Abstract
Escherichia coli is the most common cause of urinary tract infection. Elevated blood and urine interleukin-6 (IL-6) levels have been shown in inflammatory urinary tract diseases. The role of IL-6 in mediating the urodynamic dysfunction in response to E. coli-induced urinary tract infection has not yet been fully elucidated. In this study, we investigated the role of IL-6 in the nitric oxide (NO)-triggered alteration of contractile responses in the urinary bladder under an E. coli-induced inflammatory condition. The electrical field stimulation (EFS)-evoked contractions of the isolated detrusor strips, and immunoblotting for detecting protein expression in the bladders was measured short term (1 h) or long term (6 or 24 h) after intraperitoneal injection of E. coli endotoxin (lipopolysaccharide [LPS]) or intravesical instillation of human pyelonephritogenic E. coli-J96 (O4:K6) strain or LPS into mice. IL-6 and NO productions were increased in the urinary bladders of mice 1 to 24 h after LPS or E. coli-J96 treatment. Inducible NO synthase (iNOS) expression and protein kinase C (PKC) activation and EFS-evoked detrusor contractions were increased in the bladders at 6 h after LPS or E. coli-J96 treatment, which could be reversed by anti-IL-6 antibody and iNOS inhibitor aminoguanidine. At 1 h after LPS administration, bladder NO generation, endothelial NOS expression, and EFS-evoked detrusor contractions were effectively increased, whereas anti-IL-6 antibody could not reverse these LPS-induced responses. These results indicate that IL-6 may play an important role in the iNOS/NO-triggered PKC-activated contractile response in urinary bladder during E. coli or LPS-induced inflammation.
Urinary tract infections have been estimated that are the common infection acquired in hospitalized adult patients with a prevalence of 1 to 10% representing 30 to 40% of all nosocomial infections (4, 10, 14, 37). The development of urinary tract infections, including cystitis and pyelonephritis, in critically ill adult patients has been associated with considerable morbidity, prolonged hospitalization, and greater healthcare expenditures (4, 10). Urinary tract infections can also be a significant source of morbidity in the pediatric population (32). Escherichia coli is the most common cause of urinary tract infection (35). Increased frequency of micturition is a common symptom of urinary tract infection (20). The exact mechanisms of the symptoms of urinary tract infection are poorly understood.
Inflammation plays a role in most bladder pathologies (7, 30, 38). Infection of the urinary tract results in an inflammatory response characterized by increased levels of urinary cytokines and neutrophil influx (2, 6). In rodent or mouse urinary tract infection models, which involved treatment with E. coli lipopolysaccharide (LPS) by intravesical instillation or intraperitoneal injection, induced interleukin-6 (IL-6) and inducible nitric oxide synthase (iNOS) expressions occurred within 4 to 24 h (9, 23, 29). It has been found that intravesical NO donors were capable suppressing bladder hyperactivity induced by cyclophosphamide-induced cystitis (25). The iNOS was originally identified in activated murine macrophages and was induced by inflammatory mediators in a number of cell types (18). A direct relationship existed between urine nitrite levels and urinary tract infection (28). LPS is capable of inducing iNOS expression in the urinary bladder (23). A rapid upregulation of endothelial NOS (eNOS) has been demonstrated in a mouse model of E. coli LPS-induced bladder inflammation (12). Moreover, elevated levels of inflammatory cytokines such as IL-6 and IL-8 have been found in the sera and urine specimens of younger infants and children with urinary tract infections (11, 24). The IL-6 family of cytokines has been shown to play especially important roles in regulating the various biological responses through multichain receptor complex-mediated signaling (36). Many lines of evidences suggest that this family of cytokines plays important roles in regulating the immune response and inflammation (26). Although IL-6 has been reported to be elevated during urinary tract infection, the importance of IL-6 in mediating the urodynamic dysfunction in response to infection has not yet been fully elucidated. The regulatory relationship between IL-6 and NO signals in the inflammation of urinary bladder needs also to be clarified. In the present study, therefore, we hypothesize that IL-6 plays a regulatory role in the NO-triggered alteration of contractile response in the urinary bladder under an uropathogenic Escherichia coli-induced inflammatory condition.
MATERIALS AND METHODS
Bacterial strain.
The human pyelonephritogenic E. coli strain J96 serotype O4:K6 (ATCC 700336), which expresses type 1 and P-fimbrial adhesions, was used in the present study. The receptor-binding function of type I pili present on strain J96 has been identified in establishing experimental rodent bladder infections (13). Moreover, E. coli endotoxin (LPS) prepared by trichloroacetic acid extraction from E. coli serotype O26:B6 was purchased from Sigma.
Induction of inflammation.
The experimental urinary bladder infection models including intraperitoneal injection and intravesical instillation with E. coli or endotoxin were used in the present study as described previously (9, 23, 39, 40). Adult female mice (ICR strain, 25 to 30 g) were used for all experiments. Mice were purchased from the Animal Center of the College of Medicine, National Taiwan University, Taipei, Taiwan. The Animal Research Committee of College of Medicine, National Taiwan University, conducted the study in accordance with the guideline for the care and use of laboratory animals. Mice were intraperitoneally injected with LPS (7.5 mg/kg) or with pyrogen-free water (control). On the other hand, mice were anesthetized with ketamine (30 mg/kg) and xylazine (4 mg/kg) and then instilled intravesically with strain J96 (108 CFU in 100 μl of sterile phosphate-buffered saline [PBS]) or LPS (1 mg in 100 μl of sterile PBS). In some experiments, mice were intraperitoneally injected or intravesically instilled with anti-mouse IL-6 neutralizing antibody (anti-IL-6Ab [R&D Systems]; 1 μg/kg or 0.1 μg in 100 μl of sterile PBS), normal goat immunoglobulin G (IgG; a negative control for IL-6Ab [R&D Systems]; 1 μg/kg or 0.1 μg in 100 μl of sterile PBS), NG-nitro-l-arginine methyl ester (L-NAME; an NOS inhibitor; 10 mg/kg), aminoguanidine (an iNOS inhibitor; 50 mg/kg or 10 mg in 100 μl of sterile PBS), l-N6-(1-iminoethyl)lysine (L-NIL; an iNOS inhibitor; 10 mg/kg), or 1400W (N-[(3-aminomethyl)benzyl]acetamidine [Sigma]; a potent selective iNOS inhibitor, 5 mg/kg), 30 min after LPS or J96 treatment.
The choice of dosages of experimental drugs and timing to perform animal treatments in the present study was based on the experiences in references and our preliminary studies.
Preparation of detrusor strips.
Mice were sacrificed by ether anesthesia. After the animal was sacrificed, the lower abdomen was opened, and the exposed bladder was excised above the trigone. The isolated bladder was washed with several changes of modified Krebs solution (sodium chloride, 130.6 mM; potassium chloride, 4.8 mM; magnesium sulfate, 1.2 mM; sodium bicarbonate,12.5 mM; calcium chloride, 2.5 mM; and glucose, 11.1 mM) and opened by two lateral incisions to give a rectangular sheet of tissue. The electrical field stimulation (EFS)-evoked contractions was measured as described previously (39, 40). Detrusor strips (3 mm long, 1 mm wide) were suspended in a 5-ml organ bath between built-in vertical platinum electrodes. Initial tension was set at 1 g, which was the optimal tension for this preparation in preliminary experiments. Tissues were allowed to equilibrate for 60 min, during which Krebs solution was changed and replaced with fresh solution. The preparation was maintained at 37.0 ± 0.5°C and oxygenated with 95% oxygen and 5% carbon dioxide. Strips were stimulated with supramaximal trains of pulses (pulse duration, 0.2 ms; train duration, 10 s) at frequencies of 4 and 16 Hz. The tension was recorded through an isometric transducer (Grass FT.03) on a data acquisition system with analytical software (Biopac Systems, Inc.).
Western blot analysis.
Tissues were homogenized in buffer that contained 20 mM HEPES, 0.25 M sucrose, 0.5 mM EDTA, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml, and 1 mM sodium orthovanadate (Na3VO4) (pH 7.5). The cell homogenates were precleared from nuclei and cell debris by centrifugation at 10,000 × g for 15 min at 4°C. The supernatant (total cell lysate) was then ultracentrifuged at 100,000 × g for 1 h at 4°C, which resulted in a supernatant, referred to as the “cytosolic fraction” and in a pellet (the crude membrane fraction). For protein kinase C (PKC) immunoblotting, the pellet was resuspended in 250 μl of homogenizing buffer and 1% (vol/vol) of Nonidet P-40 and incubated on ice for 30 min, followed by centrifugation at 100,000 × g for 30 min at 4°C. The supernatant fraction was termed the “membrane fraction.” Western blot analysis for the presence of particular proteins or for phosphorylated forms of proteins was performed on total cell lysates; the PKC isoform distribution was performed on cytosolic and membrane fractions. Total protein containing 30 to 80 μg was separated on 8% sodium dodecyl sulfate-polyacrylamide minigels and transferred to nitrocellulose membranes (Amersham). After blocking, blots were incubated with antibodies for iNOS, eNOS, PKC isoforms (α, β, and ɛ; BD Transduction Laboratories), phospho-eNOS, and phospho-PKC (pan; Cell Signaling) in PBS-Tween 20 for 1 h, followed by two washes in PBS-Tween 20, and then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG for 30 min. Moreover, α-tubulin served as a control for sample loading and integrity. The antibody-reactive bands were revealed by using an enhanced chemiluminescence kit (Amersham) and were used to expose to Kodak radiographic film. The amount of polypeptide was quantitated by integrated densitometric analysis of the film (Kodak Gel Logic-100 Imaging System). Densitometric readings were corrected for the amount of protein in the samples loaded onto the gels. The distribution of PKC isoforms is presented as the PKC membrane/cytosolic ratio.
RT-PCR for IL-6 and iNOS expression.
The expression of IL-6 and iNOS in bladder was determined by reverse transcription-PCR (RT-PCR) analysis. Tissues were homogenized with 1 ml of TRIzol reagent (Gibco), and total RNA was isolated in accordance with the manufacturer's protocol. First-strand cDNA was synthesized by the extension of (dT) primers with 200 U of SuperScript II reverse transcriptase (Gibco) in a mixture that contained 1 μg of total RNA digested by RNase-free DNase (2 U/μg of RNA) for 15 min at 37°C. The cDNA was then used as a template in a PCR using the Perkin-Elmer DNA thermal cycler model 480. PCR was performed in a final volume of 50 μl that contained all 4 deoxynucleoside triphosphates, 1.5 mM MgCl2, 2.0 U of AmpliTaq (Gibco), and 0.4 μM concentrations of each primer (iNOS sense [5′-CCCTTCCGAAGTTTCTGGCAGCAG-3′] and antisense [5′-GGGCTCCTCCAAGGTGTTGCCC-3′]; IL-6 sense [5′-TGGAGTCACAGAAGGAGTGGCTAAG-3′] and antisense [5′-TCTGACCACAGTGAGGAATGTCCAC-3′]). The amplification cycles were 45 s at 94°C, 45 s at 65°C, and 2 min at 72°C. The PCR products were separated by electrophoresis on a 1.8% agarose gel after 30 to 35 cycles and visualized by ethidium bromide staining. The mRNA of β-actin served as control for sample loading and integrity.
Measurement of IL-6 and nitrite/nitrate (NOx) levels in urinary bladder tissue.
IL-6 and nitrite from the urinary bladder were assayed by using a mouse IL-6 ELISA kit (R&D Systems) and a NOx assay kit (R&D Systems). The procedures for sample handling and assay were performed in accordance with the manufacturer's instructions. All assays were performed in duplicate.
Statistical analysis.
Data are expressed as the means ± the standard error of the mean (SEM). Significant differences from the respective control values for each experimental test condition were assessed by using analysis of variance and the Bonferroni t test. P < 0.05 was considered to be significant.
RESULTS
IL-6 and NOx productions and IL-6 and NOS expression in urinary bladders under E. coli endotoxin- or E. coli strain J96-induced inflammation.
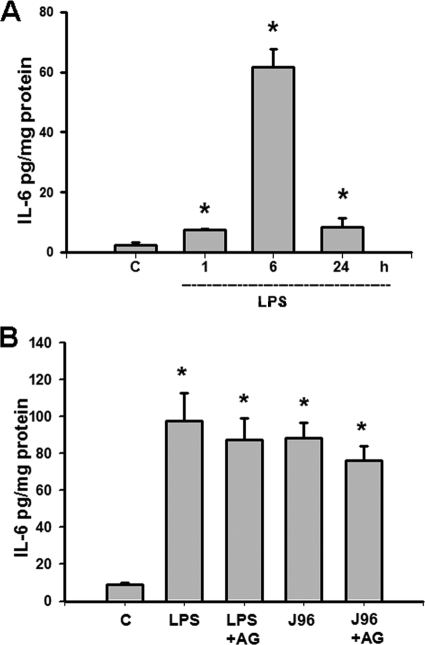
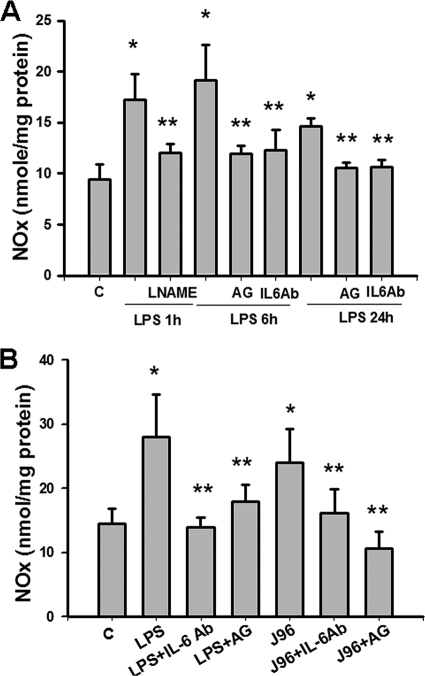
As shown in Fig. 1 and 2, the levels of IL-6 and NOx (nitrite plus nitrate) in the urinary bladders of mice injected intraperitoneally or instilled intravesically with E. coli endotoxin (LPS) or E. coli strain J96 were measured. There were the significant increases in the IL-6 (Fig. 1) and NOx (Fig. 2) levels in the urinary bladders of mice 1 to 24 h after intraperitoneal injection of LPS (Fig. 1A and 2A) or 6 h after intravesical instillation of J96 or LPS (Fig. 1B and 2B). Aminoguanidine, an iNOS inhibitor, did not affect the increase of IL-6 production in bladders instilled intravesically with J96 or LPS (Fig. 1B). Both aminoguanidine and anti-IL-6 antibody markedly attenuated the increase of NOx production in urinary bladder of mice 6 and 24 h after intraperitoneal injection of LPS (Fig. 2A) or 6 h after intravesical instillation of J96 or LPS (Fig. 2B). NOS inhibitor L-NAME could inhibit the bladder NOx production 1 h after LPS treatment (Fig. 2A), but anti-IL-6-antibody could not (data not shown). IgG, a negative control for anti-IL-6 antibody, did not affect the change of NOx production by LPS or J96 (data not shown).
FIG. 1.
Levels of IL-6 in the urinary bladders of E. coli- or endotoxin-treated mice. (A) Urinary bladders were isolated from mice 1, 6, and 24 h after intraperitoneal injection of E. coli endotoxin (LPS). (B) Bladders were isolated from mice 6 h after the intravesical instillation of E. coli strain J96 (O4:K6) or LPS. In some experiments, mice were instilled intravesically with iNOS inhibitor aminoguanidine (AG) at 30 min after E. coli treatment. IL-6 levels in the urinary bladders were assayed by using mouse IL-6 enzyme-linked immunosorbent assay kit. The data are presented as means ± the SEM for four independent experiments. *, P < 0.05 compared to the control.
FIG. 2.
Levels of NOx in the urinary bladders of E. coli or endotoxin-treated mice. (A) Urinary bladders were isolated from mice 1, 6, and 24 h after intraperitoneal injection of E. coli endotoxin (LPS). (B) Bladders were isolated from mice 6 h after intravesical instillation of E. coli strain J96 (O4:K6) or LPS. In some experiments, mice were injected intraperitoneally (A) or instilled intravesically (B) with L-NAME, anti-mouse IL-6 neutralizing antibody (anti-IL-6Ab), or aminoguanidine (AG) 30 min after E. coli treatment. NOx (nitrite+nitrate) levels in the urinary bladders were assayed by using NOx assay kit. The data are presented as the means ± the SEM for four independent experiments. *, P < 0.05 compared to the control; **, P < 0.05 compared to LPS alone.
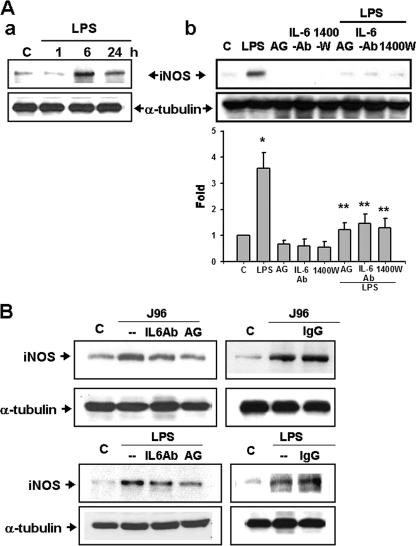
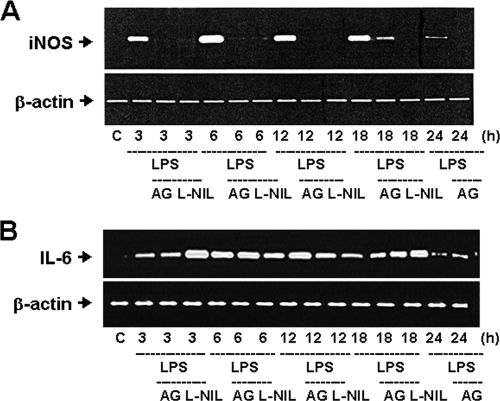
The inductions of iNOS proteins were increased in the urinary bladders of mice 6 and 24 h after intraperitoneal injection of LPS (Fig. 3A) or 6 h after intravesical instillation of J96 or LPS, which could be reversed by the treatments of iNOS inhibitors aminoguanidine or 1400W and anti-IL-6 antibody (Fig. 3). In Fig. 3B, the densitometric data of J96 (top left panel) were as follows (n-fold): control, 1.0 ± 0.0; J96 alone, 3.03 ± 0.29; J96+IL-6Ab, 1.83 ± 0.18; and J96+AG, 1.47 ± 0.2 (n = 3, P < 0.05, control versus J96 alone or J96 alone versus J96+IL-6Ab or AG). IgG did not affect the change of iNOS expression by J96 or LPS (Fig. 3B). Furthermore, the iNOS and IL-6 mRNA expressions were inducted in the urinary bladders of LPS-treated mice (Fig. 4). Treatment of iNOS inhibitors aminoguanidine and L-NIL markedly attenuated the increase of iNOS mRNA expression (Fig. 4A). Anti-IL-6 antibody could also suppress the LPS-induced iNOS mRNA expression in the urinary bladder (LPS 6 h, 270.4% ± 23.6% of control; LPS at 6 h + IL-6Ab, 122.8% ± 11.4% of control; LPS at 6 h + IgG, 281.2% ± 13.1% of control, n = 3; P < 0.05, LPS + IL-6Ab versus LPS). However, aminoguanidine and L-NIL did not affect the LPS-induced IL-6 mRNA expression (Fig. 4B). On the other hand, 1 h after injection of LPS, bladder eNOS and phosphorylated eNOS protein expressions were markedly increased, which could not be reversed by the treatment of anti-IL-6 antibody (Fig. 5A). The eNOS protein expressions were not increased in urinary bladders of mice at 6 h after the treatment of LPS with or without aminoguanidine and anti-IL-6 antibody compared to the control group (Fig. 5B). These results indicate that IL-6 signaling possesses the ability to regulate the induction of iNOS, but not eNOS, in the urinary bladder under E. coli-induced inflammatory conditions.
FIG. 3.
iNOS protein expressions in the urinary bladders of E. coli or endotoxin-treated mice. (A) Urinary bladders were isolated from mice 1, 6, or 24 h after the intraperitoneal injection of E. coli endotoxin (LPS). (B) Bladders were isolated from mice 6 h after intravesical instillation of E. coli strain J96 (O4:K6) or LPS. In some experiments, mice were injected intraperitoneally (A) or instilled intravesically (B) with iNOS inhibitors aminoguanidine (AG) and 1400W, anti-IL-6 antibody (IL-6Ab), or IgG (a negative control for IL-6Ab) 30 min after E. coli treatment. α-tubulin served as a control for sample loading and integrity. In panel Ab, quantification of the expression of iNOS protein was performed by densitometric analysis. The data are presented as the means ± the SEM for four independent experiments. *, P < 0.05 compared to the control; **, P < 0.05 compared to LPS alone. In panels Aa and B, the results shown are representative of three independent experiments.
FIG. 4.
Induction of iNOS and IL-6 mRNA expression by E. coli endotoxin in the mouse urinary bladder. Urinary bladders were isolated from mice 3 to 24 h after intraperitoneal injection of E. coli endotoxin (LPS) in the presence or absence of iNOS inhibitors aminoguanidine (AG) and L-NIL. (A) The top panel is representative of typical gel electrophoresis from a RT-PCR analysis of bladder iNOS mRNA. (B) The top panel is representative of typical gel electrophoresis from a RT-PCR analysis of bladder IL-6 mRNA. The bottom portions in panels A and B show the mRNA of β-actin served as a control for sample loading and integrity. The results shown are representative of three independent experiments.
FIG. 5.
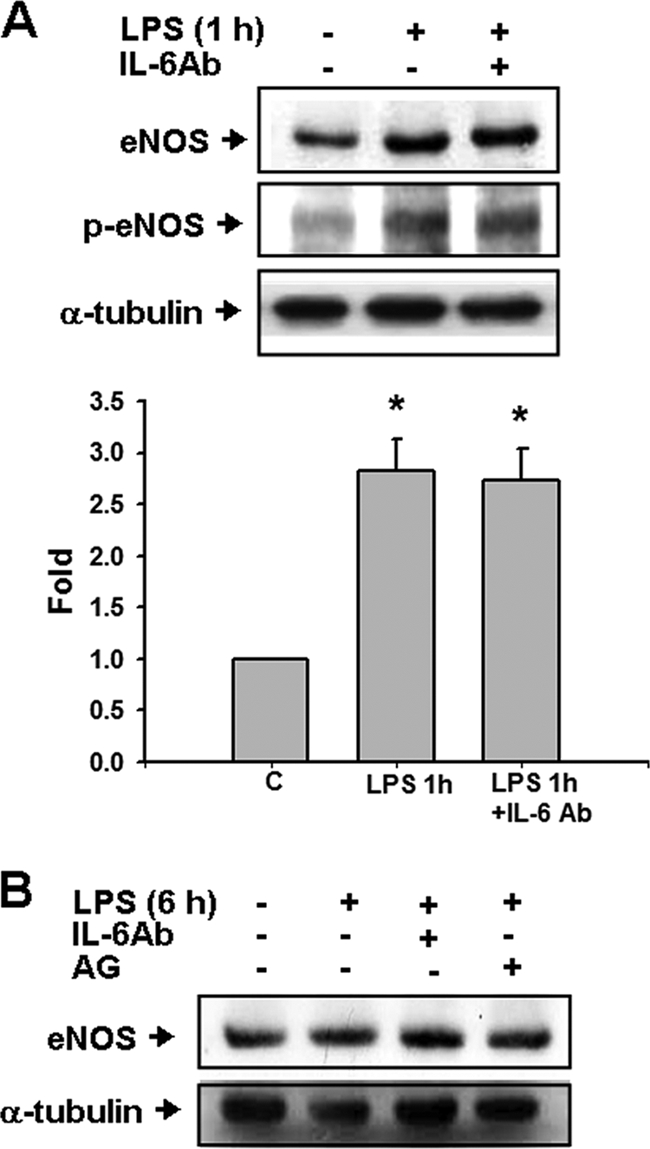
eNOS expressions in the urinary bladders of E. coli endotoxin-treated mice. Urinary bladders were isolated from mice at 1 h (A) or 6 h (B) after the intraperitoneal injection of E. coli endotoxin (LPS) in the presence or absence of anti-IL-6 antibody (IL-6Ab) or aminoguanidine (AG). Bladder eNOS and phosphorylated eNOS (p-eNOS) were detected by Western blotting. α-Tubulin served as a control for sample loading and integrity. In panel A, quantification of the expression of eNOS protein was performed by densitometric analysis. The data are presented as means ± the SEM for four independent experiments. *, P < 0.05 compared to the control. In panel B, the results shown are representative of three independent experiments.
PKC activation and contractile response in the urinary bladders of under E. coli LPS or E. coli strain J96-induced inflammation.
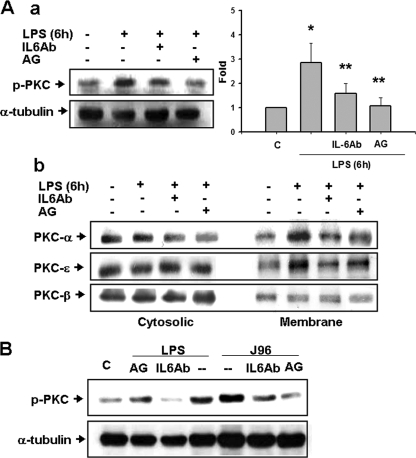
β-Phorbol-12,13-dibutyrate (a PKC activator) has previously been demonstrated to enhance the contractile responses evoked by EFS in mouse or rat urinary bladders (16, 39). We next investigated the role of IL-6 in PKC activation and contractile response in urinary bladders under E. coli LPS- or E. coli strain J96-induced inflammation. As shown in Fig. 6Aa, the phosphorylation of PKC (pan) was markedly increased in the membrane fraction of the urinary bladder at 6 h after LPS treatment, which could be reversed by aminoguanidine and anti-IL-6 antibody. Moreover, the distribution of PKC-α, -β, and -ɛ between the cytosolic and membrane fractions in the urinary bladder was analyzed by Western blotting. PKC-α and -ɛ proteins were markedly increased in the membrane fraction of bladder 6 h after LPS treatment (Fig. 6Ab). LPS did not affect the distribution of PKC-β between the cytosolic and membrane fractions in the urinary bladders (Fig. 6Ab). Both anti-IL-6 antibody and aminoguanidine treatments reversed the alteration of PKC-α and -ɛ distribution triggered by LPS (Fig. 6Ab). Similarly, the phosphorylation of PKC (pan) was markedly increased in the membrane fraction of the urinary bladder at 6 h after intravesical instillation of J96 or LPS, which could be reversed by the treatments of anti-IL-6 antibody and aminoguanidine (Fig. 6B). IgG did not affect the change of PKC activation by LPS or J96 (data not shown).
FIG. 6.
Activation of PKC in the urinary bladder of E. coli or endotoxin-treated mice. Urinary bladders were isolated from mice 6 h after intraperitoneal injection of E. coli endotoxin (LPS) (A) or 6 h after intravesical instillation of E. coli strain J96 (O4:K6) or LPS (B). In some experiments, mice were injected intraperitoneally (A) or instilled intravesically (B) with anti-IL-6 antibody (IL-6Ab) or aminoguanidine (AG, an iNOS inhibitor) at 30 min after E. coli treatment. PKC activation was assayed as the phosphorylation of PKC (Aa and B) and the translocation of PKC isoforms from cytosolic fraction to membrane fraction (Ab) by Western blot analysis using anti-phospho-PKC (pan) antibody and anti-PKC-α, -β, and -ɛ antibodies, respectively. α-Tubulin served as a control for sample loading and integrity. In Aa, the data are presented as means ± the SEM for six independent experiments. *, P < 0.05 compared to the control; **, P < 0.05 compared to LPS alone. In panels Ab and B, the results shown are representative of four independent experiments.
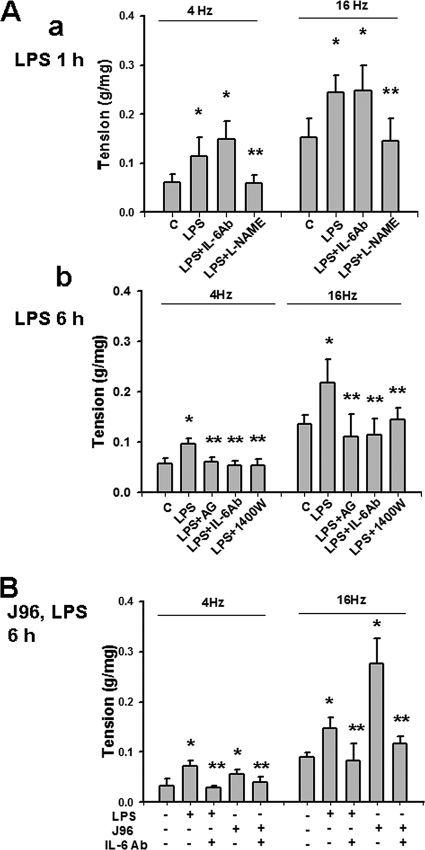
The contractile responses of the isolated mouse urinary bladder evoked by EFS (4 and 16 Hz) were significantly increased at 1 h (Fig. 7Aa) or 6 h (Fig. 7Ab) after intraperitoneal injection of LPS or at 6 h after intravesical instillation of J96 or LPS (Fig. 7B). At 1 h after LPS treatment, L-NAME but not anti-IL-6 antibody reversed the LPS-enhanced contractile responses (Fig. 7Aa). Treatments of anti-IL-6 antibody, aminoguanidine, or 1400W could inhibit the increase of EFS-evoked bladder contractile responses at 6 h after intraperitoneal injection of LPS (Fig. 7Ab) or intravesical instillation of J96 or LPS (Fig. 7B). IgG did not affect the change of contractions by E. coli (data not shown).
FIG. 7.
Effects of E. coli or endotoxin on urinary bladder contractions evoked by EFS. The contractions of detrusor muscle strips were evoked by EFS (10 s at 4 and 16 Hz). Urinary bladders were isolated from mice 1 h (Aa) or 6 h (Ab) after intraperitoneal injection of LPS (A) or 6 h after intravesical instillation of E. coli strain J96 (O4:K6) or LPS (B). In some experiments, mice were injected peritoneally (A) or instilled intravesically (B) with anti-IL-6 antibody (IL-6Ab), L-NAME (nonselective NOS inhibitor), aminoguanidine (AG), or 1400W (selective iNOS inhibitors) at 30 min after E. coli treatment. All data are presented as means ± the SEM for four independent experiments. *, P < 0.05 compared to the control; **, P < 0.05 compared to LPS alone.
DISCUSSION
In this study, we investigated the role of IL-6 in the alteration of the contractile response and its possible mechanism in a uropathogenic E. coli-induced inflammatory urinary bladder. The results indicate that IL-6 induces an iNOS/NO-triggered PKC-activated urinary bladder contraction under an E. coli-induced inflammatory condition.
IL-6 is one of the major inflammation-associated cytokines (21). Elevated plasma and urine levels of IL-6 have been demonstrated in cancer and inflammatory diseases of the urinary tract (1, 3, 17, 27). A study of interstitial cystitis patients has shown that increased IL-6 levels were detected in spontaneously voided urine but not in ureteral urine and suggested that IL-6 is the product of activated cells in the bladder (17). Recently, Bouchelouche et al. (8) demonstrated that human detrusor smooth muscle cells can release IL-6 in response to proinflammatory cytokines. Neuhaus et al. (19) have also shown that IL-6 and IL-6 receptor expression was found in urothelium, lamina propria, and detrusor cells isolated from bladder biopsies of tumor patients; these researchers further found that LPS stimulation evoked a time-dependent synthesis and/or release of IL-6, IL-6 receptor, and transcription factor signal transducer and activator of transcription 3 (Stat3) in cultured human detrusor smooth muscle cells. In the present work, we found that the IL-6 levels in the urinary bladders of mice after intraperitoneal injection of LPS or intravesical instillation of E. coli J96 strain or LPS were significantly increased. The IL-6 protein and mRNA levels were marked increased in the urinary bladders under an E. coli LPS-induced inflammation. The IL-6 levels in urine were also elevated in this condition (data not shown). Therefore, the previous findings and our results suggest that detrusor cells and urothelium may serve as a source of elevated IL-6 levels under a uropathogenic E. coli-induced inflammation.
Urine from urinary tract infections has been found to contain an isoform of NO synthase, which is an endogenous source of urine nitrite (33). Increased NO production in the bladder during urinary tract infections have previously been linked to iNOS activation, which occurs at 4 h after LPS administration (23). Recently, eNOS was shown to be an early bladder responder to intraperitoneal administered LPS, at 1 h after the LPS treatment, before the infiltration of inflammatory cells (12). Several lines of evidence, in addition to data in our study, showed the upregulation of eNOS as an early event in inflammatory processes of the bladder urothelium (12, 22, 39, 40). Kang et al. (12) further demonstrated that Akt and eNOS are both localized in the bladder urothelium and that phosphorylation of eNOS by Akt provides an attractive mechanism for rapid increases in urinary NO production. Similarly, in the present study, after short-term LPS treatment for 1 h, eNOS and phosphorylated eNOS proteins and NOx levels in the mouse urinary bladders were markedly increased, whereas iNOS could not be detected. In contrast, after LPS or E. coli J96 strain treatment for 6 h, iNOS proteins and NOx levels in the mouse urinary bladders were markedly elevated, whereas eNOS proteins were not changed. We further found that anti-IL-6 antibody can inhibit the induction of iNOS expression and NOx production in bladders by LPS or E. coli J96 strain treatment for 6 h, whereas the increase in eNOS expression in bladders by LPS treatment for 1 h is not affected by anti-IL-6 antibody. Moreover, selective iNOS inhibitors could not suppress the LPS-triggered induction in IL-6 mRNA expression in bladders. Taken together, these results indicate that IL-6 plays an important role in the regulation of iNOS, but not eNOS, expression in the urinary bladders under an E. coli-induced inflammatory condition.
The neuronal and mechanical events associated with bladder filling and micturition become hypersensitive and progressively painful during an acute urinary bladder inflammation in humans (5). It has been reported that the intravesical administration of LPS for 1 h induced rat detrusor hyperreflexia (15). Exogenous application of bacterial exotoxin N-formyl-methionyl-leucyl-phenylalanine has also been shown to cause a significantly large, monophasic contracture in rabbit detrusor during the 10-min period of toxin exposure (31). Although IL-6 and NO have been reported to be elevated during urinary tract infection, the regulatory relationship between IL-6 and NO signals in the alteration of contractile response in urinary bladder in response to E. coli-induced inflammation still remains to be clarified. In the present study, we found that, (i) for 1 h of LPS treatment, nonspecific NOS inhibitor L-NAME, but not anti-IL-6 antibody, reversed the LPS-induced NO production and LPS-enhanced contractile response in bladders; and (ii) for 6 h of LPS treatment, both anti-IL-6 antibody and selective iNOS inhibitors aminoguanidine and 1400W treatments could inhibit the increase in NO production and EFS-evoked bladder contractile responses at 6 h after LPS or E. coli J96 strain treatment. These results indicate that IL-6 activates an iNOS-regulated enhancement of contractile response in an E. coli-induced urinary bladder inflammatory condition, whereas IL-6 does not seem to be involved in the enhancement of eNOS-regulated contractile response in the urinary bladder by short-term E. coli endotoxin treatment.
A facilitatory presynaptic muscarinic mechanism in the urinary bladder, which is involved in the enhancement of acetylcholine release during continuous EFS, has been shown to be dependent on a PKC-mediated signaling pathway and influx extracellular Ca2+ into the parasympathetic nerve terminals via L and N-type Ca2+ channels (34). The PKC activator phorbol 12,13-dibutyrate has been shown to induce the contractile responses in rabbit bladder dome and urethra (41). Our previous studies have shown that the contractions of the isolated mouse and rat detrusor strips evoked by EFS were enhanced 30 min after β-phorbol 12,13-dibutyrate treatment (16, 39). The enhancement of detrusor contractions induced by short-term LPS treatment could also be inhibited by the PKC inhibitors chelerythrine and Ro32-0432 (39). In the present study, we further investigated the regulatory role of IL-6 in the PKC activation in the LPS or E. coli-J96 strain-induced urinary bladder inflammation. The results showed that the phosphorylation of PKC (pan) in the membrane fraction of urinary bladder and the translocation of PKC-α and PKC-ɛ from the cytosolic fraction to the membrane fraction in urinary bladder were markedly increased at 6 h after LPS or J96 treatment, which could be reversed by aminoguanidine and anti-IL-6 antibody. These results indicate that IL-6 is capable of activating PKC-α and -ɛ and imply that an IL-6 and iNOS/NO-activated PKC-related pathway is involved in the alteration of contractile responses in E. coli-induced urinary bladder inflammation.
In conclusion, E. coli-induced inflammation triggered the expressions of IL-6, eNOS, and iNOS in urinary bladders. Anti-IL-6 antibodies are capable of inhibiting the induction of iNOS expression, but not eNOS, the activation of PKC and the enhancement of contractile response in urinary bladders during E. coli-induced inflammation. Selective iNOS inhibitors did not suppress E. coli-triggered IL-6 expression. These results indicate that IL-6 may play an important regulatory role in the iNOS/NO-triggered PKC-activated contractile response in urinary bladder during E. coli-induced inflammation.
Acknowledgments
This study was supported by grants NSC 90-2315-B-002-009 from the National Science Council of the Taiwan and 91-N009 from the National Taiwan University Hospital.
We declare that no competing financial interests exist.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 26 May 2009.
REFERENCES
- 1.Abdel-Mageed, A. B., A. Bajwa, B. B. Shenassa, L. Human, and G. M. Ghoniem. 2003. NF-κB-dependent gene expression of proinflammatory cytokines in T24 cells: possible role in interstitial cystitis. Urol. Res. 31300-305. [DOI] [PubMed] [Google Scholar]
- 2.Agace, W. W., S. R. Hedges, M. Ceska, and C. Svanborg. 1993. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J. Clin. Investig. 92780-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, B., S. F. Shariat, J. H. Kim, T. M. Wheeler, K. M. Slawin, and S. P. Lerner. 2002. Preoperative plasma levels of interleukin-6 and its soluble receptor predict disease recurrence and survival of patients with bladder cancer. J. Urol. 1671475-1481. [PubMed] [Google Scholar]
- 4.Bagshaw, S. M., and K. B. Laupland. 2006. Epidemiology of intensive care unit-acquired urinary tract infections. Curr. Opin. Infect. Dis. 1967-71. [DOI] [PubMed] [Google Scholar]
- 5.Barnett, B. J., and D. S. Stephens. 1997. Urinary tract infection: an overview. Am. J. Med. Sci. 314245-249. [DOI] [PubMed] [Google Scholar]
- 6.Billips, B. K., S. G. Forrestal, M. T. Rycyk, J. R. Johnson, D. J. Klumpp, and A. J. Schaeffer. 2007. Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli. Infect. Immun. 755353-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorling, D. E., M. Beckman, and R. Saban. 2003. Neurogenic inflammation of the bladder. Adv. Exp. Med. Biol. 539551-583. [DOI] [PubMed] [Google Scholar]
- 8.Bouchelouche, K., S. Alvarez, T. Horn, J. Nordling, and P. Bouchelouche. 2006. Human detrusor smooth muscle cells release interleukin-6, interleukin-8, and RANTES in response to proinflammatory cytokines interleukin-1beta and tumor necrosis factor-α. Urology 67214-219. [DOI] [PubMed] [Google Scholar]
- 9.Chen, L. M., C. Wang, M. Chen, M. R. Marcello, J. Chao, L. Chao, and K. X. Chai. 2006. Prostasin attenuates inducible nitric oxide synthase expression in lipopolysaccharide-induced urinary bladder inflammation. Am. J. Physiol. Renal Physiol. 291F567-F577. [DOI] [PubMed] [Google Scholar]
- 10.Foxman, B., and P. Brown. 2003. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect. Dis. Clin. N. Am. 17227-241. [DOI] [PubMed] [Google Scholar]
- 11.Gürgöze, M. K., S. Akarsu, E. Yilmaz, A. Gödekmerdan, Z. Akça, I. Ciftçi, and A. D. Aygün. 2005. Proinflammatory cytokines and procalcitonin in children with acute pyelonephritis. Pediatr. Nephrol. 201445-1448. [DOI] [PubMed] [Google Scholar]
- 12.Kang, W. S., F. J. Tamarkin, M. A. Wheeler, and R. M. Weiss. 2004. Rapid upregulation of endothelial nitric-oxide synthase in a mouse model of Escherichia coli lipopolysaccharide-induced bladder inflammation. J. Pharmacol. Exp. Ther. 310452-458. [DOI] [PubMed] [Google Scholar]
- 13.Keith, B. R., L. Maurer, P. A. Spears, and P. E. Orndorff. 1986. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect. Immun. 53693-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klavs, I., T. Bufon Luznik, M. Skerl, M. Grgic-Vitek, T. Lejko Zupanc, M. Dolinsek, V. Prodan, M. Vegnuti, A. Kraigher, Z. Arnez, et al. 2003. Prevalence of and risk factors for hospital-acquired infections in Slovenia-results of the first national survey. J. Hosp. Infect. 54149-157. [DOI] [PubMed] [Google Scholar]
- 15.Lecci, A., M. Tramontana, S. Giuliani, M. Criscuoli, and C. A. Maggi. 1998. Effect of tachykinin NK2 receptor blockade on detrusor hyperreflexia induced by bacterial toxin in rats. J. Urol. 160206-209. [PubMed] [Google Scholar]
- 16.Liu, S. H., and S. Y. Lin-Shiau. 2000. Protein kinase c regulates purinergic component of neurogenic contractions in mouse bladder. J. Urol. 1641764-1767. [PubMed] [Google Scholar]
- 17.Lotz, M., P. Villiger, T. Hugli, J. Koziol, and B. L. Zuraw. 1994. Interleukin-6 and interstitial cystitis. J. Urol. 152869-873. [DOI] [PubMed] [Google Scholar]
- 18.Mamas, M. A., J. M. Reynard, and A. F. Brading. 2003. Nitric oxide and the lower urinary tract: current concepts, future prospects. Urology 611079-1085. [DOI] [PubMed] [Google Scholar]
- 19.Neuhaus, J., N. Schlichting, A. Oberbach, and J. U. Stolzenburg. 2007. Lipopolysaccharide-mediated regulation of interleukin-6 in cultured human detrusor smooth muscle cells. Urologe A 461193-1197. [DOI] [PubMed] [Google Scholar]
- 20.Nicolle, L. E. 2008. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol. Clin. N. Am. 351-12. [DOI] [PubMed] [Google Scholar]
- 21.Nussler, A. K., and T. R. Billiar. 1993. Inflammation, immunoregulation, and inducible nitric oxide synthase. J. Leukoc. Biol. 54171-178. [PubMed] [Google Scholar]
- 22.Oh, B. R., K. Nakajima, K. Y. Ahn, S. B. Ryu, Y. I. Park, and R. Dahiya. 2001. Nitric oxide synthase gene and protein expression are upregulated by Bacille Calmette-Guerin in the rat bladder. Eur. Urol. 39349-356. [DOI] [PubMed] [Google Scholar]
- 23.Olsson, L. E., M. A. Wheeler, W. C. Sessa, and R. M. Weiss. 1998. Bladder instillation and intraperitoneal injection of Escherichia coli lipopolysaccharide up-regulate cytokines and iNOS in rat urinary bladder. J. Pharmacol. Exp. Ther. 2841203-1208. [PubMed] [Google Scholar]
- 24.Otto, G., J. Braconier, A. Andreasson, and C. Svanborg. 1999. Interleukin-6 and disease severity in patients with bacteremic and nonbacteremic febrile urinary tract infection. J. Infect. Dis. 179172-179. [DOI] [PubMed] [Google Scholar]
- 25.Ozawa, H., M. B. Chancellor, S. Y. Jung, T. Yokoyama, M. O. Fraser, Y. Yu, W. C. de Groat, and N. Yoshimura. 1999. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J. Urol. 1622211-2216. [DOI] [PubMed] [Google Scholar]
- 26.Pan, J. Z., L. Ni, A. Sodhi, A. Aguanno, W. Young, and R. P. Hart. 2002. Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. J. Neurosci. Res. 68315-322. [DOI] [PubMed] [Google Scholar]
- 27.Peters, K. M., A. C. Diokno, and B. W. Steinert. 1999. Preliminary study on urinary cytokine levels in interstitial cystitis: does intravesical bacille Calmette-Guérin treat interstitial cystitis by altering the immune profile in the bladder? Urology 54450-453. [DOI] [PubMed] [Google Scholar]
- 28.Poljakovic, M., M. L. Svensson, C. Svanborg, K. Johansson, B. Larsson, and K. Persson. 2001. Escherichia coli-induced inducible nitric oxide synthase and cyclooxygenase expression in the mouse bladder and kidney. Kidney Int. 59893-904. [DOI] [PubMed] [Google Scholar]
- 29.Saban, M. R., H. Hellmich, N. B. Nguyen, J. Winston, T. G. Hammond, and R. Saban. 2001. Time course of LPS-induced gene expression in a mouse model of genitourinary inflammation. Physiol. Genomics 5147-160. [DOI] [PubMed] [Google Scholar]
- 30.Sant, G. R., D. Kempuraj, J. E. Marchand, and T. C. Theoharides. 2007. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology. 69(Suppl.)34-40. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz, N. T., S. Y. Jung, J. C. Kalff, M. Chancellor, and A. J. Bauer. 2002. Bacterial toxin N-formyl-methionyl-leucyl-phenylalanine acutely contracts human and rabbit detrusor through the release of eicosanoids. J. Urol. 1672603-2612. [PubMed] [Google Scholar]
- 32.Sedberry-Ross, S., and H. G. Pohl. 2008. Urinary tract infections in children. Curr. Urol. Rep. 9165-171. [DOI] [PubMed] [Google Scholar]
- 33.Smith, S. D., M. A. Wheeler, and R. M. Weiss. 1994. Nitric oxide synthase: an endogenous source of elevated nitrite in infected urine. Kidney Int. 45586-591. [DOI] [PubMed] [Google Scholar]
- 34.Somogyi, G. T., and W. C. de Groat. 1999. Function, signal transduction mechanisms and plasticity of presynaptic muscarinic receptors in the urinary bladder. Life Sci. 64411-418. [DOI] [PubMed] [Google Scholar]
- 35.Stamm, W. E. 2002. Scientific and clinical challenges in the management of urinary tract infections. Am. J. Med. 113(Suppl. 1A)1S-4S. [DOI] [PubMed] [Google Scholar]
- 36.Taga, T., and T. Kishimoto. 1997. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 15797-819. [DOI] [PubMed] [Google Scholar]
- 37.Vincent, J. L., D. J. Bihari, P. M. Suter, H. A. Bruining, J. White, M. H. Nicolas-Chanoin, M. Wolff, R. C. Spencer, and M. Hemmer. 1995. The prevalence of nosocomial infection in intensive care units in Europe: the results of the European Prevalence of Infection in Intensive Care (EPIC) study. JAMA 274639-644. [PubMed] [Google Scholar]
- 38.Warren, J. W., V. Brown, S. Jacobs, L. Horne, P. Langenberg, and P. Greenberg. 2008. Urinary tract infection and inflammation at onset of interstitial cystitis/painful bladder syndrome. Urology 711085-1090. [DOI] [PubMed] [Google Scholar]
- 39.Weng, T. I., W. J. Chen, and S. H. Liu. 2005. Bladder instillation of Escherichia coli lipopolysaccharide alters the muscle contractions in rat urinary bladder via a protein kinase C-related pathway. Toxicol. Appl. Pharmacol. 208163-169. [DOI] [PubMed] [Google Scholar]
- 40.Weng, T. I., W. J. Chen, H. Y. Wu, and S. H. Liu. 2006. Uropathogenic Escherichia coli alter muscle contractions in rat urinary bladder via a nitric oxide synthase-related signaling pathway. J. Infect. Dis. 1941774-1782. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida, M., K. Nishi, J. Machida, H. Sakiyama, K. Ikeda, and S. Ueda. 1992. Effects of phorbol ester on lower urinary tract smooth muscles in rabbits. Eur. J. Pharmacol. 222205-211. [DOI] [PubMed] [Google Scholar]