Abstract
The contribution of the inflammasome to the development of immune responses and disease during infection with the Lyme disease spirochete, Borrelia burgdorferi, is not well defined. Host defense against the spirochete is severely impaired in mice deficient in the adaptor molecule myeloid differentiation antigen 88 (MyD88), which is required not only for Toll-like receptor-mediated responses but also for the production of the proforms of interleukin 1β (IL-1β) and IL-18. These cytokines are released in active forms after cleavage by the inflammasome-associated enzyme caspase 1. To investigate the contribution of the inflammasome to host defense against B. burgdorferi, we examined Lyme borreliosis in mice deficient in either caspase 1 or apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC), a molecule upstream of caspase 1 in the inflammasome signaling cascade. We found that caspase 1-deficient mice had a mild transient elevation in pathogen burden and a trend toward an increase in the prevalence of arthritis early in infection, but these differences resolved by day 14 postinfection. Caspase 1 deficiency had no effect on B. burgdorferi-induced humoral immunity, T-cell responses, or the abilities of macrophages to ingest and degrade spirochetes. The absence of the ASC protein had no effect on the control of the spirochete or the development of immune responses and disease. These findings reveal that the caspase 1 inflammasome is not critical to host defense against the extracellular pathogen Borrelia burgdorferi.
Infection of humans with the Lyme disease spirochete, Borrelia burgdorferi, results in a characteristic pattern of skin lesions, arthritis, carditis, and neurologic abnormalities that reflect the immune response to the spirochete as it invades and disseminates in the mammalian host (7). In the murine model of Lyme borreliosis, spirochetes inoculated into the skin disseminate within days to infect all organ systems, but disease is primarily manifested in the joints and heart (4). Disease in the animal model is due largely to the innate immune response to spirochetes because histopathology reveals mainly neutrophils and macrophages within inflamed joints and hearts, respectively (5, 28, 36, 43), and occurs in the absence of adaptive (T- and B-cell-mediated) immunity (8, 28, 43).
Recent studies have further defined the role of innate immunity in Lyme borreliosis. B. burgdorferi lipoproteins activate innate immune cells through the pattern recognition molecule Toll-like receptor 2 (TLR2), which is required for innate but not adaptive immune responses to the spirochete (2, 19, 49). Spirochete components also stimulate murine cells through TLR5 and TLR9 (44). The TLR cytosolic domains contain a Toll/interleukin 1 (IL-1) receptor domain (TIR) that interacts with myeloid differentiation antigen 88 (MyD88) and results in the activation of NF-κB and the production of proinflammatory cytokines, chemokines, and costimulatory molecules that are important for host defense (6, 12, 14). We and others have previously shown that B. burgdorferi-infected MyD88-deficient (MyD88−/−) mice have significantly elevated pathogen burdens that persist through 90 days of infection despite the presence of high titers of anti-B. burgdorferi antibodies (9, 25). The elevated level of pathogen DNA in tissues was explained in part by our finding that MyD88−/− peritoneal macrophages ingested spirochetes at the same rate as wild-type (WT) cells, but the kinetics of degradation was slower, with internalized spirochetes remaining in an elongated form for a longer period. Others have found that bone marrow-derived MyD88−/− macrophages do not efficiently ingest spirochetes (44). The MyD88−/− mice developed carditis and arthritis similar to the disease in WT mice analyzed at its peak (days 14 and 28) and during regression (day 45) (9, 25). Together, these results showed that MyD88-dependent signaling pathways are not required for B. burgdorferi-induced inflammation or disease regression but are necessary for efficient control of the pathogen burden by phagocytes. These studies did not distinguish whether interruption of MyD88-dependent TLR signaling pathways was solely responsible for the impaired control of the pathogen or whether other MyD88-dependent pathways also play a role.
In addition to being a crucial signaling molecule for TLRs involved in B. burgdorferi recognition, MyD88 is required for IL-1 receptor (IL-1R)- and IL-18R-associated kinase signaling. TLR activation is a key inducer of the proforms of IL-1β and IL-18, and the secreted forms of these two cytokines require MyD88 for their receptors to mediate their effects (1, 34, 38). Behera et al. (6) have shown that IL-18 alone does not significantly contribute to host immunity in Lyme borreliosis because IL-18−/− mice exhibit no defects in pathogen clearance or the development of disease. IL-1β, however, may play a role because human peripheral blood mononuclear cells secrete IL-1β after ingestion of live B. burgdorferi spirochetes (15). In support of this hypothesis, serum levels of IL-1β were reported to be elevated in Lyme disease patients, and the levels decreased significantly after doxycycline treatment (35). IL-1β mRNA levels in erythema migrans lesions were also shown to be elevated (31).
To further delineate the role of MyD88-dependent signaling pathways in host defense against B. burgdorferi, we examined the course of Lyme borreliosis in mice deficient in either the intracellular cysteine protease caspase 1 or apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC). Caspase 1 plays a key role in inflammatory responses by cleaving pro-IL-1β and pro-IL-18 into their active secreted forms (16, 22). These cytokines are matured in a large caspase 1-containing protein complex called the inflammasome (37). ASC, a component of the inflammasome, is required for eliciting the enzymatic activity of caspase 1. Caspase 1 contains an N-terminal caspase recruitment domain (CARD) shown to be involved in the assembly of protein platforms that promote proteolytic activation of recruited caspases in the context of apoptosis and inflammation (14). In addition to cleaving pro-IL-1 and pro-IL-18, caspase 1 is also involved in other proinflammatory pathways, including NF-κB signaling pathways associated with innate and adaptive immune responses (21, 23, 41). In contrast, ASC is essential only for the secretion of IL-1β/IL-18 but dispensable for caspase 1-mediated IL-6 and tumor necrosis factor alpha secretion and NF-κB and p38 activation (40). Thus, although both caspase 1−/− mice and ASC−/− mice have defects in the production of IL-1β/IL-18, caspase 1−/− mice have additional defects in the activation of NF-κB.
Several published reports have established that the inflammasome is important for immunity to intracellular bacteria and viruses, but much less is known about the contribution of the inflammasome to host defense against extracellular pathogens that elicit cytokines activated by caspase 1 (27, 29, 30, 32, 38, 42, 48). Thus, we sought to determine whether the inflammasome is also important during infection with the B. burgdorferi spirochete as representative of a subset of extracellular pathogens. We found that while B. burgdorferi can elicit IL-1β in a caspase 1-dependent fashion from mouse macrophages in vitro, the caspase 1-dependent inflammasome is not essential for the ultimate control of B. burgdorferi infection and disease.
MATERIALS AND METHODS
Mice.
C57BL/6J caspase 1-deficient (caspase 1−/−) mice and ASC-deficient (ASC−/−) mice were kind gifts from Richard Flavell and Erol Fikrig, respectively (Yale University School of Medicine). C57BL/6J MyD88−/− mice were a kind gift from Joseph Craft (Yale University School of Medicine). Age- and sex-matched C57BL/6J WT mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used as controls. Mice were housed in microisolator cages and provided with autoclaved food, water, and bedding to reduce opportunistic infections, according to the Yale University institutional animal care and use guidelines.
Spirochetes.
Low-passage B. burgdorferi strain N40 spirochetes were expanded in modified Barbour-Stoenner-Kelly II medium and enumerated with a Petroff-Hausser counting chamber by dark-field microscopy before inoculation into mice. Mice were inoculated intradermally in the shoulder region with 104 cloned N40 spirochetes in 100 μl of Barbour-Stoenner-Kelly II medium.
Macrophage uptake of spirochetes.
Resident peritoneal macrophages were isolated from caspase 1−/− and WT mice as previously described (25). Bone marrow-derived macrophages were produced following the protocol of Mark Wooten (University of Toledo) (11, 24) by culturing bone marrow cells in RPMI medium supplemented with L929 conditioned supernatants for 6 days. At the end of the culture period, the adherent bone marrow-derived macrophages were harvested and plated onto coverslips; isolated peritoneal macrophages were similarly applied to coverslips. After overnight incubation, nonadherent cells were rinsed away and B. burgdorferi spirochetes were added at a 10:1 ratio of spirochetes to macrophages. Coverslips were incubated on ice for 5 min to allow for B. burgdorferi attachment and then were warmed to 37°C for 10 min to allow phagocytosis to proceed. Extracellular spirochetes were eliminated by washes with water. Cultures were incubated at 37°C with chase times of 0, 30, 60, 120, and 180 min. After methanol fixation, cells were labeled with a polyclonal rabbit anti-B. burgdorferi antiserum (a kind gift from Fred Kantor, Yale University) and a rat anti-mouse LAMP-1 (lysosome-associated membrane protein 1) antibody (Development Studies Hybridoma Bank, Iowa City, IA) and were visualized with tetramethyl rhodamine isocyanate-labeled F(ab′)2 goat anti-rabbit immunoglobulin G (IgG) and fluorescein isothiocyanate-labeled F(ab′)2 goat anti-rat IgG (Biosource, Camarillo, CA), respectively. Cells were examined with a Zeiss LSM 510 confocal imaging system mounted on a Zeiss Axiovert 10 microscope, using multitracking to prevent bleeding between fluorescence channels. For each time point, 100 cells associated with spirochetes were examined, and the number of cells with elongated spirochetes, a measure of spirochetes recently ingested but not yet degraded, was recorded.
Measurement of cytokines.
Cytokine production by macrophages was measured as previously described (26). Briefly, peritoneal and bone marrow-derived macrophages from uninfected WT and caspase 1−/− mice were plated into 24-well plates and allowed to adhere overnight. The wells were rinsed, and the remaining adherent cells were stimulated with the indicated amounts of B. burgdorferi lysate (peritoneal and bone marrow-derived macrophages) or live spirochetes (peritoneal macrophages only) in culture medium containing polymyxin B (10 ng/ml) for 16 h, after which supernatants were removed for subsequent measurement of cytokines produced in the absence of ATP. Fresh medium was then added containing 5 mM ATP and 10 mM HEPES for 20 min, after which time cells were washed and incubated in medium for an additional 3 h (46). The culture supernatants were removed from the ATP-stimulated cells, and the amount of IL-1β produced was quantified. We used the Ready-Set-Go! IL-1β enzyme-linked immunosorbent assay (ELISA) kit (eBiosciences, San Diego, CA) to quantify IL-1β. IL-12p40 was quantified in 16-h culture supernatants using the Ready-Set-Go! IL-12p40 ELISA kit.
On day 14 postinfection, the CD4+ T-cell populations were enriched from mouse splenocytes by negative selection using biotinylated anti-mouse CD8, B220, and Mac1 monoclonal antibodies (Pharmingen) and antibiotin antibody-coated magnetic microbeads according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). CD4+ T cells were resuspended in Click's medium supplemented with 10% heat-inactivated fetal bovine serum, 5 × 10−5 M 2-mercaptoethanol, 100 U of penicillin/ml, and 100 μg of streptomycin/ml and divided into triplicate aliquots at 2 × 106 cells/well in 24-well plates. T cells were stimulated with titrating amounts of B. burgdorferi lysate in the presence of 5 ×106 mitomycin C-treated splenocytes. Culture supernatants were harvested after 72 h of incubation, and cytokines were quantified by ELISA using the Ready-Set-Go! IFN-γ and IL-4 ELISA kits (eBiosciences, San Diego, CA). The concentrations of cytokines were determined in serial twofold dilutions using a standard curve, and the average values were determined for each sample.
Histopathology.
Hearts and bilateral hind limb joints (knee and tibiotarsal joints) were fixed in 10% neutral buffered formalin, the limbs were decalcified (decalcifying solution; Richard Allan Scientific, Kalamazoo, MI), and all tissues were processed, sectioned (5-μm-thick sections), and stained with hematoxylin and eosin by routine histologic techniques performed by Mouse Research Pathology in the Section of Comparative Medicine, Yale School of Medicine. The knee and tibiotarsal joints were scored for the presence and severity of periarticular inflammation and arthritis on a scale of 0 (negative) to 3 (severe) in a blinded fashion as described previously (3). Values for arthritis severity are the mean scores from the most inflamed tibiotarsal joints of individual mice in each group ± standard error of the mean. Carditis was considered present when inflammatory cell infiltrates were present in the heart base in tissue sections.
Measurement of B. burgdorferi-specific antibodies.
B. burgdorferi-specific antibody endpoint positive titers were determined by an ELISA using sera from infected animals. Ninety-six-well microtiter plates were coated with B. burgdorferi lysate (3 μg in 50 μl of 100% ethanol per well) by overnight incubation at 4°C. After the wells were blocked in phosphate-buffered saline containing 5% bovine serum albumin, serial twofold dilutions of sera in phosphate-buffered saline containing 0.5% bovine serum albumin and 0.5% Tween 20 were added to the wells and incubated for 1 h at room temperature. Secondary biotinylated anti-mouse IgM and IgG (Vector Labs, Burlingame, CA), anti-mouse IgG1 (Zymed, San Francisco, CA), and anti-mouse IgG3 (Southern Biotech Associates, Birmingham, AL) were used at a 1:1,000 dilution, and bound antibodies were detected with the ABC Elite peroxidase detection and 2,2′-azinobis(3-ethylbenthiazolinesulfonic acid) (ABTS) substrate kits (Vector Labs).
Quantitative PCR of B. burgdorferi DNA.
DNA was extracted from the urinary bladders of infected mice using the DNeasy tissue kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The copy number of B. burgdorferi in each sample was determined by quantitative PCR of the B. burgdorferi recA gene using an iCycler (Bio-Rad, Hercules, CA), the Brilliant SYBR green kit (Stratagene, Cedar Creek, TX), and the following primers: 5′ primer, 5′-GTGGATCTATTGTATTAGATGAGGCTCTCG-3′; 3′ primer, 5′-GCCAAAGTTCTGCAACATTAACACCTAAAG3′. The recA copy number was normalized to the mouse β-actin gene amplified using the following primers and probe: 5′ primer, 5′-ATCAGGTAGTCGGTCAGG-3′; 3′ primer, 5′-GGTATCTATCTCGACTC-3′; and probe, 6FAM-TCCAGCAGATCTGGATCAGCAAGCA-TAMRA (6FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine) (Applied Biosystems, Foster City, CA). A 60°C annealing temperature and 45 or 50 cycles were used for the recA and β-actin reactions, respectively. Standard curves were generated for both PCRs using known quantities of DNA. Reactions were performed in duplicate, and the quantities of PCR products generated were determined from the standard curves.
Statistical analysis.
The Mann-Whitney test was used to determine statistical significance in all assays except when analyzing the prevalence of disease or spirochetemia, when the Fisher's exact test was used. Arthritis severity was analyzed using the Student t test.
RESULTS
Murine macrophages secrete IL-1β in response to stimulation with B. burgdorferi.
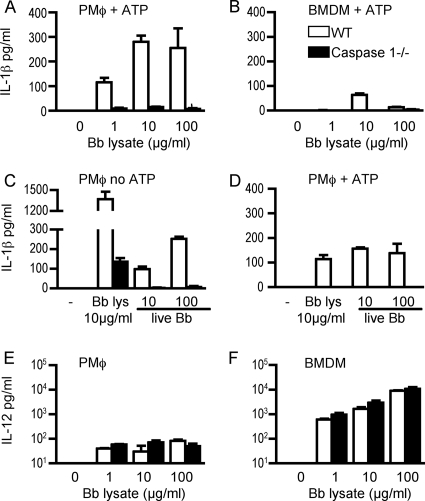
To determine whether B. burgdorferi could elicit IL-1β from murine macrophages, we analyzed the production of this cytokine by WT and caspase 1−/− macrophages derived from the peritoneal cavity and from bone marrow after stimulation with B. burgdorferi lysate. In initial experiments, cells were stimulated for 16 h, after which supernatants were removed and ATP was added, as published reports suggest that it is required for optimal secretion of IL-1β by murine cells in response to bacterial products (10, 39). Both peritoneal and bone marrow-derived macrophages produced IL-1β in a caspase 1-dependent manner (Fig. 1A and B), with larger amounts detected in the supernatants of peritoneal macrophages (Fig. 1A). To assess whether live spirochetes could also elicit IL-1β secretion, supernatants from peritoneal macrophages stimulated with B. burgdorferi lysate or live spirochetes were examined for IL-1β production (Fig. 1C and D). Both stimuli elicited IL-1β in a caspase 1-dependent manner and in the absence of exogenous ATP (Fig. 1C). In addition, a small amount of IL-1β was produced by caspase 1−/− cells in response to B. burgdorferi lysate. The amount of IL-1β was far greater when cells were stimulated with B. burgdorferi lysate than when cells were stimulated with live spirochetes at a 100:1 spirochete-to-macrophage ratio, the highest ratio used. When ATP was added to these cell cultures, additional IL-1β was detected in supernatants harvested 3 h later from WT cells but not caspase 1−/− cells (Fig. 1D). B. burgdorferi-stimulated macrophages also secrete IL-12, but equivalent amounts of this cytokine were produced by both WT and caspase 1−/− macrophages (Fig. 1E and F). We observed that bone marrow-derived macrophages secrete much higher levels of IL-12 (Fig. 1F) but lower levels of IL-1β (Fig. 1B) compared to peritoneal macrophages (Fig. 1E and A).
FIG. 1.
Murine macrophages produce IL-1β and IL-12 in response to stimulation with B. burgdorferi. Bone marrow-derived macrophages (BMDM) and peritoneal macrophages (PMφ) were plated overnight and stimulated for 16 h with B. burgdorferi lysate (Bb lysate) or viable spirochetes (PMφ only). In panels A and B, supernatants from cells stimulated for 16 h were removed for later measurement of IL-12, then ATP was added for 20 min, and cells were incubated for an additional 3 h before measurement of IL-1β. In panel C, IL-1β production was measured in culture supernatants of PMφ stimulated for 16 h with B. burgdorferi lysate or with live spirochetes at 10:1 and 100:1 spirochete-to-macrophage ratios before the addition of ATP. −, no B. burgdorferi lysate or live spirochetes. Panel D shows results after ATP stimulation as performed in panels A and B. Panels E and F show the IL-12 levels in culture supernatants from cells stimulated for 16 h in the same experiment as those in panels A and B. IL-1β and IL-12 were measured by cytokine-specific ELISAs as described in Materials and Methods. The results are reported as the means of triplicate samples plus standard errors of the means (error bars).
The absence of caspase 1 does not alter the severity of disease in B. burgdorferi-infected mice.
We examined whether caspase 1 was required in vivo for the development of inflammation by examining the evolution of arthritis (including tenosynovitis) and carditis, the two most prominent manifestations of Lyme borreliosis in the mouse. The WT mice had minimal arthritis at days 9 and 14, consistent with the known resistance of the C57BL/6 mouse strain, but the prevalence of affected joints tended to be higher in the caspase 1−/− mice (Table 1) . Although there was a statistically significant difference in arthritis severity in caspase 1−/− mice in comparison to WT mice at day 14, the overall level of inflammation was low and no joint in either group had a score of >1.0. At day 45, the arthritis incidence and severity were similar in WT and caspase 1−/− mice (Table 1). The prevalence of carditis was similar in the two groups (Table 1). Culture of blood from infected mice showed the presence of spirochetes in a subset of cultures from the caspase 1−/− mice, but not from WT mice at any of the time points tested (Table 1).
TABLE 1.
B. burgdorferi-infected caspase 1−/− mice develop mild disease
| Day postinfection | Prevalence of arthritis (no. of joints/20 joints)a
|
Severity of arthritise
|
No. of mice with active carditisf
|
No. of mice with positive blood culturesg
|
||||
|---|---|---|---|---|---|---|---|---|
| WT mice | Caspase 1−/− mice | WT mice | Caspase 1−/− mice | WT mice (n = 5) | Caspase 1−/− mice (n = 5) | WT mice (n = 5) | Caspase 1−/− mice (n = 5) | |
| 9 | 4 | 9b | 0.45 ± 0.18 | 0.55 ± 0.18 | 0 | 0 | 0 | 1h |
| 14 | 2 | 6c | 0.05 ± 0.03 | 0.23 ± 0.08 | 3 | 4 | 0 | 2i |
| 45 | 12 | 10d | 1.0 ± 0.7 | 0.4 ± 0.4 | 0 | 0 | ND | ND |
The prevalence of arthritis prevalence was reported as the number of joints with acute inflammation. The total number of joints examined in each group was 20. Differences between the values for wild-type and caspase 1−/− mice were not statistically significant.
Not statistically significantly different (P = 0.18) from the value for WT mice by Fisher's exact test.
Not statistically significantly different (P = 0.24) from the value for WT mice by Fisher's exact test.
Not statistically significantly different (P = 0.75) from the value for WT mice by Fisher's exact test.
Arthritis severity is based on the degree of leukocyte infiltration on a scale of 0 to 3. The data represent the means of scores ± standard errors of the means for the most severely affected joint. There was no statistical difference in the severity of arthritis in the mouse groups at days 9 and 45 (P = 0.41 and 0.1, respectively, by Student t test). At day 14, differences in arthritis were significant (P = 0.0015).
Carditis is reported as the number of mice with acute inflammation at the base of the heart. There were five mice in each group. There was no significant difference between the values for WT and caspase 1−/− mice (day 14, P = 1.0, Fisher's exact test).
The presence of spirochetes in blood was determined by dark-field microscopy after 14 days of culture in Barbour-Stoenner-Kelly II medium at 33°C. There were five mice in each group. Differences between the values for WT and caspase 1−/− mice were not statistically significant. ND, not determined.
Not statistically significantly different (P = 1.0) from the value for WT mice by Fisher's exact test.
Not statistically significantly different (P = 0.444) from the value for WT mice by Fisher's exact test.
B. burgdorferi-infected caspase 1−/− mice have a transient elevation in pathogen burden early in infection.
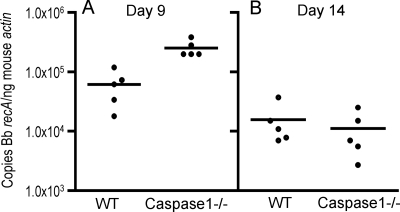
We have shown that absence of MyD88 resulted in higher B. burgdorferi burdens in mice, and we sought to determine whether caspase 1 was important for pathogen clearance. As we observed that a subset of caspase 1−/− mice had detectable spirochetemia within the first 2 weeks of infection, we chose to measure pathogen burden at infection days 9 and 14 to determine whether caspase 1 deficiency led to an early defect in pathogen control. We quantified the B. burgdorferi DNA in mouse urinary bladders, a nondiseased site reflective of disseminated infection in which the pathogen burden is less likely to be influenced by local inflammation. After 9 days of infection, caspase 1−/− mice had a statistically significant elevation in pathogen burden compared to WT mice (Fig. 2A), but no difference was seen at this site at day 14 (Fig. 2B).
FIG. 2.
Caspase 1-deficient mice have transiently elevated pathogen burdens early after infection with B. burgdorferi. The pathogen burdens in the urinary bladders of individual mice infected for 9 days (A) or 14 days (B) were determined by quantitative PCR as described in Materials and Methods. The copy number of the B. burgdorferi (Bb) recA gene was normalized to 1 ng of the mouse β-actin gene product. These results are representative of three separate experiments performed with four or five mice per group in each experiment. The pathogen burden calculated for each mouse is the average of duplicates from two separate assays. The differences between the WT and caspase 1−/− pathogen burdens at day 9 were significant (P = 0.0016, Mann-Whitney test).
Macrophages ingest and degrade B. burgdorferi in the absence of caspase 1.
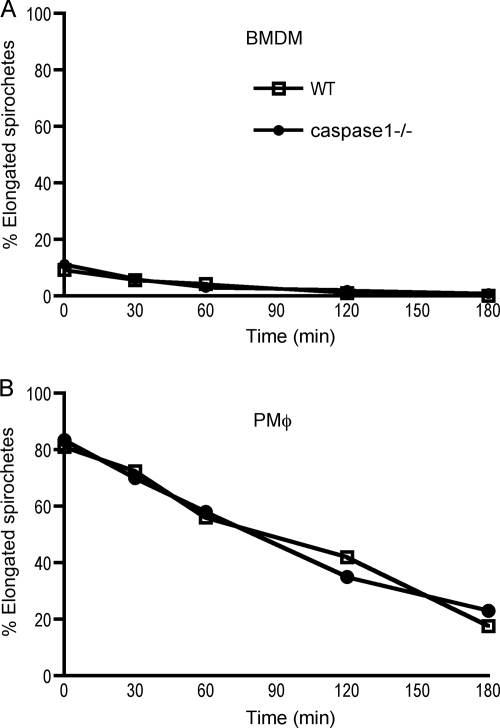
The elevated pathogen burden assessed by DNA quantification that we had previously observed in MyD88 mice was partially due to the impaired ability of macrophages to degrade spirochetes after internalization (25). A subsequent study reported that bone marrow-derived MyD88−/− macrophages could not ingest B. burgdorferi, suggesting that the competency of macrophages to eliminate spirochetes may depend on their site of derivation. As has previously been reported (44), bone marrow-derived macrophages from WT mice were much less efficient at ingesting spirochetes, with only ∼10% of cells having internalized spirochetes after the 10-min pulse (Fig. 3A), and no effect of caspase 1 deficiency on uptake could be detected. In contrast, the majority of peritoneal macrophages from both WT and caspase 1−/− mice had bound and internalized spirochetes within the first 10 min of exposure (Fig. 3B), and there was no difference in the rate at which spirochetes colocalized with LAMP-1 in the lysosomal compartment (data not shown). Internalized spirochetes were degraded at the same rate in both WT and caspase 1−/− macrophages, independent of the site of derivation, as evidenced by the decline in number of whole elongated spirochetes observed in cells over time.
FIG. 3.
Macrophage uptake and degradation of B. burgdorferi are not dependent on caspase 1. Adherent bone marrow-derived macrophages (BMDM) (A) and peritoneal macrophages (PMφ) (B) were exposed to spirochetes at a ratio of 10:1 for 10 min, and then extracellular spirochetes were eliminated by water washes. Cells were allowed to process ingested spirochetes for 0, 30, 60, 120, and 180 min as described in Materials and Methods. Cells were labeled for spirochetes and the lysosomal marker LAMP-1. The numbers of elongated spirochetes, representing ingested but not yet degraded organisms, were counted at each time point, and the percentages were plotted as a function of time. The results are representative of two separate experiments.
The humoral response to B. burgdorferi is similar in WT and caspase 1−/− mice.
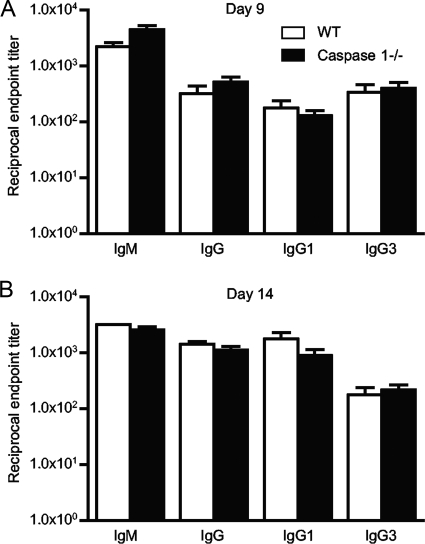
It is well established that antibody plays a crucial role in controlling B. burgdorferi infection and disease, so we examined B. burgdorferi-specific antibody titers at 9, 14, and 45 days after infection to determine whether the higher pathogen burdens at day 9 resulted from defects in humoral immunity. We did not find any significant differences in the anti-B. burgdorferi IgM and IgG titers or alterations in the IgG subtypes produced in caspase 1-deficient mice at any of the time points tested (Fig. 4 and data not shown).
FIG. 4.
Caspase 1−/− and WT mice generate similar antibody titers during B. burgdorferi infection. Antibody titers are the reciprocal endpoint positive titers of B. burgdorferi-specific antibody in serum obtained from mice infected for 9 days (A) and 14 days (B). The titers were measured by ELISAs as described in Materials and Methods. Results are representative of three experiments with five mice per group.
Th cell cytokine production in response to B. burgdorferi is not altered in the absence of caspase 1.
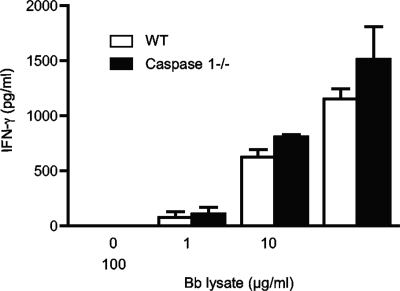
Our previous data showed a Th2 polarization of antibody and cytokine responses in B. burgdorferi-infected MyD88-deficient mice (25). As IL-1 and IL-18 are known to promote Th1 polarization, a decrease in Th1 cell cytokine production might occur in the caspase 1−/− mice in response to B. burgdorferi infection. When analyzed ex vivo, however, CD4+ T cells from infected caspase 1−/− mice secreted equivalent levels of IFN-γ compared to WT T cells after in vitro restimulation with B. burgdorferi lysate (Fig. 5). T cells from both strains secreted only very low levels of IL-4 (data not shown).
FIG. 5.
CD4+ T cells from infected caspase 1−/− mice secrete IFN-γ in response to B. burgdorferi lysate stimulation in vitro. CD4+ T cells enriched from splenocytes from WT and caspase 1−/− mice were incubated with mitomycin C-treated antigen-presenting cells and stimulated with B. burgdorferi lysate (Bb lysate). After 72 h, supernatants were collected, and the amount of IFN-γ was analyzed by ELISA. The results are reported as the means of triplicate samples plus standard errors of the means (error bars) and are representative of three separate experiments.
ASC-deficient mice do not display defects in controlling B. burgdorferi infection.
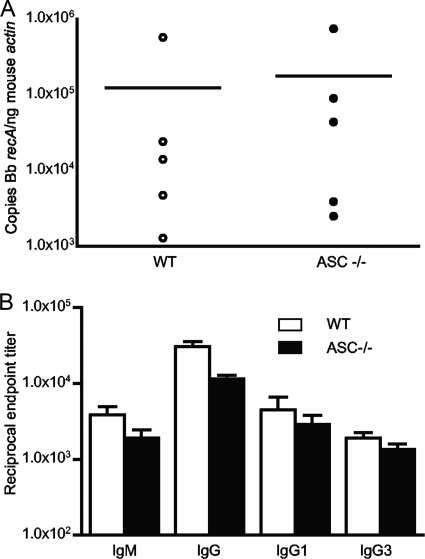
To confirm our results in the caspase 1−/− mice, we infected ASC−/− mice. These mice fail to elicit the enzymatic activity of caspase 1 to cleave pro-IL-1β and pro-IL-18, but caspase 1 retains its nonenzymatic activity, including activation of NK-κB (18, 34, 40, 47). We examined the pathogen burden, antibody responses, T-cell production of IFN-γ, and disease in ASC−/− mice 14 days after B. burgdorferi infection. The results showed that ASC−/− mice exhibit no impairment in pathogen control (Fig. 6A), production of pathogen-specific IgM and IgG subtypes (Fig. 6B), or T-cell production of IFN-γ (data not shown). ASC−/− mice also develop similar levels of disease to WT mice, which was minimal (data not shown).
FIG. 6.
ASC-deficient mice control pathogen burden and generate normal antibody responses 14 days after B. burgdorferi infection. The pathogen burdens in urinary bladders (A) were determined as described in the legend to Fig. 2, and the antibody titers (B) were determined as described in the legend to Fig. 4.
DISCUSSION
In these studies, we examined the role of the caspase 1-dependent inflammasome in murine host defense against B. burgdorferi infection. Using mice selectively deficient in either ASC, a component of the inflammasome required for caspase 1 activation, or in caspase 1 itself, we found that the inflammasome does not contribute significantly to host defense against B. burgdorferi infection in mice. Published reports have demonstrated that the absence of ASC prevents the activation of the enzymatic activity of caspase 1, thus inhibiting the production of mature IL-1β and IL-18. Our studies, therefore, demonstrate that caspase 1-dependent IL-1β and IL-18 production does not play a significant role in immune responses that help control the pathogen burden, the generation of B. burgdorferi-specific antibody responses, or the development of inflammation in the heart and joints. It is known, however, that ASC does not play a role in all caspase 1-dependent pathways, and caspase 1-deficient mice have defects in immunity in addition to the deficiencies in IL-1β and IL-18 production (23, 41).
We found that caspase 1 may play a modest role in pathogen control early after infection, but the effect on pathogen burden was transient and not comparable in magnitude to that seen in the MyD88−/− mice. We conclude that the heightened pathogen burdens in MyD88−/− mice and the alterations in the B. burgdorferi-specific antibody responses result from the absence of TLR signaling and not from the absence of IL-1/18 receptor signaling. Our results suggest that the inflammasome containing the ASC and caspase 1 proteins does not play as significant a role during B. burgdorferi infection as in infection with other pathogens. The transient perturbations that we observed in the absence of caspase 1, therefore, are most likely independent of the activation of IL-1β and may instead involve other caspase 1 functions, such as the activation of NF-κB (41).
Tsuji et al. (48) demonstrated that dendritic cells or unfractionated splenocytes from caspase 1−/− mice produced smaller amounts of IFN-γ at day 14 after infection with Listeria, an intracellular bacterium. Decreased IFN-γ mRNA levels were also detected in the caspase 1−/− cells (29). Other reports demonstrate that caspase 1 activation is required for the inflammation and disease caused by Shigella flexneri (38), Francisella tularensis (27), and Mycobacterium tuberculosis (32). In contrast to these reports with intracellular pathogens, we found that CD4+ T cells from B. burgdorferi-infected caspase 1−/− mice are capable of producing IFN-γ at levels equivalent to those from infected WT mice. These results are in accordance with a published study showing that caspase 1 deficiency does not impair CD4+ T-cell production of IFN-γ in response to infection of mice with herpes simplex virus type 2 (42), indicating that this finding is not unique to extracellular organisms.
A role for IL-1β has been suggested in Lyme borreliosis because IL-1β mRNA can be detected in erythema migrans lesions of patients with Lyme disease, and elevated levels of the cytokine can be found in their sera (31, 35). In addition, human peripheral blood mononuclear cells secrete IL-1β after ingestion of live B. burgdorferi spirochetes (15). We also found that murine peritoneal macrophages from WT but not caspase 1−/− mice secreted IL-1β in vitro in response to live B. burgdorferi, consistent with the known requirement for caspase 1 in cleavage of pro-IL-1β to produce its active form. In contrast to what has been reported for human monocytes where bacterial lysates induced only trace amounts of IL-1β (15), we found that both mouse bone marrow-derived and peritoneal macrophages are capable of producing IL-1β in a dose-dependent fashion. In the case of peritoneal macrophages stimulated with either B. burgdorferi lysate or with live spirochetes, the release of IL-1β into culture supernatants was not dependent on exogenous ATP. Our study shows that the majority of IL-1β was released during the first 16 h of culture and that ATP allowed for only modest amounts of IL-1β to be secreted thereafter. The fact that B. burgdorferi components can induce IL-1β in the absence of ATP suggests that ingestion of viable spirochetes is not required for the release of mature IL-1β from mouse peritoneal macrophages.
Our in vivo results showing minimal effect of caspase 1 and ASC deficiency on the course of murine Lyme borreliosis raise questions regarding the significance of in vitro production of IL-1β, especially because the amount elicited by viable spirochetes, even at a 100:1 spirochete-to-macrophage ratio, was quite low. Given the paucity of B. burgdorferi normally found in immunocompetent mouse tissues, it is doubtful that macrophages would ever encounter B. burgdorferi in such large numbers. This may explain why in vivo, caspase 1 deficiency had minimal effect on B. burgdorferi infection and disease. An alternative explanation, however, is that active infection provides alternative pathways for processing of pro-IL-1β to its active form (17). Another enzyme associated with processing both IL-1β and IL-18 precursors is proteinase-3 (13, 45). Pro-IL-1 can be also cleaved by cathepsin G, chymotrypsin, elastase, a mast cell chymase, different matrix metalloproteinases, and granzyme A, which can be found at the sites of neutrophil, lymphocyte, and macrophage infiltration to yield active IL-1β (20). More recently, a human mast cell chymase was shown to cleave pro-IL-18 and generate a novel and biologically active IL-18 fragment (33). Our study revealed that B. burgdorferi lysates could elicit a small amount of IL-1β from caspase 1−/− peritoneal macrophages, suggesting the existence of a caspase 1-independent pathway. If spirochete components have the ability to induce the production of IL-1β in the absence of caspase 1, this may, in part, explain why caspase 1 deficiency did not diminish IFN-γ production as it does in other bacterial infections. We did not detect IL-1β in the sera of WT or caspase 1−/− mice infected for 14 days (data not shown). If IL-1β is produced during murine infection, it is not made in sufficient amounts to be detected in the serum and may be found locally only at sites of inflammation.
In summary, our results show that although B. burgdorferi can induce murine macrophages to produce IL-1β in vitro in a caspase 1-dependent fashion, this response is not dependent on viable spirochetes. In this regard, B. burgdorferi distinguishes itself from another extracellular pathogen, Pseudomonas aeruginosa, that engages the inflammasome by releasing its components into the cytosol through a type III secretion system (30). In vivo, caspase 1 deficiency led to only a modest and transient impairment in pathogen control early in infection, which did not significantly impact the development of adaptive immunity or disease. The mechanisms by which B. burgdorferi components engage the inflammasome remain to be explored and may be applicable to other extracellular pathogens.
Acknowledgments
We thank Deborah Beck and Jialing Mao for their excellent technical assistance.
This work was supported by NIH R01AI59529 and the Jockers Award to L.K.B.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 1 June 2009.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9143-150. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R.-B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285736-739. [DOI] [PubMed] [Google Scholar]
- 3.Barthold, S., D. S. Beck, G. M. Hansen, G. A. Terwillinger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162133-138. [DOI] [PubMed] [Google Scholar]
- 4.Barthold, S. W., M. de Souza, E. Fikrig, and D. H. Persing. 1992. Lyme borreliosis in the laboratory mouse, p. 223-242. In S. E. Schutzer (ed.), Lyme disease: molecular and immunologic approaches. Cold Spring Harbor Press, Cold Spring Harbor, NY.
- 5.Barthold, S. W., C. L. Sidman, and A. L. Smith. 1992. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47605-613. [DOI] [PubMed] [Google Scholar]
- 6.Behera, A. K., E. Hildebrand, R. T. Bronson, G. Perides, S. Uematsu, S. Akira, and L. T. Hu. 2006. MyD88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin-18-independent mice infected with Borrelia burgdorferi. Infect. Immun. 741462-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bockenstedt, L. K. 2009. Lyme disease, p. 1715-1727. In G. S. Firestein, R. C. Budd, E. D. Harris, Jr., I. B. McInnes, S. Ruddy, and J. S. Sergent (ed.), Kelley's textbook of rheumatology, 8th ed., vol. II. Saunders Elsevier, Philadelphia, PA. [Google Scholar]
- 8.Bockenstedt, L. K., I. Kang, C. Chang, D. Persing, A. Hayday, and S. W. Barthold. 2001. CD4+ T helper 1 cells facilitate regression of murine Lyme carditis. Infect. Immun. 695264-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, C. J. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 1732003-2010. [DOI] [PubMed] [Google Scholar]
- 10.Brough, D., and N. J. Rothwell. 2007. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J. Cell Sci. 120772-781. [DOI] [PubMed] [Google Scholar]
- 11.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 675142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirl, C., A. Wieser, M. Yadav, S. Duerr, S. Schubert, H. Fischer, D. Stappert, N. Wantia, N. Rodriguez, H. Wagner, C. Svanborg, and T. Miethke. 2008. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat. Med. 14399-406. [DOI] [PubMed] [Google Scholar]
- 13.Coeshott, C., C. Ohnemus, A. Pilyavskaya, S. Ross, M. Wieczorek, H. Kroona, A. H. Leimer, and J. Cheronis. 1999. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc. Natl. Acad. Sci. USA 966261-6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creagh, E. M., and L. A. J. O'Neill. 2006. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 27352-357. [DOI] [PubMed] [Google Scholar]
- 15.Cruz, A. R., M. W. Moore, C. J. LaVake, C. H. Eggers, J. C. Salazar, and J. D. Radolf. 2008. Phagocytosis of Borrelia burgdorferi, the Lyme disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes. Infect. Immun. 7656-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinarello, C. A., and G. Fantuzzi. 2003. Interleukin-18 and host defense against infection. J. Infect. Dis. 187(Suppl. 2)S370-S384. [DOI] [PubMed] [Google Scholar]
- 17.Fantuzzi, G., and C. A. Dinarello. 1999. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J. Clin. Immunol. 191-11. [DOI] [PubMed] [Google Scholar]
- 18.Franciotta, D., G. Martino, E. Zardini, R. Furlan, R. Bergamaschi, M. Gironi, A. Bergami, G. Angelini, F. De Benedetti, P. Pignatti, G. Moscato, and V. Cosi. 2002. Caspase-1 levels in biological fluids from patients with multiple sclerosis and from patients with other neurological and non-neurological diseases. Eur. Cytokine Netw. 1399-103. [PubMed] [Google Scholar]
- 19.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 1632382-2386. [PubMed] [Google Scholar]
- 20.Irmler, M., S. Hertig, H. R. MacDonald, R. Sadoul, J. D. Becherer, A. Proudfoot, R. Solari, and J. Tschopp. 1995. Granzyme A is an interleukin 1 beta-converting enzyme. J. Exp. Med. 1811917-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, U., C. S. Gunther, and R. G. Roeder. 2000. Genetic analyses of NFKB1 and OCA-B function: defects in B cells, serum IgM level, and antibody responses in Nfκb12/2 Oca-b2/2 mice. J. Immunol. 1656825-6832. [DOI] [PubMed] [Google Scholar]
- 22.Kumar, S. 2007. Caspases and their many biological functions. Cell Death Differ. 141-2. [Google Scholar]
- 23.Lamkanfi, M., M. Kalai, M. Saelens, W. Declercq, and P. Vandenabeele. 2004. Caspase-1 activates nuclear factor of the κ-enhancer in B cells independently of its enzymatic activity. J. Biol. Chem. 27924785-24793. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus, J. J., M. A. Kay, A. L. McCarter, and R. M. Wooten. 2008. Viable Borrelia burgdorferi enhances interleukin-10 production and suppresses activation of murine macrophages. Infect. Immun. 761153-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 723195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariathasan, S., K. Newton, D. M. Monack, D. Vucic, D. M. French, W. P. Lee, M. Roose-Girma, S. Erickson, and V. M. Dixit. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430213-218. [DOI] [PubMed] [Google Scholar]
- 27.Mariathasan, S., D. S. Weiss, V. M. Dixit, and D. M. Monack. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 2021043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKisic, M. D., W. L. Redmond, and S. W. Barthold. 2000. T cell-mediated pathology in murine Lyme borreliosis. J. Immunol. 1656096-6099. [DOI] [PubMed] [Google Scholar]
- 29.Mencacci, A., A. Bacci, E. Cenci, C. Montagnoli, S. Fiorucci, A. Casagrande, R. A. Flavell, F. Bistoni, and L. Romani. 2000. Interleukin 18 restores defective Th1 immunity to Candida albicans in caspase 1-deficient mice. Infect. Immun. 685126-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao, E. A., R. K. Ernst, M. Dors, D. P. Mao, and A. Aderem. 2008. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc. Natl. Acad. Sci. USA 1052562-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullegger, R. R., G. McHugh, R. Ruthazer, B. Binder, H. Kerl, and A. C. Steere. 2000. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J. Investig. Dermatol. 1151115-1123. [DOI] [PubMed] [Google Scholar]
- 32.Netea, M. G., T. Azam, E. C. Lewis, L. A. B. Joosten, M. Wang, D. Langenberg, X. Meng, E. D. Chan, D.-Y. Yoon, T. Ottenhoff, S.-H. Kim, and C. A. Dinarello. 2006. Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18 interferon-gamma-dependent mechanism. PLoS Med. 31310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omoto, Y., K. Tokime, K. Yamanaka, K. Habe, T. Morioka, I. Kurokawa, H. Tsutsui, K. Yamanishi, K. Nakanishi, and H. Mizutani. 2006. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J. Immunol. 1778315-8319. [DOI] [PubMed] [Google Scholar]
- 34.Ozoren, N., J. Maumoto, L. Franchi, T.-D. Kanneganti, M. Body-Malapel, I. Erturk, R. Jagirdar, L. Zhu, N. Inohara, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Distinct roles of TLR2 and the adaptor ASC in IL-1/IL-18 secretion in response to Listeria monocytogenes. J. Immunol. 1764337-4342. [DOI] [PubMed] [Google Scholar]
- 35.Pietruczuk, A., R. Swierzbinska, S. Pancewicz, M. Pietruczuk, and T. Hermanowska-Szpakowicz. 2006. Serum levels of interleukin-18 (IL-18), interleukin-1beta (IL-1beta), its soluble receptor sIL-1RII and C-reactive protein (CRP) in patients with Lyme arthritis. Infection 34158-162. [DOI] [PubMed] [Google Scholar]
- 36.Ruderman, E. M., J. S. Kerr, S. R. Telford III, A. Spielman, L. H. Glimcher, and E. M. Gravallese. 1995. Early murine Lyme carditis has a macrophage predominance and is independent of major histocompatibility complex class II-CD4+ T cell interactions. J. Infect. Dis. 171362-370. [DOI] [PubMed] [Google Scholar]
- 37.Saleh, M., and D. R. Green. 2007. Caspase-1 inflammasomes: choosing between death and taxis. Cell Death Differ. 141559-1560. [DOI] [PubMed] [Google Scholar]
- 38.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12581-590. [DOI] [PubMed] [Google Scholar]
- 39.Sanz, J. M., and F. Di Virgilio. 2000. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. J. Immunol. 1644893-4898. [DOI] [PubMed] [Google Scholar]
- 40.Sarkar, A., M. Duncan, J. Hart, E. Hertlein, D. C. Guttridge, and M. D. Wewers. 2006. ASC directs NF-kB activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J. Immunol. 1764979-4986. [DOI] [PubMed] [Google Scholar]
- 41.Sarkar, A., M. W. Hall, M. Exline, J. Hart, N. Knatz, N. T. Gatson, and M. W. Wewers. 2006. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1β and interleukin-18. Am. J. Respir. Crit. Care Med. 1741003-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato, A., and A. Iwasaki. 2004. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc. Natl. Acad. Sci. USA 10116274-16279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaible, U. E., S. Gay, C. Museteanu, M. D. Kramer, G. Zimmer, K. Eichmann, U. Museteanu, and M. M. Simon. 1990. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am. J. Pathol. 137811-820. [PMC free article] [PubMed] [Google Scholar]
- 44.Shin, I. S., R. R. Isberg, S. Akira, S. Uematsu, A. K. Behera, and L. T. Hu. 2008. Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction. Infect. Immun. 762341-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugawara, S., A. Uehara, T. Nochi, T. Yamaguchi, H. Ueda, A. Sugiyama, K. Hanzawa, K. Kumagai, H. Okamura, and H. Takada. 2001. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J. Immunol. 1676568-6575. [DOI] [PubMed] [Google Scholar]
- 46.Sutterwala, F. S., Y. Ogura, D. S. Zamboni, C. R. Roy, and R. A. Flavell. 2006. NALP3: a key player in caspase-1 activation. J. Endotoxin Res. 12251-256. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki, T., L. Franchi, C. Toma, H. Ashida, M. Ogawa, Y. Yoshikawa, H. Mimuro, N. Inohara, C. Sasakawa, and G. Nunez. 2007. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathogens 31082-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuji, N. M., H. Tsutsui, E. Seki, K. Kuida, H. Okamura, K. Nakanishi, and R. A. Flavell. 2004. Roles of caspase-1 in Listeria infection in mice. Int. Immunol. 16335-343. [DOI] [PubMed] [Google Scholar]
- 49.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168348-355. [DOI] [PubMed] [Google Scholar]