Abstract
To colonize and cause disease at distinct anatomical sites, bacterial pathogens must tailor gene expression in a microenvironment-specific manner. The molecular mechanisms that control the ability of the human bacterial pathogen group A Streptococcus (GAS) to transition between infection sites have yet to be fully elucidated. A key regulator of GAS virulence gene expression is the CovR-CovS two-component regulatory system (also known as CsrR-CsrS). covR and covS mutant strains arise spontaneously during invasive infections and, in in vivo models of infection, rapidly become dominant. Here, we compared wild-type GAS with covR, covS, and covRS isogenic mutant strains to investigate the heterogeneity in the types of natural mutations that occur in covR and covS and the phenotypic consequences of covR or covS mutation. We found that the response regulator CovR retains some regulatory function in the absence of CovS and that CovS modulates CovR to significantly enhance repression of one group of genes (e.g., the speA, hasA, and ska genes) while it reduces repression of a second group of genes (e.g., the speB, grab, and spd3 genes). We also found that different in vivo-induced covR mutations can lead to strikingly different transcriptomes. While covS mutant strains show increased virulence in several invasive models of infection, we determined that these mutants are significantly outcompeted by wild-type GAS during growth in human saliva, an ex vivo model of upper respiratory tract infection. We propose that CovS-mediated regulation of CovR activity plays an important role in the ability of GAS to cycle between pharyngeal and invasive infections.
The bacterial pathogen group A Streptococcus (GAS) (Streptococcus pyogenes) causes many distinct human diseases. The disease manifestations range from self-limiting GAS pharyngitis to severely invasive necrotizing fasciitis (“flesh-eating” disease). The molecular mechanisms controlling GAS colonization and disease progression in distinct niches in the human host are incompletely defined. However, recent data indicate that interactions between multiple regulatory systems contribute to the control of tissue tropism and infection (3, 28, 29, 32).
One key to the ability of many bacterial pathogens to regulate gene expression is the function of two-component signal transduction systems (TCS) (19, 30). Prototypical TCS consist of a membrane-spanning sensor kinase and a cytoplasmic response regulator. Upon activation, the sensor kinase autophosphorylates and subsequently transfers the phosphate group to the response regulator. Phosphorylation of the response regulator results in activation of this molecule, leading to changes in cellular physiology, usually through activation of DNA-binding activity enabling regulation of gene expression (19).
Thirteen conserved TCS are encoded in the GAS genome, and the CovR-CovS system (control of virulence; also known as CsrR-CrsS) is the best-studied system (7-9, 12, 14, 20, 32, 34). The sensor kinase CovS and the response regulator CovR together directly or indirectly regulate ∼10% of the GAS transcriptome, including many well-described virulence factors (6, 12, 32). The CovR-CovS system is an atypical TCS as it serves mainly as a negative regulator of gene expression, and covRS mutant strains produce increased concentrations of many virulence factors (9, 20). Although phosphorylation of CovR by CovS has yet to be shown biochemically, the ratio of phosphorylated CovR to nonphosphorylated CovR is reduced in a covS mutant strain (5). Similar to several response regulators, CovR can be phosphorylated by the low-molecular-weight compound acetyl phosphate (2, 10, 22). The interactions between CovR phosphorylation by CovS, other kinases, and acetyl phosphate are currently being investigated in several laboratories (5, 16; V. Pancholi, presented at the XVII Lancefield Meeting, Porto Heli, Greece, 2008).
Recently, we and other workers provided evidence that there is in vivo selection of covRS mutant strains during invasive infections (1, 8, 32, 34). The increased resistance of covRS mutant strains to neutrophil-mediated killing, as well as their increased virulence in a murine bacteremia model of infection, raised the question of why not all GAS strains harbor covRS mutations (8, 32). Here we describe experiments aimed at addressing this question and provide data supporting the hypothesis that the CovR-CovS system facilitates the cycling of GAS strains between pharyngeal and invasive infections. In addition, we discuss why covR and covS mutant GAS strains differ phenotypically. In this study we also investigated the observed heterogeneity in the types of natural mutations that occur in covR and covS.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Strain MGAS2221 is a representative of the highly virulent M1T1 GAS clone responsible for the majority of contemporary pharyngeal and invasive GAS infections in the United States, Canada, and Western Europe (31). MGAS2221 has wild-type covR and covS alleles that encode a functional CovR-CovS system, as assessed by protein secretion and transcriptome profiles (see Fig. 2) (32). Details concerning the GAS strains used in this study are shown in Table 1. GAS strains were grown in vitro in Todd-Hewitt broth with 0.2% yeast extract (THY broth) at 37°C with 5% CO2.
FIG. 2.

Virulence factor secretion profiles are different for wild-type, covR mutant, covS mutant, and covRS mutant strains. Western immunoblots were used to assay secretion of streptococcal pyrogenic exotoxin A (SpeA), cysteine protease (SpeB), SLO, SPN, and Mac. The proteins analyzed were precipitated from filter-sterilized overnight THY broth cultures. wt, wild type.
TABLE 1.
GAS strains used in this study
| Strain | Location | Year | Disease or sourcea | Genotype |
|---|---|---|---|---|
| MGAS5005 | Ontario, Canada | 1996 | Cerebral spinal fluid | covS |
| MGAS2221 | Australia | 1988 | Scarlet fever | Wild type |
| 2221ΔcovR | NAb | NA | MGAS2221 derivative | covR |
| 2221covS::7bp | NA | NA | Mouse-passaged derivative | covS |
| 2221covSΔ1bp | NA | NA | Mouse-passaged derivative | covS |
| 2221ΔcovR/S | NA | NA | 2221covSΔ1bp derivative | covRS |
| 2221covR-R119H | NA | NA | Mouse-passaged derivative | covR(R119H) |
| 2221covR-ΔM79 | NA | NA | Mouse-passaged derivative | covR(ΔM79) |
MGAS2221 derivatives either were constructed using mutagenesis as described in Material and Methods or were isolated from mice following infection with MGAS2221 (mouse-passaged derivatives) (32).
NA, not applicable.
Construction of covR and covS isogenic mutant strains.
Isogenic covR (strain 2221ΔcovR) and covRS (strain 2221ΔcovR/S) mutants of wild-type strain MGAS2221 were constructed to augment a collection of MGAS2221 covR and covS mutants isolated in a previous study (32) (Table 1). Strain 2221ΔcovR was constructed by replacement of an internal fragment of covR with a promoterless aphA3 gene encoding kanamycin resistance. This mutation has been determined to be nonpolar (9). Strain 2221ΔcovR/S was constructed like 2221ΔcovR, except that the MGAS2221 covS mutant derivative 2221covSΔ1bp was used as the parental strain. The covS mutation in strain 2221covSΔ1bp is a 1-bp deletion that knocks the gene out of frame. Strain 2221covSΔ1bp was complemented by cloning the wild-type covS gene into plasmid pDCBB to create pCovSC. Plasmid pDCBB is a derivative of the Escherichia coli-GAS shuttle vector pDC123 that was digested with BamHI BglII and religated (4). Strain 2221covS::7bp contains a 7-bp insertion in the covS gene that knocks the gene out of frame. Whole-genome resequencing has been performed for strain 2221covS::7bp (previously designated 26PL1), and this strain differs from MGAS2221 only by the 7-bp insertion in covS (32). Strain 2221covR-R119H has a nonsynonymous mutation in covR that changes amino acid 119 from arginine to histidine. Strain 2221covR-ΔM79 has a 3-bp deletion in covR that removes one of three contiguous internal methionine codons. Strains 2221covSΔ1bp, 2221covS::7bp, 2221covR-R119H, and 2221covR-ΔM79 were previously recovered from mice experimentally infected with parental strain MGAS2221 (32). The covRS genes of GAS strains used in this study are shown in Fig. 1A.
FIG. 1.

Derivatives of GAS strain MGAS2221 used in this study. (A) The covR mutations in strains 2221ΔcovR and 2221ΔcovR/S were constructed by replacing the DNA-binding domain encoded by covR with a kanamycin resistance cassette, similar to a previously described strain (12). Strains 2221covSΔ1bp, 2221covS::7bp, 2221covR-R119H, and 2221covR-ΔM79 were isolated previously from mice experimentally infected with parental strain MGAS2221 (32). (B) Southern blot confirming correct molecular construction of isogenic mutant strains 2221ΔcovR and 2221ΔcovR/S. Genomic DNA from parental GAS strain MGAS2221, putative covR mutant strain 2221ΔcovR, and putative covRS mutant strain 2221ΔcovR/S was digested with HindIII, separated by agarose gel electrophoresis, transferred to a membrane, and probed with a region of DNA flanking covR. The expected hybridizing fragment size for the parental strain is 3.7 kb, whereas the expected size for the mutant strains is 4.2 kb. (C) Quantitative RT-PCR analysis to confirm complementation of 2221covSΔ1bp with a wild-type covS gene. RNA was isolated from exponential-phase GAS cultures, converted to cDNA, and analyzed by TaqMan quantitative RT-PCR. The experiment was performed in quadruplicate, and the bars and error bars indicate the mean ± standard deviation. WT, wild type.
Western immunoblot analysis of in vitro-grown cultures.
Supernatant proteins from GAS cultures grown overnight in THY broth were concentrated by ethanol precipitation and resuspended in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) loading buffer at 1/20 the original volume. Western immunoblots were probed with rabbit polyclonal antibodies raised against each antigen. Goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies were used to detect primary antibody binding and to generate signals.
Expression and purification of three CovR proteins.
The covR genes of wild-type strain MGAS2221 and covR mutant strains 2221covR-R119H and 2221covR-ΔM79 were PCR amplified using primers COVRXHF and COVRXHR (see Table S1 in the supplemental material). These primers incorporate an N-terminal six-His tag (see Table S1 in the supplemental material) into the CovR proteins. PCR products were digested with NcoI and BamHI (see Table S1 in the supplemental material) and ligated into pET-21b digested with the same restriction enzymes. The resultant plasmids were each transformed into E. coli BL21(DE3)/pLysS competent cells for expression of the encoded CovR proteins. The CovR expression plasmids from BL21(DE3)/pLysS cells were sequenced to confirm that no additional mutations had been introduced in the PCR and cloning steps. CovR expression was induced by addition of 500 μM isopropyl-β-d-thiogalactopyranoside (IPTG) to 250-ml LB cultures after the optical density at 600 nm reached 0.6 and incubation in a shaking incubator (200 rpm) for 4 h at 30°C. E. coli cells were collected by centrifugation, and the bacterial pellets were stored at −20°C. CovR proteins from the three E. coli cell pellets were purified using an Ni-nitrilotriacetic acid Fast Start kit (Qiagen Inc.) according to the manufacturer's instructions. Recovered proteins were analyzed by SDS-PAGE to monitor purity (see Fig. S2 in the supplemental material), and protein concentrations were determined using the Bio-Rad protein assay reagent (Bio-Rad).
EMSAs.
Whether the CovR proteins produced by covR mutant GAS strains 2221covR-R119H and 2221covR-ΔM79 were able to bind DNA in a sequence-dependent manner was tested using electrophoretic mobility shift assays (EMSAs). EMSAs were performed using a LightShift chemiluminescent EMSA kit (Pierce) according to the manufacturer's instructions. Briefly, unlabeled 210-bp probes spanning the promoter region of the CovR-regulated spyCEP genes or the non-CovR-regulated dnaN gene were amplified by PCR using primers SPYCEPF and SPYCEPR or primers DNANF and DNANR (see Table S1 in the supplemental material). Labeled probes were generated using primers that had identical sequences but were 5′ labeled with biotin (Sigma-Genosys). Reaction mixtures containing 40 pg of labeled probe and 5 μM mutant or wild-type CovR were prepared using 2× binding buffer [40 mM Tris-HCl (pH 7.4), 2 mM CaCl2, 200 μg bovine serum albumin, 20 μg poly(dI-dC), 2 mM dithiothreitol, 32 mM acetyl phosphate]. The reaction mixtures were incubated at room temperature for 15 min before they were loaded on 5% Tris-borate-EDTA-PAGE gels (Bio-Rad) and electrophoresed at 100 V for 65 min. DNA was electroblotted onto a positively charged nylon membrane (Hybond-N+; GE Healthcare), probed with a streptavidin-horseradish peroxidase conjugate, and developed.
Expression microarray analysis.
A custom Affymetrix microarray was designed for monitoring GAS gene expression. More than 90% of the MGAS2221 genes were represented on our custom array, and 16 probe pairs for each gene were included. A probe pair is a perfect-match (PM) probe and a corresponding mismatch (MM) probe. The sequences of MM probes are identical to those of PM probes except that the central base of each 25-mer probe is substituted. Subtracting the MM probe hybridization signal intensity from the PM probe hybridization signal intensity reduces the background noise, increasing the sensitivity.
GAS strains MGAS2221, 2221covS::7bp, 2221covR-R119H, and 2221covR-ΔM79 were grown at 37°C with 5% CO2 in THY broth. Samples were recovered during the early exponential phase of growth, which corresponded to an optical density at 600 nm of ∼0.2. Recovered GAS samples were incubated at room temperature for 5 min following addition of 2 volumes of RNAprotect (Qiagen Inc.) to maintain RNA integrity. GAS strains were harvested by centrifugation, and total RNA was isolated, converted to cDNA, labeled, and hybridized to our custom array as described previously (13). Single microarrays were used for each of the four strains. Estimates of gene expression were calculated using GCOS software v1.4 (Affymetrix Inc.). Data were normalized across samples to minimize discrepancies that can arise due to experimental variables (e.g., probe preparation or hybridization). Genes with average expression values less than 10 were manually removed from the data. To reduce the complexity of the data set, principal component analysis (PCA) was performed (Partek Genomics Suite; Partek Inc.) (11). A PCA plot was generated to enable visualization of the variation in the data set, and clustered data points indicated similar transcriptomes. The expression microarray data have been deposited in the Gene Expression Omnibus database at NCBI (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE16436.
Quantitative PCR analysis.
TaqMan quantitative reverse transcription (RT)-PCR assays were performed using an ABI 7500 Fast system (Applied Biosystems). Gene transcript levels were compared using the ΔΔCT method (27). TaqMan primers and probes for genes of interest and the internal control gene proS are shown in Table S1 in the supplemental material. All experiments were performed in triplicate on two separate occasions.
GAS growth in human saliva.
Human saliva was isolated from donor individuals, pooled, cleared by centrifugation, and filter sterilized as previously described (28). To compare the abilities of wild-type strain MGAS2221 and the isogenic covS mutant 2221covS::7bp to grow in human saliva, we first grew overnight cultures in THY broth. The following morning, 1 ml of each overnight culture was diluted 1:100 with sterile phosphate-buffered saline (PBS). Subsequently, 100-μl portions of the diluted cultures were inoculated into 9.9-ml aliquots of filter-sterilized human saliva. Samples (100 μl) of the GAS-saliva solutions were recovered over time, serial dilutions were prepared, and the numbers of GAS CFU were determined following overnight incubation on blood agar plates. The experiment was repeated three times, and mean values are reported below.
Assays of GAS competition during growth in human saliva.
Two competition assays were performed. In the first competition assay wild-type strain MGAS2221 and covS mutant strain 2221covS::7bp were added to the same saliva sample. In the second competition assay covS mutant strain 2221covSΔ1bp and the complemented derivative 2221covSΔ1bp/pCovSC were added to the same saliva sample. Briefly, 1-ml portions of overnight THY broth cultures of the two strains to be compared were diluted 1:100 in 99 ml sterile PBS. The two strains were mixed together and further diluted by adding 100 μl of each of the PBS-diluted GAS to 9.8 ml of human saliva. After vortexing to ensure homogeneity, a 100-μl sample was removed, serially diluted, and plated on blood agar plates (three plates per dilution). The remainder of the GAS-inoculated saliva was incubated at 37°C for 12 h. After incubation a 100-μl sample was removed, serially diluted, and plated on blood agar plates as described above. In addition, a 10-μl sample was inoculated into 9.99 ml of fresh human saliva, the preparation was incubated for 12 h at 37°C, a 100-μl aliquot was removed, the titer was determined, and 10 μl was inoculated into 9.99 ml of fresh saliva. This cycle was repeated for a total of 72 h (six saliva aliquots) (see Fig. S3 in the supplemental material). The number of CFU of each strain in the saliva was determined using colony morphology on blood agar plates; the GAS containing a functional CovR-CovS system produced small nonmucoid colonies, while the covS mutant GAS produced large mucoid colonies. Importantly, when we performed experiments in which MGAS2221 or 2221covS::7bp was grown by itself in human saliva (see Fig. 7A), we found no evidence that GAS strains were able to switch colony morphology. However, to ensure that the colonies we identified as strain MGAS2221 or strain 2221covS::7bp colonies were indeed colonies of these strains, we performed colony PCR and sequenced the covS gene for at least 12 colonies of each strain recovered at the 0-, 12-, 24-, 36-, and 72-h time points. As expected, all mucoid colonies from the saliva inoculated with MGAS2221 and 2221covS::7bp had the 7-bp insertion in covS indicative of strain 2221covS::7bp, while all nonmucoid colonies had a wild-type covS allele. As strain 2221covSΔ1bp/pCovSC is chloramphenicol resistant, we confirmed that colonies that we identified as 2221covSΔ1bp or 2221covSΔ1bp/pCovSC colonies from the saliva inoculated with 2221covSΔ1bp and 2221covSΔ1bp/pCovSC were indeed colonies of these strains by patching colonies onto THY agar plates with and without chloramphenicol (4 μg/ml). For the 0-, 12-, 24-, 36-, and 72-h time points we patched at least 25 colonies (average, 41 colonies) of each strain. As expected, all nonmucoid colonies grew on plates with or without chloramphenicol, while mucoid colonies grew only on THY agar plates lacking chloramphenicol.
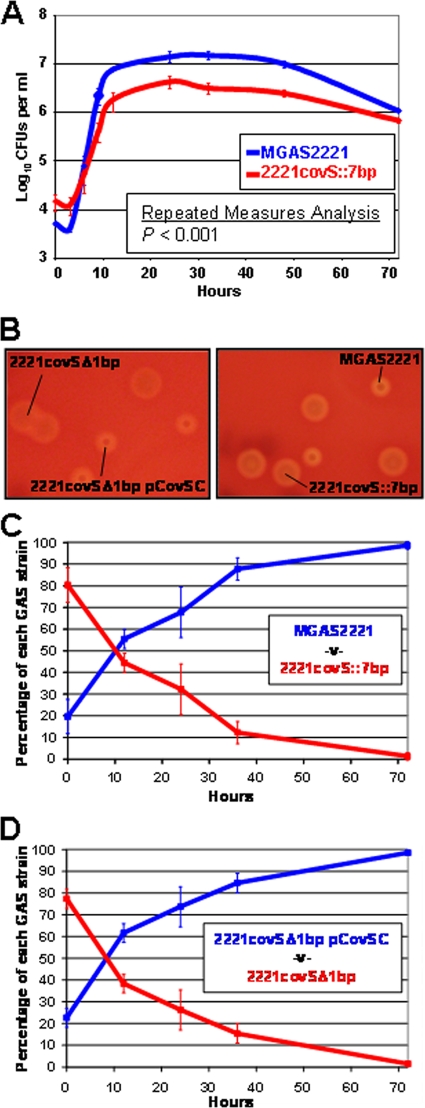
FIG. 7.
GAS containing a functional CovR-CovS system significantly outcompete covS mutant GAS during growth in human saliva. (A) Wild-type GAS strain MGAS2221 and isogenic covS mutant 2221covS::7bp were grown in separate 10-ml aliquots of human saliva, and the numbers of CFU were determined over time. The data are the mean ± standard error of the mean number of GAS CFU for each strain from triplicate experiments. (B) Colonies on representative blood agar plates showing distinct large mucoid (strains 2221covSΔ1bp and 2221covS::7bp) and small nonmucoid (strains MGAS2221 and 2221covSΔ1bp/pCovSC) colony morphologies. (C) Growth competition assay with wild-type strain MGAS2221 and isogenic covS mutant 2221covS::7bp. (D) Growth competition assay with the covS mutant strain 2221covSΔ1bp and the complemented derivative 2221covSΔ1bp/pCovSC. For panels C and D the two GAS strains were added to the same aliquot of human saliva (time zero). Every 12 h 1:1,000 dilution in fresh saliva was performed, and the number of CFU of each strain in the saliva was determined. The results are expressed as the relative percentages of the two strains in the saliva at the indicated time points. The experiments were performed in quadruplicate, and the bars and error bars indicate the mean ± standard deviation. The data in panels C and D are statistically significantly different as determined by repeated measures analysis (P < 0.0001).
RESULTS
Construction of covR, covS, and covRS isogenic mutant strains.
To facilitate investigation of the relationship between CovR and CovS in regulating serotype M1 GAS gene expression, we created a collection of single and double covR and covS mutant derivatives of wild-type strain MGAS2221 (Fig. 1A). The covS mutant strains 2221covS::7bp and 2221covSΔ1bp and the covR mutant strains 2221covR-R119H and 2221covR-ΔM79 were previously isolated from mice experimentally infected with parental strain MGAS2221 (32). An additional covR mutant strain and a covRS double-mutant strain were created by insertional replacement of an internal region of the covR gene with a kanamycin resistance cassette in strains MGAS2221 and 2221covSΔ1bp, respectively. The covR and covS mutations in strains 2221ΔcovR and 2221ΔcovR/S were confirmed by PCR, DNA sequence, and Southern blot analyses (Fig. 1B and data not shown).
To confirm that the covS mutation in strain 2221covSΔ1bp disrupted CovS function, we complemented 2221covSΔ1bp with a plasmid-encoded wild-type covS allele. Quantitative RT-PCR analysis showed that plasmid pCovSC, but not empty vector pDCBB, restored the gene expression to that observed for wild-type strain MGAS2221 (Fig. 1C). While complementation of strain 2221covS::7bp was not performed, the whole genome of this strain has been resequenced, and the 7-bp insertion in covS was identified as the only genetic difference between this strain and MGAS2221 (32). Despite extensive efforts we were not able to complement any of our covR mutant strains. The lack of complementation was due to our inability to clone the wild-type covR gene into shuttle vector pDCBB, regardless of whether ligation preparations were transformed into E. coli or directly into GAS. Only mutated covR alleles were recovered, suggesting that the covR gene is lethal in E. coli and GAS when multiple copies are present.
Western immunoblot analyses of proteins secreted by wild-type and covR, covS, and covRS mutant GAS strains.
Western immunoblot analyses were performed to identify differences in protein secretion between MGAS2221 and isogenic covR, covS, and covRS mutant strains. Supernatant proteins from overnight GAS cultures were assayed for reactivities with antibodies directed against the secreted virulence factors S. pyogenes exotoxin A (SpeA), SpeB, streptolysin O (SLO), S. pyogenes NAD glycohydrolase (SPN), and Mac (Fig. 2). The cysteine protease SpeB was the only protein detected by these antibodies using secreted proteins from wild-type strain MGAS2221. In part, this pattern of reactivity may be due to the SpeB-mediated degradation of SLO, SPN, and Mac (note that SpeB also has low-level activity against SpeA) (25, 32). The covS mutant strain 2221covSΔ1bp secreted all of the proteins assayed other than SpeB, a secretion pattern identical to that of the well-described clinical covS mutant isolate MGAS5005 (32). Secreted proteins from the covR mutant strain 2221ΔcovR exhibited reactivity only with SpeA and SpeB antibodies, and SpeB was expressed at a concentration higher than that observed for parental strain MGAS2221 (Fig. 2). Given the high level of SpeB produced by 2221ΔcovR, it is possible that this strain also secretes SLO, SPN, and Mac but that these proteins are degraded by SpeB and hence not visible when Western analysis is performed.
The finding that SpeB was not expressed following covS mutation but was highly expressed following covR mutation indicated that SpeB was negatively regulated by CovR and/or positively regulated by CovS. To distinguish between these possibilities, we utilized the covRS double mutant strain 2221ΔcovR/S. The virulence factor secretion profile of strain 2221ΔcovR/S was identical to that of 2221ΔcovR (Fig. 2). Thus, the data indicate that CovR negatively regulates SpeB expression and that CovS attenuates the CovR-mediated repression of SpeB.
Interestingly, the virulence factor secretion profiles of the in vivo-selected covR mutant strains 2221covR-R119H and 2221covR-ΔM79 differed from one another (Fig. 2). Strain 2221covR-ΔM79, which has a 3-bp deletion within covR that removes one of three contiguous internal methionine codons (see Fig. S1 in the supplemental material), had a secretion profile identical to that of the constructed covR mutant strain 2221ΔcovR. In contrast, strain 2221covR-R119H, which has a nonsynonymous mutation in covR (see Fig. S1 in the supplemental material), had a secretion profile identical to that of the covS mutant strain 2221covSΔ1bp. The covR mutations had no noticeable effect on the stability or solubility of CovR, as assessed by native PAGE and SDS-PAGE of purified recombinant proteins (see below) (data not shown; see Fig. S2 in the supplemental material). We hypothesize that the two different secretion profiles are a consequence of the covR mutation in strain 2221covR-ΔM79 being a null mutation, while the mutation in strain 2221covR-R119H prevents CovR from being regulated by CovS and hence this strain is phenotypically a covS mutant.
EMSA comparisons of wild-type and mutant CovR proteins.
Our Western immunoblot data are consistent with the hypotheses that the CovR protein of strain 2221covR-ΔM79 has no DNA-binding activity and the CovR protein of strain 2221covR-R119H has DNA-binding activity but is not regulated by CovS. To test these hypotheses, we overexpressed and purified His-tagged derivatives of the wild-type and mutant CovR proteins and assayed their DNA-binding activities using EMSAs. Positive control (spyCEP promoter) and negative control (dnaN promoter) DNA probes were generated by PCR. The wild-type CovR protein bound and shifted the spyCEP promoter probe but not the dnaN probe, confirming previous data (Fig. 3) (33). Similarly, the R119H mutant CovR protein was also able to bind and shift the spyCEP promoter probe but not the dnaN probe, indicating that the R119H CovR mutant protein has sequence-specific DNA-binding activity. In contrast, the ΔM79 CovR mutant protein failed to bind either DNA probe, indicating that the ΔM79 mutation disrupts CovR DNA binding.
FIG. 3.

The CovR protein of strain 2221covR-R119H, but not the CovR protein of strain 2221covR-ΔM79, exhibits DNA-binding activity. An EMSA was performed to compare the DNA-binding abilities of wild-type (MGAS2221) and mutant (2221covR-R119H and 2221covR-ΔM79) recombinant CovR proteins. Recombinant CovR protein with the R119H mutation was able to bind the spyCEP promoter region (33), whereas the ΔM79 mutation eliminated DNA-binding activity. The dnaN probe served as a negative (no binding) control. The arrow indicates the shift observed when CovR proteins from strains MGAS2221 and 2221covR-R119H were used.
Comparison of gene expression of wild-type and covR and covS mutant strains.
To further investigate the difference between covR mutant strains 2221covR-R119H and 2221covR-ΔM79, we performed a genome-wide analysis of GAS gene expression. Gene transcription during the early exponential phase of growth in THY broth was monitored for wild-type strain MGAS2221, the isogenic covS mutant 2221covS::7bp, and covR mutants 2221covR-R119H and 2221covR-ΔM79. PCA of the expression data confirmed that despite having mutations in covR, strains 2221covR-R119H and 2221covR-ΔM79 have very distinct transcriptomes (as shown by the lack of clustering of these strains in Fig. 4A). In addition, the microarray data showed that strain 2221covR-R119H not only has a protein secretion pattern similar to that of a covS mutant strain (Fig. 2) but also has a similar transcriptome (Fig. 4A). To confirm the expression microarray data, we performed a TaqMan quantitative RT-PCR analysis. RNA from GAS strains grown to the exponential (Fig. 4B) and late stationary (Fig. 4C) phases was analyzed. The quantitative PCR data confirmed that strain 2221covR-R119H has a transcription pattern similar to that of strain 2221covS::7bp and different from that of strain 2221covR-ΔM79.
FIG. 4.
The transcriptome of strain 2221covR-R119H, but not the transcriptome of strain 2221covR-ΔM79, mirrors the transcriptome of a covS mutant strain. The transcriptomes of parental strain MGAS2221, covS mutant derivative 2221covS::7bp, and covR mutant derivatives 2221covR-R119H and 2221covR-ΔM79 were compared by performing expression microarray and quantitative RT-PCR analyses. (A) Expression microarray data. The PCA plot shows clustering of gene expression in strain 2221covR-R119H with gene expression in strain 2221covS::7bp but not with gene expression in 2221covR-ΔM79 or parental strain MGAS2221. PCA assesses the variance in a data set in terms of principal components (11). The two most significant principal components are shown on the x and y axes. (B and C) Quantitative RT-PCR data. The transcript levels were determined for mutant strains 2221covS::7bp, 2221covR-R119H, and 2221covR-ΔM79 and compared to those for parental strain MGAS2221. Strains were assayed after they grew to the exponential (B) and late stationary (C) phases in THY broth. The experiment was performed in triplicate, and the bars and error bars indicate the mean ± standard deviation.
Quantitative RT-PCR analysis of the relationship between CovR and CovS.
For a prototypical TCS a mutation in the sensor kinase-encoding gene would result in the same phenotype as a mutation in the response regulator gene, since the response regulator is inactive in the absence of phosphorylation by the sensor kinase (30). The CovR-CovS system does not function in this manner, however, as shown by the different transcription (Fig. 4) and protein secretion (Fig. 2) patterns of covR and covS mutant strains. To investigate the regulatory relationship between CovR and CovS in more detail, we compared wild-type and covR, covS, and covRS mutant strains using quantitative RT-PCR. Compared to wild-type strain MGAS2221, the three mutant strains all transcribed speA at similar higher levels during exponential (∼4-fold) (Fig. 5A) and early stationary (∼32-fold) (Fig. 5B) phases of growth. In contrast, while the covR and covRS mutant strains transcribed the speB and grab genes at higher levels than MGAS2221, the covS mutant strain transcribed these genes at lower levels during exponential (∼4-fold for speB and ∼21-fold for grab) and early stationary (∼9,000-fold for speB and ∼ 24-fold for grab) phases. Similar, although not as dramatic, differences between the four GAS strains were also observed for the spd3, SPy0430, and rivR genes (Fig. 5A and 5B). Our data indicate that virulence factor-encoding genes regulated by the CovR-CovS system fall into two groups. The first group, exemplified by speA, is significantly repressed by CovR only in the presence of CovS. The second group, exemplified by speB, is significantly repressed by CovR only in the absence of CovS.
FIG. 5.

CovS simultaneously activates and represses the ability of CovR to regulate distinct subgroups of virulence-associated genes. Quantitative RT-PCR was used to compare the transcript levels of the virulence-associated speA, speB, spd3, SPy0430, grab, and rivR genes. The transcript levels were determined for mutant strains 2221ΔcovR, 2221ΔcovS::7bp, and 2221ΔcovR/S and compared to those for parental strain MGAS2221. Cells from the exponential (A) and early stationary (B) phases of growth in THY broth were assayed. The experiment was performed in triplicate, and the bars and error bars indicate the mean ± standard deviation.
Analysis of the CovR-CovS system during GAS growth in human saliva.
To gain insight into the role of CovR and CovS during upper respiratory tract infection, we grew strains MGAS2221, 2221covS::7bp, 2221ΔcovR, and 2221ΔcovR/S in human saliva and assayed the transcription of select GAS virulence factors. Genes encoding the virulence factor SpeA (∼70-fold), the putative virulence factor SPy0430 (∼290-fold), and the virulence gene regulator RivR (∼2.5-fold) were all upregulated in the covR, covS, and covRS mutant strains compared to parent strain MGAS2221 (Fig. 6). Importantly, while the transcript levels of the speB, spd3, and grab genes in strains MGAS2221, 2221ΔcovR, and 2221ΔcovR/S were similar, in strain 2221covS::7bp the transcript levels of these genes were 30-fold, 11-fold, and 180-fold lower, respectively. These data show that, similar to our in vitro observations, there appear to be two classes of CovR-CovS-regulated genes during GAS growth in an ex vivo model of upper respiratory tract infection.
FIG. 6.

CovS-mediated regulation of CovR activity is observed using an ex vivo model of upper respiratory tract infection. GAS strains were grown in human saliva for 24 h and subjected to quantitative RT-PCR analysis. The speA, speB, spd3, SPy0430, grab, and rivR transcript levels were determined for mutant strains 2221ΔcovR, 2221ΔcovS::7bp, and 2221ΔcovR/S and compared to those for parental strain MGAS2221. The experiment was performed in triplicate, and the bars and error bars indicate the mean ± standard deviation.
Growth assays comparing wild-type and covS mutant strains in human saliva.
Despite the hypervirulence of covS mutant GAS strains in ex vivo and in vivo models of invasive infection (8, 32) and despite the isolation of covS mutant strains from human invasive infections (8, 32), there have been no reports of covS mutant strains causing large outbreaks of GAS infections. One possible explanation for this observation is that covS mutant strains are attenuated in the ability to disseminate throughout the population. As the main portal of GAS transmission is the upper respiratory tract (17, 26) and as dissemination commonly occurs through dispersal of aerosolized saliva (17, 18), covS mutant strains may be attenuated in the ability to colonize, proliferate, and/or persist in the upper respiratory tract. Using growth and persistence in human saliva as a surrogate for growth and persistence in the upper respiratory tract, we compared parental strain MGAS2221 and isogenic mutant 2221covS::7bp. Each strain was inoculated into filter-sterilized 10-ml aliquots of human saliva, and over time the number of GAS CFU per ml of saliva was determined. A statistically significant difference was observed between the abilities of the two strains to grow in saliva, and between 9 and 48 h the number of CFU of the wild-type strain was four- to fivefold greater than the number of CFU of the covS mutant (Fig. 7A).
Assays of competition between wild-type and covS mutant GAS strains during growth in human saliva.
The biological significance of the difference in growth in human saliva between strains MGAS2221 and 2221covS::7bp was investigated by performing competition assays (see Fig. S3 in the supplemental material). The competition assays differed from the saliva growth assays described above because (i) both strains tested were incubated in the same saliva sample and (ii) the GAS-inoculated saliva was diluted 1:1,000 with fresh saliva every 12 h for 72 h to more closely resemble the constant flow of saliva that occurs during upper respiratory tract infection. Two competition assays were performed, one comparing MGAS2221 with 2221covS::7bp and the other comparing 2221covSΔ1bp with the complemented derivative 2221covSΔ1bp/pCovSC. The strains could be distinguished from one another, after serial dilution and plating on blood agar plates, by differences in colony morphology. GAS strains with a functioning CovR-CovS system produce small nonmucoid colonies, while covS mutant strains produce large mucoid colonies (Fig. 7B). In both the competition assays with MGAS2221 and 2221covS::7bp (Fig. 7C) and the competition assays with 2221covSΔ1bp/pCovSC and 2221covSΔ1bp (Fig. 7D) the strain with a functional CovR-CovS system significantly outcompeted the covS mutant strain. Indeed, the covS mutant GAS strain accounted for less than 1.5% of the total GAS at the 72-h time point, despite the fact that it accounted for >75% of the total GAS at time zero. The data show that a functioning CovR-CovS system enhances the ability of GAS to grow in human saliva.
DISCUSSION
The CovR-CovS TCS has been extensively studied due to its key role in regulating GAS virulence. Using animal models of infection, we and other workers have shown that strains having mutations in the covR and covS genes arise spontaneously during invasive infections and rapidly become dominant (8, 32, 34). While the rate and importance of covRS mutation during human invasive infections are unknown, the isolation of covRS mutant strains from human infections indicates that they contribute to disease outcome. The selection of covRS mutant strains during invasive infections, coupled with the absence of any report describing the isolation of covRS mutant GAS from upper respiratory tract infections, suggests that wild-type strains may have a survival advantage in the upper respiratory tract environment, while covRS mutant strains may have a survival advantage during invasive infections. Here, we show that CovS simultaneously activates and inhibits the CovR-mediated repression of distinct subsets of virulence factor-encoding genes, that different covR mutations may have dramatically different effects on transcription, and that GAS strains with a functional CovR-CovS system outcompete covS mutant strains during growth in human saliva.
The types of mutations that spontaneously occur in covR and covS differ, which was observed initially by Engleberg and colleagues (8) and was confirmed by our laboratory (32). The majority of covR mutations that have been identified are nonsynonymous mutations resulting in amino acid substitutions. In contrast, the majority of covS mutations that have been identified are small insertions or deletions that knock the covS gene out of frame. Such a mutation pattern may be explained by selection against SpeB production, and hence selection for disruption of CovS activity (Fig. 2), to prevent SpeB-mediated degradation of the many immune evasion proteins liberated by GAS. While a covS null mutant strain has dramatically reduced SpeB expression, a covR null mutant strain expresses SpeB at levels even greater than the levels expressed by wild-type GAS (Fig. 2). Therefore, rather than selection for covR null mutations, we believe that there is selection for mutations that enable CovR to retain DNA-binding activity (strain 2221covR-R119H) (Fig. 3) while it loses the ability to be regulated by CovS and hence the strain is phenotypically identical to covS mutant strains (strain 2221covR-R119H) (Fig. 4A). It should be noted, however, that some covR null mutant strains have been isolated from invasive infections (e.g., strain 2221covR-ΔM79). We envision that the significant upregulation of virulence factors by covR null mutant strains, coupled with a covRS-independent decrease in SpeB production (13), accounts for the selection of these strains over wild-type GAS during invasive infections.
The results obtained for the MGAS2221 derivatives 2221covR-R119H and 2221covR-ΔM79 support the notion that different covR mutant strains can have significantly different transcript profiles and corresponding virulence factor secretion profiles. As strain 2221covR-ΔM79 is phenotypically identical to a covR deletion mutant strain (strain 2221ΔcovR) (Fig. 2) and given that the ΔM79 CovR protein is unable to bind DNA (Fig. 3), there is strong evidence that this strain has a null covR allele. The ΔM79 deletion in CovR removes one of three methionine residues that are located immediately adjacent to an amino acid that, based on homology to other response regulators, forms part of the CovR active site (see Fig. S1 in the supplemental material) (21). In contrast, the R119H mutation in strain 2221covR-R119H is located in the linker region of CovR that connects the receiver and response domains (see Fig. S1 in the supplemental material). Interestingly, the same amino acid is also substituted in the CovR protein of the serotype M3 strain SSI-1 (24), a hypervirulent strain isolated in Japan whose genome has been fully sequenced, although in this strain the amino acid change is R119C. This mutation may help explain the hypervirulent phenotype of strain SSI-1, as the hypervirulent phenotype of M1 strain MGAS5005 is explained by mutation in covS (32). A recent report also describes the importance of a single nonsynonymous mutation in covR for the virulence of a different serotype M3 GAS strain (23).
One extracellular factor known to regulate the CovR-CovS system is the human antimicrobial peptide LL-37 (15). In a process that requires functional CovS, the presence of subinhibitory levels of LL-37 increases transcription of several CovR-CovS-regulated genes (e.g., the has operon and mac). We agree with the hypothesis of Gryllos and colleagues that LL-37 inhibits covS activity and plays a major role in silencing CovS during invasive infections (15). We hypothesize that selection of covS mutant strains occurs only when other methods of attenuating CovS function, such as via LL-37-mediated signaling, are insufficient.
Using in vitro-grown GAS, we found that a covR covS double-mutant strain has a transcription pattern highly similar to that of a covR mutant strain but different from that of a covS mutant strain (Fig. 5). Thus, the data indicate that CovS functions solely through CovR, while CovR functions in the presence or absence of CovS, although different groups of genes are targeted. The mechanism by which CovS activates CovR to repress one set of genes (the “speA group” [e.g., speA, hasA, and ska]) while it simultaneously inhibits CovR's ability to repress a second set of genes (the “speB group” [e.g., speB, grab, and spd3]) is unknown. The simplest explanation is that CovS phosphorylates CovR and that only phosphorylated CovR is able to significantly repress speA group promoters, while only nonphosphorylated CovR is able to significantly repress speB group promoters. This is almost certainly an oversimplification, however, and individual genes are regulated by the cumulative effects of not only the CovR phosphorylation status but also additional (CovR-CovS-regulated) transcription factors. In addition, it has been proposed that CovR may bind to the promoter region of genes in one of two different orientations based on the promoter sequence (5). Thus, the number and orientation of CovR binding sites within a promoter region may also be important contributing factors.
Mixing a covS mutant GAS strain with a strain that has a functional CovR-CovS system enabled determination that the CovR-CovS system confers a significant competitive advantage during growth in human saliva (Fig. 7C and 7D), as shown by our ex vivo model of upper respiratory tract infection. Differences in the type and amount of freely secreted proteins liberated by the two strains must not account for the competitive difference as the strains are grown in the same saliva sample and hence proteins freely secreted by one strain would impact the two strains equally. The data support our hypothesis that while covS mutant strains are positively selected during invasive infections, they are negatively selected during upper respiratory tract infections and hence are attenuated in the ability to disseminate via the upper respiratory tract route. While CovS is required for GAS growth under general stress conditions (e.g., high temperature, high osmolarity, low pH) in some strains (7), we observed no difference in growth for several isogenic wild-type and covS mutant pairs grown under these conditions (data not shown). Thus, the decreased ability of covS mutant strains to grow in human saliva is not simply due to a significant defect in their ability to resist stress. It is possible that serotype-specific differences account for the difference between our data and those of Dalton and Scott (7).
Our laboratory is interested in determining the transcriptional, translational, and functional differences which distinguish GAS during invasive and noninvasive infections. We believe that further analysis of the phenotypic differences between wild-type, covR mutant, and covS mutant GAS strains may uncover genes and proteins important in colonization, dissemination, or disease at distinct infection sites. The discovery of factors that promote GAS upper respiratory tract infections, an understudied area of GAS research, would enable development of targeted strategies to enhance GAS eradication and/or therapeutic protocols.
Supplementary Material
Acknowledgments
We thank June R. Scott (Emory University) for providing GAS strain JRS950, which was used to construct covR mutations in MGAS2221 and derivatives of this strain.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 18 May 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aziz, R. K., M. J. Pabst, A. Jeng, R. Kansal, D. E. Low, V. Nizet, and M. Kotb. 2004. Invasive M1T1 group A Streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol. Microbiol. 51123-134. [DOI] [PubMed] [Google Scholar]
- 2.Bernish, B., and I. van de Rijn. 1999. Characterization of a two-component system in Streptococcus pyogenes which is involved in regulation of hyaluronic acid production. J. Biol. Chem. 2744786-4793. [DOI] [PubMed] [Google Scholar]
- 3.Bessen, D. E., A. Manoharan, F. Luo, J. E. Wertz, and D. A. Robinson. 2005. Evolution of transcription regulatory genes is linked to niche specialization in the bacterial pathogen Streptococcus pyogenes. J. Bacteriol. 1874163-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaffin, D. O., and C. E. Rubens. 1998. Blue/white screening of recombinant plasmids in Gram-positive bacteria by interruption of alkaline phosphatase gene (phoZ) expression. Gene 21991-99. [DOI] [PubMed] [Google Scholar]
- 5.Churchward, G. 2007. The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci. Mol. Microbiol. 6434-41. [DOI] [PubMed] [Google Scholar]
- 6.Coye, L. H., and C. M. Collins. 2004. Identification of SpyA, a novel ADP-ribosyltransferase of Streptococcus pyogenes. Mol. Microbiol. 5489-98. [DOI] [PubMed] [Google Scholar]
- 7.Dalton, T. L., and J. R. Scott. 2004. CovS inactivates CovR and is required for growth under conditions of general stress in Streptococcus pyogenes. J. Bacteriol. 1863928-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engleberg, N. C., A. Heath, A. Miller, C. Rivera, and V. J. DiRita. 2001. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J. Infect. Dis. 1831043-1054. [DOI] [PubMed] [Google Scholar]
- 9.Federle, M. J., K. S. McIver, and J. R. Scott. 1999. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J. Bacteriol. 1813649-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao, J., A. A. Gusa, J. R. Scott, and G. Churchward. 2005. Binding of the global response regulator protein CovR to the sag promoter of Streptococcus pyogenes reveals a new mode of CovR-DNA interaction. J. Biol. Chem. 28038948-38956. [DOI] [PubMed] [Google Scholar]
- 11.Girolami, M., and R. Breitling. 2004. Biologically valid linear factor models of gene expression. Bioinformatics 203021-3033. [DOI] [PubMed] [Google Scholar]
- 12.Graham, M. R., L. M. Smoot, C. A. Migliaccio, K. Virtaneva, D. E. Sturdevant, S. F. Porcella, M. J. Federle, G. J. Adams, J. R. Scott, and J. M. Musser. 2002. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. USA 9913855-13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham, M. R., K. Virtaneva, S. F. Porcella, W. T. Barry, B. B. Gowen, C. R. Johnson, F. A. Wright, and J. M. Musser. 2005. Group A Streptococcus transcriptome dynamics during growth in human blood reveals bacterial adaptive and survival strategies. Am. J. Pathol. 166455-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gryllos, I., R. Grifantini, A. Colaprico, S. Jiang, E. Deforce, A. Hakansson, J. L. Telford, G. Grandi, and M. R. Wessels. 2007. Mg2+ signalling defines the group A streptococcal CsrRS (CovRS) regulon. Mol. Microbiol. 65671-683. [DOI] [PubMed] [Google Scholar]
- 15.Gryllos, I., H. J. Tran-Winkler, M. F. Cheng, H. Chung, R. Bolcome III, W. Lu, R. I. Lehrer, and M. R. Wessels. 2008. Induction of group A Streptococcus virulence by a human antimicrobial peptide. Proc. Natl. Acad. Sci. USA 10516755-16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gusa, A. A., J. Gao, V. Stringer, G. Churchward, and J. R. Scott. 2006. Phosphorylation of the group A streptococcal CovR response regulator causes dimerization and promoter-specific recruitment by RNA polymerase. J. Bacteriol. 1884620-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamburger, M., Jr., and O. H. Robertson. 1948. Expulsion of group A hemolytic streptococci in droplets and droplet nuclei by sneezing, coughing and talking. Am. J. Med. 4690-701. [DOI] [PubMed] [Google Scholar]
- 18.Katzenell, U., J. Shemer, and Y. Bar-Dayan. 2001. Streptococcal contamination of food: an unusual cause of epidemic pharyngitis. Epidemiol. Infect. 127179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laub, M. T., and M. Goulian. 2007. Specificity in two-component signal transduction pathways. Annu. Rev. Genet. 41121-145. [DOI] [PubMed] [Google Scholar]
- 20.Levin, J. C., and M. R. Wessels. 1998. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol. Microbiol. 30209-219. [DOI] [PubMed] [Google Scholar]
- 21.Marchler-Bauer, A., J. B. Anderson, M. K. Derbyshire, C. DeWeese-Scott, N. R. Gonzales, M. Gwadz, L. Hao, S. He, D. I. Hurwitz, J. D. Jackson, Z. Ke, D. Krylov, C. J. Lanczycki, C. A. Liebert, C. Liu, F. Lu, S. Lu, G. H. Marchler, M. Mullokandov, J. S. Song, N. Thanki, R. A. Yamashita, J. J. Yin, D. Zhang, and S. H. Bryant. 2007. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 35D237-D240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, A. A., N. C. Engleberg, and V. J. DiRita. 2001. Repression of virulence genes by phosphorylation-dependent oligomerization of CsrR at target promoters in S. pyogenes. Mol. Microbiol. 40976-990. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi-Akiyama, T., T. Ikebe, H. Watanabe, T. Uchiyama, T. Kirikae, and Y. Kawamura. 2006. Use of DNA arrays to identify a mutation in the negative regulator, csrR, responsible for the high virulence of a naturally occurring type M3 group A streptococcus clinical isolate. J. Infect. Dis. 1931677-1684. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa, I., K. Kurokawa, A. Yamashita, M. Nakata, Y. Tomiyasu, N. Okahashi, S. Kawabata, K. Yamazaki, T. Shiba, T. Yasunaga, H. Hayashi, M. Hattori, and S. Hamada. 2003. Genome sequence of an M3 strain of Streptococcus pyogenes reveals a large-scale genomic rearrangement in invasive strains and new insights into phage evolution. Genome Res. 131042-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nooh, M. M., R. K. Aziz, M. Kotb, A. Eroshkin, W. J. Chuang, T. Proft, and R. Kansal. 2006. Streptococcal mitogenic exotoxin, SmeZ, is the most susceptible M1T1 streptococcal superantigen to degradation by the streptococcal cysteine protease, SpeB. J. Biol. Chem. 28135281-35288. [DOI] [PubMed] [Google Scholar]
- 26.Shelburne, S. A., III, C. Granville, M. Tokuyama, I. Sitkiewicz, P. Patel, and J. M. Musser. 2005. Growth characteristics of and virulence factor production by group A Streptococcus during cultivation in human saliva. Infect. Immun. 734723-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shelburne, S. A., III, D. B. Keith, M. T. Davenport, N. Horstmann, R. G. Brennan, and J. M. Musser. 2008. Molecular characterization of group A Streptococcus maltodextrin catabolism and its role in pharyngitis. Mol. Microbiol. 69436-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shelburne, S. A., III, P. Sumby, I. Sitkiewicz, C. Granville, F. R. DeLeo, and J. M. Musser. 2005. Central role of a bacterial two-component gene regulatory system of previously unknown function in pathogen persistence in human saliva. Proc. Natl. Acad. Sci. USA 10216037-16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sitkiewicz, I., and J. M. Musser. 2006. Expression microarray and mouse virulence analysis of four conserved two-component gene regulatory systems in group A streptococcus. Infect. Immun. 741339-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69183-215. [DOI] [PubMed] [Google Scholar]
- 31.Sumby, P., S. F. Porcella, A. G. Madrigal, K. D. Barbian, K. Virtaneva, S. M. Ricklefs, D. E. Sturdevant, M. R. Graham, J. Vuopio-Varkila, N. P. Hoe, and J. M. Musser. 2005. Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J. Infect. Dis. 192771-782. [DOI] [PubMed] [Google Scholar]
- 32.Sumby, P., A. R. Whitney, E. A. Graviss, F. R. DeLeo, and J. M. Musser. 2006. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumby, P., S. Zhang, A. R. Whitney, F. Falugi, G. Grandi, E. A. Graviss, F. R. Deleo, and J. M. Musser. 2008. A chemokine-degrading extracellular protease made by group A Streptococcus alters pathogenesis by enhancing evasion of the innate immune response. Infect. Immun. 76978-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker, M. J., A. Hollands, M. L. Sanderson-Smith, J. N. Cole, J. K. Kirk, A. Henningham, J. D. McArthur, K. Dinkla, R. K. Aziz, R. G. Kansal, A. J. Simpson, J. T. Buchanan, G. S. Chhatwal, M. Kotb, and V. Nizet. 2007. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13981-985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.