Abstract
Pneumocystis infection causes increased intracellular levels of reactive oxygen species (ROS) and the subsequent apoptosis of alveolar macrophages (Amø). Assessments of key prosurvival molecules in Amø and bronchoalveolar lavage fluids from infected rats and mice showed low levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) and reduced activation of phosphoinositide-3 kinase (PI-3K). Ubiquitous calcium-sensing protein calmodulin protein and mRNA levels were also reduced in Amø during Pneumocystis pneumonia (Pcp). Calmodulin has been implicated in control of GM-CSF production and PI-3K activation in other immune cell types. Experiments to determine the control of GM-CSF and PI-3K by calmodulin in Amø showed that GM-CSF expression and PI-3K activation could not be induced when calmodulin was inhibited. Calmodulin inhibition also led to increased levels of ROS and apoptosis in cells exposed to bronchoalveolar lavage fluids from infected animals. Supplementation of Amø with exogenous calmodulin increased survival signaling via GM-CSF and PI-3K and reduced ROS and apoptosis. These data support the hypotheses that calmodulin levels at least partially control survival signaling in Amø and that restoration of GM-CSF or PI-3K signaling will improve host response to the organism.
Alveolar macrophages (Amø) are an important cell type for the clearance of Pneumocystis carinii, P. murina, and P. jiroveci organisms from the lungs of animals and humans (33, 35, 38). Loss of Amø renders animals susceptible to Pneumocystis pneumonia (Pcp) (47), while increased Amø numbers retard progression of the disease (33; M. E. Lasbury submitted for publication). Low Amø numbers in animals with Pcp are caused by increased apoptosis, which is related to the catabolism of intracellular polyamines and production of hydrogen peroxide (35, 37). Reduced survival pathway signaling and antioxidant expression also contribute to the apoptosis of Amø during Pcp (Lasbury, submitted). Elucidation of the mechanisms of reduced apoptotic resistance is necessary to design immunomodulatory therapies to increase the host response to the organism.
Many systems that combat apoptotic stimulation via reactive oxygen species (ROS) exist in mammalian cells, including granulocyte-macrophage colony-stimulating factor (GM-CSF) and phosphoinositide kinase 3 (PI-3K). GM-CSF has antiapoptotic and anti-Pneumocystis effects. Previous studies have shown that GM-CSF knockout mice are prone to Pcp (54) and that GM-CSF is involved in the adaptive immune response to Pneumocystis through augmentation in the killing ability of CD8+ T lymphocytes (43) and expansion of CD4+ populations (51). GM-CSF overexpression in a CD4+ T-lymphocyte-depleted, GM-CSF−/− mouse model of Pcp resulted in less inflammation and reduced infection at 4 weeks (49), showing that GM-CSF also plays a role in the innate immune response to the organism.
Phosphatidylinositol(3,4,5)-triphosphate, the product of PI-3K enzymatic activity, mediates Akt-1 (also called protein kinase B) (1, 18, 29) activation. Akt-1 controls many prosurvival functions (9, 10, 11, 13, 23), making PI-3K activation a linchpin of survival signaling. Studies indicate that GM-CSF participates in the control of active phospho-PI-3K (pPI-3K) levels. Induction of PI-3K activation is lost if the cells are not pretreated with GM-CSF (30), and GM-CSF activates neutrophils via PI-3K (26). Therefore, mechanisms that control GM-CSF production may also control survival signaling.
Both GM-CSF expression and PI-3K activation are linked to the ubiquitous calcium-sensing molecule calmodulin. However, calmodulin can both stimulate and inhibit these molecules, depending on the cellular environment. For example, the action of a calmodulin-dependent phosphatase, calcineurin, is required for GM-CSF transcription in T lymphocytes (61), but elimination of a calmodulin-dependent kinase II binding site in the Ets1 transcription factor actually enhanced GM-CSF transcription in T cells (39). Similarly, inhibition of calmodulin prevents PI-3K-mediated phosphorylation of phosphatidylinositol in Chinese hamster ovarian (CHO) cell lysates (24), but calmodulin controls the PI-3K-mediated downstream phosphorylation of Raf1 at Ser338, which is critical for Raf1 activation in green monkey kidney cells (44). The role of calmodulin and the downstream enzymes that are dependent on it in Amø GM-CSF expression and PI-3K activation has not been investigated.
In the current study, we hypothesized that Amø apoptosis during Pcp involves GM-CSF and the calmodulin-mediated mechanisms that control it. We also theorized that changes in calmodulin and GM-CSF levels would affect downstream antiapoptotic molecules, such as PI-3K. We found that GM-CSF, calmodulin, and pPI-3K levels were low in Amø and bronchoalveolar lavage (BAL) fluids from rats and mice with Pcp. A calmodulin inhibitor reduced Amø expression of GM-CSF and PI-3K activation. Amø incubated with BAL fluids from Pneumocystis-infected animals had higher levels of ROS and apoptosis when calmodulin activity was inhibited. Exogenous calmodulin introduced into the Amø reduced ROS and apoptosis, while also increasing GM-CSF and PI-3K activation. These data indicate that Pcp-induced downregulation of calmodulin plays a role Amø susceptibility to apoptosis during infection via GM-CSF and PI-3K.
MATERIALS AND METHODS
Reagents and antibodies.
An enzyme-linked immunosorbent assay (ELISA) for the measurement of total PI-3K and pPI-3K (Active Motif, Carlsbad, CA) and GM-CSF (R&D Systems, Minneapolis, MN) for rats and mice was performed on Amø lysates in accordance with the manufacturers’ instructions. Antibodies against the following proteins were obtained from Abcam (Cambridge, MA): rat and mouse cleaved caspase-3, rat and mouse calmodulin, and rat and mouse GM-CSF. Anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) was purchased from Research Diagnostics (Flanders, NJ). The calmodulin inhibitor W-7 [N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide·HCl] was purchased from Biomol (Plymouth Meeting, PA), and the PI-3K inhibitor wortmannin was purchased from Calbiochem (San Diego, CA), while the antioxidant N-acetylcysteine (NAC) and bovine testis calmodulin protein were obtained from Sigma-Aldrich (St. Louis, MO). Caspase-9 inhibitor was purchased from R&D Systems. Chariot reagent for protein transfection was purchased from Active Motif. The fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) for assessment of ROS levels and cell culture reagents were purchased from Invitrogen (Carlsbad, CA).
Rodent models of Pcp and isolation of Pneumocystis organisms.
All rodents (120- to 140-g Sprague-Dawley rats and 18- to 20-g BALB/c mice) used were females, obtained from Harlan (Indianapolis, IN), and were given antibiotics as previously described to prevent extraneous infections (5, 34). Mice were immunosuppressed by weekly intraperitoneal injection of 0.3 mg anti-CD4 monoclonal antibody (clone GK1.5; Harlan, Indianapolis, IN) and transtracheally inoculated with purified Pneumocystis murina organisms as previously described (31, 34). Rats were treated continuously with 1.8 mg/liter dexamethasone (Dex) in drinking water and transtracheally inoculated with Pneumocystis carinii organisms as previously described (5). Animal studies were approved by the Indiana University Animal Care and Use Committee and carried out under the supervision of veterinarians. Animals were housed in the Indiana University Laboratory Animal Resource Center, an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility.
Isolation of Amø and BAL fluids.
Five milliliters of sterile saline was used to lavage each rat (1 ml for mice) as previously described (34). Cells and organisms were removed from the BAL fluids by centrifugation at 300 × g for 10 min, and the clarified BAL fluids were stored at −70°C until used. Amø were isolated from BAL fluids, identified, and enumerated as previously described (31). Purified Amø were used immediately for protein or RNA isolation or were incubated in complete medium (RPMI 1640 supplemented with 10% fetal bovine serum, 1 mM pyruvate, 1% nonessential amino acids, 14 mM glucose, 17.9 mM NaHCO3, 10 mM HEPES, 100 U/ml penicillin, and 0.1 mg/ml streptomycin) or BAL fluids containing live organisms, heat-killed organisms (1 h at 96°C), or chemical modulators for specific time periods in 24-well plates.
Calmodulin was introduced into Amø by first complexing the protein (1 μg in 100 μl phosphate-buffered saline, pH 7.6) with 0.6 μl Chariot reagent in 100 μl H2O for 30 min at 25°C and then layering the complex over 1 ×106 Amø in a well of a six-well plate. Serum-free medium (400 μl) was added to each well, and the plates were incubated for 3 h at 37°C and 5% CO2. Test solutions were then added, and the plates were incubated overnight. The cells were released from the tissue culture plastic with xylocaine as previously described (35) and harvested by gentle scraping.
RNA isolation and real-time reverse transcription-PCR.
Total RNA was isolated from Amø with the TRIzol reagent according to the manufacturer's instructions (Invitrogen). RNA concentration and purity were determined by spectrophotometry. First-strand cDNA was synthesized from the total RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) primed by oligo(dT) and random primers; 0.2 μg of total RNA was used for each reaction, with a total reaction volume of 20 μl. Two microliters of each cDNA product was used for quantitative PCR analysis. Real-time reverse transcription-PCR for rat and mouse calmodulin 1, 2, and 3 genes was performed using Assays-on-Demand gene expression kits (Applied Biosystems, Foster City, CA) on a Smartcycler (Cepheid, Sunnyvale, CA). Ribosomal protein S8 (RPS8) mRNA was assayed in an identical manner as a control, since its mRNA levels are not affected by the infection (65). Primers for RPS8 PCR were as described previously (65). Data were normalized to RPS8 mRNA levels in each sample and were shown as increases relative to levels for control cells from uninfected animals.
Flow cytometry.
To determine ROS levels in Amø during Pcp, the cells were incubated with 10 μM of H2DCFDA and fluorescence was examined. H2DCFDA, a cell-permeable indicator for ROS, is cleaved to a nonfluorescent diol by intracellular esterases and then oxidized by ROS to a fluorescent form. Cells were analyzed on an Epics 500 flow cytometer (Beckman Coulter, Miami, FL) with a forward and side scatter gate applied to select Amø. The median whole-cell fluorescein isothiocyanate fluorescence of oxidized H2DCFDA was captured.
Immunoblotting.
Amø isolated from animals were resuspended in 100 μl cold (4°C) cell lysis buffer (150 mM NaCl, 1.0% Triton X-100, 1% deoxycholate, 5 mM EDTA, 10 mM Tris, pH 7.2) containing 1% protease inhibitor cocktail (Sigma) and sonicated four times for 10 s each on ice. Soluble protein concentration was determined by using the Coomassie Plus protein reagent (Pierce, Rockford, IL) in accordance with the manufacturer's instructions. Equal amounts of soluble protein from each condition were electrophoresed in a 10% polyacrylamide gel and then transferred onto a polyvinylidene difluoride membrane using the NuPAGE system (Invitrogen). Membranes were blocked and then reacted with the primary antibody in blocking buffer for 2 h. After a washing, the blots were reacted with secondary antibody anti-mouse immunoglobulin G or anti-rabbit immunoglobulin G conjugated with horseradish peroxidase for 1 h at 25°C, followed by chemiluminescence analysis with the ECL Plus reagent kit (Amersham Biosciences, Piscataway, NJ). Densitometry analysis of results on X-ray films was performed by using ImageJ software (http://rsb.info.nih.gov/ij/).
Statistics.
Data are presented as means ± standard deviations (SD). Differences between groups were determined using the two-tail Student t test and were considered statistically significant if P was <0.05. Comparisons between three or more treatment groups were made by one-way analysis of variance (ANOVA). Comparison tests as follow-up to ANOVA included both Bonferroni's test and multiple paired t tests for groups identified as statistically different. A 5% significance level was used for all ANOVA tests and posttests.
RESULTS
GM-CSF levels in the BAL fluids of rats and mice with Pcp.
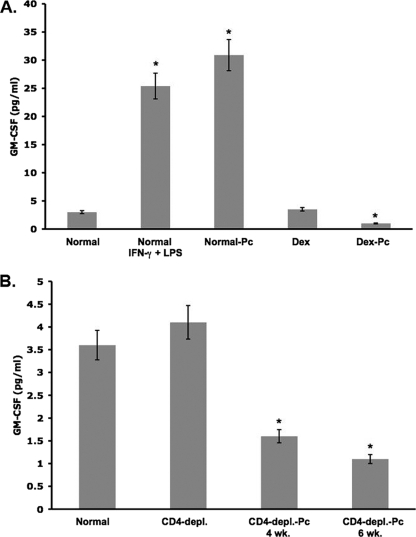
To determine whether Pneumocystis infection affects GM-CSF production, we assessed the levels of this growth factor in the BAL fluids of rats and mice with Pcp. Alveolar lining fluids from control and infected animals were recovered by BAL. Dilution of the alveolar lining fluid was minimized by lavaging with small volumes of sterile saline (5 ml for rats, 1 ml for mice). In an ELISA, normal rat BAL fluids showed low levels of GM-CSF. BAL fluids from Dex-treated rats had similar levels of GM-CSF, indicating that immunosuppression did not significantly alter levels (Fig. 1A). GM-CSF production was found to be greatly increased (eightfold) in immunocompetent rats 48 h after transtracheal instillation of 1 μg gamma interferon (IFN-γ) (48) and 0.5 μg lipopolysaccharide (LPS) (40). Transtracheal inoculation of normal rats with 7.8 × 106 P. carinii organisms also greatly induced (10-fold increase) GM-CSF production, measured 7 days after the inoculation. However, 4 weeks of Pneumocystis infection reduced the BAL fluid GM-CSF levels by 67% compared to the values for normal rats (P < 0.05) (Fig. 1A).
FIG. 1.
Levels of GM-CSF in BAL fluid. Soluble rat or mouse BAL fluid proteins were assessed by ELISA for total GM-CSF. All GM-CSF values are averages ± SD from triplicate samples of six individual rats or eight individual mice from each condition. (A) Normal rats were immunocompetent, Dex-treated rats were immunosuppressed with Dex, Dex-Pc rats were immunosuppressed and Pneumocystis infected for 4 weeks. Normal-Pc rats were immunocompetent, transtracheally inoculated with P. carinii, and sacrificed 7 days later. Normal IFN-γ + LPS rats were intranasally instilled with 1 μg IFN-γ and 0.5 μg LPS and were sacrificed 48 h later. (B) The same ELISA kit was used to assess soluble Amø protein from mice. Normal mice were immunocompetent, CD4-depl. mice were immunosuppressed by anti-CD4 antibody administration, and CD4-depl.-Pc mice were immunosuppressed and infected for 4 or 6 weeks. * (both panels), P < 0.05 versus the normal condition.
The infection had a similar effect on GM-CSF production in mice. Figure 1B shows that GM-CSF levels in BAL fluids from immunocompetent mice were much higher than those in BAL fluids from mice infected with Pneumocystis for either 4 or 6 weeks (56% decrease at 4 weeks and 69.5% at 6 weeks; P < 0.05 for both time points versus normal). Immunosuppression by depletion of CD4+ lymphocytes did not have a significant effect on GM-CSF levels; therefore, the infection was responsible for the losses noted.
GM-CSF levels in Amø from Pneumocystis-infected rats and mice.
Amø are a significant source of GM-CSF in the lung. To determine if a loss of Amø GM-CSF production contributes to the low BAL fluid GM-CSF levels, we measured GM-CSF levels in Amø from control and infected animals. In the sample of Amø from normal rats, a single 14-kDa GM-CSF band was noted, as shown in Fig. 2A for a representative of six rats tested. In protein samples of Amø from Dex-treated rats, two GM-CSF bands were noted and the total signal was slightly increased (Fig. 2A) (P > 0.05 versus normal). In blots of Amø protein from Dex-treated, Pneumocystis-infected (Dex-Pc) rats, only the higher-molecular-weight band was observed but the intensity of the band was low. The average signal was reduced by 65% (P < 0.05 versus normal) (Fig. 2A), as assessed by image analysis of independent immunoblots of four individual rats for each condition. Previous results indicate that doublet or variable-size bands for GM-CSF represent changes in glycosylation of the protein (46). Our results suggest that the infection can induce changes in GM-CSF glycosylation.
FIG. 2.
GM-CSF protein in Amø during Pcp. Soluble proteins from rat or mouse Amø were blotted and probed for GM-CSF. (A) Conditions for rats are as described for normal, Dex-treated, and Dex-Pc rats in the Fig. 1 legend. (B) Conditions for mice are as described in the legend of Fig. 1. For both panels, representative immunoblots are shown from four trials of individual rats and three independent trials of Amø pooled from three animals for each condition for mice. Changes from normal values are average signal strengths ± SD for all trials, after normalization of GM-CSF to the GAPDH control signal. *, P < 0.05 versus normal condition.
In the mouse model of infection, immunosuppression by CD4 lymphocyte depletion did not have a significant effect on Amø GM-CSF levels. However, Western blotting of the sample from pooled Amø of three mice for each condition showed a 69% to 71% decrease in the amount of GM-CSF after 4 or 6 weeks of infection (Fig. 2B). Image analysis of immunoblots from three independent trials showed that the decreases at both time points were significant (P < 0.05 versus normal) (Fig. 2B). The immunoblot results revealed that Amø proteins from normal mice had a doublet of GM-CSF bands, while those from infected mice showed only the lower band of the doublet.
GM-CSF in caspase-9 inhibitor-treated rats.
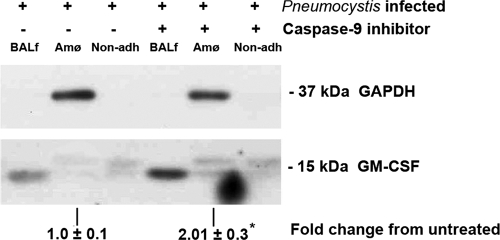
Caspase-9 inhibitor treatment has been shown to increase Amø number in rats and mice with Pcp (33). To obtain further evidence for the importance of Amø in alveolar GM-CSF production, a caspase-9 inhibitor was used to treat rats with Pcp. After 3 weeks of treatment, Amø, nonadherent cells, and cell-free BAL fluids were isolated from six untreated, infected rats and six caspase-9 inhibitor-treated infected rats. No Dex control was used, as both the untreated and treated groups were Dex treated and since previous results indicated that Dex had no effects on Amø apoptosis (33). Counting of Amø revealed that caspase-9 inhibitor treatment increased the numbers of the cells by 58.4% ± 4.1% ([1.94 ± 0.2] × 106 and [3.32 ± 0.1] × 106 cells for untreated and caspase-9 inhibitor-treated animals, respectively), which agreed well with our previous results (33). Protein samples from each rat were probed for GM-CSF. A representative immunoblot result is shown in Fig. 3. With suppression of Amø apoptosis and recovery of Amø numbers by caspase-9 inhibitor treatment (33), levels of GM-CSF in the cell-free BAL fluid and in the Amø were increased in all trials. Image analysis of the GM-CSF signal from Amø, normalized to the GAPDH signal, indicated a greater-than-twofold increase in cellular GM-CSF levels (P < 0.05 versus untreated control) (Fig. 3). Nonadherent cell fractions from untreated and caspase-9 inhibitor-treated animals had similar levels of GM-CSF, suggesting that they contribute similar amounts of the growth factor. Image analysis was not carried out for BAL fluid or nonadherent fractions because no normalizing GAPDH signal was detected; however, Fig. 3 shows a distinct increase in the level of BAL fluid GM-CSF between the samples and little or no change in GM-CSF signal in the nonadherent fraction. This result suggests that Amø are a significant source of GM-CSF in BAL fluids and that Pcp-mediated effects on Amø are responsible for the low GM-CSF levels seen during pneumonia.
FIG. 3.
GM-CSF protein level after caspase-9 inhibition. Dex-Pc rats were treated with caspase-9 inhibitor (33) or diluent only for 3 weeks. BALf samples are cell-free BAL fluids. Amø were isolated as described in Materials and Methods. Non-adh refers to the nonadherent fraction of the BAL fluid particulate matter after isolation of Amø. Blotted proteins were probed for GM-CSF and GAPDH. GAPDH was detected in Amø fractions only. The BAL fluid fraction was cell free, and the cell number in the non-adh fraction was too low to allow for detection of GAPDH. The change in GM-CSF signal strength in Amø, normalized to the GAPDH signal, was calculated from the average ± SD. Results are representative of three separate trials, each trial composed of samples pooled from two rats for each condition. *, P < 0.05 versus untreated condition.
PI-3K activity levels in Amø from rats and mice with Pcp.
GM-CSF has been shown to control PI-3K activation (26, 30). Low GM-CSF levels in the lungs and Amø of animals with Pcp may suggest that PI-3K activation (phosphorylation) is low in Amø during Pcp. To test this possibility, total PI-3K and pPI-3K levels in Amø from rats and mice with Pcp were determined by ELISA.
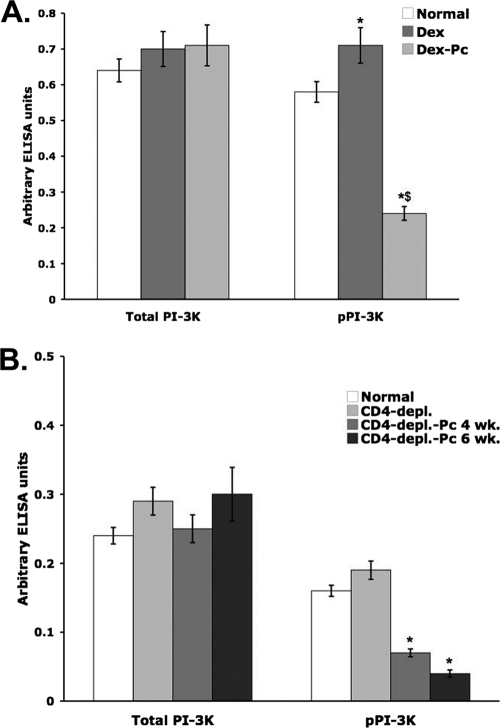
After 4 weeks, both immunosuppression alone and Pcp conditions produced small but significant increases in total Amø PI-3K protein levels (Fig. 4A). The level of pPI-3K was also significantly higher in Amø from Dex-treated rats, but Pcp negatively affected the activation of PI-3K. The pPI-3K level in Amø from Dex-Pc rats was 65.8% lower than in Amø from normal rats (Fig. 4A) (P < 0.05), indicating that the infection inhibits the activation of the PI-3K pathway but not the expression of PI-3K protein.
FIG. 4.
Levels of total PI-3K and pPI-3K in Amø during Pcp. Soluble rat or mouse Amø proteins were assessed by ELISA for total PI-3K and active PI-3K (pPI-3K). Triplicate samples were assessed for each of six individual rats or eight individual mice, and average values ± SD for arbitrary ELISA units are shown. (A) Conditions were as described for normal, Dex-treated, and Dex-Pc rats in the Fig. 1 legend. (B) The same ELISA kit was used to assess soluble Amø protein from mice. Conditions for mice were as described in the legend of Fig. 1. * (both panels), P < 0.05 versus the normal condition for the same target, total PI-3K or pPI-3K; $, P < 0.05 versus the Dex pPI-3K condition.
In the CD4-depleted-mouse model of Pcp, immunosuppression caused increased levels of total PI-3K and pPI-3K, but the changes did not reach the level of statistical significance. After 4 weeks of infection, there were small increases in total PI-3K protein, but these increases were smaller than the changes in the rat model and not statistically significant. In contrast, there was a 63.2% decrease in pPI-3K levels 4 weeks after initiation of Pcp in mice. An additional 15.8% decrease was observed at 6 weeks of infection (Fig. 4B) (P < 0.05 for both time points versus the CD4-depleted-mouse model).
Calmodulin mRNA levels in Amø from rats and mice with Pcp.
Calmodulin has been implicated in the control of both GM-CSF expression and PI-3K activation; therefore, the low levels of GM-CSF and PI-3K activation in Amø during Pcp suggest a defect in calmodulin levels or activity. To investigate this possibility, we first assessed calmodulin mRNA levels in Amø from rats and mice.
Mammalian calmodulin is encoded by three separate genes (CaM1, CaM2, and CaM3), but their gene products are identical (45). The mRNAs from the three different genes can be discerned by real-time PCR with appropriate probes based on sequence differences in codon usage (66). Differences in mRNA levels were assessed in six individual rats for each condition in triplicate reactions, and the average difference ± SD from normal was calculated after normalization to the RPS8 mRNA level, which does not change during Pcp (65). Amø from Dex-treated rats did not show a significant difference in mRNA levels for any of the three calmodulin genes; however, Dex-Pc rat Amø had lower levels of all three mRNAs (Table 1). The changes in CaM1 gene mRNA levels did not reach statistical significance, but changes in CaM2 (70% decrease) and CaM3 (40% decrease) gene mRNA levels were significant (P < 0.05 versus normal).
TABLE 1.
Calmodulin mRNA levels in Amø during Pcp
| Species | Conditiona | mRNA levelb for:
|
||
|---|---|---|---|---|
| CaM1 | CaM2 | CaM3 | ||
| Rat | Normal | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| Rat | Dex treated | 1.3 ± 0.3 | 0.9 ± 0.1 | 0.9 ± 0.4 |
| Rat | Dex-Pc | 0.7 ± 0.2 | 0.3 ± 0.2c | 0.6 ± 0.2c |
| Mouse | Normal | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| Mouse | CD4 depl. | 1.1 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.1 |
| Mouse | CD4 depl. Pc | 0.7 ± 0.4 | 0.4 ± 0.3c | 0.4 ± 0.2c |
Normal, immunocompetent animals; Dex treated and CD4 depl., immunosuppressed animals; Dex-Pc and CD4 depl. Pc, immunosuppressed and Pneumocystis-infected animals.
Results are averages ± SD for six rats or eight mice for each condition.
P < 0.05 versus normal condition for the same species.
In the mouse model, CD4 depletion did not change mRNA levels for any of the three genes, based on average threshold cycle values (±SD) from triplicate reactions for five individual mice. Mice infected for 4 weeks had large decreases in CaM2 and CaM3 mRNA levels (60% decreases for each; P < 0.05 for each versus normal) (Table 1). Levels of CaM1 mRNA were also decreased, but, as was the case for rats, this change did not reach the level of statistical significance.
To determine if the factor that negatively affects calmodulin mRNAs was present in the alveolar lining fluid, normal rat Amø were incubated with BAL fluids from control or infected rats for 18 h and then assessed for CaM mRNA levels. Real-time PCR for each of the three calmodulin genes showed that only BAL fluids from infected rats were capable of decreasing the calmodulin mRNA levels (Table 2). Similar to Amø from infected animals, Amø incubated with BAL fluids from infected animals showed a significant decrease in the CaM2 (50% decrease) and CaM3 (30% decrease) mRNA levels (P < 0.05) (Table 2). CaM1 mRNA levels were low, but not significantly so, also similar to what was found for Amø from infected animals. BAL fluids from Dex-treated but uninfected rats did not alter calmodulin mRNA levels, indicating that Dex was not responsible for the altered calmodulin levels (Table 2), and similar results were obtained from infected rats and infected mice despite different immunosuppressive regimens, suggesting that the method of immunosuppression did not play a role in the effect.
TABLE 2.
Effects of BAL fluids on Amø calmodulin mRNAs
| BAL fluid sourcea | mRNA levelb for:
|
||
|---|---|---|---|
| CaM1 | CaM2 | CaM3 | |
| Normal | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| Dex treated | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.0 |
| Dex-Pc | 0.8 ± 0.2 | 0.5 ± 0.2c | 0.7 ± 0.2c |
Normal, immunocompetent animals; Dex treated, immunosuppressed animals; Dex-Pc, immunosuppressed, Pneumocystis-infected animals.
Results are averages ± SD for six independent assays of each condition.
P < 0.05 versus normal condition for the same species.
Since remnants of lysed and dead organisms are present in cell-free BAL fluids, we also sought to determine if these were responsible for the calmodulin defects. Normal Amø (1 × 106) were incubated with viable or heat-killed P. carinii organisms (5 × 106) for 18 h before real-time PCR determination of calmodulin mRNA levels. In six independent incubation trials, heat-killed organisms had no effect on the mRNA levels from any of the CaM genes. Likewise, incubation of viable organisms with Amø for 18 h did not alter the Amø calmodulin mRNA levels (P > 0.05 for CaM1, CaM2, and CaM3 versus the no-organism control; n = 6).
Calmodulin protein levels in Amø from rats and mice with Pcp.
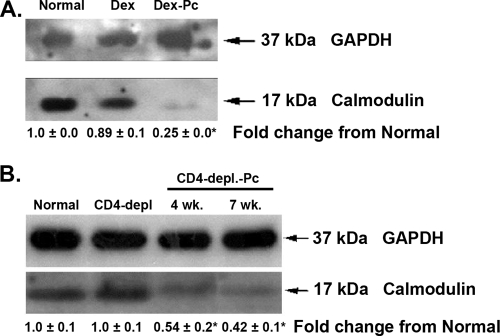
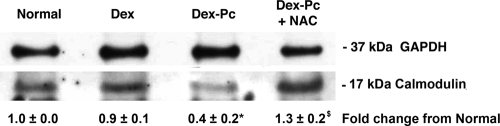
We next determined if reduced calmodulin mRNA levels were indicative of reduced calmodulin protein levels. Rat Amø proteins were blotted and probed for calmodulin. With GAPDH probing to ensure equal protein loading from each condition, the calmodulin signals for normal rats, immunosuppressed rats, and rats with Pcp were compared. As shown in Fig. 5A, Amø from normal and Dex-treated rats had similar levels of calmodulin protein, but Dex-Pc rat Amø had very low levels of calmodulin.
FIG. 5.
Calmodulin protein in Amø during Pcp. Soluble proteins from rat or mouse Amø were blotted and probed for calmodulin. (A) Conditions are as described for normal, Dex-treated, and Dex-Pc rats in the Fig. 1 legend. (B) Conditions for mice are as described in the legend of Fig. 1, except that mice were assessed after 4 and 7 weeks of infection. For both panels, representative immunoblots are shown from four trials for individual rats and three independent trials of Amø pooled from three animals for each condition for mice. Changes from normal values are average signal strengths ± SD for all trials, after normalization of GM-CSF to the GAPDH control signal. *, P < 0.05 versus the normal condition.
Representative immunoblots of mouse Amø showed a similar decrease in calmodulin levels. Amø from normal and CD4-depleted mice had high levels of calmodulin, while Amø from infected mice, at both 4 weeks and 7 weeks of infection, showed substantial decreases in the level of calmodulin (Fig. 5B). At 4 weeks of infection, the number of Amø in mice with Pcp had not dropped significantly (35), but the loss of calmodulin correlates well with the level of apoptosis noted in these cells during Pcp (33, 35).
NAC treatment of Amø alters calmodulin levels.
Low levels of calmodulin in the Amø could be due solely to decreased expression. The results showing low calmodulin mRNA levels (Table 1) and protein levels (Fig. 5) suggest that downregulation of expression contributes to the decrease. However, increased calmodulin degradation by the 26S proteasome through oxidation of methionines by ROS would also contribute to lower calmodulin levels (16, 59). To investigate whether ROS may contribute to low calmodulin levels, we treated rat Amø with BAL fluids from normal, Dex-treated, or Dex-Pc rats for 18 h. Some Dex-Pc rat BAL fluids were supplemented with 2.5 mM NAC. As shown in Fig. 6, Amø incubated with BAL fluids from Dex-Pc rats had 60% lower levels of calmodulin than Amø incubated with BAL fluids from normal or immunosuppressed animals. NAC treatment resulted in significantly increased calmodulin protein levels in the Amø, a 3.2-fold increase compared to Amø that did not get the oxidant, and 1.3-fold higher than the level in control macrophages (P < 0.05 versus control) (Fig. 6). This result shows that calmodulin protein levels are positively affected by reduction of ROS in the Amø, so ROS may contribute to the low calmodulin levels in Amø during Pcp.
FIG. 6.
Calmodulin in rat Amø after treatment with antioxidant. Normal Amø were incubated with BAL fluids from normal, Dex-treated, or Dex-Pc rats for 18 h. Some Amø were incubated with BAL fluids from Dex-Pc rats supplemented with 2.5 mM NAC, an antioxidant. Soluble Amø proteins were probed for GAPDH and calmodulin. The change in calmodulin signal strength, normalized to the GAPDH signal, was calculated from the average ± SD. Signal strength values were from three individual trials. *, P < 0.05 versus Amø incubated with normal rat BAL fluids; $, P < 0.05 versus Amø incubated with Dex-Pc rat BAL fluids.
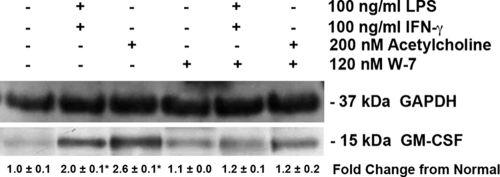
Effects of calmodulin inhibition on GM-CSF.
Our results described above suggest that calmodulin and GM-CSF levels are low in Amø during Pcp. Published results suggest that calmodulin can control GM-CSF expression in some cell types (61). Therefore, we tested if inhibition of calmodulin could affect GM-CSF production in Amø. Normal rat Amø were incubated with the calmodulin inhibitor W-7 (120 nM) for 30 min (58), with subsequent addition of saline or 100 ng/ml LPS and IFN-γ (36) or 200 nM acetylcholine (53) for 2 h to induce GM-CSF production. Total cellular protein was then probed for GM-CSF on immunoblots. The growth factor was induced in Amø incubated with IFN-γ/LPS or with acetylcholine, resulting in a twofold increase in GM-CSF signal when normalized for GAPDH signal (P < 0.05 versus unstimulated Amø) (Fig. 7). However, no increase in GM-CSF was noted when Amø were pretreated with the calmodulin inhibitor prior to stimulation with IFN-γ/LPS or acetylcholine (P > 0.05 versus unstimulated Amø) (Fig. 7). These results indicate that calmodulin controls GM-CSF levels in Amø, as in other cell types (55, 61).
FIG. 7.
Amø GM-CSF protein levels after calmodulin inhibition. Amø from normal rats were incubated with saline, IFN-γ, LPS, or acetylcholine (Ach) for 2 h at the concentrations stated. Some samples were also treated with the calmodulin inhibitor W-7. Soluble Amø proteins were probed for GAPDH and GM-CSF detection. The change in GM-CSF signal strength, normalized to GAPDH signal, was calculated from the average ± SD. Signal strength values were from three individual trials; each trial consisted of Amø pooled from four rats. *, P < 0.05 versus Amø incubated with saline.
Effects of calmodulin on apoptosis of Amø incubated with Dex-Pc rat BAL fluids.
Amø were incubated with BAL fluids from immunosuppressed or infected rats, with or without a calmodulin inhibitor. In each of three trials, BAL fluids from Dex-treated rats did not increase apoptosis or decrease pPI-3K levels. BAL fluids from infected rats had a significant effect on apoptosis, as assessed by image analysis of activated caspase-3 immunoblots (increased 9.3-fold; P < 0.05 versus BAL fluids from Dex-treated rats). These same BAL fluids also reduced pPI-3K levels in Amø by 31.8% as measured by ELISA (P < 0.05 versus control).
Further inhibition of calmodulin by inclusion of the inhibitor W-7 significantly increased apoptosis (3.5-fold increase over that for Dex-Pc rat BAL fluid-treated samples; P < 0.05). pPI-3K levels were reduced an additional 39.1% by calmodulin inhibition in addition to Dex-Pc rat BAL fluid treatment (P < 0.05). These data suggest that calmodulin plays a role in survival signaling and apoptosis resistance. However, in each case, incubation with W-7 alone did not negatively affect apoptosis or pPI-3K levels, suggesting that calmodulin inhibition or low pPI-3K levels alone do not induce cell death in the absence of an apoptotic stimulus.
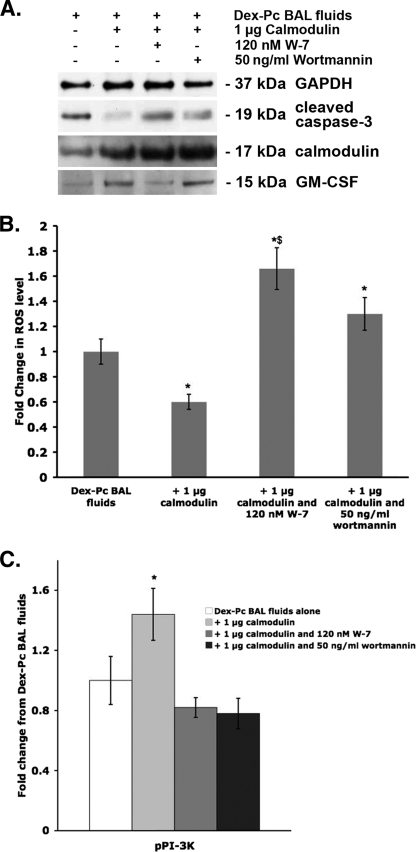
To confirm the importance of calmodulin in apoptosis of Amø during Pcp, we introduced exogenous calmodulin into Amø 3 hours prior to incubation with BAL fluids from infected rats. Some samples also included 120 nM calmodulin inhibitor W-7 or 50 ng/ml wortmannin (47), which inhibits PI-3K activity. Some cells were incubated with BAL fluids and Chariot reagent only as a control. Amø were harvested after 12 h and assessed for calmodulin, cleaved caspase-3, GAPDH, and GM-CSF levels, as well as intracellular ROS and pPI-3K. Amø from three wells of the same condition were pooled for Western blotting, while individual wells were used for ROS and pPI-3K assessment.
As shown in Fig. 8A, cells incubated with Dex-Pc rat BAL fluid had low levels of calmodulin and GM-CSF proteins but high levels of cleaved caspase-3. Chariot alone and other controls had no effect on the parameters assessed. Pretreatment of Amø with calmodulin increased intracellular calmodulin levels, as shown by the three calmodulin treatment lanes in Fig. 8A. Exogenous calmodulin also increased GM-CSF levels and decreased active caspase-3 levels, while inhibition of calmodulin eliminated these effects (Fig. 8A). This result indicates that calmodulin can affect GM-CSF production in Amø. In cells incubated with calmodulin and a PI-3K inhibitor, calmodulin and GM-CSF levels were unchanged but the levels of cleaved caspase-3 were increased (Fig. 8A). These results indicate that active PI-3K was necessary for inhibition of some apoptosis but did not control calmodulin or GM-CSF levels.
FIG. 8.
Effects of exogenous calmodulin on survival signaling and apoptosis. Normal rat Amø (1 × 106/well) were incubated with 1 μg calmodulin protein complexed to Chariot transfection reagent. After 3 h, the cells were exposed to Dex-Pc rat BAL fluids, and some were exposed to chemical inhibitors as shown. (A) Representative immunoblot analysis of Amø protein pooled from three wells for each condition. Two independent trials were conducted. (B) ROS levels were assessed as described in Materials and Methods. One well of Amø was used for assessment for each condition. Results are averages ± SD for two independent trials. *, P < 0.05 versus Amø treated with Dex-Pc rat BAL fluid alone; $, P < 0.05 versus Amø treated with Dex-Pc rat BAL fluids plus 1 μg calmodulin. (C) ELISA-based assessment of pPI-3K levels from individual wells of Amø treated as described for panel B. Results shown are average changes ± SD from two independent trials. The level of pPI-3K in Dex-Pc rat BAL fluid-treated cells was arbitrarily set to 1.0. *, P < 0.05 versus treatment with Dex-Pc rat BAL fluids alone; $, P < 0.05 versus treatment with Dex-Pc rat BAL fluids plus 1 μg calmodulin.
ROS levels were high in Amø incubated with BAL fluids from infected animals but were reduced by 42% when the cells were pretreated with calmodulin (P < 0.05 versus Dex-Pc rat BAL fluids alone) (Fig. 8B). Inhibition of calmodulin eliminated the suppressive effect of calmodulin on ROS production and resulted in a significantly higher level of ROS in these cells than in those incubated with Dex-Pc rat BAL fluids alone (P < 0.05 versus Dex-Pc rat BAL fluids only) (Fig. 8B). Inhibition of PI-3K with wortmannin also eliminated the positive effect of exogenous calmodulin, but not to the same degree as calmodulin inhibition. These data indicate that both calmodulin and pPI-3K play a role in control of ROS production.
Finally, pPI-3K levels were significantly increased with calmodulin transfection (increased 1.54-fold ± 0.2-fold; P < 0.05 versus Dex-Pc rat BAL fluid-treated Amø) (Fig. 8C). This increase was lost with inhibition of calmodulin or PI-3K (P > 0.05 versus Dex-Pc rat BAL fluid-treated Amø), suggesting that calmodulin controls PI-3K activation in Amø.
DISCUSSION
GM-CSF is important for the host response to Pneumocystis, including cytokine production (41) and clearance of organisms by Amø (51), but our data show that GM-CSF levels in BAL fluids (Fig. 1) and Amø (Fig. 2) are reduced during Pcp. Previous reports have suggested that fibroblasts and alveolar epithelial cells are producers of GM-CSF (7, 19). We have shown that suppression of Amø apoptosis by inhibition of caspase-9 activation leads to increased GM-CSF production (Fig. 3), suggesting that Amø are also an important source of GM-CSF in the lung. This implies that the loss of Amø due to apoptosis during Pcp (33, 35) has a deleterious effect on GM-CSF levels in the alveolar space, which in turn may contribute to Pcp progression. These data also suggest that the loss of Amø-produced GM-CSF is not compensated for through increased GM-CSF production by pulmonary or inflammatory cells, rendering the alveolar environment unable to respond to infection with normal GM-CSF signaling events.
Amø defects during Pcp are similar to those in primary alveolar proteinosis (PAP), a condition in which GM-CSF levels and actions are reduced. PAP is caused by circulating GM-CSF-neutralizing antibodies or defective GM-CSF receptor activity (14, 27). During both Pcp and PAP, phagocytosis and recycling of surfactant lipids and surfactant proteins by Amø are defective. Surfactant proteins and lipids build up in the alveolar spaces, and there are losses of Amø functions in both Pcp (4, 32, 35) and PAP (21).
Pcp is often found in association with secondary PAP (56, 60), which may be due to dysfunction in surfactant uptake or low GM-CSF levels, leading to decreased Amø survival. Administration of GM-CSF leads to increased macrophage survival (8, 20, 62) and organism clearance in neonate mice with Pcp (51). Data presented in this study correlating reduced GM-CSF levels with decreased Amø survival signaling, increased intracellular Amø ROS levels (Fig. 8), and increased apoptosis suggest that Pcp pathogenesis is linked to Amø survival.
Our data also show that PI-3K activation is low in Amø during Pcp (Fig. 4). This is important because PI-3K plays a central role in cell survival signaling (13), and survival signaling in Amø during Pcp is reduced (Lasbury, submitted). However, low PI-3K signaling is not the source of Amø apoptosis, since PI-3K inhibition by wortmannin alone does not induce apoptosis (52).
Since the calcium sensing protein calmodulin has been shown to be involved in the control of both GM-CSF levels (61) and PI-3K activation (44), we determined calmodulin levels in Amø from animals with Pcp. During Pcp, levels of calmodulin were down (Fig. 5), and this change was seen in both rat and mouse models of infection. We also assessed calmodulin mRNAs in Amø during Pcp. Calmodulin is coded for by three genes; they diverge in the 5′ and 3′ untranslated regions and other noncoding sequences but are translated to form the same protein (17). The CaM1 and CAM3 genes have more than one polyadenylation site, and a total of five calmodulin transcripts may be produced (45). Evidence shows that these transcripts are differentially regulated and transcribed because their promoter sequences possess different combinations of transcription factor binding sites (3, 22, 57). Only the CaM2 and CaM3 gene mRNA levels in Amø showed changes (Tables 1 and 2) during Pcp, suggesting that there is differential control of calmodulin gene transcription in Amø.
Aged calmodulin, that is, calmodulin with oxidized C-terminal methionine residues, is targeted for proteasomal degradation at a higher rate than reduced calmodulin or calmodulin bound by calcium (16, 59). Since exogenous antioxidants increased the levels of calmodulin in Amø (Fig. 6), the low calmodulin pools in Amø during Pcp may also be due to an increase in calmodulin degradation, not merely reduced production.
Cell-free BAL fluids from infected animals were capable of inducing the changes in calmodulin mRNA levels (Table 2), while purified organisms could not. Viability of the organisms was also not a factor in altering calmodulin mRNA levels; therefore, the molecule(s) responsible for downregulating the expression of calmodulin may be a soluble factor(s) from the host or organism. Previous reports have implicated β-glucan (15, 37), the major surface glycoprotein (6, 34), and polyamines from the organism (35, 37) in modulation of host cell functions. However, our data showed that incubation of organisms with Amø for 18 h did not induce changes in CaM mRNA levels, suggesting that, if an organism-excreted substance is responsible, the levels of this agent are not sufficiently high in the media after 18 h to produce change. Analysis of concentration differences of candidate molecules in BAL fluids from infected animals, which can induce CaM mRNA reductions, and conditioned media from Pneumocystis and Amø coincubations, which cannot alter CaM mRNA levels, may identify the responsible agent.
Calmodulin is important to the Amø response during Pcp, as shown by both inhibition (Fig. 7) and add-back experiments (Fig. 8). These are the first data showing that calmodulin mediates GM-CSF expression in Amø; this mediation was previously shown only in T lymphocytes and fibroblasts (55, 61). In our studies, calmodulin controlled GM-CSF levels (Fig. 7 and 8) and PI-3K activation (Fig. 8) and these changes correlated with changes in Amø ROS and apoptosis levels (Fig. 8). Suppression of apoptosis by calmodulin is a specific response to the apoptotic stimulus induced by Pcp or Dex-Pc animal BAL fluids, since calmodulin alone did not alter apoptosis or pPI-3K levels in the absence of an apoptotic stimulus (data not shown). Whether calmodulin addition mediates its effects as a general response to apoptosis was not assessed but is possible, since previous data indicate that other apoptosis-stimulating mechanisms (Fas, staurosporine) involve calmodulin-mediated events (50, 64).
Our data indicated that calmodulin is an important signaling molecule for survival and ROS control in Amø during Pcp. However, modulation or overexpression of calmodulin is not an attractive therapeutic target because of the myriad systems in which it acts (2, 12, 25, 28, 42, 63). Therefore, stimulation of calmodulin or calcium signaling as a possible treatment for Pcp carries the high probability of disruption of other delicately balanced regulatory systems. In vitro evidence in this study indicated that factors that increase GM-CSF and PI-3K activation result in reduced ROS and apoptosis and suggests that further investigations into the signaling that led to their downregulation and methods for increasing their activity will lead to new treatments for Pcp.
Acknowledgments
This study was supported in part by funds from the National Institutes of Health (RO1 HL 65170 and RO1 A1062259).
The authors have no conflicting financial interests.
Editor: A. Casadevall
Footnotes
Published ahead of print on 1 June 2009.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 156541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Ali, M., F. Ponchel, K. E. Wilson, M. J. D. Francis, X. Wu, A. Verhoef, A. W. Boylston, D. J. Veale, P. Emery, A. F. Markham, J. R. Lamb, and J. D. Isaacs. 2001. Rheumatoid arthritis synovial T cells regulate transcription of several genes associated with antigen-induced anergy. J. Clin. Investig. 107519-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold, D. B., and N. Heintz. 1997. A calcium responsive element that regulates expression of two calcium binding proteins in Purkinje cells. Proc. Natl. Acad. Sci. USA 948842-8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atochina, E. N., J. M. Beck, S. T. Scanlon, A. M. Preston, and M. F. Beers. 2001. Pneumocystis carinii pneumonia alters expression and distribution of lung collectins SP-A and SP-D. J. Lab. Clin. Med. 137429-439. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett, M. S., J. A. Fishman, S. F. Queener, M. M. Durkin, M. A. Jay, and J. W. Smith. 1988. New rat model of Pneumocystis carinii infection. J. Clin. Microbiol. 261100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benfield, T. L., B. Lundgren, J. H. Shelhamer, and J. D. Lundgren. 1999. Pneumocystis carinii major surface glycoprotein induces interleukin-8 and monocyte chemoattractant protein-1 release from a human alveolar epithelial cell line. Eur. J. Clin. Investig. 29717-722. [DOI] [PubMed] [Google Scholar]
- 7.Blau, H., S. Riklis, V. Kravtsov, and M. Kalina. 1994. Secretion of cytokines by rat alveolar epithelial cells: possible regulatory role for SP-A. Am. J. Physiol. 266L148-L155. [DOI] [PubMed] [Google Scholar]
- 8.Bratton, D. L., Q. Hamid, M. Boguniewicz, D. E. Doherty, J. M. Kailey, and D. Y. Leung. 1995. Granulocyte macrophage colony-stimulating factor contributes to enhanced monocyte survival in chronic atopic dermatitis. J. Clin. Investig. 95211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96857-868. [DOI] [PubMed] [Google Scholar]
- 10.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvasen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 2821318-1321. [DOI] [PubMed] [Google Scholar]
- 11.Cho, H.-Y., S. P. Reddy, and S. R. Kleeberger. 2006. Nrf2 defends the lung from oxidative stress. Antioxid. Redox Signal. 876-87. [DOI] [PubMed] [Google Scholar]
- 12.Colomer, J., N. Agell, P. Engel, J. Alberola-Ila, and O. Bachs. 1993. Calmodulin expression during proliferative activation of human T lymphocytes. Cell Calcium 14609-618. [DOI] [PubMed] [Google Scholar]
- 13.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91231-241. [DOI] [PubMed] [Google Scholar]
- 14.Dirksen, U., R. Nishinakamura, P. Groneck, U. Hattenhorst, L. Nogee, R. Murray, and S. Burdach. 1997. Human pulmonary alveolar proteinosis associated with a defect in GM-CSF/IL-3/IL-5 receptor common beta chain expression. J. Clin. Investig. 1002211-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, S. E., P. Y. Hahn, F. McCann, T. J. Kottom, Z. Vuk-Pavlovic, and A. H. Limper. 2005. Pneumocystis cell wall β-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-κB-dependent mechanisms. Am. J. Respir. Cell Mol. Biol. 32490-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrington, D. A., H. Sun, K. K. Murray, J. Costa, T. D. Williams, D. J. Bigelow, and T. C. Squier. 2001. Selective degradation of oxidized calmodulin by the 20S proteasome. J. Biol. Chem. 276937-943. [DOI] [PubMed] [Google Scholar]
- 17.Fischer, R., M. Koller, M. Flura, S. Mathews, M. A. Strehler-Page, J. Krebs, J. T. Penniston, E. Carafoli, and E. E. Strehler. 1988. Multiple divergent mRNAs code for a single human calmodulin. J. Biol. Chem. 26317055-17062. [PubMed] [Google Scholar]
- 18.Franke, T. F., D. R. Kaplan, L. C. Cantley, and A. Toker. 1997. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275665-668. [DOI] [PubMed] [Google Scholar]
- 19.Gasson, J. C. 1991. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood 771131-1145. [PubMed] [Google Scholar]
- 20.Gehrmann, J. 1995. Colony-stimulating factors regulate programmed cell death of rat microglia/brain macrophages in vitro. J. Neuroimmunol. 6355-61. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Rothi, R. J., and J. O. Harris. 1986. Pulmonary alveolar proteinosis. Further evaluation of abnormal alveolar macrophages. Chest 90656-661. [DOI] [PubMed] [Google Scholar]
- 22.Ikeshima, H., K. Shimoda, K. Matsuo, J. Hata, K. Maejima, and T. Takano. 1994. Spermatocyte-specific transcription by calmodulin gene II promoter in transgenic mice. Mol. Cell. Endocrinol. 9949-53. [DOI] [PubMed] [Google Scholar]
- 23.Jain, A. K., and A. K. Jaiswal. 2007. GSK-3β acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 28216502-16510. [DOI] [PubMed] [Google Scholar]
- 24.Joyal, J. L., D. J. Burks, S. Pons, W. F. Matter, C. J. Vlahos, M. F. White, and D. B. Sacks. 1997. Calmodulin activates phosphatidylinositol 3-kinase. J. Biol. Chem. 27228183-28186. [DOI] [PubMed] [Google Scholar]
- 25.Jurado, L. A., P. S. Chockalingam, and H. W. Jarrett. 1999. Apocalmodulin. Physiol. Rev. 79661-682. [DOI] [PubMed] [Google Scholar]
- 26.Kamata, N., H. Kutsuna, F. Hato, T. Kato, N. Oshitani, T. Arakawa, and S. Kitagawa. 2004. Activation of human neutrophils by granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor alpha: role of phosphatidylinositol 3-kinase. Int. J. Hematol. 80421-427. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura, T., N. Tanaka, J. Watanabe, K. Uchida, S. Kanegasaki, Y. Yamada, and K. Nakata. 1999. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 190875-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klee, C. 1988. Interaction of calmodulin with Ca2+ and target proteins, p. 35-56. In C. B. Cohen and C. B. Klee (ed.), Calmodulin. Elsevier, New York, NY.
- 29.Klippel, A., W. M. Kavanaugh, D. Pot, and L. T. Williams. 1997. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol. Cell. Biol. 17338-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodama, T., K. Hazeki, O. Hazeki, T. Okada, and M. Ui. 1999. Enhancement of chemotactic peptide-induced activation of phosphoinositide 3-kinase by granulocyte-macrophage colony-stimulating factor and its relation to the cytokine-mediated priming of neutrophil superoxide-anion production. Biochem. J. 337201-209. [PMC free article] [PubMed] [Google Scholar]
- 31.Lasbury, M. E., P. J. Durant, M. S. Bartlett, J. W. Smith, and C. H. Lee. 2003. Correlation of organism burden and alveolar macrophage counts during infection with Pneumocystis carinii and recovery. Clin. Diagn. Lab. Immunol. 10293-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lasbury, M. E., P. J. Durant, S. H. Wang, C. Zhang, C. P. Liao, D. Tschang, and C. H. Lee. 2006. Alterations in surfactant protein A form and clearance during Pneumocystis pneumonia. J. Eukaryot. Microbiol. 53(Supp. 1)S119-S121. [DOI] [PubMed] [Google Scholar]
- 33.Lasbury, M. E., P. J. Durant, C. A. Ray, D. Tschang, R. Schwendener, and C. H. Lee. 2006. Suppression of alveolar macrophage apoptosis prolongs survival of rats and mice with Pneumocystis pneumonia. J. Immunol. 1766443-6453. [DOI] [PubMed] [Google Scholar]
- 34.Lasbury, M. E., P. Lin, D. Tschang, P. J. Durant, and C. H. Lee. 2004. Effect of bronchoalveolar lavage from Pneumocystis carinii-infected hosts on phagocytic activity of alveolar macrophages. Infect. Immun. 722140-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lasbury, M. E., S. Merali, P. J. Durant, D. Tschang, C. A. Ray, and C. H. Lee. 2007. Polyamine-mediated apoptosis of alveolar macrophages during Pneumocystis pneumonia. J. Biol. Chem. 28211009-11020. [DOI] [PubMed] [Google Scholar]
- 36.Lee, M. T., K. Kaushansky, P. Ralph, and M. B. Ladner. 1990. Differential expression of M-CSF, G-CSF, and GM-CSF by human monocytes. J. Leukoc. Biol. 47275-282. [DOI] [PubMed] [Google Scholar]
- 37.Liao, C. P., M. E. Lasbury, S. H. Wang, C. Zhang, P. J. Durant, Y. Murakami, S. Matsufuji, and C. H. Lee. 2009. Pneumocystis mediates overexpression of antizyme inhibitor resulting in increased polyamine levels and apoptosis in alveolar macrophages. J. Biol. Chem. 2848174-8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 992110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, H., and T. Grundström. 2002. Calcium regulation of GM-CSF by calmodulin-dependent kinase II phosphorylation of Ets1. Mol. Biol. Cell 134497-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundy, S. K., A. A. Berlin, and N. W. Lukacs. 2003. Interleukin-12-independent down-modulation of cockroach antigen-induced asthma in mice by intranasal exposure to bacterial lipopolysaccharide. Am. J. Pathol. 1631961-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandujano, J. F., N. B. D'Souza, S. Nelson, W. R. Summer, R. C. Beckerman, and J. E. Shellito. 1995. Granulocyte-macrophage colony stimulating factor in Pneumocystis carinii pneumonia in mice. Am. J. Respir. Crit. Care Med. 1511233-1238. [DOI] [PubMed] [Google Scholar]
- 42.Mayer, B., and B. Hemmens. 1997. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem. Sci. 22477-481. [DOI] [PubMed] [Google Scholar]
- 43.McAllister, F., C. Steele, M. Zheng, J. E. Shellito, and J. K. Kolls. 2005. In vitro effector activity of Pneumocystis murina-specific T-cytotoxic-1 CD8+ T cells: role of granulocyte-macrophage colony-stimulating factor. Infect. Immun. 737450-7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moretó, J., A. Lladó, M. Vidal-Quadras, M. Calvo, A. Pol, C. Enrich, and F. Tebar. 2008. Calmodulin modulates H-Ras mediated Raf-1 activation. Cell. Signal. 201092-1103. [DOI] [PubMed] [Google Scholar]
- 45.Nojima, H. 1987. Molecular evolution of the calmodulin gene. FEBS Lett. 217187-190. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto, M., M. Nakai, C. Nakayama, H. Yanagi, H. Matsui, H. Noguchi, M. Namiki, J. Sakai, K. Kadota, M. Fukui, and H. Hara. 1991. Purification and characterization of three forms of differently glycosylated recombinant human granulocyte-macrophage colony-stimulating factor. Arch. Biochem. Biophys. 286562-568. [DOI] [PubMed] [Google Scholar]
- 47.Otsuka, M., Y. Negishi, and Y. Aramaki. 2007. Involvement of phosphatidylinositol-3-kinase and ERK pathways in the production of TGF-beta1 by macrophages treated with liposomes composed of phosphatidylserine. FEBS Lett. 581325-330. [DOI] [PubMed] [Google Scholar]
- 48.Ouellet, N., Y. Nadeau, Y. Bergeron, and M. G. Bergeron. Enhancement of host resistance to pneumococcal pneumonia by the combination of interferon-gamma and ceftriaxone, abstr. B-984. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 16 to 19 December 2001.
- 49.Paine, R., III, A. M. Preston, S. Wilcoxen, H. Jin, B. B. Siu, S. B. Morris, J. A. Reed, G. Ross, J. A. Whitsett, and J. M. Beck. 2000. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J. Immunol. 1642602-2609. [DOI] [PubMed] [Google Scholar]
- 50.Pan, Z., W. Radding, T. Zhou, E. Hunter, J. Mountz, and J. M. McDonald. 1996. Role of calmodulin in HIV-potentiated Fas-mediated apoptosis. Am. J. Pathol. 149903-910. [PMC free article] [PubMed] [Google Scholar]
- 51.Qureshi, M. H., K. M. Empey, and B. A. Garvy. 2005. Modulation of proinflammatory responses to Pneumocystis carinii f. sp. muris in neonatal mice by granulocyte-macrophage colony-stimulating factor and IL-4: role of APCs. J. Immunol. 174441-448. [DOI] [PubMed] [Google Scholar]
- 52.Reddy, S. A. G., J. H. Huang, and W. S.-L. Liao. 2000. Phosphatidylinositol 3-kinase as a mediator of TNF-induced NF-κB activation. J. Immunol. 1641355-1363. [DOI] [PubMed] [Google Scholar]
- 53.Sato, E., S. Koyama, Y. Okubo, K. Kubo, and M. Sekiguchi. 1998. Acetylcholine stimulates alveolar macrophages to release inflammatory cell chemotactic activity. Am. J. Physiol. 274L970-L979. [DOI] [PubMed] [Google Scholar]
- 54.Seymour, J. F. 2006. Extra-pulmonary aspects of acquired pulmonary alveolar proteinosis as predicted by granulocyte-macrophage colony-stimulating factor-deficient mice. Respirology 11S16-S22. [DOI] [PubMed] [Google Scholar]
- 55.Shannon, M. F., L. S. Coles, M. A. Vadas, and P. N. Cockerill. 1997. Signals for activation of the GM-CSF promoter and enhancer in T cells. Crit. Rev. Immunol. 17301-323. [DOI] [PubMed] [Google Scholar]
- 56.Shibasaki, M., K. Hashimoto, M. Okamoto, Y. Hayashi, K. Imaizumi, N. Hashimoto, N. Ozaki, T. Yokoi, K. Takagi, Y. Hasegawa, K. Shimokata, and T. Kawabe. 2009. Up-regulation of surfactant protein production in a mouse model of secondary pulmonary alveolar proteinosis. Am. J. Respir. Cell Mol. Biol. 40536-542. [DOI] [PubMed] [Google Scholar]
- 57.Shimoda, K., H. Ikeshima, K. Matsuo, J. Hata, K. Maejima, and T. Takano. 1995. Spatial and temporal regulation of the rat calmodulin gene III directed by a 877-base promoter and 103-base leader segment in the mature and embryonal central nervous system of transgenic mice. Brain Res. Mol. Brain Res. 3161-70. [DOI] [PubMed] [Google Scholar]
- 58.Suda, J., and N. Aoki. 1981. Inhibition of platelet function by a calmodulin interacting agent, W-7. Thromb. Res. 21447-455. [DOI] [PubMed] [Google Scholar]
- 59.Tarcsa, E., G. Szymanska, S. Lecker, C. M. O'Connor, and A. L. Goldberg. 2000. Ca2+-free calmodulin and calmodulin damaged by in vitro aging are selectively degraded by 26 S proteasomes without ubiquitination. J. Biol. Chem. 27520295-20301. [DOI] [PubMed] [Google Scholar]
- 60.Tran Van Nhieu, J., A. M. Vojtek, J. F. Bernaudin, E. Escudier, and J. Fleury-Feith. 1990. Pulmonary alveolar proteinosis associated with Pneumocystis carinii. Ultrastructural identification in bronchoalveolar lavage in AIDS and immunocompromised non-AIDS patients. Chest 98801-805. [DOI] [PubMed] [Google Scholar]
- 61.Tsuboi, A., M. Muramatsu, A. Tsutsumi, K. Arai, and N. Arai. 1994. Calcineurin activates transcription from the GM-CSF promoter in synergy with either protein kinase C or NF-kappa B/AP-1 in T cells. Biochem. Biophys. Res. Commun. 1991064-1072. [DOI] [PubMed] [Google Scholar]
- 62.Ujihara, M., K. Nomura, O. Yamada, N. Shibata, M. Kobayashi, and K. Takano. 2001. Granulocyte-macrophage colony-stimulating factor ensures macrophage survival and generation of the superoxide anion: a study using a monocytic-differentiated HL60 subline. Free Radic. Biol. Med. 311396-1404. [DOI] [PubMed] [Google Scholar]
- 63.Van Eldik, L., and D. Watterson (ed.). 1998. Calmodulin and signal transduction, Academic Press, New York, NY.
- 64.Wang, R. H., M. Fang, and S. B. Xue. 1996. Changes of intracellular calcium, calmodulin in normal and tumor cells triggered by staurosporine. Shi Yan Sheng Wu Xue Bao 29133-139. [PubMed] [Google Scholar]
- 65.Zhang, C., S. H. Wang, M. E. Lasbury, D. Tschang, C. P. Liao, P. J. Durant, and C. H. Lee. 2006. Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina. Infect. Immun. 741857-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang, S. P., N. Natsukari, G. Bai, R. A. Nichols, and B. Weiss. 1993. Localization of the multiple calmodulin messenger RNAs in differentiated PC12 cells. Neuroscience 55571-582. [DOI] [PubMed] [Google Scholar]