Abstract
Periodontitis and Chlamydia pneumoniae infection are independent risk factors for cardiovascular diseases. The aim of this study was to investigate the effect of C. pneumoniae and Aggregatibacter actinomycetemcomitans infection on hepatic inflammation and lipid homeostasis of apolipoprotein E-deficient mice. Mice were infected with viable C. pneumoniae intranasally three times for chronic infection or once for acute infection. Viable A. actinomycetemcomitans was administered 10 times intravenously alone or in concert with C. pneumoniae. Hepatic alterations were assessed by histochemistry, lipid quantification, and fatty acid profile analysis. The RNA expression levels and the presence of pathogens in the livers and lungs were detected by quantitative real-time PCR. Both pathogens were detected in the livers of the infected animals. Chronic C. pneumoniae infection induced marked changes in hepatic lipid homeostasis. A. actinomycetemcomitans infection resulted in inflammatory cell infiltration into the liver, accompanied by elevated hepatic RNA expression levels of inflammation-related genes and higher serum amyloid A and lipopolysaccharide concentrations. Our results indicate that proatherogenic pathogens infect the liver, causing proinflammatory alterations and lipid disturbances. This infection may maintain chronic systemic inflammation attributable to atherogenesis.
Periodontitis, a chronic infection affecting the tooth-supporting tissues, and persistent Chlamydia pneumoniae infection, e.g., in the lungs and vasculature, are associated with an increased risk of cardiovascular diseases (CVD) (1, 14). A major periodontal pathogen, Aggregatibacter actinomycetemcomitans, and C. pneumoniae are gram-negative bacteria containing a potent proatherogenic outer membrane component, lipopolysaccharide (LPS). Seropositivity to these pathogens and their carriage are highly prevalent (15, 21, 38). Prospective studies have demonstrated that systemic exposure to A. actinomycetemcomitans is associated with a twofold risk for incident CVD (22). A recent survey showed that the periodontal pathogen burden, and especially the amount of A. actinomycetemcomitans, is associated with prevalent coronary heart disease (28). Similarly, reports have indicated an association between chronic C. pneumoniae infection and CVD and, further, a role of the total pathogen burden in the risk for coronary heart disease (2, 41).
Many infections affecting the gastrointestinal tract, such as viral hepatitis and small intestinal bacterial overgrowth, may be associated with nonalcoholic fatty liver disease (NAFLD) (5, 39). However, the connection between infections by proatherogenic pathogens and liver damage is not well known. A recent report showed a significantly elevated seropositivity rate for C. pneumoniae among men with nonalcoholic steatohepatitis (4). In addition, C. pneumoniae replicates in mouse Kupffer cells and is able to induce a local proinflammatory state in the liver (20). Some epidemiological studies have reported an association between periodontitis and NAFLD (10, 25). In experimental animal studies, Escherichia coli LPS-induced periodontal inflammation generated NAFLD in a rat model (30). Moreover, in vitro studies have shown the capacity of various spirochetes to stimulate rat Kupffer cells to produce reactive oxygen species related to hepatic injury (18, 19).
The aim of this study was to investigate the effect of acute and chronic C. pneumoniae infections, as well as A. actinomycetemcomitans infection, on the hepatic lipid and inflammation status of atherosclerosis-susceptible apolipoprotein E (apoE)-deficient mice. The degree and nature of hepatic lipid balance and inflammation were assessed by histochemistry, gene expression, total lipid quantification, and fatty acid profile analyses.
MATERIALS AND METHODS
Bacterial strains.
The clinical A. actinomycetemcomitans strain AT445b (serotype b, fimbriated and aggregative phenotype) was cultivated in a tryptic soy-serum-bacitracin-vancomycin medium. Cultures were incubated at 37°C with 5% CO2 for 48 h. The C. pneumoniae isolate Kajaani 7, a Finnish epidemic strain (8), was isolated and cultivated as previously described (31).
Mice.
Male apoE-deficient mice (B6.129P2-Apoetm1Unc/Crl) were purchased from Charles River Laboratories (Belgium). All animal care and experimentation were conducted with ethical permission and were in accordance with the current guidelines of the Council of Europe. The mice were fed standard mouse chow and maintained at the National Public Health Institute Animal Facilities (Helsinki, Finland) in a germfree environment.
A. actinomycetemcomitans and C. pneumoniae infections. (i) Short-term experiment (14 weeks).
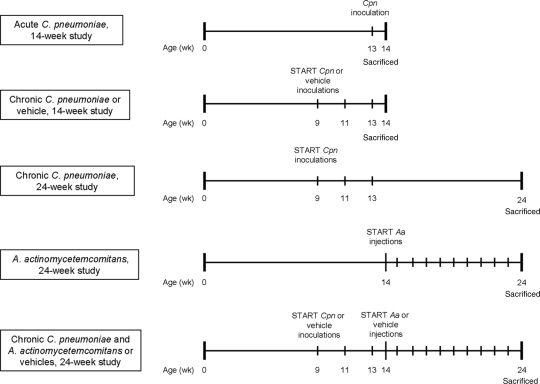
For the short-term experiment, the mice (aged 9 weeks, n = 20) were divided randomly into three groups (Fig. 1). The group acutely infected with C. pneumoniae (acute CPN group) received viable C. pneumoniae (2 × 106 inclusion-forming units [IFU]/40 μl sucrose-phosphate-glutamic acid buffer [SPG]) intranasally once at the age of 13 weeks. The chronically infected CPN group (chronic CPN group) was inoculated with viable C. pneumoniae (2 × 106 IFU/40 μl SPG) intranasally three times at 2-week intervals from the age of 9 weeks. The control group received vehicle (40 μl SPG) three times. The mice were sacrificed at the age of 14 weeks.
FIG. 1.
Experimental design of the short-term (14-week) and the long-term (24-week) mouse experiments. Aa, A. actinomycetemcomitans; Cpn, C. pneumoniae.
(ii) Long-term experiment (24 weeks).
For the long-term experiment, the mice (aged 9 weeks, n = 39) were divided randomly into four groups (Fig. 1). The AA infection group was inoculated intravenously with live A. actinomycetemcomitans (107 CFU/50 μl 0.9% NaCl) once a week for 10 consecutive weeks starting from the age of 14 weeks. The chronic CPN group received viable C. pneumoniae (2 × 106 IFU/40 μl SPG) intranasally three times at 2-week intervals starting from the age of 9 weeks. The group with combined chronic C. pneumoniae and A. actinomycetemcomitans infection (chronic CPN+AA group) was inoculated in the same manner as the groups with a single infection, starting with C. pneumoniae inoculations and followed by A. actinomycetemcomitans injections. The control group received a combination of vehicles (40 μl SPG and 50 μl 0.9% NaCl). The mice were sacrificed at the age of 24 weeks.
Detection of bacteria in lung, liver, and heart tissues.
A. actinomycetemcomitans was detected in lung, liver, and heart tissues by a quantitative real-time PCR; the detection limit for the A. actinomycetemcomitans assay is 2 genome equivalents (GE), calculated from the lowest standard used (12). C. pneumoniae was detected in lung, liver, and heart tissues first by quantitative real-time PCR (9, 24). The negative samples were further examined by a semiconventional nested PCR with larger template volumes (32). Briefly, the first round of the nested PCR was conventional, with outer primers that amplified a 192-bp product of the C. pneumoniae PstI fragment. A heat-labile uracil-DNA N-glycosylase enzyme was added, and dTTP was replaced with dUTP in this conventional PCR to prevent carryover contamination. The second round of the PCR was performed as a real-time PCR with a LightCycler instrument (Roche). The inner primer pair detected a 128-bp product of the PstI fragment. The real-time PCR was adapted from the method described by Ciervo et al. (7). Repetitiously in every run, both PCR methods detected 8 GE, which was the lowest standard used.
Histological analysis.
Liver paraffin sections (5 μm) were stained with hematoxylin and eosin. The hepatic morphology was evaluated by two specialists (I.L.B. and K.L.) in a manner blinded to the grouping. Inflammatory changes (i.e., level of inflammatory cell infiltration) were graded from 0 to 4, with 0 depicting incidental or minimal changes and 4 depicting severe or extensive changes. Microvesicular appearance was graded from 0 to 4 according to the percentage of cells containing excess vesicles as follows: 1, <25%; 2, 26 to 50%; 3, 51 to 75%; 4, >75%. In addition, the sections were immunostained for CD68 (catalog no. sc-7084, dilution 1:50; Santa Cruz) and interleukin 1β (IL-1β) (catalog no. AF-401-NA, dilution 1:100; R&D Systems) using anti-goat secondary antibody (Vector Laboratories) and 3-amino-9-ethyl carbazole detection. The degree of positive staining was graded from 0 to 3.
Frozen liver sections (10 μm) from the short experiment were stained with Oil Red O and Mayer's hematoxylin, and the degree of staining was evaluated by three specialists (M.J., P.T.K., and P.J.P) in a manner blinded to the grouping. The results of Oil Red O staining for neutral triacylglycerols and lipids were graded from 0 to 2.
Liver lipid analyses.
Frozen liver tissue (10 mg) was homogenized in methanol-chloroform. The extract was dried under nitrogen, and, after it was dissolved in methanol, the concentrations of choline-containing phospholipids (enzymatic colorimetric method; phospholipids B kit 999-54006; Wako Chemicals GmbH) and cholesterol (cholesterol oxidase-peroxidase-amidopyrine method; kit 11489232; Roche) were determined. The liver triacylglycerols were measured as previously described (6) and quantified using a glycerol phosphate oxidase-peroxidase-amidopyrine method (kit 11488872; Roche).
Tissue fatty acid analysis.
The total lipids from aliquots of 50 to100 mg of liver tissue were extracted with hexane-isopropanol (3:2) and transmethylated by heating with acidic methanol (5% H2SO4) (11). The compositions of the recovered fatty acid methyl esters were determined by gas-liquid chromatography (Hewlett Packard 6890 gas chromatograph and Chemstation software [revision B.01.03]) using a DB225 capillary column (30 m, 0.32-mm inside diameter, 0.25-mm phase layer; Agilent Technologies). Split injection and hydrogen as the carrier gas were used, and the oven temperature was programmed to rise from 160°C to 230°C. The proportions of the methylated fatty acids were expressed as percentage of weight normalized to 100%.
Quantitative real-time PCR.
RNA from the liver tissue was isolated using a GenElute mammalian total RNA purification kit (Sigma-Aldrich) and cDNA synthesized using the ImProm-II reverse transcription system (Promega). We designed primers (Thermo Scientific) for mouse IL-1β, monocyte chemoattractant protein 1 (MCP-1), CD68, sterol regulatory element binding protein 1c (SREBP-1c), fatty acid synthase (FAS), acetyl coenzyme A carboxylase β (ACC2), low-density lipoprotein receptor (LDLr), and glyceraldehyde-3-phosphate dehydrogenase (GADPH) with Beacon Designer (Premier Biosoft International). The primer sequences are displayed in Table 1. A quantitative real-time PCR using the Mx3005P real-time quantitative PCR (qPCR) system (Stratagene) was performed using primer concentrations of 100 nM. The cDNA was amplified for 40 cycles using Brilliant SYBR green qPCR master mix (Stratagene). The results were analyzed using software provided by Stratagene, and the expression levels were calculated using GADPH as an endogenous reference gene. The changes in the expression levels were defined by the 2−ΔΔCt method (17).
TABLE 1.
Quantitative PCR primer sequences
| Target gene | Sequence (5′-3′)
|
Reference sequence no.a | |
|---|---|---|---|
| Forward primer | Reverse primer | ||
| IL-1β | GATCCTCTCCAGCCAAGC | GGGTCCGTCAACTTCAAAG | NM 008361 |
| MCP-1 | GAGCCAGACGGGAGGAAG | ATGGTGGTGGAGGAAGAGAG | NM 011333 |
| CD68 | GCGGCTCCCTGTGTGTCTG | TTCTGTGGCTGTAGGTGTCATCG | NM 009853 |
| SREBP-1c | TGGCTTGGTGATGCTATGTTGAG | CTGGTGGAGGGCTGGAAGG | NM 011480 |
| FAS | AGGTGCTGGCTGGAGAAGG | CGGTCGGTGGCTGTGTATTC | NM 007988 |
| ACC2 | TCAAGTATGCTCTCAAGGTAG | TAACTGCTGCCATTGTAGG | NM 133904 |
| LDLr | GGTTCCTGTCCATCTTCTTCC | TCTTCAGCCGCCAGTTCC | NM 010700 |
| GADPH | TTCAACGGCACAGTCAAGG | CTCCACGACATACTCAGCAC | AK144690 |
National Center of Biotechnology Information (http://www.ncbi.nlm.nih.gov/).
Serum analyses.
Serum LPS activity was determined by a Limulus amebocyte lysate assay coupled with a chromogenic substrate (HyCult biotechnology b.v.), and LPS binding protein (HyCult biotechnology b.v.), serum amyloid A (SAA) (Biosource), alanine aminotransferase (Abbot), adiponectin (R&D Systems), nonesterified fatty acid (Wako Chemicals GmbH), phospholipid (Wako Chemicals GmbH), triacylglycerol (Roche), and cholesterol (Roche) concentrations were measured using commercially available kits. Serum phospholipid transfer protein activity (13), apoA-I concentration (36), and A. actinomycetemcomitans immunoglobulin G (IgG) and IgA (23) were measured as reported previously.
Statistical analyses.
Results are expressed as the means and standard errors of the mean (SEM) for normally distributed variables or as median and interquartile ranges for skewed variables. The statistical significance of the differences between the infected mice and the respective controls was analyzed using the nonparametric Mann-Whitney U two-independent-samples test. Two-tailed bivariate correlations were determined using Spearman correlation analysis. Statistical analyses were performed with the Statistical Package for Social Sciences (SPSS) version 15.0.1.
RESULTS
Detection of bacteria in tissues.
In the short-term experiment, C. pneumoniae was quantitatively detectable in the lungs of 14/14 (100%) animals. The median pathogen levels in tissues were 306 (range, 12 to 1,246) and 24,875 (range, 8,292 to 41,250) GE/mg in the chronic and acute infection models, respectively. In the nonquantitative semiconventional PCR, 1/7 (14%) and 2/7 (29%) livers of the mice with chronic and acute infections were positive for the pathogen. In the long-term experiment, C. pneumoniae was detected by semiconventional PCR in the lungs of 11/20 (55%) mice but not detected in the liver samples. A. actinomycetemcomitans was quantitatively detected in the livers of 6/20 (30%) mice, with a median pathogen level of 10 (range, 3 to 1,654) GE/mg tissue. C. pneumoniae was not detected in the heart tissues, and A. actinomycetemcomitans was not detected in the lung or heart tissues. The control mice were free of these pathogens.
Histological changes in the hepatic tissue.
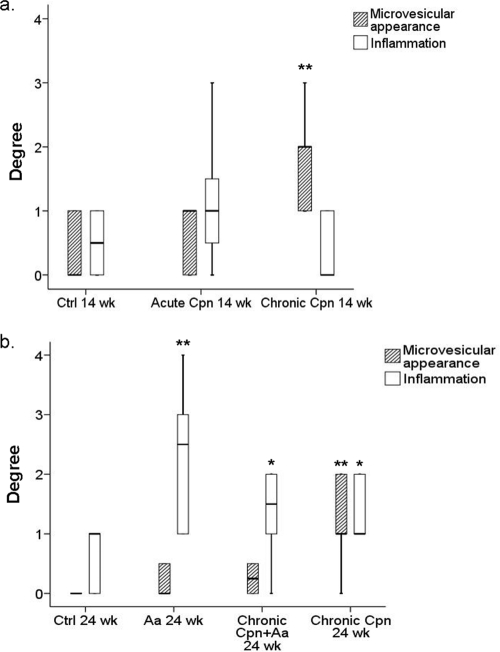
Figure 2 presents the quantitative data on the degree of microvesicular appearance and inflammation. In the short-term experiment, the chronic C. pneumoniae infection group displayed an increased microvesicular appearance (P = 0.007). Acute C. pneumoniae infection had only minor effects on liver morphology or inflammation status. The degree of Oil Red O staining for neutral triacylglycerols and lipids remained the same in the chronic group but was lower in the acute group (P = 0.005). In the long-term experiment, chronic C. pneumoniae infection induced mild inflammation (P = 0.017) and, in addition, an increased microvesicular appearance was observed (P = 0.001). A. actinomycetemcomitans administered alone (P = 0.002), and in concert with C. pneumoniae (P = 0.023), induced moderate hepatic inflammation, as demonstrated by infiltration of perivascular mononuclear inflammatory cells and neutrophils into the liver tissue.
FIG. 2.
Degree of hepatic microvesicular appearance and inflammation, as evaluated from hematoxylin-and-eosin-stained liver paraffin cross-sections. (a) Short-term experiment. Control (Ctrl), n = 6; acute CPN group, n = 7; chronic CPN group, n = 7. (b) Long-term experiment. Ctrl, n = 9; AA group, n = 10; chronic CPN+AA group, n = 10; chronic CPN group, n = 10. Data are represented as median values including interquartile ranges. **, P < 0.01; *, P < 0.05 (compared to the respective control groups by the Mann-Whitney U test).
Changes in the hepatic lipid content and fatty acid profiles.
The hepatic lipid contents are summarized in Table 2 . Compared to levels in the control mice, the phospholipid levels were lower in all infected mice. The changes in cholesterol and triacylglycerol levels were not significant, except that the concentration of triacylglycerols was lower in the acute C. pneumoniae infection group (P = 0.001) than in the control. The ratio of triacylglycerols to phospholipids was lowered in the acute C. pneumoniae infection group in the short-term experiment and increased in both chronic C. pneumoniae infection groups in both experiments as well as in the A. actinomycetemcomitans-infected mice (Table 2).
TABLE 2.
Hepatic lipid concentrations and the cellular triacyglycerol/phospholipid ratio
| Exptl group (no. of mice) | Lipid level (mmol/mg tissue) ± SEMa
|
Triacylglycerol/ phospholipid ratioa | ||
|---|---|---|---|---|
| Triacylglycerols | Cholesterol | Phospholipids | ||
| Short term, 14 wk | ||||
| Acute CPN (7) | 13.4 ± 2.8 (0.001) | 5.1 ± 0.3 (0.445) | 68.4 ± 2.1 (0.005) | 0.2 ± 0.0 (0.002) |
| Chronic CPN (7) | 23.5 ± 3.6 (0.073) | 5.1 ± 0.6 (0.366) | 50.0 ± 3.4 (0.001) | 0.5 ± 0.1 (0.366) |
| Control (6) | 31.3 ± 2.5 | 4.8 ± 0.2 | 78.8 ± 1.6 | 0.4 ± 0.0 |
| Long term, 24 wk | ||||
| AA (10) | 36.2 ± 3.3 (0.065) | 6.8 ± 0.5 (0.968) | 19.5 ± 2.2 (<0.001) | 2.1 ± 0.2 (<0.001) |
| Chronic CPN (10) | 43.5 ± 4.8 (0.549) | 4.4 ± 0.2 (0.079) | 40.9 ± 3.8 (<0.001) | 1.2 ± 0.2 (0.006) |
| Chronic CPN+AA (10) | 30.9 ± 4.0 (0.447) | 5.0 ± 0.4 (0.604) | 73.8 ± 4.8 (0.027) | 0.4 ± 0.1 (0.905) |
| Control (9) | 38.9 ± 6.3 | 5.8 ± 0.6 | 88.9 ± 4.3 | 0.5 ± 0.2 |
P values (given in parentheses) indicate significance of results compared to those of their respective control groups by the Mann-Whitney U two-independent-samples test. Boldface values indicate statistically significant difference compared to the control group.
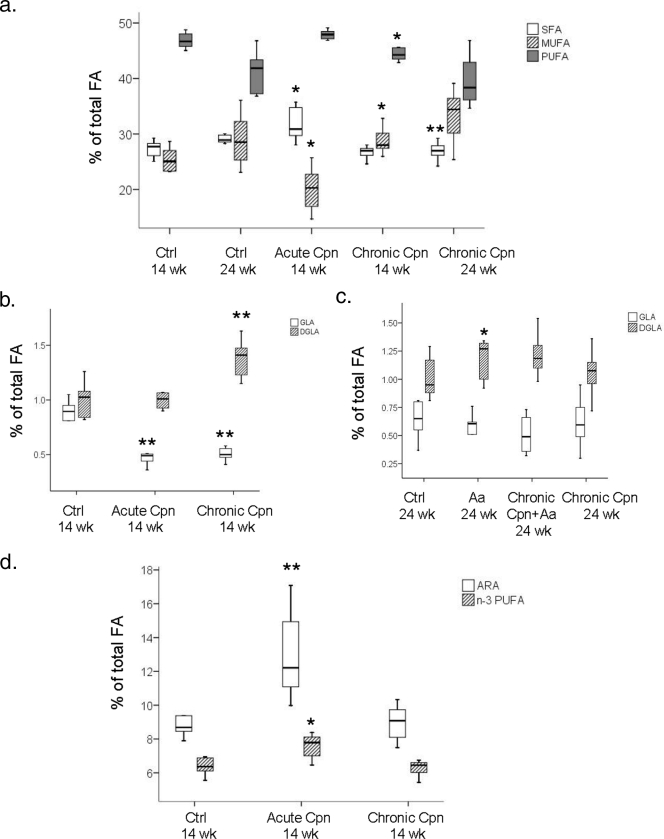
Compared to levels in the control mice, the proportions of saturated fatty acids (SFA) were higher in the mice with acute C. pneumoniae infection (P = 0.010) and lower in the mice with chronic C. pneumoniae infection in the long-term experiment (P = 0.006) (Fig. 3). Correspondingly, proportions of monounsaturated fatty acids (MUFA) were lower in the mice with acute C. pneumoniae infection (P = 0.032) and higher in the mice with chronic C. pneumoniae infection in the short-term (P = 0.022) and long-term (P = 0.086) experiments (Fig. 3a). Furthermore, the proportions of polyunsaturated fatty acids (PUFA) were significantly lower in mice with chronic C. pneumoniae infection in the short-term experiment (P = 0.010) (Fig. 3a). Changes in the SFA, MUFA, and PUFA proportions in A. actinomycetemcomitans-infected mice were minor.
FIG. 3.
Composition of hepatic fatty acids. (a) SFA, MUFA, and PUFA proportions in C. pneumoniae-infected mice; (b) liver GLA and DGLA proportions in the long-term experiment; (c) liver GLA and DGLA proportions in the short-term experiment; (d) ARA and n−3 PUFA proportions in the short-term experiment. The results are median percentages (by weight) of total liver fatty acids including interquartile ranges. **, P < 0.01; *, P < 0.05 (compared to the respective control groups by the Mann-Whitney U test). Ctrl, control.
Compared to levels in their respective controls, the proportion of anti-inflammatory dihomo-γ-linolenic acid (DGLA) (20:3n−6) was higher in the short-term experiment in the mice with chronic C. pneumoniae infection (P = 0.008) (Fig. 3b) and in the long-term experiment in the mice with A. actinomycetemcomitans infection (P = 0.025) (Fig. 3c). In addition, the proportion of the DGLA precursor γ-linolenic acid (GLA) (18:3n−6) was decreased in the short-term experiment in mice with acute (P = 0.003) and chronic (P = 0.003) C. pneumoniae infections (Fig. 3b). The proportions of anti-inflammatory n−3 PUFA (P = 0.022) and proinflammatory arachidonic acid (ARA) (20:4n−6) (P = 0.007) were elevated in mice with acute C. pneumoniae infection (Fig. 3d).
RNA and protein expression levels in liver tissue.
Acute C. pneumoniae infection (P = 0.002 and P = 0.002) as well as A. actinomycetemcomitans administered alone (P < 0.001 and P = 0.002) and together with C. pneumoniae (P < 0.001 and P = 0.120) induced elevated relative RNA expression levels of genes encoding inflammation markers, IL-1β, and the macrophage marker CD68, respectively (Table 3). The production of these proteins was verified by immunohistochemical stainings of the paraffin sections. Compared to levels in the controls, the IL-1β production was 1.1- to 1.3-fold higher, whereas the CD68 production was 2.2- to 2.6-fold higher (data not shown). Acute C. pneumoniae (P = 0.005) and A. actinomycetemcomitans (P = 0.003) infection also gave rise to increased relative RNA expression of MCP-1. The FAS, SREBP-1c, ACC2, and LDLr levels remained unchanged (Table 3).
TABLE 3.
Hepatic gene expression changes
| Exptl group | Fold change in expression ± SEMa
|
||||||
|---|---|---|---|---|---|---|---|
| IL-1β | MCP-1 | CD68 | SREBP-1c | FAS | ACC2 | LDLr | |
| Short term (14 wk) | |||||||
| Acute CPN | 3.9 ± 1.1 (0.002) | 2.8 ± 0.8 (0.004) | 3.2 ± 0.5 (0.002) | 1.9 ± 0.5 (0.731) | 1.0 ± 0.2 (1.000) | 1.3 ± 0.2 (0.394) | 1.1 ± 0.2 (1.000) |
| Chronic CPN | 0.9 ± 0.2 (0.035) | 1.1 ± 0.3 (0.731) | 1.2 ± 0.2 (0.234) | 1.5 ± 0.3 (0.234) | 1.3 ± 0.3 (0.731) | 1.4 ± 0.4 (0.731) | 1.1 ± 0.2 (0.731) |
| Long term (24 wk) | |||||||
| AA | 3.0 ± 0.7 (<0.001) | 1.5 ± 0.2 (0.008) | 2.4 ± 0.7 (0.002) | 1.1 ± 0.2 (0.258) | 1.5 ± 0.3 (0.028) | 1.0 ± 0.1 (1.000) | 1.2 ± 0.1 (1.000) |
| Chronic CPN | 1.0 ± 0.2 (0.156) | 1.1 ± 0.1 (0.497) | 1.2 ± 0.2 (1.000) | 1.0 ± 0.1 (0.050) | 1.0 ± 0.1 (0.497) | 0.9 ± 0.1 (0.720) | 1.0 ± 0.2 (0.156) |
| Chronic CPN+AA | 2.2 ± 0.5 (<0.001) | 0.7 ± 0.1 (0.050) | 1.3 ± 0.1 (0.156) | 1.1 ± 0.1 (0.730) | 1.5 ± 0.3 (0.497) | 0.9 ± 0.1 (0.156) | 1.1 ± 0.1 (0.497) |
ΔΔCt compared to the results for their respective control mice. P values (given in parentheses) indicate significance of results compared to those of their respective control groups by the Mann-Whitney U two-independent-samples test. Boldface values indicate statistically significant difference compared to the control group.
Serum analyses.
The mean serum A. actinomycetemcomitans IgG class antibody levels, expressed in absorbance units (AU), were clearly elevated (2.2 AU) in the mice infected with the pathogen compared to the noninfected mice (0.02 AU). The serum A. actinomycetemcomitans IgA class antibodies were undetectable. Serum triacylglycerol levels were lower in mice with A. actinomycetemcomitans infection (P = 0.043). Serum alanine aminotransferase (32.4 ± 3.4 versus 56.6 ± 7.5 U/liter; P = 0.010) and adiponectin (5.9 ± 0.2 versus 6.9 ± 0.3 U/liter, P = 0.032) concentrations were lower in mice acutely infected with C. pneumoniae. Serum LPS activities tended to be higher in all infected mice both in the short-term experiment (21.3 ± 6.0 versus 10.2 ± 3.4 endotoxin units [EU]/ml, P = 0.458) and in the long-term experiment (57.1 ± 4.7 versus 41.5 ± 4.9 EU/ml, P = 0.075). SAA concentrations were higher in mice acutely infected with C. pneumoniae (296.4 ± 58.1 versus 20.4 ± 10.7 μg/ml, P = 0.003) and A. actinomycetemcomitans (217.1 ± 127.0 versus 61.1 ± 28.8 μg/ml, P = 0.368). Phospholipid transfer protein activity was significantly higher only in mice acutely infected with C. pneumoniae (16.1 ± 1.6 versus 11.1 ± 1.3 μmol/ml/h, P = 0.015).
Correlation analysis between the parameters.
Hepatic microvesicular appearance displayed a negative correlation with serum SAA concentration (r = −0.443; P < 0.001) and the ratio of hepatic n−6 PUFA and n−3 PUFA (r = −0.394; P = 0.002). Hepatic inflammation correlated positively with serum A. actinomycetemcomitans IgG class antibody levels (r = 0.437; P = 0.005), serum LPS activity (r = 0.269; P = 0.040), the ratio of hepatic n−6 PUFA to n−3 PUFA (r = 0.472; P < 0.001) or to hepatic MUFA (r = 0.320; P = 0.013). Liver inflammation correlated negatively with serum phospholipids (r = −0.473; P = 0.035) and proportions of hepatic n−3 PUFA (r = −0.448; P < 0.001) and total PUFA (r = −0.290; P = 0.026).
DISCUSSION
This study demonstrates that in apoE-deficient mice, chronic C. pneumoniae infection is associated with hepatic fatty acid imbalance, disturbances in the hepatocellular total triacylglycerol/phospholipid balance, and the appearance of microvesicles in the liver, whereas A. actinomycetemcomitans infection results in hepatic inflammation.
In recent years, the role of pathogens and infections has become of utmost importance in the etiology of many common diseases, which previously have been unrelated to pathogenic microbes. In our study, apoE-deficient mice predisposed to chronic C. pneumoniae infection had marked hepatic morphological and lipid homeostatic changes. This outcome was analogous in both short-term and long-term experiments in animals with chronic infections. The pathogen was detectable in the lungs of mice with both acute and chronic infections; the major difference in the GE levels clearly indicates the favorable outcome of the infection models. Most importantly, C. pneumoniae was also detectable in the livers of animals in the short-term experiment, in which the livers also displayed the most distinct microvesicular morphology. In this 14-week mouse experiment, the morphological changes were accompanied by clear alterations in the proportions of liver fatty acids compared to those of the control group: increases in total MUFA and DGLA and corresponding decreases in total PUFA and GLA. The entire hepatic fatty acid profiles of the mice with chronic C. pneumoniae infections in the short-term and long-term experiments resembled each other, whereas they both clearly differed from that of the group with acute infection. This indicates that the duration of the infection has a major impact on the liver fatty acid profile. A hepatoprotective role of n−3 PUFA has been reported (26). Similarly, our data demonstrate a significant negative correlation between liver n−3 PUFA and liver inflammation.
In contrast to chronic C. pneumoniae infection, acute C. pneumoniae infection resulted in more inflammation-related responses; i.e., serum inflammation markers as well as liver RNA expression levels of certain inflammatory genes were higher than those found in the controls. The concentration of hepatic triacylglycerols was decreased. In these livers, total SFA, proinflammatory ARA, and anti-inflammatory n−3 PUFA were more abundant than in the livers of the control group. The upregulation of these mediators may be explained by the direct acute response to the bacterial challenge or by systemic stimulation by bacterial compounds such as LPS (35).
The administration of A. actinomycetemcomitans has been reported to induce systemic inflammation in mice with apoE-deficient backgrounds (34). In accordance, our study demonstrated that A. actinomycetemcomitans infection resulted in the infiltration of perivascular mononuclear inflammatory cells into the liver. The elevated inflammation status was verified also by upregulated RNA expression levels of inflammatory genes in the liver and elevated serum levels of the infection-related markers SAA and LPS. The pathogen was detectable in the liver tissue, and the size of the spleen was markedly increased (data not shown). All these effects are likely due to continuous exposure to antigens via A. actinomycetemcomitans inoculations for 10 weeks. Similar responses were observed in mice infected with both C. pneumoniae and A. actinomycetemcomitans. However, the double-infection model displayed reduced inflammatory response. This may be due to priming of the mouse immune defense with the first infection with C. pneumoniae, thereby suppressing the overall effect of the following A. actinomycetemcomitans infection.
When analyzing serum lipid levels, we observed a negative correlation between serum phospholipid content and liver inflammation. Additionally, all the infected mice had lower hepatic phospholipid contents, particularly those with A. actinomycetemcomitans or chronic C. pneumoniae infection alone. This is most probably due to an LPS-induced increase in the activity of phospholipases A1 and A2 in the liver (16). Consequently, the ratio of hepatic triacylglycerols to phospholipids was increased in both groups with chronic C. pneumoniae or A. actinomycetemcomitans infection. Such a change may imply that prolonged infection leads to a decrease in the amount of structural cell membrane phospholipids, as observed earlier for mice infected by a hepatitis virus strain (3). Lipid alterations may cause severe problems, since an appropriate membrane lipid composition is required for several major functions, e.g., membrane fluidity, signaling, and topology of attached proteins (29). Therefore, the effect of the pathogens on liver phospholipids needs to be studied further.
There are certain obvious limitations of the present study. We used apoE-deficient mice to examine the systemic impact of proatherogenic microbes in animals susceptible to atherosclerosis. This mouse model has been designed to be sensitive to spontaneous and especially to Western diet-induced atherosclerosis. The mouse model has, in addition, been utilized in a study of diet-induced nonalcoholic steatohepatitis (33). However, the selection of the mouse strain can be considered a limitation of the present study, because the apoE deficiency influences both the liver lipid metabolism and the overall response to the bacterially induced endotoxemia (27, 37). On the other hand, since rodents are in general highly resistant to the deleterious effects of LPS, the increased susceptibility to infection in apoE-deficient mice makes this animal model more human-like. Another limitation concerns gender. Many genes involved in lipid metabolism are expressed and regulated sexually dimorphically in the liver (40). As our study comprises only male animals, further research is needed to verify whether the present observations are also repeatable with female mice. The molecular mechanism underlying the observed morphological alterations induced by chronic C. pneumoniae infection needs further study as well.
In conclusion, we observed several significant metabolic effects caused by exposure to two proatherogenic microbes, A. actinomycetemcomitans and C. pneumoniae, on the livers of mice prone to atherosclerosis. The two pathogens had different impact profiles when the mice were infected separately, whereas the combined infection had suppressing effects. Based on our results, chronic C. pneumoniae infection may cause significant disturbances in hepatic fatty acid homeostasis. Such a condition was preceded by an inflammatory response that was verified in the animal group with acute infection. The A. actinomycetemcomitans infection displayed more severe inflammatory changes in the liver, which correlated with serum markers of inflammation. Both mechanisms contribute to the development of atherosclerosis. These observations are of general interest, since both pathogens are highly common among the middle-aged and elderly populations.
Acknowledgments
We thank Anne Hakala, Pirjo Nurmi, Tiina Karvonen, Tiina Keippilä, Jari Metso, and Sari Nuutinen for excellent technical assistance. The personnel in the animal facilities at the National Public Health Institute are acknowledged.
This work was supported by grants from the Academy of Finland (grants 211129 and 118391 to P.J.P. and 111261 and 129148 to R.K.), the Aarne Koskelo Foundation (to K.H.), the Ida Montin Foundation (to K.H.), the Sigrid Juselius Foundation (to M.J. and P.J.P.), and the Finnish Foundation for Cardiovascular Research (to M.J.). Wihuri Research Institute is maintained by the Jenny and Antti Wihuri Foundation.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Beck, J. D., and S. Offenbacher. 1998. Oral health and systemic disease: periodontitis and cardiovascular disease. J. Dent. Educ. 62859-870. [PubMed] [Google Scholar]
- 2.Belland, R. J., S. P. Ouellette, J. Gieffers, and G. I. Byrne. 2004. Chlamydia pneumoniae and atherosclerosis. Cell. Microbiol. 6117-127. [DOI] [PubMed] [Google Scholar]
- 3.Bingen, A., J. P. Martin, F. Klein, and M. Pessah. 1992. Modification of the amount of cholesterol in hepatic steatosis induced in susceptible and resistant mice infected with MHV3: a biochemical and ultrastructural study. Hepatology 151137-1146. [DOI] [PubMed] [Google Scholar]
- 4.Bolukbas, F. F., C. Bolukbas, F. Zeyrek, M. Aslan, H. I. Bahcecioglu, and I. Ozardali. 2005. High rate of seropositivity of Chlamydia pneumoniae IgA in male patients with nonalcoholic steatohepatitis. Dig. Dis. Sci. 501141-1145. [DOI] [PubMed] [Google Scholar]
- 5.Brown, A. J. 2008. Viral hepatitis and fatty liver disease: how an unwelcome guest makes pâté of the host. Biochem. J. 416e15-7. [DOI] [PubMed] [Google Scholar]
- 6.Bykov, I., S. Junnikkala, M. Pekna, K. O. Lindros, and S. Meri. 2006. Complement C3 contributes to ethanol-induced liver steatosis in mice. Ann. Med. 38280-286. [DOI] [PubMed] [Google Scholar]
- 7.Ciervo, A., A. Petrucca, and A. Cassone. 2003. Identification and quantification of Chlamydia pneumoniae in human atherosclerotic plaques by LightCycler real-time-PCR. Mol. Cell. Probes 17107-111. [DOI] [PubMed] [Google Scholar]
- 8.Ekman, M. R., J. T. Grayston, R. Visakorpi, M. Kleemola, C. C. Kuo, and P. Saikku. 1993. An epidemic of infections due to Chlamydia pneumoniae in military conscripts. Clin. Infect. Dis. 17420-425. [DOI] [PubMed] [Google Scholar]
- 9.Gaydos, C. A., T. C. Quinn, and J. J. Eiden. 1992. Identification of Chlamydia pneumoniae by DNA amplification of the 16S rRNA gene. J. Clin. Microbiol. 30796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgiou, T. O., R. I. Marshall, and P. M. Bartold. 2004. Prevalence of systemic diseases in Brisbane general and periodontal practice patients. Aust. Dent. J. 49177-184. [DOI] [PubMed] [Google Scholar]
- 11.Hara, A., and N. S. Radin. 1978. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 90420-426. [DOI] [PubMed] [Google Scholar]
- 12.Hyvarinen, K., S. Laitinen, S. Paju, A. Hakala, L. Suominen-Taipale, M. Skurnik, E. Kononen, and P. J. Pussinen. Detection and quantification of five major periodontal pathogens by single copy gene-based real-time PCR. Innate Immun., in press. [DOI] [PubMed]
- 13.Jauhiainen, M., and C. Ehnholm. 2005. Determination of human plasma phospholipid transfer protein mass and activity. Methods 3697-101. [DOI] [PubMed] [Google Scholar]
- 14.Kalayoglu, M. V., P. Libby, and G. I. Byrne. 2002. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA 2882724-2731. [DOI] [PubMed] [Google Scholar]
- 15.Könönen, E., S. Paju, P. J. Pussinen, M. Hyvönen, P. Di Tella, L. Suominen-Taipale, and M. Knuuttila. 2007. Population-based study of salivary carriage of periodontal pathogens in adults. J. Clin. Microbiol. 452446-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, M. S., G. F. Kang, and S. Ghosh. 1988. Activation of phospholipases A1 and A2 in heart, liver, and blood during endotoxin shock. J. Surg. Res. 45472-480. [DOI] [PubMed] [Google Scholar]
- 17.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔ CT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 18.Marangoni, A., S. Accardo, R. Aldini, M. Guardigli, F. Cavrini, V. Sambri, M. Montagnani, A. Roda, and R. Cevenini. 2006. Production of reactive oxygen species and expression of inducible nitric oxide synthase in rat isolated Kupffer cells stimulated by Leptospira interrogans and Borrelia burgdorferi. World J. Gastroenterol. 123077-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marangoni, A., R. Aldini, M. Guardigli, V. Sambri, L. Giacani, M. Montagnani, A. Roda, and R. Cevenini. 2003. Phagocytosis of Treponema pallidum and reactive oxygen species production by isolated rat Kupffer cells. Med. Microbiol. Immunol. 192183-188. [DOI] [PubMed] [Google Scholar]
- 20.Marangoni, A., M. Donati, F. Cavrini, R. Aldini, S. Accardo, V. Sambri, M. Montagnani, and R. Cevenini. 2006. Chlamydia pneumoniae replicates in Kupffer cells in mouse model of liver infection. World J. Gastroenterol. 126453-6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paldanius, M. 2007. Serological studies on Chlamydia pneumoniae infections. Thesis. University of Oulu, Oulu, Finland.
- 22.Pussinen, P. J., K. Nyyssonen, G. Alfthan, R. Salonen, J. A. Laukkanen, and J. T. Salonen. 2005. Serum antibody levels to Actinobacillus actinomycetemcomitans predict the risk for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 25833-838. [DOI] [PubMed] [Google Scholar]
- 23.Pussinen, P. J., T. Vilkuna-Rautiainen, G. Alfthan, K. Mattila, and S. Asikainen. 2002. Multiserotype enzyme-linked immunosorbent assay as a diagnostic aid for periodontitis in large-scale studies. J. Clin. Microbiol. 40512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reischl, U., N. Lehn, U. Simnacher, R. Marre, and A. Essig. 2003. Rapid and standardized detection of Chlamydia pneumoniae using LightCycler real-time fluorescence PCR. Eur. J. Clin. Microbiol. Infect. Dis. 2254-57. [DOI] [PubMed] [Google Scholar]
- 25.Saito, T., Y. Shimazaki, T. Koga, M. Tsuzuki, and A. Ohshima. 2006. Relationship between periodontitis and hepatic condition in Japanese women. J. Int. Acad. Periodontol. 889-95. [PubMed] [Google Scholar]
- 26.Schmocker, C., K. H. Weylandt, L. Kahlke, J. Wang, H. Lobeck, G. Tiegs, T. Berg, and J. X. Kang. 2007. Omega-3 fatty acids alleviate chemically induced acute hepatitis by suppression of cytokines. Hepatology 45864-869. [DOI] [PubMed] [Google Scholar]
- 27.Sehayek, E., S. Shefer, L. B. Nguyen, J. G. Ono, M. Merkel, and J. L. Breslow. 2000. Apolipoprotein E regulates dietary cholesterol absorption and biliary cholesterol excretion: studies in C57BL/6 apolipoprotein E knockout mice. Proc. Natl. Acad. Sci. USA 973433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spahr, A., E. Klein, N. Khuseyinova, C. Boeckh, R. Muche, M. Kunze, D. Rothenbacher, G. Pezeshki, A. Hoffmeister, and W. Koenig. 2006. Periodontal infections and coronary heart disease: role of periodontal bacteria and importance of total pathogen burden in the coronary event and periodontal disease (CORODONT) study. Arch. Intern. Med. 166554-559. [DOI] [PubMed] [Google Scholar]
- 29.Sprong, H., P. van der Sluijs, and G. van Meer. 2001. How proteins move lipids and lipids move proteins. Nat. Rev. Mol. Cell Biol. 2504-513. [DOI] [PubMed] [Google Scholar]
- 30.Tomofuji, T., D. Ekuni, R. Yamanaka, H. Kusano, T. Azuma, T. Sanbe, N. Tamaki, T. Yamamoto, T. Watanabe, M. Miyauchi, and T. Takata. 2007. Chronic administration of lipopolysaccharide and proteases induces periodontal inflammation and hepatic steatosis in rats. J. Periodontol. 781999-2006. [DOI] [PubMed] [Google Scholar]
- 31.Törmäkangas, L., H. Alakärppä, D. Bem David, M. Leinonen, and P. Saikku. 2004. Telithromycin treatment of chronic Chlamydia pneumoniae infection in C57BL/6J mice. Antimicrob. Agents Chemother. 483655-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Törmäkangas, L., L. Erkkilä, T. Korhonen, T. Tiirola, A. Bloigu, P. Saikku, and M. Leinonen. 2005. Effects of repeated Chlamydia pneumoniae inoculations on aortic lipid accumulation and inflammatory response in C57BL/6J mice. Infect. Immun. 736458-6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tous, M., N. Ferre, J. Camps, F. Riu, and J. Joven. 2005. Feeding apolipoprotein E-knockout mice with cholesterol and fat enriched diets may be a model of non-alcoholic steatohepatitis. Mol. Cell. Biochem. 26853-58. [DOI] [PubMed] [Google Scholar]
- 34.Tuomainen, A. M., M. Jauhiainen, P. T. Kovanen, J. Metso, S. Paju, and P. J. Pussinen. 2008. Aggregatibacter actinomycetemcomitans induces MMP-9 expression and proatherogenic lipoprotein profile in apoE-deficient mice. Microb. Pathog. 44111-117. [DOI] [PubMed] [Google Scholar]
- 35.Van Amersfoort, E. S., T. J. C. Van Berkel, and J. Kuiper. 2003. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 16379-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Haperen, R., A. van Tol, P. Vermeulen, M. Jauhiainen, T. van Gent, P. van den Berg, S. Ehnholm, F. Grosveld, A. van der Kamp, and R. de Crom. 2000. Human plasma phospholipid transfer protein increases the antiatherogenic potential of high density lipoproteins in transgenic mice. Arterioscler. Thromb. Vasc. Biol. 20:1082-1088. [DOI] [PubMed] [Google Scholar]
- 37.Van Oosten, M., P. C. Rensen, E. S. Van Amersfoort, M. Van Eck, A. M. Van Dam, J. J. Breve, T. Vogel, A. Panet, T. J. Van Berkel, and J. Kuiper. 2001. Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. A new therapeutic approach to treat gram-negative sepsis. J. Biol. Chem. 2768820-8824. [DOI] [PubMed] [Google Scholar]
- 38.Vilkuna-Rautiainen, T., P. J. Pussinen, M. Roivainen, T. Petays, P. Jousilahti, T. Hovi, E. Vartiainen, and S. Asikainen. 2006. Serum antibody response to periodontal pathogens and herpes simplex virus in relation to classic risk factors of cardiovascular disease. Int. J. Epidemiol. 351486-1494. [DOI] [PubMed] [Google Scholar]
- 39.Wigg, A. J., I. C. Roberts-Thomson, R. B. Dymock, P. J. McCarthy, R. H. Grose, and A. G. Cummins. 2001. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 48206-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, X., E. E. Schadt, S. Wang, H. Wang, A. P. Arnold, L. Ingram-Drake, T. A. Drake, and A. J. Lusis. 2006. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, J., A. A. Quyyumi, J. E. Norman, G. Csako, M. A. Waclawiw, G. M. Shearer, and S. E. Epstein. 2000. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am. J. Cardiol. 85140-146. [DOI] [PubMed] [Google Scholar]