Abstract
Pseudomonas aeruginosa can invade corneal epithelial cells and translocates multilayered corneal epithelia in vitro, but it does not penetrate the intact corneal epithelium in vivo. In healthy corneas, the epithelium is separated from the underlying stroma by a basement membrane containing extracellular matrix proteins and pores smaller than bacteria. Here we used in vivo and in vitro models to investigate the potential of the basement membrane to defend against P. aeruginosa. Transmission electron microscopy of infected mouse corneas in vivo showed penetration of the stroma by P. aeruginosa only where the basement membrane was visibly disrupted by scratch injury, suggesting that the intact basement membrane prevented penetration. This hypothesis was explored using an in vitro Matrigel Transwell model to mimic the corneal basement membrane. P. aeruginosa translocation of multilayered corneal epithelia grown on Matrigel was ∼100-fold lower than that of cells grown without Matrigel (P < 0.005, t test). Matrigel did not increase transepithelial resistance. Matrigel-grown cells blocked translocation by a P. aeruginosa protease mutant. Without cells, Matrigel also reduced traversal of P. aeruginosa and the protease mutant. Fluorescence microscopy revealed a relative accumulation of bacteria at the superficial epithelium of cells grown on Matrigel at 3 h compared to cells grown on uncoated filters. By 5 h, bacteria accumulated beneath the cells, suggesting direct trapping by the Matrigel. These findings suggest that the basement membrane helps defend the cornea against infection via physical barrier effects and influences on the epithelium and that these roles could be compromised by P. aeruginosa proteases.
Pseudomonas aeruginosa is an important opportunistic pathogen, commonly affecting burn victims, individuals with cystic fibrosis, patients in hospital intensive care units, and contact lens wearers (9-11). In the absence of contact lens wear, the cornea is remarkably resistant to infection, with P. aeruginosa effectively colonizing this tissue only if it is injured or otherwise compromised (41). To initiate clinically significant corneal pathology, P. aeruginosa (and almost all other microbes) must first access the corneal stroma, which is normally protected by a multilayered epithelium and associated basement membrane (1).
The corneal epithelial basement membrane is secreted by the overlying epithelia and is comprised of sheets of extracellular matrix constituents, including type IV collagen, heparan sulfate proteoglycan, and various glycoproteins (laminin, entactin, nidogen, and fibronectin) and growth factors that mediate cellular function (1). Quantitative imaging of the corneal epithelial basal membrane in the rhesus macaque has shown a complex cross-linking of fibers and proteins intermingled with pores ranging from 30 to 400 nm in size (2). As shown for other basement membranes in the body (17, 19, 33), the corneal basement membrane anchors epithelial cells and provides positional information for healing, tissue regeneration, and repair (44). In vitro, Matrigel forms an artificial basement membrane with pores ranging from 26 to 359 nm in size, is composed of laminin, collagen IV, heparan sulfate proteoglycans, entactin, nidogen, and naturally occurring growth factors, and closely resembles the natural corneal basement membrane (2, 26).
It has been shown that P. aeruginosa isolates can translocate MDCK cell monolayers (6, 22) and that translocation and virulence were reduced by mutation of genes encoding multidrug resistance efflux systems (23). Purified elastase and exotoxin A from P. aeruginosa have each been shown to increase the permeability of MDCK cell monolayers (4), and purified elastase increases alveolar permeability in vivo (5). However, the role of the basement membrane in P. aeruginosa translocation has not been studied.
We have previously shown that P. aeruginosa can translocate multilayered corneal epithelia in vitro and that human tear fluid reduced both translocation in vitro and virulence in vivo in murine models of corneal infection (28). While this and other studies have focused on the role of the tear film and corneal epithelium in defense against P. aeruginosa keratitis (14), little attention has been given to the basement membrane. Previous studies have reported that the epithelial basement membranes of other tissues can form a physical barrier to potential pathogens, including human papillomavirus, herpes simplex virus, and Rift Valley fever virus (25, 42, 43, 47). In this study, it was hypothesized that the corneal basement membrane forms a physical barrier to defend against the penetration of P. aeruginosa, since its pores are smaller than the size of bacteria, and that P. aeruginosa proteases can functionally overcome that defense. This hypothesis was examined correlatively using transmission electron microscopy of in vivo-infected mouse corneas and tested directly using a quantitative in vitro Matrigel-based model system to mimic the corneal epithelium and its associated basement membrane.
MATERIALS AND METHODS
Bacterial strains and mutants.
The invasive P. aeruginosa strains 6294, PA01, and an isogenic triple protease mutant of PA01 (lasA lasBA prA) (which lacks LasA, LasB, and alkaline protease) (12) were used. Strains 6294 and PA01 behave similarly with respect to invasion and translocation of corneal epithelial cells in vitro and are equally virulent in murine models of corneal infection (28, 30). Bacteria were cultured on Trypticase soy agar plates at 37°C and then resuspended in SHEM (supplemented hormonal epithelial media) without antibiotics to a concentration of 108 CFU/ml (optical density at 650 nm of 0.1) for use during in vivo experiments. For in vitro assays, the bacterial suspension was subsequently diluted to a final concentration of 106 CFU/ml. Bacteria were enumerated by viable counts after serial dilution to confirm bacterial concentrations.
Murine in vivo infection model and transmission electron microscopy.
Female C57BL/6 mice (5 to 7 weeks old) were infected using the scarification model of corneal infection as described previously (40). Briefly, three full-thickness epithelial abrasions were produced on the left corneas of six mice with a 26-gauge needle after anesthesia. Eyes were immediately inoculated with 5 μl of SHEM inoculum containing strain 6294. After 24 h, mice were euthanized and the left eye enucleated. Eyes were then prepared for transmission electron microscopy. Briefly, whole eyes were fixed in 3% gluteraldehyde in 0.1 M phosphate buffer (pH = 7.2) at room temperature for 3 h and then postfixed in 1% osmium tetroxide in phosphate buffer at room temperature for 2 h. Samples were dehydrated in a graded series of ethanol and embedded in Spurr's resin. Thin sections of the cornea were examined with a Zeiss EM 100A electron microscope set at 60 kV.
Cell culture.
Immortalized rabbit corneal epithelial cells (36) were grown to confluence on 8-μm-pore-size filters that were either uncoated or coated with Matrigel (BD Biosciences, Bedford, MA) and then air-lifted as previously described (27). Briefly, the cells were cultured on the filters for 3 days with both sides of the filter submerged in SHEM and then air-lifted by removing media from the top chamber from day 4 to day 7. Cells were used for experiments on day 7 after passage.
In vitro assay of bacterial traversal.
Corneal epithelial cells were grown on Transwell filters with or without Matrigel-coating (BD Biosciences, San Jose, CA). Bacterial inocula were added only to the apical chamber, such that bacteria could reach the basal chamber only by translocating the cells and the matrix on which they were grown. The use of 8-μm-pore-size filter supports ensured that bacteria could reach the lower chamber if they were able to penetrate the cells and/or Matrigel coating. Matrigel-coated and uncoated filters used without cells were also included as controls. At hourly time points after inoculation, viable bacteria in the upper and lower chambers were enumerated by viable counts. Transepithelial resistance (TER) was also recorded using an Evom meter (World Precision Instruments Inc., Sarasota, FL). Additional samples were included to enumerate bacteria retained by the cells and/or Matrigel at various time points postinfection. For this purpose, Transwell filters were removed from their plastic inserts after one wash with phosphate-buffered saline (PBS) and then treated with 0.25% (vol/vol) Triton X-100 in PBS for 15 min to lyse cells before enumerating them by viable counts.
Fluorescence microscopy.
PA01 and its protease mutant (lasA lasBA prA) were transformed with plasmid pSMC2, constitutively expressing green fluorescent protein (GFP). At 3 h and 5 h postinfection, filters were rinsed once with PBS and fixed for 5 min with 4% (wt/vol) paraformaldehyde in PBS. The filters were then washed three times (for 5 min each) with PBS, excised, and flat-mounted for microscopy. Z-stack analysis using the Volocity software (Improvision Inc., Lexington, MA) and an IX-70 microscope (Olympus, Melville, NY) were used to visualize bacteria expressing GFP relative to the apical cell surface, the underlying filter, and the Matrigel coating between them if present.
Statistical analysis.
Data were expressed as means ± standard deviations. Statistical significance of differences between groups was determined using Student's t test. P values of <0.05 were considered significant. Experiments were repeated at least three times with at least three samples used in each group.
RESULTS
In vivo, P. aeruginosa bacteria were detected in the underlying stroma only where the basement membrane was discontinuous.
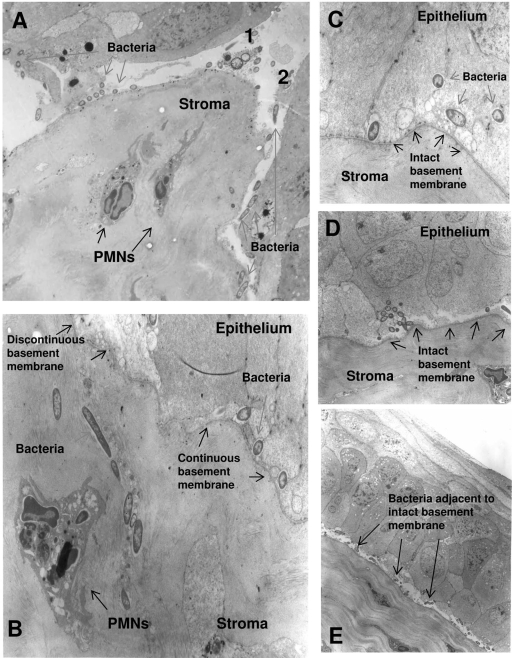
Transmission electron microscopy images of infected corneas showed extensive epithelial and basement membrane disruption where the scratches of the central cornea had been made to enable the initiation of disease (Fig. 1). Areas of basement membrane disruption were characterized by massive stromal swelling, epithelial detachment, and polymorphonuclear (PMN) leukocyte infiltration (Fig. 1A and B). In areas where the basement membrane was discontinuous, bacteria were seen both in the subbasal epithelia and within the stroma (Fig. 1A and B). Regions distant from the scratched area showed intact basement membrane with or without epithelial disruption (Fig. 1C, D, and E). While bacteria were found both inside and between intact epithelial cells (Fig. 1C), most were found adjacent to the upper (epithelial) side of the intact basement membrane (Fig. 1B and E). In these areas where the basement membrane was intact, bacteria were not found in the underlying stroma (Fig. 1C, D, and E).
FIG. 1.
Transmission electron micrograph of scratch-injured and infected mouse cornea after 24 h of incubation with P. aeruginosa invasive strain 6294. (A) Scratched area where the basement membrane had been breached (1, 2) and the stroma infiltrated by PMNs. (B) Section showing discontinuous and intact basement membrane, the former correlating with bacterial and PMN infiltration of the stroma. (C to E) Different sections peripheral to the scratched area showing intact basement membrane and the absence of bacterial infiltration of the stroma.
Matrigel reduced P. aeruginosa traversal of corneal epithelial cells in vitro.
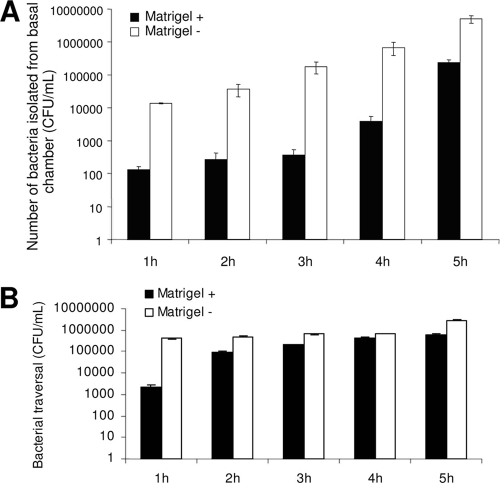
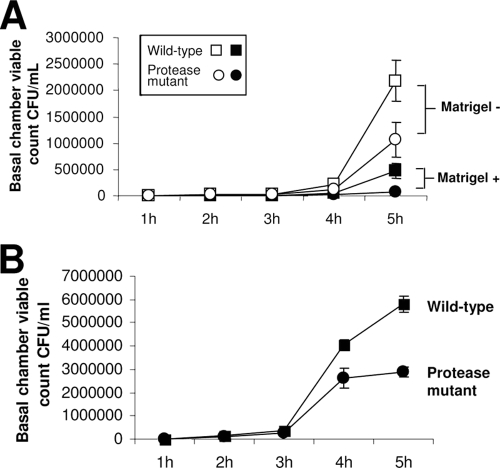
Qualitative evidence from in vivo experiments suggested that the corneal basement membrane could restrict bacterial penetration into the corneal stroma. To test this directly, we developed an in vitro model system of bacterial traversal that enabled quantification of data (see Methods). Artificial basement membrane (Matrigel) was used since it has been shown to resemble corneal basement membrane in vivo both physically and biochemically. Bacterial traversal rates through corneal epithelia grown on Transwell filters were compared for cells grown with or without a Matrigel coating on the filter. The number of bacteria that reached the basal chamber when cells were grown on Matrigel was reduced ∼100-fold at each time point up to 4 h postinoculation and by ∼10-fold at the 5-h time point compared to the number for cells grown without Matrigel (Fig. 2A). Matrigel alone (without cells) also significantly reduced bacterial traversal through the filters (∼175-fold at 1 h and <5-fold at each of the remaining time points) (Fig. 2B). Growth of cells on Matrigel did not significantly affect TER at most time points (Table 1).
FIG. 2.
(A) Traversal by P. aeruginosa strain PA01 of corneal epithelial cells grown on filters with and without Matrigel coating. Cells grown on Matrigel showed increased resistance to bacterial traversal compared to cells grown without Matrigel over the 5-h assay (P ≤ 0.04, t test at all time points). (B) Matrigel-coated filters without cells reduced traversal of PA01 compared to that with uncoated filters over the 5-h assay (P ≤ 0.01, t test at all time points).
TABLE 1.
TER across multilayers of rabbit corneal epithelial cells grown with or without Matrigel and previously inoculated with P. aeruginosa strain PA01a
| Condition | Mean TER reading (Ω/cm2) from cells infected with PA01 at indicated time point postinoculation
|
|||||
|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | 5 h | |
| With Matrigel | 141 ± 14 | 144 ± 20 | 146 ± 4 | 147 ± 8 | 144 ± 9 | 136 ± 15 |
| Without Matrigel | 161 ± 11 | 174 ± 4 | 166 ± 6 | 165 ± 3 | 157 ± 5 | 156 ± 5 |
TER of Matrigel-grown cells was significantly reduced from that of cells grown without Matrigel only at 2 and 3 h (P = 0.004 and 0.017 respectively, t test). Baseline readings for Matrigel only or filters alone were significantly lower than TER values for cells grown with and without Matrigel (data not shown).
Matrigel promotes retention of P. aeruginosa within the Transwell filter system with and without epithelial cells.
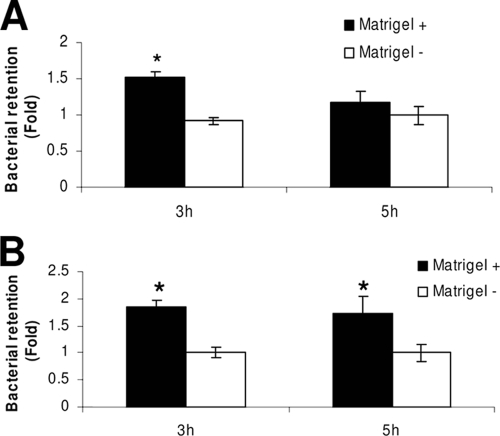
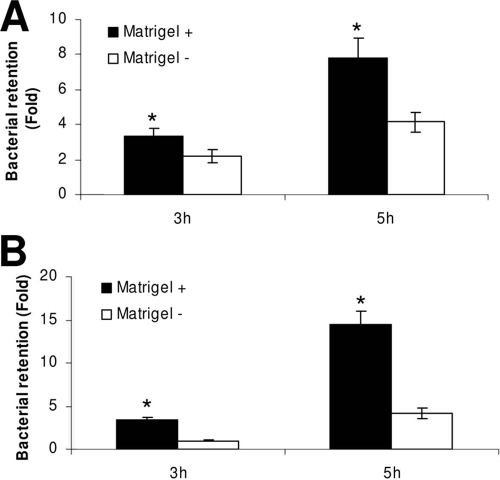
Reduced penetration of P. aeruginosa through Matrigel-coated filters (irrespective of whether cells were present) suggested that Matrigel could physically retain the bacteria. To test that directly, the numbers of bacteria retained by filter-grown corneal epithelial cells (or filters alone) were compared with and without Matrigel (Fig. 3). Retention of viable bacteria was observed at 3 h postinfection for cells grown on Matrigel compared to results for cells grown without Matrigel, which showed no retentive effect when normalized to retention by filters alone (Fig. 3A). By 5 h, no significant difference in retention of viable bacteria was found, suggesting that bacteria could overcome this retentive effect with time. Matrigel-coated filters (without cells) also showed greater accumulation of bacteria than uncoated filters at 3 h (Fig. 3B). However, in this instance, the retentive effect of Matrigel was also observed at 5 h postinfection.
FIG. 3.
(A) Quantification of P. aeruginosa strain PA01 bacteria recovered from epithelial cells grown with and without Matrigel at 3 and 5 h postinfection. Matrigel-grown cells showed a greater retention of P. aeruginosa than cells alone at 3 h (*, P < 0.001, t test). By 5 h postinfection, the difference in bacterial retention was not seen (P > 0.05, t test). (B) Matrigel-coated filters without cells also showed retention of bacteria compared to uncoated filters at 3 and 5 h postinfection (*, P ≤ 0.02, t test) (viable count data were normalized to filters alone). For each time point, viable count data were normalized to data for uncoated filters (i.e., bacteria retained by the filter without cells or Matrigel).
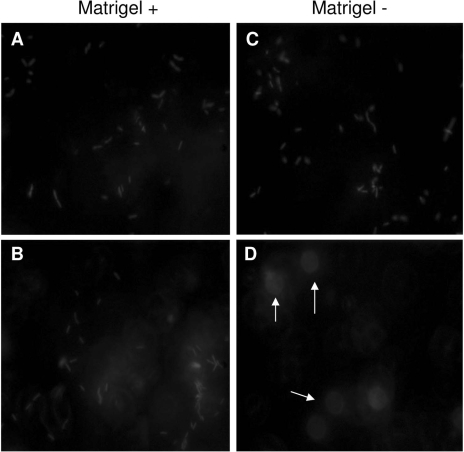
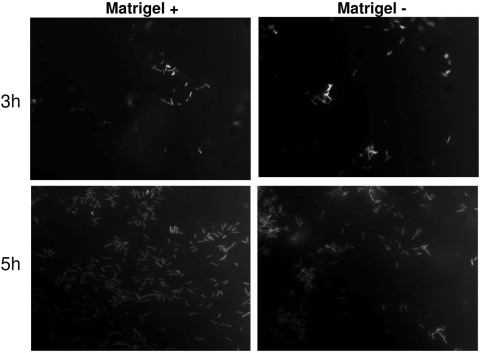
Fluorescence microscopy of Matrigel-grown cells (Fig. 4) showed two distinct zones of bacterial retention, the first being at the apical cell surface (Fig. 4A) and the other at the filter surface (Fig. 4B). Without Matrigel, apical cell surface retention of bacteria was also observed (Fig. 4C) but filter surface retention was absent (Fig. 4D).
FIG. 4.
Fluorescence microscopy with Z-stack analysis of corneal epithelial cells grown with and without Matrigel at 5 h postinfection with P. aeruginosa strain PA01 expressing GFP. Cells grown on Matrigel showed bacterial retention in two distinct zones: at the apical cell surface (A) and the filter surface (B). Cells grown without Matrigel showed apical surface retention of bacteria (C) but no filter surface retention (D). Arrows indicate filter pores.
P. aeruginosa protease activity modulates traversal through epithelial cells and Matrigel.
Since basement membrane proteins and epithelial cells could be susceptible to P. aeruginosa protease activity, we compared traversal of corneal epithelial cells grown with and without Matrigel by wild-type P. aeruginosa and a protease mutant (PA01 lasA lasBA prA). Wild-type bacteria showed increased traversal of corneal epithelial cells compared to results for the protease mutant with and without Matrigel (Fig. 5A). It was also observed that the presence of Matrigel significantly reduced the traversal of both the wild-type and mutant bacteria. Indeed, in the presence of Matrigel, traversal of the protease mutant was almost completely blocked (Fig. 5A). Wild-type and protease mutants were also compared for their ability to traverse Matrigel without cells (Fig. 5B). Matrigel was equally effective at blocking traversal of both wild-type and protease mutant bacteria for the first 3 h. Thereafter, wild-type bacteria had a significant advantage in their ability to penetrate the Matrigel (Fig. 5B).
FIG. 5.
Traversal of corneal epithelial cells grown with or without Matrigel (A) or Matrigel-coated filters without cells (B) by P. aeruginosa PA01 or its protease mutant (PA01 lasA lasBA prA). (A) With (closed symbols) or without (open symbols) Matrigel, wild-type PA01 (squares) showed increased traversal of corneal epithelial cells compared to results for the protease mutant (circles) (P < 0.05, t test). Both wild-type and protease mutant bacteria each showed reduced traversal of corneal epithelial cells in the presence of Matrigel (P = 0.002 and 0.006 for the wild type and the mutant, respectively, t test). (B) Without cells, wild-type bacteria showed a ∼2-fold increase in traversal of Matrigel-coated filters compared to results for the protease mutant, but only after 3 h postinfection (P = 0.006 and 0.0002 at 4 and 5 h, respectively, t test).
Matrigel promotes retention of the protease mutant within the Transwell filter system with and without epithelial cells.
Matrigel was associated with increased retention of the protease mutant on the filter system with cells (Fig. 6A) and without cells (Fig. 6B). Growth of cells on Matrigel was associated with 1.54-fold and 1.87-fold increases in bacterial retention at 3 and 5 h, respectively, compared to results for cells grown without Matrigel (Fig. 6A). In the presence of corneal epithelial cells, retention of the protease mutant also significantly increased between 3 and 5 h with and without Matrigel (Fig. 6A), contrasting with previously observed behavior of wild-type bacteria under the same conditions (Fig. 3A). Fluorescence microscopy also showed increased retention of the protease mutant between 3 and 5 h for cells grown with and without Matrigel (Fig. 7).
FIG. 6.
(A) Quantification of P. aeruginosa protease mutant (PA01 lasA lasBA prA) recovered from epithelial cells grown with or without Matrigel at 3 and 5 h postinfection. Retention of this mutant was increased when cells were grown on Matrigel (P = 0.03 and 0.005 at 3 and 5 h, respectively, t test). Greater retention of bacteria was observed at 5 h than at 3 h (P = 0.001 and P = 0.005 with and without Matrigel, respectively, t test). (B) Matrigel alone without cells also caused retention of the protease mutant compared to results with uncoated filters (P < 0.001 at each time point, t test). For each time point, viable count data were normalized to data for uncoated filters (i.e., bacteria retained by the filter without cells or Matrigel).
FIG. 7.
Fluorescence microscopy of corneal epithelial cells grown with (left column) or without (right column) Matrigel at 3 and 5 h postinfection with P. aeruginosa (PA01 lasA lasBA prA) expressing GFP. Increased bacterial retention was observed between 3 and 5 h with or without Matrigel. Greater retention of bacteria was observed with Matrigel-grown cells at 5 but not at 3 h.
DISCUSSION
Microscopy of in vivo-infected eyes suggested that bacteria penetrated into the corneal stroma from the overlying corneal epithelium only in regions where the basement membrane had become discontinuous. That data supported the hypothesis that the basement membrane acts as a barrier against bacterial traversal in vivo. Quantitative in vitro experiments confirmed that the basement membrane could reduce bacterial penetration, that the mechanism involved a physical barrier effect, and that P. aeruginosa proteases have the potential to reduce this protective activity.
Our results are consistent with those of previous studies, which have reported that epithelial basement membranes of other tissues, such as columnar genital epithelium and epidermal layers of the skin and lining of the gut, can act as a barrier to herpes simplex virus and Rift Valley fever virus to limit initial disease onset and dissemination in mouse and insect models of flank scarification and Rift Valley fever infection, respectively (25, 43, 47). Our results also add to our knowledge of P. aeruginosa translocation gained from previous studies (4, 6, 22, 23) by showing that the basement membrane contributes to reducing P. aeruginosa translocation of multilayered corneal epithelial cells and directly showing the involvement of P. aeruginosa proteases in the bacterial translocation process.
Our data comparing wild-type PA01 and the triple protease mutant in their ability to penetrate the basement membrane in vitro directly show that P. aeruginosa protease expression can enhance bacterial penetration through the basement membrane alone and in combination with cells (Fig. 5). Protease expression by P. aeruginosa is an important virulence mechanism (8, 13, 24, 45). Previous studies have examined the roles of elastase (LasB) and alkaline protease on corneal pathology using purified proteases and/or bacterial mutants deficient in one or multiple proteases to establish those contributing to P. aeruginosa's virulence in vivo (32, 35, 46). While the mechanism for involvement of P. aeruginosa proteases in vivo has not been established, basement membrane components are known to be targeted (7, 20, 21, 34). In addition, purified P. aeruginosa elastase increases airway epithelial permeability in vivo (5) and in vitro (6), the latter through an effect on tight junctions. Thus, it has been assumed that proteases allow the pathogen to gain access to tissues.
Bacterial traversal across Transwell filters was shown to be most effectively reduced when Matrigel and cells were both present (Fig. 2A). Yet bacterial retention by cells grown on filters was increased by Matrigel for only the first 3 h; by 5 h, similar numbers of bacteria were retained with or without Matrigel (Fig. 3A). Other data collected in this study could explain this apparent paradox. As discussed above, protease activity enhanced bacterial penetration through both cells and Matrigel. Indeed, for Matrigel used alone, traversal was effectively prevented only for the first 3 h (Fig. 5B). After that, wild-type bacteria showed a significant increase in penetration compared to protease mutants (Fig. 5A and Fig. 2A), which corresponded to a reduced retentive capacity of Matrigel for wild-type bacteria at 5 h compared to that in the initial 3-h period (Fig. 3A) and to an increased retentive capacity of Matrigel for the protease mutant at the same time points (Fig. 6A). Taken together, those data suggested that after the first 3 h, proteases had compromised the protective activity of Matrigel against bacterial penetration in these assays. Since protease production by P. aeruginosa is regulated by quorum sensing and other regulatory systems (16, 37, 38), the capacity to penetrate might depend upon the bacterial concentration and the surrounding environmental conditions. Indeed, all of the experiments performed with wild-type bacteria, with or without epithelial cells, showed that traversal was delayed rather than prevented; once traversal began, additional traversal at each hourly time point appeared quantitatively similar. Thus, by 5 h, bacterial traffic in and out of the cell layers in these in vitro assays might have reached a steady state.
It is also possible that Matrigel contributed to protecting cells against traversal by mechanisms independent of direct physical trapping, for example, by modulating antimicrobial activity of epithelial cells. Epithelial cells can make antimicrobial peptides in response to bacteria (31). Basement membrane proteins are known to play roles in epithelial growth, differentiation, and polarization that could influence antimicrobial expression and provide an alternative/additional explanation for the data obtained in the present study. Data supporting a more complex role for Matrigel beyond simple barrier activity include that traversal was reduced by ∼2 log (Fig. 2A at 3 h) while in the same experiments retention by the cells with Matrigel was only 1.51-fold higher than that of cells alone (Fig. 3A at 3 h). This discrepancy could potentially be explained by reduced bacterial viability. Alternatively, there might be a reduced propensity for bacteria to associate with cells when grown on Matrigel. A further explanation for less-than-expected bacterial retention by cells/Matrigel several hours postinfection could be that protease production influences other cellular responses to bacteria which are in turn impacted by the Matrigel. For example, if bacterial proteases enhance the innate immune response(s) of the epithelial cells to enable them to more easily resist bacteria (3), then protease mutants would be expected to persist longer via their reduced ability to trigger a robust cellular response.
In conclusion, the data collected in this study suggest that the corneal epithelial basement membrane may function as an important defense against infection. In vivo observations strongly suggest that the intact basement membrane prevents bacteria from entering the corneal stroma. Quantitative data collected in vitro showed that an artificial basement membrane (Matrigel) that is physically and biochemically similar to the corneal basement membrane can provide a barrier to bacterial traversal when used alone or in combination with corneal epithelial cells. While the mechanism for protection was found to involve direct physical trapping within the basement membrane, which is likely due to a filtering effect of its small pore size, there may also be effects of epithelial cell permeability, antimicrobial activity, or other epithelial changes. These data are consistent with animal models of ocular infection, which have shown that a deep penetrating injury to the anterior stroma is required to make otherwise healthy corneas susceptible to infection by a wide range of microbes, including bacteria, viruses, amoebas, and fungi (18, 29, 39, 48). The data are also consistent with clinical observations of patients with corneal disease which support the probability of a broad-spectrum defense against infection independent of epithelial cell barrier function, since not all types of epithelial injury/disease predispose to corneal infection (15). However, our results also show that basement membrane defenses against infection can be compromised, by P. aeruginosa proteases in this instance. Further in vivo studies would help elucidate the role of the basement membrane and multilayered epithelia in defending the cornea and other tissues against infection. However, given the difficulty of in vivo basement membrane studies, the in vitro Matrigel system used in the present study provides a useful model for investigating the cellular and molecular basis of epithelial barrier function. Research aimed at exploring the role of barriers that prevent bacterial penetration could lead to a better understanding of how tissues become susceptible to infection and may ultimately lead toward a means for restoring resistance.
Acknowledgments
This work was supported by NIH grants EY11221 (to S.M.J.F.); NEI fellowship F31 EY016328-03 (to I.A.) and the ASM Robert D. Watkins Graduate Research fellowship (to I.A.); and Student OD project grants from the California Chapter of the American Academy of Optometry (to L.K. and C.Y.).
Special thanks go to Eugenia Zhang for her technical assistance.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 8 June 2009.
REFERENCES
- 1.Abrahamson, D. R. 1986. Recent studies on the structure and pathology of basement membranes. J. Pathol. 149257-278. [DOI] [PubMed] [Google Scholar]
- 2.Abrams, G. A., S. L. Goodman, P. F. Nealey, M. Franco, and C. J. Murphy. 2000. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 29939-46. [DOI] [PubMed] [Google Scholar]
- 3.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1113-118. [DOI] [PubMed] [Google Scholar]
- 4.Azghani, A. O. 1996. Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A. Am. J. Respir. Cell Mol. Biol. 15132-140. [DOI] [PubMed] [Google Scholar]
- 5.Azghani, A. O., J. C. Connelly, B. T. Peterson, L. D. Gray, M. L. Collins, and A. R. Johnson. 1990. Effects of Pseudomonas aeruginosa elastase on alveolar epithelial permeability in guinea pigs. Infect. Immun. 58433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azghani, A. O., L. D. Gray, and A. R. Johnson. 1993. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect. Immun. 612681-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bejarano, P. A., J. P. Langeveld, B. G. Hudson, and M. E. Noelken. 1989. Degradation of basement membranes by Pseudomonas aeruginosa elastase. Infect. Immun. 573783-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackwood, L. L., R. M. Stone, B. H. Iglewski, and J. E. Pennington. 1983. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect. Immun. 39198-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourcier, T., F. Thomas, V. Borderie, C. Chaumeil, and L. Laroche. 2003. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 87834-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers, D., F. Scott, R. Bangur, R. Davies, A. Lim, S. Walters, G. Smith, T. Pitt, D. Stableforth, and D. Honeybourne. 2005. Factors associated with infection by Pseudomonas aeruginosa in adult cystic fibrosis. Eur. Respir. J. 26651-656. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, K. H., S. L. Leung, H. W. Hoekman, W. H. Beekhuis, P. G. Mulder, A. J. Geerards, and A. Kijlstra. 1999. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet 354181-185. [DOI] [PubMed] [Google Scholar]
- 12.Cowell, B. A., S. S. Twining, J. A. Hobden, M. S. Kwong, and S. M. Fleiszig. 2003. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology 1492291-2299. [DOI] [PubMed] [Google Scholar]
- 13.Engel, L. S., J. M. Hill, A. R. Caballero, L. C. Green, and R. J. O'Callaghan. 1998. Protease IV, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa. J. Biol. Chem. 27316792-16797. [DOI] [PubMed] [Google Scholar]
- 14.Evans, D. J., N. A. McNamara, and S. M. Fleiszig. 2007. Life at the front: dissecting bacterial-host interactions at the ocular surface. Ocul. Surf. 5213-227. [DOI] [PubMed] [Google Scholar]
- 15.Fleiszig, S. M. 2006. The Glenn A. Fry award lecture 2005. The pathogenesis of contact lens-related keratitis. Optom. Vis. Sci. 83866-873. [DOI] [PubMed] [Google Scholar]
- 16.Gambello, M. J., S. Kaye, and B. H. Iglewski. 1993. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect. Immun. 611180-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gipson, I. K., S. Spurr-Michaud, A. Tisdale, and M. Keough. 1989. Reassembly of the anchoring structures of the corneal epithelium during wound repair in the rabbit. Investig. Ophthalmol. Vis. Sci. 30425-434. [PubMed] [Google Scholar]
- 18.Girgis, D. O., G. D. Sloop, J. M. Reed, and R. J. O'Callaghan. 2003. A new topical model of Staphylococcus corneal infection in the mouse. Investig. Ophthalmol. Vis. Sci. 441591-1597. [DOI] [PubMed] [Google Scholar]
- 19.Hay, E. D. 1985. Matrix-cytoskeletal interactions in the developing eye. J. Cell Biochem. 27143-156. [DOI] [PubMed] [Google Scholar]
- 20.Heck, L. W., K. Morihara, and D. R. Abrahamson. 1986. Degradation of soluble laminin and depletion of tissue-associated basement membrane laminin by Pseudomonas aeruginosa elastase and alkaline protease. Infect. Immun. 54149-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heck, L. W., K. Morihara, W. B. McRae, and E. J. Miller. 1986. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect. Immun. 51115-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirakata, Y., B. B. Finlay, D. A. Simpson, S. Kohno, S. Kamihira, and D. P. Speert. 2000. Penetration of clinical isolates of Pseudomonas aeruginosa through MDCK epithelial cell monolayers. J. Infect. Dis. 181765-769. [DOI] [PubMed] [Google Scholar]
- 23.Hirakata, Y., R. Srikumar, K. Poole, N. Gotoh, T. Suematsu, S. Kohno, S. Kamihira, R. E. Hancock, and D. P. Speert. 2002. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J. Exp. Med. 196109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobden, J. A. 2002. Pseudomonas aeruginosa proteases and corneal virulence. DNA Cell Biol. 21391-396. [DOI] [PubMed] [Google Scholar]
- 25.Huard, J., W. G. Feero, S. C. Watkins, E. P. Hoffman, D. J. Rosenblatt, and J. C. Glorioso. 1996. The basal lamina is a physical barrier to herpes simplex virus-mediated gene delivery to mature muscle fibers. J. Virol. 708117-8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinman, H. K., M. L. McGarvey, L. A. Liotta, P. G. Robey, K. Tryggvason, and G. R. Martin. 1982. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 216188-6193. [DOI] [PubMed] [Google Scholar]
- 27.Kruszewski, F. H., T. L. Walker, and L. C. DiPasquale. 1997. Evaluation of a human corneal epithelial cell line as an in vitro model for assessing ocular irritation. Fundam. Appl. Toxicol. 36130-140. [PubMed] [Google Scholar]
- 28.Kwong, M. S., D. J. Evans, M. Ni, B. A. Cowell, and S. M. Fleiszig. 2007. Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infect. Immun. 752325-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larkin, D. F., and D. L. Easty. 1990. Experimental Acanthamoeba keratitis: I. Preliminary findings. Br. J. Ophthalmol. 74551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, E. J., D. J. Evans, and S. M. Fleiszig. 2003. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Investig. Ophthalmol. Vis. Sci. 445220-5227. [DOI] [PubMed] [Google Scholar]
- 31.McNamara, N. A., R. Van, O. S. Tuchin, and S. M. Fleiszig. 1999. Ocular surface epithelia express mRNA for human beta defensin-2. Exp. Eye Res. 69483-490. [DOI] [PubMed] [Google Scholar]
- 32.Miyajima, S., T. Akaike, K. Matsumoto, T. Okamoto, J. Yoshitake, K. Hayashida, A. Negi, and H. Maeda. 2001. Matrix metalloproteinases induction by pseudomonal virulence factors and inflammatory cytokines in vitro. Microb. Pathog. 31271-281. [DOI] [PubMed] [Google Scholar]
- 33.Mollard, R., and M. Dziadek. 1998. A correlation between epithelial proliferation rates, basement membrane component localization patterns, and morphogenetic potential in the embryonic mouse lung. Am. J. Respir. Cell Mol. Biol. 1971-82. [DOI] [PubMed] [Google Scholar]
- 34.Nagano, T., J. L. Hao, M. Nakamura, N. Kumagai, M. Abe, T. Nakazawa, and T. Nishida. 2001. Stimulatory effect of pseudomonal elastase on collagen degradation by cultured keratocytes. Investig. Ophthalmol. Vis. Sci. 421247-1253. [PubMed] [Google Scholar]
- 35.Nicas, T. I., and B. H. Iglewski. 1985. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Can. J. Microbiol. 31387-392. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto, S., M. Ohji, K. Kosaku, N. Sundarraj, J. R. Hassell, R. A. Thoft, and J. M. Pipas. 1995. Characterization of immortalized rabbit corneal epithelial cells with SV40 large T antigen. Jpn. J. Ophthalmol. 39323-333. [PubMed] [Google Scholar]
- 37.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 2601127-1130. [DOI] [PubMed] [Google Scholar]
- 38.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1795756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira, S. R., M. A. Guimaraes, L. V. Neto, D. Segenreich, R. B. Varella, V. L. Antunes Chagas, and F. P. Camara. 2001. Herpes simplex virus ophthalmic disease induced using two different methods of mice inoculation. Braz. J. Infect. Dis. 5183-191. [DOI] [PubMed] [Google Scholar]
- 40.Preston, M. J., S. M. Fleiszig, T. S. Zaidi, J. B. Goldberg, V. D. Shortridge, M. L. Vasil, and G. B. Pier. 1995. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect. Immun. 633497-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramphal, R., M. T. McNiece, and F. M. Polack. 1981. Adherence of Pseudomonas aeruginosa to the injured cornea: a step in the pathogenesis of corneal infections. Ann. Ophthalmol. 13421-425. [PubMed] [Google Scholar]
- 42.Roberts, J. N., C. B. Buck, C. D. Thompson, R. Kines, M. Bernardo, P. L. Choyke, D. R. Lowy, and J. T. Schiller. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 13857-861. [DOI] [PubMed] [Google Scholar]
- 43.Romoser, W. S., M. J. Turell, K. Lerdthusnee, M. Neira, D. Dohm, G. Ludwig, and L. Wasieloski. 2005. Pathogenesis of Rift Valley fever virus in mosquitoes—tracheal conduits & the basal lamina as an extra-cellular barrier. Arch. Virol. Suppl. (19):89-100. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki, K., J. Saito, R. Yanai, N. Yamada, T. Chikama, K. Seki, and T. Nishida. 2003. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog. Retin. Eye Res. 22113-133. [DOI] [PubMed] [Google Scholar]
- 45.Thibodeaux, B. A., A. R. Caballero, M. E. Marquart, J. Tommassen, and R. J. O'Callaghan. 2007. Corneal virulence of Pseudomonas aeruginosa elastase B and alkaline protease produced by Pseudomonas putida. Curr. Eye Res. 32373-386. [DOI] [PubMed] [Google Scholar]
- 46.Twining, S. S., S. E. Kirschner, L. A. Mahnke, and D. W. Frank. 1993. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Investig. Ophthalmol. Vis. Sci. 342699-2712. [PubMed] [Google Scholar]
- 47.Weeks, B. S., R. S. Ramchandran, J. J. Hopkins, and H. M. Friedman. 2000. Herpes simplex virus type-1 and -2 pathogenesis is restricted by the epidermal basement membrane. Arch. Virol. 145385-396. [DOI] [PubMed] [Google Scholar]
- 48.Wu, T. G., K. R. Wilhelmus, and B. M. Mitchell. 2003. Experimental keratomycosis in a mouse model. Investig. Ophthalmol. Vis. Sci. 44210-216. [DOI] [PubMed] [Google Scholar]