Abstract
Systemic anthrax manifests as toxemia, rapidly disseminating septicemia, immune collapse, and death. Virulence factors include the anti-phagocytic γ-linked poly-d-glutamic acid (PGA) capsule and two binary toxins, complexes of protective antigen (PA) with lethal factor (LF) and edema factor. We report the characterization of LF, PA, and PGA levels during the course of inhalation anthrax in five rhesus macaques. We describe bacteremia, blood differentials, and detection of the PA gene (pagA) by PCR analysis of the blood as confirmation of infection. For four of five animals tested, LF exhibited a triphasic kinetic profile. LF levels (mean ± standard error [SE] between animals) were low at 24 h postchallenge (0.03 ± 1.82 ng/ml), increased at 48 h to 39.53 ± 0.12 ng/ml (phase 1), declined at 72 h to 13.31 ± 0.24 ng/ml (phase 2), and increased at 96 h (82.78 ± 2.01 ng/ml) and 120 h (185.12 ± 5.68 ng/ml; phase 3). The fifth animal had an extended phase 2. PGA levels were triphasic; they were nondetectable at 24 h, increased at 48 h (2,037 ± 2 ng/ml), declined at 72 h (14 ± 0.2 ng/ml), and then increased at 96 h (3,401 ± 8 ng/ml) and 120 h (6,004 ± 187 ng/ml). Bacteremia was also triphasic: positive at 48 h, negative at 72 h, and positive at euthanasia. Blood neutrophils increased from preexposure (34.4% ± 0.13%) to 48 h (75.6% ± 0.08%) and declined at 72 h (62.4% ± 0.05%). The 72-h declines may establish a “go/no go” turning point in infection, after which systemic bacteremia ensues and the host's condition deteriorates. This study emphasizes the value of LF detection as a tool for early diagnosis of inhalation anthrax before the onset of fulminant systemic infection.
Bacillus anthracis is the gram-positive spore-forming organism that causes anthrax. Spores gain entry through three routes, dermal, ingestion, and inhalation, causing three distinct disease forms (31). Inhalation anthrax has a rapid onset and is usually fatal. In the anthrax attacks of 2001, the fatality rate was 45% despite the use of antibiotics and supportive treatment (16, 17). Recent studies in a murine model using a nontoxigenic encapsulated strain indicate that for all three routes of entry, bacterial growth may occur at the site of inoculation (11). Vegetative organisms may then disseminate to the draining lymph node, the spleen, and finally to the lungs and blood (11). The guinea pig model for dissemination of fully pathogenic B. anthracis indicates that spores may be engulfed by lung macrophages and then transported to mediastinal thoracic lymph nodes where they germinate and subsequently disseminate to the bloodstream (35). In all models of untreated fulminant anthrax, the virulent B. anthracis infection results in systemic bacteremia, sepsis-induced shock, respiratory distress, and extensive hemorrhage and is often fatal (31).
The vegetative anthrax bacilli circumvent host defenses by producing a poly-d-glutamic acid (PGA) capsule that may protect them from phagocytosis (36) and two major binary toxins which disrupt immune signaling (1, 41, 47). Protective antigen (PA), which binds cell receptors to internalize the toxins, combines with both of the catalytic toxin components: lethal factor (LF), a zinc-dependent endoproteinase, and edema factor (EF), an adenylate cyclase that forms lethal toxin (LTx) and edema toxin, respectively (31). The known targets of LF include five members of the family of mitogen-activated protein kinase kinase (MAPKK) response regulators (31). LF cleaves and inactivates MAPKKs, which plays an intermediate role in MAPK immune activation (41). LTx affects a broad range of immune cells, including macrophages, neutrophils, dendritic cells, and T and B lymphocytes, activities which are central to the ability of B. anthracis to disable host defenses (1, 41, 47). The other catalytic protein, EF, converts ATP to cyclic AMP, causing an efflux of fluid from the cell and localized edema (31). In addition, the EF-induced rise in cyclic AMP inhibits phagocytosis, microbiocidal activity, neutrophil chemotaxis, and superoxide production, contributing to extensive physiological immune dysfunction (41). Edema toxin was also shown to contribute to increased transcription of the anthrax toxin receptor genes in host cells, theoretically contributing to enhanced receptor expression and toxin uptake (48).
Since LTx is integral to the pathogenesis of anthrax, understanding its levels over the disease course and during treatment may help to understand the clinical course and to devise strategies for and timing of therapeutic intervention (44). The PGA capsule antigen is also a key virulence factor in anthrax and is an abundant antigen produced on the surfaces of B. anthracis vegetative cells. During infection, PGA is shed from the bacterium, and serum PGA levels have been shown to correlate with bacterial load (23). Previous studies quantifying PA and LF in rabbits and guinea pigs succeeded in measuring these toxins in late infection or during the final 24 h of the infection cycle (22, 25, 40). While these studies provide important information about toxin levels prior to death, they do not offer a detailed understanding of the dynamics of toxemia immediately postexposure and at the onset of infection—critical time points for diagnosis and early intervention.
We have previously reported the development of a rapid, high-specificity, high-sensitivity mass spectroscopy (MS) method for the quantification of LF in serum and plasma (4). The aim of this study, therefore, was to evaluate the MS technology for early and rapid diagnosis of disease in a rhesus macaque model of inhalation anthrax. We also sought to compare detection of LF with other B. anthracis antigens secreted or released during infection, specifically PA and PGA, and to compare these with hematological parameters and the more established diagnostic tools for anthrax, detection of the pagA gene and bacteremia (http://www.bt.cdc.gov/agent/anthrax/lab-testing/approvedlrntests.asp). This is the first study to demonstrate the early and extended kinetics of anthrax LF toxemia and its relationship to innate immune cell changes and the infection cycle in rhesus macaques with inhalation anthrax.
MATERIALS AND METHODS
Materials.
All chemicals and reagents were from Sigma-Aldrich (Saint Louis, MO) except where indicated.
Animal care.
The inhalation anthrax study in rhesus macaques (Macaca mulatta) was done at the Battelle Biomedical Research Center (Columbus, OH). The study protocol was approved by both Battelle and the Centers for Disease Control and Prevention (CDC) Institutional Animal Care and Use Committees (IACUC) and followed the stringent requirements for care and use set down by CDC IACUC guidelines, which adhere to the National Institutes of Health guidelines for the care and use of laboratory animals. All aspects of the study were designed to minimize stress in the animals. Five female rhesus macaques, 3.3 to 4.0 kg prior to challenge, were obtained from Covance (Alice, TX). Animals were quarantined for 6 weeks and tuberculin tested every 2 weeks with negative results. Animals judged to be moribund after the spore challenge were humanely euthanized.
Challenge protocol.
Bacillus anthracis Ames strain was prepared and characterized at Battelle. Anesthetized rhesus macaques were challenged via head-only exposure in a class III biosafety cabinet with approximately 300 to 400 50% lethal dose (LD50) equivalents of B. anthracis Ames spores by methods described previously (approximately 1.7 × 107 to 2.2 × 107 spores, where 1 × LD50 equals 55,000 spores or CFU) (14). The spore dose corresponds to that which has been used to yield high infection rates (43). Aerosol exposure time was adjusted so that each animal inhaled the required cumulative volume of air to attain the targeted dose, assuming 100% retention of the inhaled dose. Exposure times ranged from 10 to 30 min, and animals were anesthetized for the entire period to minimize stress. Actual total spore exposure doses ranged from 308 to 460 LD50 equivalents of B. anthracis Ames spores (geometric mean ± percent SE = 378 ± 8 LD50).
Blood collection.
Blood was collected in serum separation tubes at 42 days preexposure and postexposure at 2, 4, 6, 12, 24, 48, 72, 96, and 120 h for determining LF, PA, and PGA concentrations. Serum was filter sterilized, and sterility was confirmed by culture before other analyses. For bacteremia and pagA PCR, whole blood was collected in EDTA tubes at 42 days preexposure, at 2, 4, 6, 12, 24, 48, and 72 h postexposure, and at euthanasia. Whole blood was also collected for hematology at 42 days preexposure and postexposure at 2, 24, 48, and 72 h.
Bacteremia.
Approximately 10 to 40 μl of whole blood was inoculated by loop onto a blood agar plate which was incubated at 37°C ± 2°C for a minimum of 48 h. A positive bacteremia for B. anthracis was indicated by the presence of white colonies, 4 to 10 mm in diameter with a rough appearance and irregular edges. The specific grading of bacteremia is indicated in “Data reporting and statistical analyses.”
Hematology.
Complete blood counts and differentials were determined using an Advia 120 hematology system (Siemens Healthcare Diagnostics, Deerfield, IL) according to the manufacturer's guidelines. Normal reference range blood levels were obtained from a review of the existing literature, which gave the means and standard deviations (SD) for several hematological variables (24). Normal ranges for the relevant hematological variables were calculated as the mean ± the SD.
LF quantification.
LF activity was quantified by methods reported previously (4). Briefly, LF in a sample was captured by anti-LF monoclonal antibodies (MAb) on magnetic protein G beads (Invitrogen Corporation, Carlsbad, CA) and then mixed with 40 μl of buffer and a synthetic peptide substrate optimized for cleavage by LF. This mixture was incubated at 37°C for 2 h, and then a 2-μl volume was analyzed by isotope dilution matrix-assisted laser desorption ionization-mass spectrometry. The remaining LF-substrate mixture was incubated for 21 h and then analyzed by MS again. To achieve the necessary dynamic range and to account for high-sensitivity rapid-response sample analysis, two standard curves were prepared, one for 20 ng/ml, μl (“low volume” [LV]) and the other for 200 ng/ml, μl (“high volume” [HV]) serum volumes. In addition, three quality control samples (QCs) were prepared in human serum pools (Interstate Blood Bank, Memphis, TN) using recombinant LF (List Biological Laboratory, Campbell, CA) and validated. The LV standard curve ranged from 0.025 to 100 ng/ml, with QCs at 2 and 20 ng/ml. LV analysis at a 2-h incubation covered 0.5 to 100 ng/ml, and at a 21-h incubation it covered 0.05 to 25 ng/ml. The HV standards and samples were analyzed at 2 h for high sensitivity qualitative diagnostics (the limit of detection for HV analysis at 2 h was 0.05 ng/ml) and at 21 h for quantification, which covered 0.005 to 2.5 ng/ml and included QCs at 0.2 and 2.0 ng/ml. The lowest standard for each analytical range represents the detection limit for that range. The relative SD for QC validation ranged from 8.5 to 14.7% (A. E. Boyer et al., unpublished data).
In an anthrax emergency, a high volume of sample would be analyzed for the most sensitive results in less than 4 h. However, to conserve usage of animal study samples, smaller volumes were analyzed initially. Specifically, 20-μl volumes of sera from rhesus macaques were assayed for LF at all time points using the LV standard curve and QCs. Test samples which were negative after 21 h using 20 μl were reevaluated using a 200-μl sample to increase analytic sensitivity using the HV standards and QCs. Unknown samples were quantified from the appropriate 2-h or 21-h standard curve when responses were within the specified standard curve range. Samples with responses higher than 50 ng/ml after the LV analysis at a 2-h incubation were diluted appropriately, and analysis was repeated with the 20-μl standards and QCs.
PA antigen capture immunoassay.
Microtiter plates (Thermo Labsystems, Franklin, MA) were coated with monoclonal anti-PA antibody AVR1046 obtained by the same methodologies used for anti-LF monoclonal described previously (4) at 1.28 μg/ml in 0.01 M phosphate-buffered saline (PBS), pH 7.4. Coated plates were washed with a wash buffer consisting of PBS and 0.1% Tween 20, pH 7.4. Recombinant PA (rPA; List Biological Laboratories, Inc., Campbell, CA) was serially diluted twofold using a 1/25 or a 1/100 dilution of normal rhesus macaque serum (RMS). The rPA dilutions formed a seven-point standard curve from 0.6 to 37.5 ng/ml. Samples were serially diluted twofold in dilution buffer (DB; PBS, 5% skim milk, 0.5% Tween 20, pH 7.4). Sample and rPA standard dilutions were incubated for 30 min at 37°C and then washed. Captured antigen was detected by adding human standard reference serum AVR801 (109.4 μg/ml anti-PA immunoglobulin G) (37) diluted 100-fold in DB and incubated for 60 min at 37°C. Plates were washed and then incubated with horseradish peroxidase-conjugated mouse anti-human immunoglobulin G Fc PAN MAb HP6043 (Hybridoma Reagent Laboratory, Baldwin, MD) diluted 1:10,000 for 60 min at 37°C. Washed plates were developed with 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)-diammonium salt (ABTS)-H2O2 (Kirkegaard and Perry Laboratories, Gaithersburg, MD) for 15 min or 30 min for starting test sera dilutions of 1/25 and 1/100, respectively, and stopped with peroxidase stop solution. The optical density at 410 nm (OD410) was read with a 490-nm reference filter. Samples were analyzed in duplicate in two or more separate analytical runs. Data were analyzed using a four-parameter logistic-curve fitting model with enzyme-linked immunosorbent assay (ELISA) for Windows software version 2.15 (34; http://www.cdc.gov/Ncidod/dbmd/bimb/ELISA/downloadelisa_2.htm).
The reactivity threshold (RT) of the PA ELISA was determined from duplicate analyses of 88 PA-negative RMS diluted 1/25 in DB, yielding a mean OD of 0.047 and an SD of 0.021. The RT (mean OD plus 2 SD) was 0.089, below the average OD of 0.142 (n = 27) for the lowest rPA standard (0.6 ng/ml). The RT was below the minimum detectable concentration (MDC) of the assay; therefore, the MDC was used as the default RT. The MDC (32) was determined from 27 independent calibration curves. The analytic sensitivity of the antigen capture ELISA using the MDC was calculated as 4.84 ng/ml PA analyte after a 1/25 dilution.
PGA antigen capture immunoassay.
A quantitative antigen capture ELISA for PGA was constructed using a 50:50 mixture of PGA MAbs F24F2 and F26G3 (23). Microtiter plates were coated overnight with 100 μl of PGA MAb (1 μg/ml), washed with 0.05% Tween 20 in PBS (PT), and blocked for 90 min with PT. Serum samples were initially diluted 1/40 or 1/10 and then twofold serially diluted in PT and incubated for 90 min in the MAb-coated wells (100 μl/well). Plates were washed with PT, incubated for 90 min with 100 μl of horseradish peroxidase-labeled PGA MAb (1 μg/ml), washed again, and then incubated with tetramethylbenzidine substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD). The reaction was stopped with 1 M H3PO4. The plates were read using a VersaMax plate reader (Molecular Devices, Sunnyvale, CA). Concentrations of PGA in the serum were calculated using purified B. anthracis PGA as a standard with the assistance of SoftMax Pro (Molecular Devices). An OD450 of 0.5 was used as the end point. Values were adjusted as described previously to correct for the background (33). The sensitivity of the antigen capture ELISA was approximately 9 ng/ml of serum after a 1/40 dilution and 2.25 ng/ml for a 1:10 dilution. Due to limitations with sample volumes, PGA analyses were from single readings.
PCR assay.
DNA was extracted from whole-blood samples of rhesus macaques preexposure (−42 days), at 24, 48, and 72 h, and at euthanasia using the Qiagen DNA mini kit (Germantown, MD). Isolated DNA was analyzed by PCR for the presence of the PA gene (pagA) of B. anthracis using the LightCycler Bacillus anthracis detection kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions.
Data reporting and statistical analyses.
Values for PA, LF, and PGA were reported in nanograms per milliliter for 24 to 120 h. The LF assay reported values of ≥1 ng/ml to the nearest 0.01 ng/ml and <1 ng/ml to the nearest 0.001 ng/ml. Both the PA and PGA assay measurements were reported to the nearest nanogram per milliliter. Animal survival times were rounded to the nearest full day. Due to limitations on allowable blood sample volumes from animals, PGA data were from a single analysis per animal per time point. Qualitative results for PCR of pagA were reported as positive or negative. Culture results were graded as positive if six or more colonies were present, low positive if one to five colonies were present in the primary phase, or negative if no colonies were present. Due to standard limitations for blood collection during animal studies, samples for animal D from days 6 through 9 (144 to 216 h) were not available for PCR, LF, PA, and PGA testing. Standard formulas for calculating the geometric mean and percent SE were generated in Microsoft Excel for comparing measurements per time point. LF and PGA results collected after 24 h were transformed using log10 and analyzed using least squares regression and the correlation coefficient calculated.
RESULTS
Animal exposures and survival.
The mean B. anthracis spore aerosol dose (LD50 equivalents ± percent SE) for the five rhesus macaques was 378 LD50 ± 8%. Animals A, C, and E survived to 96 h and received 308, 434, and 326 LD50, respectively. Animal B received 385 LD50 and survived to 120 h. Animal D, which received 460 LD50, survived to day 9. The geometric mean time to death for the entire cohort was 118.06 h ± 0.17 h (4.92 days).
Detection and kinetics of B. anthracis antigens in rhesus macaques.
Tables 1 and 2 summarize the quantitative and qualitative results for parameters of infection in rhesus macaques and include the serum levels of the proteins, PA and LF, the capsule antigen PGA, detection of the pagA gene by PCR, and bacteremia status. These parameters were not detected at time points earlier than 24 h. Therefore, the 2, 4, 6, and 12 h time points were excluded from Tables 1 and 2.
TABLE 1.
Quantitative values for parameters of infection in rhesus macaques infected by spore aerosol exposure to B. anthracis Ames strain: PA, LF, PGAa
| Factor analyzed | Animal ID | Concn (ng/ml) at indicated time point
|
|||||
|---|---|---|---|---|---|---|---|
| −42 days | 24 h | 48 h | 72 h | 96 h | 120 h | ||
| PA | A | − | − | − | − | 147* | |
| B | − | − | − | − | − | 3,153* | |
| C | − | − | − | − | −* | ||
| D | − | − | − | − | − | − | |
| E | − | − | − | − | 19,434* | ||
| LF | A | − | 0.006 | 45.16 | 10.29 | 103.08* | |
| B | − | − | 25.87 | 18.97 | 63.58 | 1,235.76* | |
| C | − | 0.200 | 51.66 | 10.57 | 34.07* | ||
| D | − | 0.018 | 38.10 | 8.06 | 4.48 | 27.73 | |
| E | − | − | 42.00 | 25.08 | 3,885.89* | ||
| GM | 0.028 | 39.53 | 13.31 | 82.78 | 185.12 | ||
| SE | 1.82 | 0.12 | 0.24 | 2.01 | 5.68 | ||
| PGA | A | − | − | 10,394 | 20 | 32,000* | |
| B | − | − | 48 | 17 | 681 | 1,126,388* | |
| C | − | − | 7,061 | 16 | 3,541* | ||
| D | − | − | 1,523 | 7 | 3 | 32 | |
| E | − | − | 6,527 | 18 | 1,965,663* | ||
| GM | 2,037 | 14 | 3,401 | 6004 | |||
| SE | 2 | 0.2 | 8 | 187 | |||
Values for PA, LF, and PGA are shown in nanograms per milliliter for 24, 48, 72, 96, and 120 h. The LF assay reported values of ≥1 ng/ml to the nearest 0.01 ng/ml and <1 ng/ml to the nearest 0.001 ng/ml. Both the PA and PGA assay measurements were reported to the nearest nanograms per milliliter. Asterisks indicate the measurement at euthanasia. Geometric means (GM) and SE were calculated by standard methods. Measurements for postexposure time points at 2, 4, 6, and 12 h were negative for all parameters and thus were not included. Due to standard requirements for blood collection during animal studies, samples for animal D from days 6 through 9 were not available for LF, PA, and PGA testing. −, negative values obtained for PA, LF, and PGA.
TABLE 2.
Qualitative values for parameters of infection in rhesus macaques infected by spore aerosol exposure to B. anthracis Ames strain: pagA and bacteremia status
| Quantitative assay | Animal ID | Resulta at indicated time point
|
|||||
|---|---|---|---|---|---|---|---|
| −42 days | 24 h | 48 h | 72 h | 96 h | 120 h | ||
| pagA PCR | A | − | − | + | + | +* | |
| B | − | − | − | + | NS | +* | |
| C | − | − | + | + | +* | ||
| D | − | − | + | − | NS | NS | |
| E | − | − | + | + | +* | ||
| Bacteremia status | A | − | − | + | − | +* | |
| B | − | − | + | ± | NS | +* | |
| C | − | − | + | − | +* | ||
| D | − | − | + | − | NS | NS | |
| E | − | − | + | + | +* | ||
Qualitative results for pagA PCR are indicated as positive (+) or negative (−). Culture results were graded as positive (+) if there were more than five colonies, negative (−) if there were no colonies, and low positive (±) if there were one to five colonies in primary phase. NS, no sample was available. Asterisks indicate the measurement at euthanasia. Measurements for postexposure time points at 2, 4, 6, and 12 h were negative for all parameters and thus were not included. Due to standard requirements for blood collection during animal studies, samples for animal D from days 6 through 9 were not available for pagA PCR. At euthanasia on day 9, animal D was found to be bacteremic.
PA detection in rhesus macaques.
The earliest time point for which PA was detected in serum was 96 to 120 h. Thus, samples for time points earlier than 96 to 120 h had levels lower than the detection limit, an MDC of 4.8 ng/ml. PA was detected at the latest time point in three of the five rhesus macaques which had the highest levels of LF and PGA (Table 1). PA levels were consistently higher than those of LF at these late time points, with the highest at 19,434 ng/ml in animal E at 96 h (Table 1). Animals A and B had PA levels of 147 ng/ml at 96 h and 3,153 ng/ml at 120 h, respectively.
LF detection and kinetics in rhesus macaques.
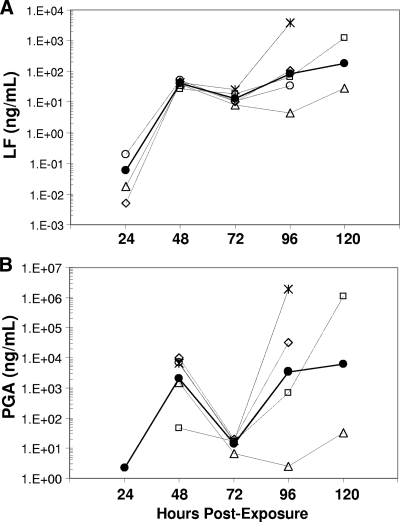
LF was first detected in serum at 24 h postexposure in three of five animals and in all animals at 48 h and through 120 h (Table 1). The kinetic profile for LF was triphasic during infection (Fig. 1A). LF levels were low at 24 h, ranging from 0.006 to 0.200 ng/ml (Table 1). By 48 h, LF levels increased more than 100-fold (phase 1) and were similar in all animals (range, 25.87 to 51.66 ng/ml). At 72 h, LF decreased 1.3-fold to 4.8-fold (phase 2) and remained similar among all animals (range, 8.06 to 25.08 ng/ml). At 96 and 120 h, LF levels increased (phase 3) in animals A, B, C, and E and varied by as much as 100-fold at euthanasia. For example, at 96 h, animal C had 34.07 ng/ml and animal E had 3,885.89 ng/ml (Table 1). LF levels in animal D declined at 72 h and continued to decline at 96 h before increasing slightly at 120 h. Thus, animal D had an extended phase 2 compared to the other animals. LF levels for animal D were also the lowest among all animals at 72, 96, and 120 h (the last data point available), possibly due to a lower severity of disease resulting in the animal's survival to day 9. The kinetic profile for LF illustrates the three phases, the similarity of LF levels among animals at 48 h, their decline at 72 h, rapid increases and divergence by 96 h until euthanasia, and the lower LF levels from 72 to 120 h in animal D (Fig. 1A).
FIG. 1.
Kinetics profile for LF and PGA in rhesus macaques. (A) The geometric mean and individual values of LF (ng/ml) for each animal infected with B. anthracis Ames strain were plotted on a log10 scale for each time point in hours to compare the kinetic profiles over time. (B) The geometric mean and individual values of PGA (ng/ml) were plotted on a log10 scale for each time point. The same symbols are used for panels A and B: animal A, ⋄; animal B, □; animal C, ○; animal D, ▵; animal E, ×; and geometric mean, •. The negative value for PGA at 24 h was assigned a value of the limit of detection divided by two of 1.125 ng/ml according to Hornung et al. (13a).
PGA detection and kinetics in rhesus macaques.
PGA was detectable in sera of all five animals by 48 h postexposure and at all subsequent time points (Table 1). The kinetics of PGA were similar to those of LF, with a triphasic profile (Fig. 1B). At 48 h (phase 1), PGA ranged from 48 to 10,394 ng/ml (Table 1). At 72 h (phase 2), PGA fell by as much as 500-fold (ranging from 7 to 20 ng/ml). At 96 and 120 h (phase 3), PGA increased substantially in animals A, B, C, and E, ranging from 3,541 to 1,965,663 ng/ml at death (Table 1). However, in animal D, which survived to day 9, a decline in PGA was observed at 72 and 96 h, similar to that seen for LF in this animal. PGA levels in this animal declined from 1,523 ng/ml at 48 h to 7 ng/ml at 72 h and to 3 ng/ml at 96 h, suggesting an initial rapid and extended (48-h) clearance of PGA-producing bacilli. A subtle increase in PGA levels to 32 ng/ml at 120 h may indicate the beginning of a resurgence in bacterial load. The triphasic kinetics of PGA are clearly depicted with the variable extremes in PGA at 48, 96, and 120 h compared to the sharp decline at 72 h and the extended decline and substantially lower PGA for animal D at 72, 96, and 120 h (Fig. 1B).
PCR and culture status in rhesus macaques complement LF and PGA levels.
PCR of pagA was positive in four animals by 48 h and by 72 h and in the four animals tested at euthanasia by 96 h and 120 h (Table 2). Blood was culture positive in all five animals by 48 h (Table 2). However, at 72 h, blood cultures were negative for three animals, A, C, and D, and low positive in a fourth animal, B. All animals were bacteremic at euthanasia. PCR for pagA also reverted to negative at 72 h in animal D, the 9-day survivor with the lowest PGA and LF levels at that time point (Table 2). The change in bacteremia from positive at 48 h to negative at 72 h, together with the falling levels of both LF and PGA, is a significant finding and suggests an active process of microbial clearance. The threefold reduction in the geometric mean concentrations from 48 to 72 h for LF compared to 143-fold for PGA is also important (Table 1) and indicates that, despite the significant systemic changes during the course of disease, LF remains a consistent and reliable marker of infection from at least 24 h onwards. LF analysis of additional sampling time points between 12 and 24 h may extend this time frame to even earlier.
The qualitative results for bacteremia and the pagA PCR are in agreement with the observed changes in LF and PGA over time, as well as the differences between animals (Tables 1 and 2). For example, at 48 h, a negative PCR result that was observed for animal B was paralleled by much lower PGA level in this animal (48 ng/ml) than in others (1,523 to 10,394 ng/ml). Also, the change in bacteremic status from positive at 48 h to negative at 72 h corresponded with 72-h declines in LF and PGA. Both bacteremia and pagA PCR analysis were negative at 72 h for animal D, which also exhibited the lowest 72-h LF and PGA levels (Tables 1 and 2).
Correlation of LF and PGA.
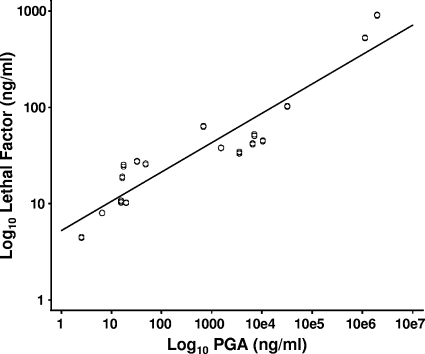
To determine whether there was a correlation between LF and PGA, all measurements after 24 h were paired and the 51 paired values were plotted and subjected to least squares analysis. The data indicate a strong positive correlation between the log10-transformed LF and PGA levels after 24 h, with a slope significantly different from 0 (P < 0.0001) and a correlation coefficient (r2) of 0.869 (Fig. 2).
FIG. 2.
Correlation between levels of LF and PGA in sera from rhesus macaques infected with B. anthracis Ames strain. LF and PGA results collected after 24 h were paired, and the 51 paired values were transformed using log10 and analyzed using least squares regression analysis, and the correlation coefficient was calculated. The slope was significantly different from 0 (P < 0.0001), and the correlation coefficient (r2) was 0.869.
Neutrophil and lymphocyte frequencies and neutrophil-to-lymphocyte (N/L) ratios.
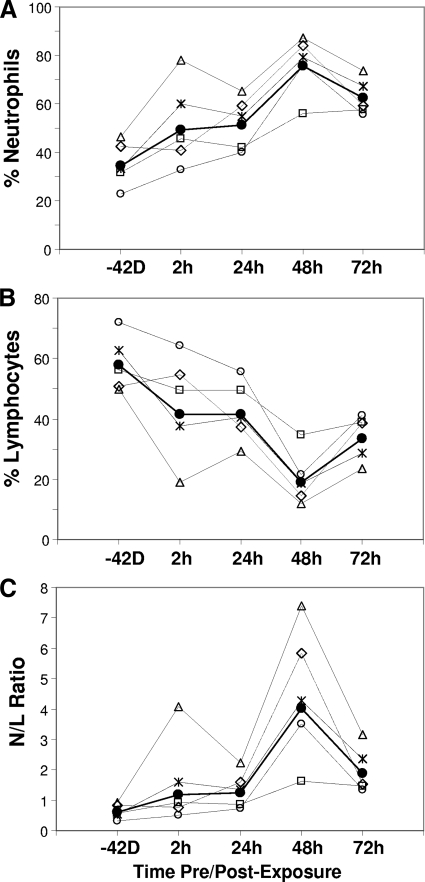
The frequencies (%) and ratios of neutrophils and lymphocytes were determined (Table 3). Normal rhesus macaque neutrophil levels range from 19.1 to 52.5% (24). These five animals exhibited normal preexposure levels ranging from 22.8 to 46.4% (Table 3). Levels increased as early as 2 h postexposure in animals B, C, D, and E to levels ranging from 32.8 to 77.9%. Animal A exhibited no increase in neutrophils at 2 h. By 48 h, neutrophils increased in all five animals to levels ranging from 56.1 to 87.1% and then declined slightly in animals A, C, D, and E at 72 h to levels ranging from 55.7 to 73.8% (Table 3). The kinetic profile for percent neutrophils illustrates their increase from preexposure to 48 h followed by declines at 72 h (Fig. 3A). The elevated percent neutrophils at 48 h and decline at 72 h (Fig. 3A) mirrored the 48-h surge and 72-h decline in LF and PGA levels (Fig. 1). The percent neutrophils in animal D, the 9-day survivor, were higher than those in all other animals for all time points, especially at 2 h postexposure (Fig. 3A). It is worth noting that this animal had the highest starting percent neutrophils and received the highest spore dose of 460 LD50. Despite higher-than-normal neutrophil levels at 48 and 72 h, the total white blood cell (WBC) count remained within normal limits (9.17 × 103/μl to 21.13 × 103/μl) in three of five animals at 48 h and four of five animals at 72 h (Table 3). Neutrophils were the primary phagocytic innate cell type with a kinetic profile that matched the profiles for LF and PGA, with a 48-h increase and a 72-h decline. The kinetic profile was also consistent among all five animals (Fig. 3A). Levels of other phagocytic cell types represented by monocytes were not consistent between animals (data not shown).
TABLE 3.
Values for hematological parameters in rhesus macaques infected with B. anthracis Ames straina
| Animal ID | WBC count (103/μl)
|
% of:
|
N/L ratio
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophils
|
Lymphocytes
|
|||||||||||||||||||
| −42 days | 2 h | 24 h | 48 h | 72 h | −42 days | 2 h | 24 h | 48 h | 72 h | −42 days | 2 h | 24 h | 48 h | 72 h | −42 days | 2 h | 24 h | 48 h | 72 h | |
| A | 10.35 | 10.22 | 11.95 | 21.96 | 11.47 | 42.6 | 41.0 | 59.2 | 84.1 | 59.2 | 50.7 | 54.6 | 37.2 | 14.4 | 38.6 | 0.84 | 0.75 | 1.59 | 5.84 | 1.53 |
| B | 7.51 | 10.81 | 9.19 | 11.77 | 13.17 | 31.8 | 45.8 | 41.9 | 56.1 | 57.6 | 56.1 | 49.4 | 49.5 | 34.6 | 38.9 | 0.57 | 0.93 | 0.85 | 1.62 | 1.48 |
| C | 8.34 | 9.11 | 7.05 | 13.46 | 13.34 | 22.8 | 32.8 | 40.1 | 76.1 | 55.7 | 72.1 | 64.2 | 55.5 | 21.6 | 41.2 | 0.32 | 0.51 | 0.72 | 3.52 | 1.35 |
| D | 6.36 | 11.67 | 7.75 | 13.93 | 16.41 | 46.4 | 77.9 | 65.4 | 87.1 | 73.8 | 49.7 | 19.1 | 29.3 | 11.8 | 23.5 | 0.93 | 4.08 | 2.23 | 7.38 | 3.14 |
| E | 11.08 | 12.66 | 11.35 | 24.67 | 34.35 | 33.4 | 60.1 | 54.8 | 79.1 | 67.4 | 62.8 | 37.5 | 40.6 | 18.5 | 28.5 | 0.53 | 1.60 | 1.35 | 4.28 | 2.36 |
| GM | 8.55 | 10.83 | 9.26 | 16.43 | 16.26 | 34.4 | 49.2 | 51.3 | 75.6 | 62.4 | 57.7 | 41.6 | 41.4 | 18.8 | 33.4 | 0.60 | 1.18 | 1.24 | 4.02 | 1.87 |
| SE | 0.11 | 0.06 | 0.11 | 0.16 | 0.22 | 0.13 | 0.16 | 0.10 | 0.08 | 0.05 | 0.07 | 0.24 | 0.12 | 0.20 | 0.11 | 0.21 | 0.43 | 0.23 | 0.30 | 0.18 |
The primary hematological parameters showing important trends relating to B. anthracis infection are included for −42 days preexposure and 2, 24, 48, and 72 h postexposure. The frequencies of neutrophils and lymphocytes as percentages and white blood cells (WBC) as 103/μl are given for each animal over time. Geometric means (GM) and SE were calculated by standard methods. Bold indicates values that are above normal, and underlining refers to values that are below normal levels. The normal ranges are as follows: 19.1 to 52.5% for neutrophils, 43.3 to 77.8% for lymphocytes, and 9.17 × 103/μl to 21.13 × 103/μl for WBC (24).
FIG. 3.
Kinetic profile of immune responses in rhesus macaques infected with B. anthracis Ames strain. Percent neutrophils (A), percent lymphocytes (B), and the N/L ratio (C) were plotted over time for the geometric mean and individual values for all five animals. The same symbols are used for panels A, B, and C: animal A, ⋄; animal B, □; animal C, ○; animal D, ▵; animal E, ×; and geometric mean, •. The time points for −42 days (−42D) and 2 h are not to scale.
The normal range for percent lymphocytes in rhesus macaques is 43.3 to 77.8% (24). Lymphocytes for all five rhesus macaques in this study were within normal limits preexposure, ranging from 49.7 to 72.1%. Following spore exposure, percent lymphocytes declined from 2 to 48 h in all animals to below normal levels, ranging from 11.8 to 34.6%. This was followed by increases at 72 h in all animals to levels ranging from 23.5 to 41.2% (Table 3). The 72-h increases in percent lymphocytes coincided with the declines in LF and PGA and negative bacteremia in some animals (Tables 1 to 3). The plotted geometric mean and individual levels for percent lymphocytes over time illustrate the general decline from the time of exposure to 48 h postchallenge and increase at 72 h (Fig. 3B). The 9-day survivor, animal D, had lower-than-normal percent lymphocytes compared to the other animals for all time points measured, in particular at 2 h postexposure (Fig. 3B).
An elevated N/L ratio is a common marker of inflammation and microbial infection (49). Given the normal ranges for percent neutrophils and percent lymphocytes (24), the expected normal range for the N/L ratio is 0.25 to 1.21. Thus, an N/L ratio greater than 1.21 might indicate an inflammatory response and infection. Compared to preexposure levels, the N/L ratios for animals A, B, and C changed only slightly at 2 h postexposure and were still within normal limits (Table 3). However, the N/L ratios for animals D and E at 2 h were 4.08 and 1.60, respectively, 4.4-fold and threefold higher than preexposure ratios which were within the normal range. Changes from 2 h to 24 h were mixed. For animals A and C, the N/L ratio from 2 h to 24 h increased slightly by 2.1- and 1.4-fold to 1.59 (above normal) and 0.72 (within normal), respectively. For D and E, the N/L ratio declined from 2 h to 24 h by 1.8- to 1.2-fold to 2.23 and 1.35, respectively. By 48 h, the N/L ratios for all five animals increased and were above normal, ranging from 1.62 to 7.38. By 72 h, the N/L ratio in all five animals declined from 1.1- to 3.8-fold and ranged from 1.35 to 3.14 (Table 3).
The kinetic profile for the N/L ratio mirrored that for neutrophils (Fig. 3C). In animal D, the N/L ratio was substantially higher at 2 h postexposure than in other animals and had a substantial decline at 24 h. At 48 h, the N/L ratio increased substantially in all but animal B (Fig. 3C). The N/L ratio declined at 72 h in all five animals, emphasizing the declines in neutrophils and recovery in lymphocytes as well as mirroring the 72-h declines in specific parameters of infection (LF, PGA, culture). In addition to the substantial increase at 2 h, animal D had the highest N/L ratio for all time points. As with neutrophils, the higher N/L ratio in animal D corresponds to the lowest levels of LF and PGA at 72 and 96 h postinfection (Fig. 1A and B) and an extended survival of 9 days (216 h) compared to 96 and 120 h for the other four animals (Table 1).
DISCUSSION
This study is the first to track a comprehensive set of parameters indicative of toxemia and bacteremia over the course of inhalation anthrax in rhesus macaques. It used the most sensitive assays for toxin detection (LF) and bacterial load (PGA) available. The results yield important insights that will support anthrax diagnostics, therapeutics development, and clinical treatment. The results also lead to an improved understanding of anthrax pathogenesis and innate cell changes which may reflect the host's early immune responses to B. anthracis nascent infection.
The results indicate that the MS detection of functional LF provides an improved diagnostic tool for inhalation anthrax in nonhuman primates. The detection of LF in rhesus macaques as early as 24 h postexposure and over the course of a 96-h to 120-h (4- to 5-day) infection period demonstrates the importance of this approach for early diagnosis of inhalation anthrax (Table 1). Kobiler et al. (22) previously reported using a PA capture immunoassay to monitor progression of anthrax toxemia in serum from infected rabbits and guinea pigs. However, measurements with this assay were achieved only over the last 24 h of infection and ranged from 1 ng/ml up to approximately 105 ng/ml (22). Mabry et al. used capture immunoassays for PA and LF in infected guinea pigs and rabbits (25). However, PA was measured only in late infection or at death (<9 h before death) in guinea pigs (0.1 to 1.7 μg/ml), and PA and LF were measured only in rabbits at terminal time points at 80 to 100 μg/ml and 11 to 15 μg/ml, respectively (25). A recent report on a europium nanoparticle-based ELISA for PA has described detection limits of 0.01 ng/ml (40), but in agreement with previous studies (22, 25), PA was detectable only in the later stages of infection in mice (40). With the improved detection limits of the functional LF MS assay at 0.005 ng/ml and higher levels of LF demonstrated in early infection, we have demonstrated a clear advantage of using LF for earlier detection of systemic anthrax. Functional LF levels as low as 0.006 ng/ml were detected at 24 h using MS, levels that would not be detectable using previously published technologies (25). These data emphasize that LF is the better target for early detection of anthrax.
PA in this study was not detectable until euthanasia and in only three animals, which indicates that PA levels at all time points preceding death were below the 4.84 ng/ml detection limit for the PA ELISA reported here. Nonetheless, PA detection provided an additional confirmation of anthrax-specific toxemia. The late-infection PA levels reported here of 147 to 19,434 ng/ml correspond with those reported previously for guinea pigs at 100 to 1,700 ng/ml but are much lower than reported for rabbits at 80 and 100 μg/ml (25).
Although there are differences in detection limits between the LF and PA assays described here, the measured LF levels at the early time points 48 to 72 h were higher than the 4.84 ng/ml detection limit for PA, and therefore, LF levels were higher than PA at these early time points. These data suggest that either more LF is produced or this antigen is less rapidly sequestered by host tissues than PA at earlier time points, and thus the LF/PA ratios in serum are high. Conversely, we observed that LF/PA ratios were lower in late infection. These lower ratios during late infection in rhesus macaques may be due to saturation of host receptors, decreased cellular uptake, and thus accumulation of PA in serum (30). Accumulation of serum PA in late infection is in agreement with previous studies in rabbits (22, 25), guinea pigs (25), and mice (40). Consequently, lower serum LF/PA ratios will reflect the uptake of LF via the PA-primed host cells.
These findings raise interesting questions about appropriate interventions after exposure to or infection with B. anthracis spores. One interpretation of the low serum PA levels at 24 to 72 h is that PA is rapidly adsorbed to host tissues (30), and thus circulating PA is the rate-limiting toxin component for potentiating early infection and the associated bacteremia of anthrax. In this context, a potential concern might be raised that an rPA vaccine given postexposure during this phase might facilitate additional uptake of LF and thus exacerbate the infection.
The perceived risk of potentiating the infection due to postexposure vaccination with the current U.S. licensed anthrax vaccine and for rPA vaccines in development is low. First, in the course of the overall infection the rate limitation is most likely PA processing and turnover at the host cell receptor rather than the levels in serum. Second, PA is not readily released from an aluminum hydroxide vaccine formulation (15). Third, the quantity of PA contributed by a vaccine given intramuscularly to a patient would be insignificant in comparison to the quantity produced by the bacterium at that stage of infection. Fourth, the anthrax vaccine is formalin treated which is anticipated to inactivate its protein components (18), thereby reducing the risk of additional toxin uptake. Furthermore, the likelihood of vaccination during infection is low; the anthrax vaccine is contraindicated in persons who have recovered from anthrax (9), and where vaccination is recommended for use in a postexposure setting prior to onset of infection, it is coadministered with antibiotics (8).
The PGA capture immunoassay also represents a valuable diagnostic tool and facilitates a surrogate measurement of bacterial load (23). PGA was consistently detected from 48 h onwards postexposure. The rapid increases in PGA at 48 h and declines at 72 h are reflected in the positive 48-h and negative 72-h bacteremia. Compared to LF, PGA was much higher than LF at 48, 96, and 120 h. This probably reflects the accumulation of PGA in the blood when bacteremia is positive, whereas some LF secreted by the organism is sequestered intracellularly. PGA declined much more rapidly at 72 h than LF, which may be due to the rapid clearance of bacilli; the cellular uptake of LF may be slower, allowing a portion of LF to linger in the blood in the absence of bacteremia (44). Future studies planned may define how quickly PGA clears from the blood with antibiotic treatment.
LF and PGA were highly correlated with a coefficient of linearity (r2) of 0.869, which is similar to that reported previously for PA and bacteremia in rabbits (r2 = 0.864) (22). Since PGA correlates with bacterial levels (23) and LF correlates with PGA, it is likely that LF may also correlate with bacterial levels and therefore may represent an alternative measurement for the stage or severity of infection. Studies in progress may yield additional measurements to support this association.
While LF and PGA were consistently detectable in RMS, reversion in bacteremia and pagA PCR reactivity over time provides a key insight to the onset and progression of systemic anthrax. Although most animals were bacteremic and had detectable pagA at 48 h, some reverted to a negative result at 72 h. These changing response patterns for pagA detection and bacteremia define a potential window of diagnostic uncertainty for these methods. Culture-dependent methods may fail if a patient is presented during this critical window of infection. This illustrates the importance of applying multiple analyses for diagnosis of anthrax and emphasizes the central role that the high-sensitivity and high-throughput capability of MS provides as a diagnostic technology.
A triphasic kinetics profile for B. anthracis infection in rhesus macaques was defined with a 48-h increase in LF, PGA, and percent neutrophils, followed by 72-h declines, before the final surge in parameters of infection and subsequent death. Importantly, the reversal of bacteremia from positive detection at 48 h to negative detection at 72 h provides compelling evidence supporting these quantitative declines. All elements define a 72-h decline in infection and represent tangible measures of antigen (LF) and microbial (PGA and culture) clearance in early anthrax infection. A similar but shorter triphasic profile for LF was also observed in rabbits and suggests that they may also be mounting innate cell changes that lead to antigen clearance (unpublished data).
Our data from rhesus macaques suggest an important contribution by neutrophils in the innate cell changes leading to temporary reduction in bacterial burden and antigenemia. The 48- and 72-h kinetics of neutrophils mirrored those of the parameters of infection with a 48-h increase and 72-h decline. Since the percent neutrophil kinetic profiles were consistent between all five infected animals, it is the most likely immune source responsible for the temporary clearance of microbial products observed at 72 h. We conclude from our data that there are two critical time periods during the infection process in rhesus macaques. The earliest period is at the spore-tissue interface. This first interaction of microbes with tissues initiates signaling events that sequentially recruit neutrophils (1 to 2 h) and then macrophages and other phagocytic cells to the site of tissue inflammation (10, 13, 21, 39). The second critical period coincides with the appearance of bacilli in the blood. Mature neutrophils are released from the marrow pool into the blood in response to increased demands for neutrophils during tissue inflammation (12). Thus, blood neutrophil levels may be used as an indicator of increases in neutrophils at the inflammatory sites, similar to that reported to occur for monocytes (13). In this context, the increase in neutrophils at 2 h in four of the five animals suggests a role for neutrophil involvement in the earliest stages of infection at the spore-tissue interface. However, the second increase in the neutrophils at 48 h in all five animals was consistent with the onset of bacteremia and increase in LF and PGA. This suggests that the neutrophils responded appropriately to the initial infection threat at 2 h and to the escalating sepsis at 48 h, which was followed by comprehensive declines in parameters of infection. It is interesting to note that animal D, with the highest neutrophil response at 2 h and throughout infection, had the lowest parameters of infection and survived an additional 96 to 120 h (4 to 5 days) longer than the other animals, indicating that the neutrophils may have contributed to the delay of fulminant bacteremia in this animal.
Data supporting the importance of neutrophils and their products in anthrax infection (3, 19, 20, 28, 29, 45, 46) and other important infections (26, 27, 42) are substantial. In vitro, human neutrophils engulf B. anthracis spores, induce germination, and kill vegetative B. anthracis (29). Neutrophils also produce abundant antimicrobial peptides called defensins (20, 29), which can kill vegetative bacilli (29), neutralize anthrax lethal toxin, and protect against lethal toxin killing (20). Related θ defensins, found in primates, kill both spores and vegetative bacilli and neutralize lethal toxin (45). In cutaneous anthrax, neutrophils are the first cell type recruited to infected tissues (46). Resistant mice with enhanced neutrophil and reduced macrophage function had an accumulation of neutrophils at the infection site (3). Neutrophils were also prominent in the cutaneous anthrax cases from 2001 (38). For inhalation anthrax, neutrophil recruitment in the lungs of dogs and pigs was associated with survival after high exposure doses (28). Furthermore, the inhalation anthrax patients from the 2001 event had elevated circulating neutrophils or band forms even though overall WBC counts were normal (17), consistent with other microbial infections (2). Most rhesus macaques in this study also fit this description, with elevated neutrophils and normal WBC counts as late as 72 h. Therefore, current evidence suggests that early recruitment of neutrophils is an important feature of cutaneous and inhalation anthrax. It may be presumed that this is also the case for gastrointestinal anthrax (28).
Our study provides additional information on neutrophil involvement in the early stages of inhalation anthrax. We can deduce that the substantial neutrophil recruitment observed at 2 h in animal D may have contributed to its extended survival. Importantly, since it is well established that neutrophils are the first cell type recruited (within 1 to 2 h) to the lung tissues in other infections (10, 13), it is reasonable to assume that the same is true for anthrax.
Whereas neutrophils increased during inhalation anthrax in this study, we observed that the percentage of lymphocytes decreased over time. Many microbial infections, including those caused by B. anthracis, Yersinia spp., Streptococcus spp., Staphylococcus aureus, and Listeria monocytogenes, are characterized by decreased lymphocytes along with increased neutrophils (7, 49). The lymphocyte declines occur because microbial products trigger lymphocyte cell death by apoptosis (6). In our study, lymphocyte profiles declined early and at 48 h, which was consistent with the expected response (7). The increase in lymphocytes observed at 72 h may reflect their recovery, concurrent with declines in bacterial load.
In this study, we have observed a classical response to microbial infections with elevated neutrophils and reduced lymphocytes during B. anthracis infection. Their combination, expressed as the N/L ratio (49), yielded a clear measure of the changes in inflammation over time. While excess inflammation is considered detrimental, capable of causing widespread tissue damage, it is often associated with resistance to serious infections (26, 27, 42). This study included one animal with enhanced early inflammation and extended survival. The enhanced inflammation at 48 h in all five macaques led to a temporary clearance of microbial products. The temporary clearance of microbial products was longer in the 9-day survivor and might be viewed clinically as a “false” recovery, which has also been reported for human inhalation anthrax cases (5). Ongoing animal studies are suggesting that the levels of circulating LF are associated with the length of survival and may be of prognostic value. These studies may also further explain the triphasic kinetics of toxemia described here.
Conclusions.
This study is the first to track both LF and PGA levels in inhalation anthrax in rhesus macaques and to compare these with bacteremia and innate immune cell recruitment. A triphasic kinetic profile of LF and PGA, but not PA antigenemia, was observed in rhesus macaques. This study also identified a potential source of early innate defense, an association between neutrophil recruitment and temporary clearance of infection. Our data support the previously defined role for neutrophils in protection from cutaneous infections (3, 29). These data also suggest that neutrophils may attempt to clear bacilli early in systemic B. anthracis inhalation infection, before they are overwhelmed or “disarmed” by the effects of the anthrax toxins, which leads to the characteristic exponential increase in bacteremia, antigenemia, and death. Brachman described human inhalation anthrax as a “biphasic” disease with two distinct periods of illness (5). The first period is manifest as mild and flu-like and is followed by an improvement in clinical status (the second phase described in our study). The final clinical stage is acute, sudden, and usually fatal (5). We have defined the central stage of clinical improvement observed by Brachman as a brief remission with reduced PGA and LF and absence of bacteremia. This period also represents a window of potential false negative diagnoses for culture-based tests and illustrates the importance of using a combination of tests for diagnosis. The sensitivity of the MS LF assay circumvents this problem with early and consistent detection of infection. The MS LF assay has provided an improved understanding of anthrax infection dynamics with this first description of the initial acute phase, through the remission phase, and beyond. The ability to detect infection in the initial phase prior to the onset of high-density bacteremia is important for successful intervention. Use of the LF MS assay in emergencies may give the earliest and most reliable measure of anthrax, leading to earlier and more successful intervention.
Acknowledgments
We thank Ed Nuzum and Judith Hewitt of the Office of Biodefense Research Affairs, DMID/NIAID/NIH, and Gabriel Meister at the Battelle Biomedical Research Center for their critical review of the manuscript. Additional thanks go to Hercules Moura, Suzanne Kalb, Jarad Schiffer, and Stephen D. Soroka of the CDC for assistance.
This study was supported in part by Public Health Service grants AI059348 (T.R.K. and A.P.) and AI061200 (T.R.K. and A.P.). The research described in this publication was supported by funds made available from the Centers for Disease Control and Prevention, Coordinating Office for Terrorism Preparedness and Emergency Response.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 8 June 2009.
REFERENCES
- 1.Agrawal, A., J. Lingappa, S. H. Leppla, S. Agrawal, A. Jabbar, C. Quinn, and B. Pulendran. 2003. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424329-334. [DOI] [PubMed] [Google Scholar]
- 2.Ardron, M. J., J. C. Westengard, and T. F. Dutcher. 1994. Band neutrophil counts are unnecessary for the diagnosis of infection in patients with normal total leukocyte counts. Am. J. Clin. Pathol. 102646-649. [DOI] [PubMed] [Google Scholar]
- 3.Bischof, T. S., B. L. Hahn, and P. G. Sohnle. 2007. Experimental cutaneous Bacillus anthracis infections in hairless HRS/J mice. Int. J. Exp. Pathol. 8875-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer, A. E., C. P. Quinn, A. R. Woolfitt, J. L. Pirkle, L. G. McWilliams, K. L. Stamey, D. A. Bagarozzi, J. C. Hart, Jr., and J. R. Barr. 2007. Detection and quantification of anthrax lethal factor in serum by mass spectrometry. Anal. Chem. 798463-8470. [DOI] [PubMed] [Google Scholar]
- 5.Brachman, P. S. 1980. Inhalation anthrax. Ann. N. Y. Acad. Sci. 35383-93. [DOI] [PubMed] [Google Scholar]
- 6.Carrero, J. A., B. Calderon, and E. R. Unanue. 2006. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J. Exp. Med. 203933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrero, J. A., and E. R. Unanue. 2006. Lymphocyte apoptosis as an immune subversion strategy of microbial pathogens. Trends Immunol. 27497-503. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2002. Use of anthrax vaccine in response to terrorism: supplemental recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly. Rep. 511024-1026. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2000. Use of anthrax vaccine in the United States: recommendations of the advisory committee on immunization practices. MMWR Recommend. Rep. 49(RR-15)1-20. [Google Scholar]
- 10.Doherty, D. E., G. P. Downey, G. S. Worthen, C. Haslett, and P. M. Henson. 1988. Monocyte retention and migration in pulmonary inflammation. Requirement for neutrophils. Lab. Investig. 59200-213. [PubMed] [Google Scholar]
- 11.Glomski, I. J., A. Piris-Gimenez, M. Huerre, M. Mock, and P. L. Goossens. 2007. Primary involvement of pharynx and Peyer's patch in inhalational and intestinal anthrax. PLoS Pathog. 3e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gossett, K. A., P. S. MacWilliams, and B. Cleghorn. 1985. Sequential morphological and quantitative changes in blood and bone marrow neutrophils in dogs with acute inflammation. Can. J. Comp. Med. 49291-297. [PMC free article] [PubMed] [Google Scholar]
- 13.Goto, Y., J. C. Hogg, T. Suwa, K. B. Quinlan, and S. F. van Eeden. 2003. A novel method to quantify the turnover and release of monocytes from the bone marrow using the thymidine analog 5′-bromo-2′-deoxyuridine. Am. J. Physiol. Cell Physiol. 285C253-C259. [DOI] [PubMed] [Google Scholar]
- 13a.Hornung, R. W., and L. D. Reed. 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hug. 546-51. [Google Scholar]
- 14.Ivins, B. E., P. F. Fellows, M. L. M. Pitt, J. E. Estep, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Efficacy of a standard human anthrax vaccine against Bacillus anthracis aerosol spore challenge in rhesus monkeys. Salisbury Med. Bull. 87(Suppl. 7)125-126. [Google Scholar]
- 15.Jendrek, S., S. F. Little, S. Hem, G. Mitra, and S. Giardina. 2003. Evaluation of the compatibility of a second generation recombinant anthrax vaccine with aluminum-containing adjuvants. Vaccine 213011-3018. [DOI] [PubMed] [Google Scholar]
- 16.Jernigan, D. B., P. L. Raghunathan, B. P. Bell, R. Brechner, E. A. Bresnitz, J. C. Butler, M. Cetron, M. Cohen, T. Doyle, M. Fischer, C. Greene, K. S. Griffith, J. J. Guarner, J. L. Hadler, J. A. Hayslett, R. Meyer, L. R. Petersen, M. Phillips, R. Pinner, T. Popovic, C. P. Quinn, J. Reefhuis, D. Reissman, N. Rosenstein, A. Schuchat, W. J. Shieh, L. Siegal, D. L. Swerdlow, F. C. Tenover, M. Traeger, J. W. Ward, I. Weisfuse, S. Wiersma, K. Yeskey, S. Zaki, D. A. Ashford, B. A. Perkins, S. Ostroff, J. Hughes, D. Fleming, J. P. Koplan, J. L. Gerberding, and the National Anthrax Epidemiologic Investigation Team. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 81019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jernigan, J. A., D. S. Stephens, D. A. Ashford, C. Omenaca, M. S. Topiel, M. Galbraith, M. Tapper, T. L. Fisk, S. Zaki, T. Popovic, R. F. Meyer, C. P. Quinn, S. A. Harper, S. K. Fridkin, J. J. Sejvar, C. W. Shepard, M. McConnell, J. Guarner, W. J. Shieh, J. M. Malecki, J. L. Gerberding, J. M. Hughes, B. A. Perkins, and the Anthrax Bioterrorism Investigation Team. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joellenbeck, L. M., L. L. Zwanziger, J. S. Durch, and B. S. Strom. 2002. The anthrax vaccine. Is it safe? Does it work? National Academy Press, Washington, DC. [PubMed]
- 19.Kaufmann, S. H. E., A. Sher, and R. Ahmed. 2002. Immunology of infectious diseases. ASM Press, Washington, DC.
- 20.Kim, C., N. Gajendran, H. W. Mittrücker, M. Weiwad, Y. H. Song, R. Hurwitz, M. Wilmanns, G. Fischer, and S. H. Kaufmann. 2005. Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc. Natl. Acad. Sci. USA 1024830-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi, S. D., J. M. Voyich, C. Burlak, and F. R. DeLeo. 2005. Neutrophils in the innate immune response. Arch. Immunol. Ther. Exp. (Warsaw) 53505-517. [PubMed] [Google Scholar]
- 22.Kobiler, D., S. Weiss, H. Levy, M. Fisher, A. Mechaly, A. Pass, and Z. Altboum. 2006. Protective antigen as a correlative marker for anthrax in animal models. Infect. Immun. 745871-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozel, T. R., W. J. Murphy, S. Brandt, B. R. Blazar, J. A. Lovchik, P. Thorkildson, A. Percival, and C. R. Lyons. 2004. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc. Natl. Acad. Sci. USA 1015042-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krise, G. M. 1960. Hematology of the normal monkey. Ann. N. Y. Acad. Sci. 85803-810. [DOI] [PubMed] [Google Scholar]
- 25.Mabry, R., K. Brasky, R. Geiger, R. Carrion, Jr., G. B. Hubbard, S. Leppla, J. L. Patterson, G. Georgiou, and B. L. Iverson. 2006. Detection of anthrax toxin in the serum of animals infected with Bacillus anthracis by using engineered immunoassays. Clin. Vaccine Immunol. 13671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martineau, A. R., S. M. Newton, K. A. Wilkinson, B. Kampmann, B. M. Hall, N. Nawroly, G. E. Packe, R. N. Davidson, C. J. Griffiths, and R. J. Wilkinson. 2007. Neutrophil-mediated innate immune resistance to mycobacteria. J. Clin. Investig. 1171988-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maung, A. A., S. Fujimi, M. P. MacConmara, G. Tajima, A. M. McKenna, A. J. Delisle, C. Stallwood, A. B. Onderdonk, J. A. Mannick, and J. A. Lederer. 2008. Injury enhances resistance to Escherichia coli infection by boosting innate immune system function. J. Immunol. 1802450-2458. [DOI] [PubMed] [Google Scholar]
- 28.Mayer-Scholl, A. 2006. A review of the interaction of Bacillus anthracis with cells of the innate immune response. Berl. Munch. Tierarztl. Wochenschr. 119216-221. [PubMed] [Google Scholar]
- 29.Mayer-Scholl, A., R. Hurwitz, V. Brinkmann, M. Schmid, P. Jungblut, Y. Weinrauch, and A. Zychlinsky. 2005. Human neutrophils kill Bacillus anthracis. PLoS Pathog. 1e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moayeri, M., J. F. Wiggins, and S. H. Leppla. 2007. Anthrax protective antigen cleavage and clearance from the blood of mice and rats. Infect. Immun. 755175-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mock, M., and T. Mignot. 2003. Anthrax toxins and the host: a story of intimacy. Cell. Microbiol. 515-23. [DOI] [PubMed] [Google Scholar]
- 32.O'Connell, M. A., B. A. Belanger, and P. D. Haaland. 1993. Calibration and assay development using the four-parameter logistic model. Chemometrics Intelligent Lab. Syst. 2097-114. [Google Scholar]
- 33.Peterman, J. H. 1991. Immunochemical considerations in the analysis of data from non-competitive solid-phase immunoassays, p. 47-65. In J. E. Butler (ed.), Immunochemistry of solid-phase immunoassay. CRC Press, Inc., Boca Raton, FL.
- 34.Plikaytis, B. D., P. F. Holder, and G. M. Carlone. 1996. Program ELISA for Windows user's manual, version 1.00. Centers for Disease Control and Prevention, Atlanta, GA.
- 35.Ross, J. M. 1957. The pathogenesis of anthrax following the administration of spores by the respiratory route. J. Pathol. Bacteriol. 73485-494. [Google Scholar]
- 36.Scorpio, A., D. J. Chabot, W. A. Day, D. K. O'Brien, N. J. Vietri, Y. Itoh, M. Mohamadzadeh, and A. M. Friedlander. 2007. Poly-γ-glutamate capsule-degrading enzyme treatment enhances phagocytosis and killing of encapsulated Bacillus anthracis. Antimicrob. Agents Chemother. 51215-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semenova, V. A., E. Steward-Clark, K. L. Stamey, T. H. Taylor, Jr., D. S. Schmidt, S. K. Martin, N. Marano, and C. P. Quinn. 2004. Mass value assignment of total and subclass immunoglobulin G in a human standard anthrax reference serum. Clin. Diagn. Lab. Immunol. 11919-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shieh, W. J., J. Guarner, C. Paddock, P. Greer, K. Tatti, M. Fischer, M. Layton, M. Philips, E. Bresnitz, C. P. Quinn, T. Popovic, B. A. Perkins, and S. R. Zaki, and the Anthrax Bioterrorism Investigation Team. 2003. The critical role of pathology in the investigation of bioterrorism-related cutaneous anthrax. Am. J. Pathol. 1631901-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soehnlein, O., E. Kenne, P. Rotzius, E. E. Eriksson, and L. Lindbom. 2008. Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages. Clin. Exp. Immunol. 151139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang, S., M. Moayeri, Z. Chen, H. Harma, J. Zhao, H. Hu, R. H. Purcell, S. H. Leppla, and I. K. Hewlett. 2009. Detection of anthrax toxin by an ultrasensitive immunoassay using europium nanoparticles. Clin. Vaccine Immunol. 16408-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turk, B. E. 2007. Manipulation of host signaling pathways by anthrax toxins. Biochem. J. 402405-417. [DOI] [PubMed] [Google Scholar]
- 42.van Faassen, H., R. KuoLee, G. Harris, X. Zhao, J. W. Conlan, and W. Chen. 2007. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect. Immun. 755597-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vietri, N. J., B. K. Purcell, S. A. Tobery, S. L. Rasmussen, E. K. Leffel, N. A. Twenhafel, B. E. Ivins, M. D. Kellogg, W. M. Webster, M. E. Wright, and A. M. Friedlander. 2009. A short course of antibiotic treatment is effective in preventing death from experimental inhalational anthrax after discontinuing antibiotics. J. Infect. Dis. 199336-341. [DOI] [PubMed] [Google Scholar]
- 44.Walsh, J. J., N. Pesik, C. P. Quinn, B. Urdaneta, C. A. Dykewicz, A. E. Boyer, J. Guarner, P. Wilkins, K. J. Norville, J. R. Barr, S. R. Zaki, J. B. Patel, S. P. Reagan, J. L. Pirkle, T. A. Treadwell, N. R. Messonnier, L. D. Rotz, R. F. R. F. Meyer, and D. S. Stephens. 2007. A case of naturally acquired inhalation anthrax: clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin. Infect. Dis. 44968-971. [DOI] [PubMed] [Google Scholar]
- 45.Wang, W., C. Mulakala, S. C. Ward, G. Jung, H. Luong, D. Pham, A. J. Waring, Y. Kaznessis, W. Lu, K. A. Bradley, and R. I. Lehrer. 2006. Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J. Biol. Chem. 28132755-32764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welkos, S. L., R. W. Trotter, D. M. Becker, and G. O. Nelson. 1989. Resistance to the Sterne strain of B. anthracis: phagocytic cell responses of resistant and susceptible mice. Microb. Pathog. 715-35. [DOI] [PubMed] [Google Scholar]
- 47.Xu, L., and D. M. Frucht. 2007. Bacillus anthracis: a multi-faceted role for anthrax lethal toxin in thwarting host immune defenses. Int. J. Biochem. Cell Biol. 3920-24. [DOI] [PubMed] [Google Scholar]
- 48.Xu, Q., E. D. Hesek, and M. Zeng. 2007. Transcriptional stimulation of anthrax toxin receptors by anthrax edema toxin and Bacillus anthracis Sterne spore. Microb. Pathog. 4337-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zahorec, R. 2001. Ratio of neutrophil to lymphocyte counts: rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy. 1025-14. [PubMed] [Google Scholar]