bacteria were quantified and expressed as the number of bacteria per cell in the tissue culture. *, P < 0.05; **, P < 0.005. WT, wild type; nanA (pJH0311), nanA mutant; nanA (pHG0704), complemented strain. Error bars represent the SEM.
NanA is required for intestinal colonization in vivo.
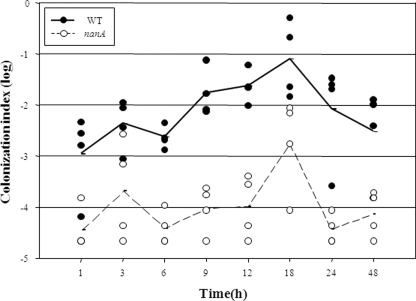
Because NanA is obviously crucial for the growth and adhesion of V. vulnificus during infection of INT-407 cells in vitro, it is reasonable to hypothesize that NanA also might play a similar role in vivo during infection of mice. To address this hypothesis, mice were coinoculated intragastrically with the nanA mutant and the wild type, and the strains colonized on the intestine were recovered and enumerated (Fig. 7). Both strains appeared to colonize the mouse intestine as early as 1 h after inoculation, and the colonization index increased as the period of colonization was prolonged and reached a maximum level at 18 h. The colonization index of the nanA mutant ranged from 10−5 to 10−3 and was consistently and significantly (about 100-fold) lower than that of the wild-type strain, demonstrating that the nanA mutant is severely defective in colonizing the intestines of mice.
FIG. 7.
Colonization dynamics of the V. vulnificus strains. A competition assay was performed by inoculation of a mouse with the bacterial suspension prepared by mixing equal numbers of the wild type and the nanA mutant and then by enumeration of the bacterial cells colonized on the mouse intestine at the indicated time intervals. Each filled (wild type) and open (nanA mutant) circle represents a colonization index value (number of CFU recovered/number of CFU inoculated) calculated from the result of the individual competition assay; mean values are displayed as a line on the graph.
DISCUSSION
Sialic acids are a family of over 40 naturally occurring N- or O-substituted neuraminic acids, and they mediate a wide range of biological processes, including cell-cell and cell-small molecule interactions (39). Sialic acid is also the name for the most abundant and the best-studied sialic acid, Neu5Ac. Sialic acids are found widely distributed in animal tissues, especially in mucus glycoproteins, such as mucins. The mucins are the main component of the mucus layer, which is faced consistently and persistently with invading bacteria. Therefore, it is perhaps not surprising that sialic acids play an important role in mediating host-pathogen interactions. It appears that many pathogenic bacteria have evolved multiple routes to capture sialic acid from environments and to incorporate the sialic acid into cell surface macromolecules that modulate the pathogen's interaction with the host (34, 39, 40). The incorporation of sialic acid (sialylation) into bacterial cell surface molecules (such as sialylated lipopolysaccharide or polysialic acid capsule) is presumed to allow pathogens to disguise themselves as host cells and thus evade or counteract the host's immune responses (9, 34, 40). Although there is a great diversity in compositions and structure of the V. vulnificus capsular polysaccharide (CPS) (29, 33), researchers have been not successful in finding Neu5Ac residues in the CPS. But the genes involved in biosynthesis of Neu5Ac and the polysialic acid capsule, such as neuB and neuA, are found for V. vulnificus CMCP6 and YJ016 in the GenBank genome sequence database, indicating that V. vulnificus can synthesize Neu5Ac and the polysialic acid capsule. However, the question of whether Neu5Ac accumulates on its surface to evade the host immune system has not yet been addressed.
Many pathogenic bacteria also utilize sialic acid as carbon, nitrogen, and energy sources (35, 39, 40). In the present study, the growth rate of the V. vulnificus nanA mutant in MEM was not significantly different from that of the wild type. However, the growth rate of the mutant in the INT-407 tissue culture was significantly lower than that of the wild type. This result suggests that V. vulnificus, as an enteropathogenic bacterium, is able to metabolize host sialic acid in those growth environments. However, until now, there has been no definitive analysis of the biochemical pathway of sialic acid metabolism in V. vulnificus, and the genes encoding the components of the pathways have not yet been characterized at a molecular level. Extensive searches for amino acid sequences similar to those of the E. coli nan system predicted the putative nan system from V. vulnificus entries in the genome sequence database (data not shown; GenBank accession numbers AE016795 and AE016796). Although the nanA and nanEK genes encoding ManNAc-6-P epimerase and ManNAc kinase are organized in the same orientation in E. coli (39), the presumed V. vulnificus nanEK is transcribed divergently from nanA. The differences in nanAEK genetic organization, along with the low level of homology in NanA amino acid sequences (26% identical), indicate that the sialic acid catabolism of V. vulnificus would be evolved through its own host-microbe interactions that might be different from those for E. coli. This lack of overall homology in genetic organizations may be a common feature inherited in the various bacterial nan systems as proposed by Vimr and colleagues (39).
In the present study, the nanA mutant that is not able to catabolically utilize Neu5Ac revealed reduced virulence and impaired growth and adhesion in tissue cultures (Fig. 3 to 6). Furthermore, the nanA mutant revealed a reduced colonization index (Fig. 7), reflecting the impaired ability of the mutant to not only grow, survive, and persist in but also adhere to the intestine (5). Growth of the nanA mutant was not inhibited by the presence of Neu5Ac in the growth medium (Fig. 5C), demonstrating that the growth defect of the nanA mutant may result from its inability to utilize Neu5Ac rather than a toxic accumulation of Neu5Ac. Expression of the V. vulnificus nan system, including the nanT gene encoding a Neu5Ac transporter, is tightly repressed by NanR, as is that of the H. influenzae nan system (S. H. Choi, unpublished data; 14). Growth of the V. vulnificus nanR mutant was inhibited by the presence of Neu5Ac (data not shown), indicating that the derepression of nanT accumulates Neu5Ac to the level of toxicity. Although other explanations are possible, it is reasonable to hypothesize that the intracellular level of Neu5Ac in the wild type and the nanA mutant is precisely controlled by NanR and that the toxicity of the Neu5Ac is not apparent.
We examined colony opacity of V. vulnificus grown with LBS and M9 medium (plus glucose) in the presence or absence of Neu5Ac. The colony morphotypes of the strains were all opaque and not significantly different from each other regardless of the presence of Neu5Ac (data not shown). Although it has been observed that colony opacity of the bacteria and the production of CPS are directly related, very little has been known about the biosynthetic pathway for the V. vulnificus CPS (12, 27, 28, 45, 49). However, Pseudomonas aeruginosa and E. coli, for which CPS synthesis is well characterized biochemically and genetically, use versatile pathways for the synthesis of CPS (1, 39). Therefore, it is perhaps not surprising that V. vulnificus also uses versatile pathways (and versatile substrates, such as glucose) and does not depend entirely on Neu5AC for the synthesis of CPS.
If a biosynthetic pathway similar to that for E. coli and P. aeruginosa (1, 39) operates in V. vulnificus, ManNAc, the product of Neu5Ac cleavage by NanA, would be converted to ManNAc-6-P and, ultimately, to UDP-N-acetyl-d-glucosamine (UDP-GlcNAc), which is the main activated precursor of surface-associated carbohydrate (such as CPS and lipopolysaccharide) synthesis (1, 6). One possible explanation is that loss of the ability to degrade Neu5Ac to ManNAc in the nanA mutant can impair the optimal synthesis of CPS and change its morphotype to reduced opacity in the presence of Neu5Ac, as shown in Fig. 1B. It is generally believed that less CPS are produced in bacteria with reduced opacity, and the adhesion abilities of bacteria are affected by the amount of CPS (12, 47). Therefore, it is likely that reduced opacity may be a reason, if not the sole reason, for the impaired adherence exhibited by the nanA mutant (Fig. 6). However, the exact role of NanA in CPS biosynthesis and alteration of adhesion activity of V. vulnificus still remains to be determined.
In summary, it is apparent that V. vulnificus is able to utilize Neu5Ac as a nutrient during infection. The nanA mutant that was not able to catabolically utilize Neu5Ac revealed a less-opaque colony morphotype and exhibited a reduced virulence in tissue cultures and in mice, indicating that NanA plays an important role in V. vulnificus pathogenesis. In addition, growth with and adherence to epithelial cells and intestinal colonization of the nanA mutant were significantly impaired, indicating that NanA and the capability of catabolic utilization of Neu5Ac could contribute to V. vulnificus pathogenesis by ensuring survival and multiplication during infection rather than directly aggravating damage or injury of the host.
Acknowledgments
This study was supported by grants to S.H.C. from the Marine Biotechnology Program funded by the Ministry of Land, Transport, and Maritime Affairs; the Korean Research Foundation (KRF-2008-314-C0036); and the National Research Laboratory, Korea Science and Engineering Foundation (R0A-2007-000-20039-0), Republic of Korea.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 1 June 2009.
REFERENCES
- 1.Belanger, M., L. L. Burrows, and J. S. Lam. 1999. Functional analysis of genes responsible for the synthesis of the B-band O antigen of Pseudomonas aeruginosa serotype O6 lipopolysaccharide. Microbiology 1453505-3521. [DOI] [PubMed] [Google Scholar]
- 2.Biosca, E. A., H. Llorens, E. Garay, and C. Amaro. 1993. Presence of a capsule in Vibrio vulnificus biotype 2 and its relationship to virulence for eels. Infect. Immun. 611611-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 4.Bulai, T., D. Bratosin, V. Artenie, and J. Montreuil. 2002. Characterization of a sialate pyruvate-lyase in the cytosol of human erythrocytes. Biochimie 84655-660. [DOI] [PubMed] [Google Scholar]
- 5.Chang, D. E., D. J. Smalley, D. L. Tucker, M. P. Leatham, W. E. Norris, S. J. Stevenson, A. B. Anderson, J. E. Grissom, D. C. Laux, P. S. Cohen, and T. Conway. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. USA 1017427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creuzenet, C., M. Belanger, W. W. Wakarchuk, and J. S. Lam. 2000. Expression, purification, and biochemical characterization of WbpP, a new UDP-GlcNAc C4 epimerase from Pseudomonas aeruginosa serotype O6. J. Biol. Chem. 27519060-19067. [DOI] [PubMed] [Google Scholar]
- 7.Goo, S. Y., H.-J. Lee, W. H. Kim, K.-L. Han, D.-K. Park, H.-J. Lee, S. M. Kim, K.-S. Kim, K.-H. Lee, and S.-J. Park. 2006. Identification of OmpU of Vibrio vulnificus as a fibronectin-binding protein and its role in bacterial pathogenesis. Infect. Immun. 745586-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulig, P. A., K. L. Bourdage, and A. M. Starks. 2005. Molecular pathogenesis of Vibrio vulnificus. J. Microbiol. 43118-131. [PubMed] [Google Scholar]
- 9.Harvey, H. A., W. E. Swords, and M. A. Apicella. 2001. The mimicry of human glycolipids and glycosphingolipids by the lipooligosaccharides of pathogenic Neisseria and Haemophilus. J. Autoimmun. 16257-262. [DOI] [PubMed] [Google Scholar]
- 10.Hase, C. C., and R. A. Finkelstein. 1993. Bacterial extracellular zinc-containing metalloproteases. Microbiol. Rev. 57823-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper, L. V., T. Midtvedt, and J. I. Gordon. 2002. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 22283-307. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh, Y.-C., S.-M. Liang, W.-L. Tsai, Y.-H. Chen, T.-Y. Liu, and C.-M. Liang. 2003. Study of capsular polysaccharide from Vibrio parahaemolyticus. Infect. Immun. 713329-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong, H. S., M. H. Lee, K. H. Lee, S. J. Park, and S. H. Choi. 2003. SmcR and cyclic AMP receptor protein coactivate Vibrio vulnificus vvpE encoding elastase through the RpoS-dependent promoter in a synergistic manner. J. Biol. Chem. 27845072-45081. [DOI] [PubMed] [Google Scholar]
- 14.Johnston, J. W., A. Zaleski, S. Allen, J. M. Mootz, D. Armbruster, B. W. Gibson, M. A. Apicella, and R. S. Munson. 2007. Regulation of sialic acid transport and catabolism in Haemophilus influenzae. Mol. Microbiol. 6626-39. [DOI] [PubMed] [Google Scholar]
- 15.Lång, H., G. Jonson, J. Holmgren, and E. T. Palva. 1994. The maltose regulon of Vibrio cholerae affects production and secretion of virulence factors. Infect. Immun. 624781-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, J. H., M. W. Kim, B. S. Kim, S. M. Kim, B. C. Lee, T. S. Kim, and S. H. Choi. 2007. Identification and characterization of the Vibrio vulnificus rtxA essential for cytotoxicity in vitro and virulence in mice. J. Microbiol. 45146-152. [PubMed] [Google Scholar]
- 17.Lim, M. S., M. H. Lee, J. H. Lee, H. M. Ju, N. Y. Park, H. S. Jeong, J. E. Rhee, and S. H. Choi. 2005. Identification and characterization of the Vibrio vulnificus malPQ operon. J. Microbiol. Biotechnol. 15616-625. [Google Scholar]
- 18.Linkous, D. A., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174207-214. [DOI] [PubMed] [Google Scholar]
- 19.Makin, S. A., and T. J. Beveridge. 1996. The influence of A-band and B-band lipopolysaccharide on the surface characteristics and adhesion of Pseudomonas aeruginosa to surfaces. Microbiology 142299-307. [DOI] [PubMed] [Google Scholar]
- 20.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 1741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, C. P., and M. Bohnhoff. 1963. Changes in the mouse's enteric microflora associated with enhanced susceptibility to Salmonella infection following streptomycin treatment. J. Infect. Dis. 11359-66. [DOI] [PubMed] [Google Scholar]
- 22.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1702575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milton, D. L., R. O'Toole, P. Horstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 1781310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nesper, J., S. Schild, C. M. Lauriano, A. Kraiss, K. E. Klose, and J. Reidl. 2002. Role of Vibrio cholerae O139 surface polysaccharides in intestinal colonization. Infect. Immun. 705990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman, J. V., R. Kolter, D. C. Laux, and P. S. Cohen. 1994. Role of leuX in Escherichia coli colonization of the streptomycin-treated mouse large intestine. Microb. Pathog. 17301-311. [DOI] [PubMed] [Google Scholar]
- 26.Oka, A., H. Sugisaki, and M. Takanami. 1981. Nucleotide sequence of the kanamycin resistance transposon Tn903. J. Mol. Biol. 147217-226. [DOI] [PubMed] [Google Scholar]
- 27.Park, N. Y., J. H. Lee, B. C. Lee, T. S. Kim, and S. H. Choi. 2006. Identificatioin and characterization of the Vibrio vulnificus wbpO gene essential for lipopolysaccharide synthesis in Vibrio vulnificus. J. Microbiol. Biotechnol. 16808-816. [Google Scholar]
- 28.Park, N. Y., J. H. Lee, M. W. Kim, H. G. Jeong, B. C. Lee, T. S. Kim, and S. H. Choi. 2006. Identificatioin of the Vibrio vulnificus wbpP gene and evaluation of its role in virulence. Infect. Immun. 74721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy, G. P., U. Hayat, Q. W. Xu, K. V. Reddy, Y. H. Wang, K. W. Chiu, J. G. Morris, and C. A. Bush. 1998. Structure determination of the capsular polysaccharide from Vibrio vulnificus strain 6353. Eur. J. Biochem. 255279-288. [DOI] [PubMed] [Google Scholar]
- 30.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 2739-497. [Google Scholar]
- 31.Reissig, J. L., J. L. Storminger, and L. F. Leloir. 1955. A modified colorimetric method for the estimation of N-acetylamino sugars. J. Biol. Chem. 217959-966. [PubMed] [Google Scholar]
- 32.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 33.Senchenkova, S. N., A. S. Shashkov, Y. A. Knirel, C. Esteve, E. Alcaide, S. Merino, and J. M. Tomas. 2009. Structure of a polysaccharide from the lipopolysaccharide of Vibrio vulnificus clinical isolate YJ016 containing 2-acetimidoylamino-2-deoxy-l-galacturonic acid. Carbohydr. Res. 3441009-1013. [DOI] [PubMed] [Google Scholar]
- 34.Severi, E., D. W. Hood, and G. H. Thomas. 2007. Sialic acid utilization by bacterial pathogens. Microbiology 1532817-2822. [DOI] [PubMed] [Google Scholar]
- 35.Severi, E., G. Randle, P. Kivlin, K. Whitfield, R. Young, R. Moxon, D. Kelly, D. Hood, and G. H. Thomas. 2005. Sialic acid transport in Haemophilus influenzae is essential for lipopolysaccharide sialylation and serum resistance and is dependent on a novel tripartite ATP-independent periplasmic transporter. Mol. Microbiol. 581173-1185. [DOI] [PubMed] [Google Scholar]
- 36.Shinoda, S., S. Miyoshi, H. Tamanaka, and N. N. Miyoshi. 1985. Some properties of Vibrio vulnificus hemolysin. Microbiol. Immunol. 29583-590. [DOI] [PubMed] [Google Scholar]
- 37.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2177-188. [DOI] [PubMed] [Google Scholar]
- 38.Vimr, E. R., and F. A. Troy. 1985. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J. Bacteriol. 164845-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vimr, E. R., K. A. Kalivoda, E. L. Deszo, and S. M. Steenbergen. 2004. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 68132-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vimr, E. R., K. Lichtensteiger, and S. M. Steenbergen. 2000. Sialic acid metabolism's dual function in Haemophilus influenzae. Mol. Microbiol. 361113-1123. [DOI] [PubMed] [Google Scholar]
- 41.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiggins, R., S. Hicks, P. Soothill, M. Millar, and A. Corfield. 2001. Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex. Transm. Infect. 77402-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson, M., R. McNab, and B. Henderson. 2002. Bacterial disease mechanisms, 1st ed. Cambridge University Press, Cambridge, United Kingdom.
- 44.Wright, A. C., J. G. Morris, Jr., D. R. Maneval, Jr., K. Richardson, and J. B. Kaper. 1985. Cloning of the cytotoxin-hemolysin gene of Vibrio vulnificus. Infect. Immun. 50922-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright, A. C., J. L. Powell, J. B. Kaper, and J. G. Morris, Jr. 2001. Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect. Immun. 696893-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright, A. C., L. M. Simpson, J. D. Oliver, and J. G. Morris, Jr. 1990. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 581769-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto, T., M. J. Albert, and R. B. Sack. 1994. Adherence to human small intestines of capsulated Vibrio cholerae O139. FEMS Microbial. Lett. 119229-235. [DOI] [PubMed] [Google Scholar]
- 48.Yoon, H., S. Lim, S. Heu, S. Choi, and S. Ryu. 2003. Proteome analysis of Salmonella enterica serovar Typhimurium fis mutant. FEMS Microbiol. Lett. 226391-396. [DOI] [PubMed] [Google Scholar]
- 49.Zuppardo, A. B., and R. J. Siebeling. 1998. An epimerase gene essential for capsule synthesis in Vibrio vulnificus. Infect. Immun. 662601-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]