Abstract
Current evidence suggests that protective antigen (PA)-based anthrax vaccines may elicit a narrow neutralizing antibody repertoire, and this may represent a vulnerability with PA-based vaccines. In an effort to identify neutralizing specificities which may complement those prevalent in PA antiserum, we evaluated whether sequences within the 2β2-2β3 loop of PA, which are apparent in the crystal structure of heptameric but not monomeric PA, might represent a target for an epitope-specific vaccine for anthrax and, further, whether antibodies to these sequences are induced in rabbits immunized with monomeric PA. We evaluated the immunogenicity in rabbits of a multiple antigenic peptide (MAP) displaying copies of amino acids (aa) 305 to 319 of this region. Overall, four out of six rabbits vaccinated with the MAP peptide in Freund's adjuvant developed high-titer, high-avidity antibody responses which cross-reacted with the immobilized peptide sequence comprising aa 305 to 319 and with PA, as determined by an enzyme-linked immunosorbent assay, and which displayed potent and durable neutralization of lethal toxin (LeTx) in vitro, with peak titers which were 452%, 100%, 67%, and 41% of the peak neutralization titers observed in positive-control rabbits immunized with PA. Importantly, analysis of sera from multiple cohorts of rabbits with high-titer immunity to PA demonstrated a virtual absence of this potent antibody specificity, and work by others suggests that this specificity may be present at only low levels in primate PA antiserum. These results highlight the potential importance of this immunologically cryptic neutralizing epitope from PA as a target for alternative and adjunctive vaccines for anthrax.
Bacillus anthracis has a long and storied history as the causative agent of anthrax in wildlife, livestock, and human hosts. More recently, the easy distribution and extreme toxicity associated with inhalation of its endospores have positioned B. anthracis as an accessible yet formidable bioweapon for use in warfare and terrorism. The morbidity and mortality associated with inhalation of anthrax spores among humans is largely a direct result of the elaboration of lethal toxin (LeTx) during vegetative growth of virulent strains of B. anthracis. The anthrax toxins LeTx and edema toxin are classic A-B toxins, where lethal factor (LF) and edema factor (EF) represent the active moieties and protective antigen (PA) the binding moiety. PA binds the anthrax toxin receptor (ATR), either CMG2 or TEM8, and forms a heptameric prepore which binds EF or LF. Under the acidic conditions of the late endosome, EF and LF are transported into the cell cytosol, where they exert their enzymatic activities as edema toxin and LeTx, respectively (9, 11, 24).
PA-specific humoral immunity has been demonstrated to protect animals from experimental challenge with anthrax even in the absence of LF and EF immunity (25, 37, 58). Animal model studies have shown that anthrax vaccine adsorbed (AVA), the currently licensed anthrax vaccine in the United States, provides protection by stimulating antibodies against PA (38, 57), and AVA has been shown to confer a high degree of protection from an inhalation spore challenge in rabbits and primates (14, 20, 38). However, the multiple injections and yearly boosts required for establishment and maintenance of immunity, and the reactogenicity of and potential adverse reactions to AVA, have raised broad concern and have motivated commitment to the development of next-generation anthrax vaccines (10, 21, 39, 52).
Most of the efforts to develop new vaccines for anthrax have focused on the elicitation of immunity to PA. Ongoing research is also focused on the design and testing of vaccines targeting antigens other than PA in an effort to broaden the breadth of immunity induced through vaccination (8, 15, 30, 40). This was motivated in part by the realization, informed primarily by analysis of PA-specific monoclonal antibodies (MAbs) in mice and humans, that the antibody specificities responsible for LeTx neutralization may be limited to only a few dominant specificities (1, 7, 28, 29, 44). These are focused primarily on domains 1′ and 4 of PA63, which are involved in binding to LF and EF and to the anthrax toxin receptors, respectively. The limited breadth of the neutralizing repertoire induced through PA immunization could leave vaccinees vulnerable to possible maliciously altered or selected B. anthracis strains resistant to the neutralizing specificities, a contingency for which proof of principle has now been demonstrated (2, 48).
The solution of the 1TZN crystal structure revealed the PA heptamer bound to the CMG2 cell receptor, including sequences within the 2β2-2β3 loop of PA which were unresolved in previous crystal structures (23). The 2β2-2β3 loop had previously been identified as containing the chymotrypsin cleavage site and was shown to be critical for LeTx function, specifically for translocation of EF and LF into the cytosol (33, 34, 54, 55). The surface-exposed nature of this sequence, as deduced through protein-structure algorithms and through experimental demonstration that the site is accessible to protease cleavage, led us to believe that it might represent an effective target for an epitope-specific vaccine for anthrax.
To date, efforts to develop vaccines targeting specific epitopes within PA or LF have been limited, and there are no published accounts of efficacious peptide vaccines targeting PA. Our hypothesis that this site in domain 2 of PA might represent a neutralizing determinant was confirmed when two groups independently reported mouse MAbs specific for the 2β2-2β3 loop region, which possessed LeTx-neutralizing activity (16, 61). Here, we demonstrate that a multiple antigenic peptide (MAP) consisting of four copies per molecule of amino acids (aa) 305 to 319 of PA can elicit humoral immunity in rabbits that is specific for the 2β2-2β3 loop neutralizing determinant (LND) and which demonstrates potent neutralization of LeTx in vitro. We further show that antibody specific for the LND is not induced in rabbits immunized with full-length PA.
MATERIALS AND METHODS
Synthetic peptides and recombinant proteins.
The synthetic peptides and recombinant proteins used in the study are listed in Table 1. A four-branch MAP displaying four copies per molecule of aa 305 to 319 of PA (single-letter code, GNAEVHASFFDIGGS; GenBank accession no. P13423) linked at the C terminus to the branching lysine core of the MAP was synthesized using standard F-moc chemistry. The linear synthetic peptides used for the analysis of peptide inhibition include aa 305 to 319 of PA and an irrelevant peptide consisting of aa 1 to 16 of the Aβ42 peptide deposited pathologically in Alzheimer's disease (single-letter code, DAEFRHDSGYEVHHQK) (50). All synthetic peptides were synthesized commercially (Sigma-Genosys, The Woodlands, TX). Two recombinant proteins were used for assessing immunity specific for the peptide comprising aa 305 to 319 (305-319 peptide) of PA by an enzyme-linked immunosorbent assay (ELISA). Both recombinant proteins were molecularly constructed, expressed, and purified essentially as previously described (35). The recombinants display two tandemly repeated copies of either aa 299 to 327 (single-letter code, HTSEVHGNAEVHASFFDIGGSVSAGFSNS) or aa 305 to 319 (single-letter code, GNAEVHASFFDIGGS) of PA linked to maltose binding protein. The DNA sequences encoding the proteins were validated with automated dideoxy sequencing of the sense and antisense DNA (49), and the purified proteins were found to be in excess of 90% pure by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis.
TABLE 1.
Peptide and recombinant proteins used in this study
| Description | Sequence |
|---|---|
| Synthetic peptides | |
| MAP peptidea | GNAEVHASFFDIGGS\ /GNAEVHASFFDIGGS |
| K-K-K | |
| GNAEVHASFFDIGGS/ \GNAEVHASFFDIGGS | |
| PA 305-319 peptide | GNAEVHASFFDIGGS |
| Irrelevant peptideb | DAEFRHDSGYEVHHQK |
| Recombinant proteins | |
| MBP-PA 305-319c | MBP-(GNAEVHASFFDIGGS)2 |
| MBP-PA 299-327c | MBP-(HTSEVHGNAEVHASFFDIGGSVSAGFSNS)2 |
The MAP peptide consists of four PA 305-319 peptide arms extending from the α- and ε-amino groups of a branching lysine core indicated by “K-K-K.”
The irrelevant peptide sequence is from the Aβ peptide comprising aa 1 to 16, derived from the amyloid precursor protein.
The recombinant proteins consist of maltose binding protein (MBP) expressed as fusions with two tandem copies of the peptide sequences shown.
Cell lines.
RAW 264.7 is a mouse macrophage cell line derived from Abelson murine leukemia virus-induced tumors in BALB/c mice (ATCC, Manassas, VA) (42).
Immunization of rabbits and sample collection.
Female New Zealand White rabbits were immunized on day 0 with 500 to 250 μg of the MAP peptide in an emulsion with complete Freund's adjuvant (CFA) (Covance Research Products, Denver, PA). Rabbits were then boosted at 2-week intervals with 125 μg of the MAP peptide in an emulsion with incomplete Freund's adjuvant (IFA) (Sigma Biochemicals, St. Louis, MO). Serum samples were collected prior to the first immunization (day 0), 10 days after each booster immunization, and, for some rabbits, approximately 2.5 months after the final booster immunization. Two PA-immune control rabbits were immunized with 250 μg of soluble PA in an emulsion with CFA (PA83; List Biological Laboratories, Inc., Campbell, CA) and then boosted four times at 2-week intervals with 125 μg of PA in IFA. Sera were obtained from the control PA-immune rabbits approximately 10 days after the fifth immunization. In two additional experiments, seven rabbits were immunized with 250 μg of soluble PA in an emulsion with CFA and then boosted 2 weeks later with 125 μg of PA in IFA. Separately, four rabbits were immunized with 250 μg of PA by using Alhydrogel adjuvant (Brenntag Biosector, Denmark) and boosted twice at 2-week intervals with 125 μg of PA in Alhydrogel. In both experiments, sera were obtained 10 days after the final booster immunization for analysis. Rabbits inoculated with recombinant adeno-associated virus (rAAV) vectors expressing PA63 are described elsewhere (30). All animal procedures were approved by the Institutional Animal Care and Use Committee and were performed in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International.
ELISA analysis.
Individual rabbit antisera were analyzed in duplicate by ELISA as described previously (35). For analysis of antibodies specific for PA, wells of microtiter plates (Immulon 2; Thermo Labsystems, Franklin, MA) were coated overnight at 4°C with 100 ng of PA (PA83; List Biological Laboratories, Inc., Campbell, CA) in a 0.05 M carbonate buffer (pH 9.5). For analysis of antipeptide binding, wells were coated with 100 ng of a recombinant protein displaying two tandemly repeated copies of either aa 299 to 327 or aa 305 to 319 of PA, both expressed as fusions with maltose binding protein. Bound antibody was detected with secondary biotinylated antibody specific for rabbit immunoglobulin G (Southern Biotechnology, Birmingham, AL), followed by streptavidin-alkaline phosphatase and 4-nitrophenylphosphate (Roche, Indianapolis, IN). Absorbance at 405 nm minus absorbance at 650 nm was determined using an ELISA reader (Emax microplate reader; Molecular Devices, Menlo Park, CA). Antibody titers were determined from serial twofold dilutions of individual rabbit sera and represent the reciprocal dilution at the 50% effective concentration (EC50) established using nonlinear regression to fit a variable slope sigmoidal equation to the serial dilution data with Prism 5.0 (GraphPad Software, Inc., San Diego, CA). The lower limit of assay detection was 16.
Avidity analysis.
Avidity assays were based on ELISA quantitation of bound antibody in the presence or absence of chaotrope as described by Anttila et al., with slight modification (3). Briefly, after initial exploratory studies with several chaotropes at a range of concentrations, 2 M NH4SCN was selected based on the maximal discrimination of antibody binding with this chaotrope (31, 41). For the chaotrope titration, duplicate plates were coated with PA and ELISA procedures were followed as described above. After antiserum incubation, the plates were washed in wash buffer and chaotrope plates received 100 μl of 2 M NH4SCN (Sigma Biochemicals, St. Louis, MO) and were incubated for 30 min at room temperature. Nonchaotrope plates were handled in parallel but received only wash buffer in place of chaotrope. After the chaotrope step, both plates were washed and processed as usual for the remainder of the ELISA. The avidity index, which represents the fraction of bound antibody resistant to chaotrope, was determined for each serum sample and is defined as the EC50 antibody titer in the presence of a chaotrope elution, divided by the EC50 titer observed without chaotrope elution, multiplied by 100. EC50 titers were determined as described above.
TNAs.
The ability of antibody to block LeTx action in vitro was assessed in a toxin neutralization assay (TNA), using the RAW 264.7 cell line (ATCC, Manassas, VA) essentially as described previously (30). Briefly, cells were grown in culture in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, penicillin-streptomycin, and 50 μM 2-mercaptoethanol (complete medium) in a humidified 6.5% C02 incubator. Complete medium was used for dilution of all assay reagents. For each experiment, cells were harvested using 3 mM EDTA, washed with Dulbecco's modified Eagle's medium, and plated at 30 × 103 cells/well in 96-well flat-bottom plates for overnight culture (Costar 3596; Corning, Inc., Corning, NY). The following day, heat-denatured rabbit antisera in duplicate were serially diluted in polypropylene round-bottom 96-well plates in a final volume of 50 μl per well. LeTx reagent containing PA83 and LF in complete medium was prepared at a 2× (twice the final) concentration, with the final concentration representing 2.5 to 3.5 multiples of the amount needed to kill 50% of the RAW 264.7 cells. Each TNA was validated by a contemporaneous PA titration. For each neutralization assay, 110 ng/ml PA83 was used along with 150 ng of LF. The diluted rabbit antiserum was added to the LeTx, and the mixture was incubated for 30 min before being transferred to the RAW 264.7 cells in exchange for the preexisting medium. Following a 4 h of incubation, 20 μl of MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethylphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] reagent was added to each well (CellTiter96 AQ; Promega Corp., Madison, WI), and after an additional 2 h of incubation, absorbance at 405 nm minus absorbance at 650 nm was determined for each plate by using a Vmax plate reader. Neutralization 50% effective dose (ED50; the effective dilution at which 50% of cells are protected from cytotoxicity) titers were determined from serial twofold dilutions of individual rabbit sera and represent the reciprocal dilution at the EC50 established using nonlinear regression to fit a variable slope sigmoidal equation to the serial dilution data with Prism 5.0 (GraphPad Software, Inc., San Diego, CA) (19). The lower limit of assay detection was 16. For the analysis of peptide inhibition of TNA, experimental serum samples were preincubated with 20 μM peptide for 30 min at room temperature prior to analysis in the TNA.
Statistical analysis.
For determination of ELISA EC50 titers and TNA ED50 titers, four-parameter logistic regression was used to fit variable slope sigmoidal equations to the serial dilution data. Student's t test was used for comparisons of data between groups. All statistical analysis was performed using GraphPad Prism software version 5.0 (GraphPad Software, Inc., San Diego, CA).
RESULTS
MAP-specific rabbit antiserum is immunoreactive with the immobilized 305-319 peptide and with PA and neutralizes LeTx in vitro.
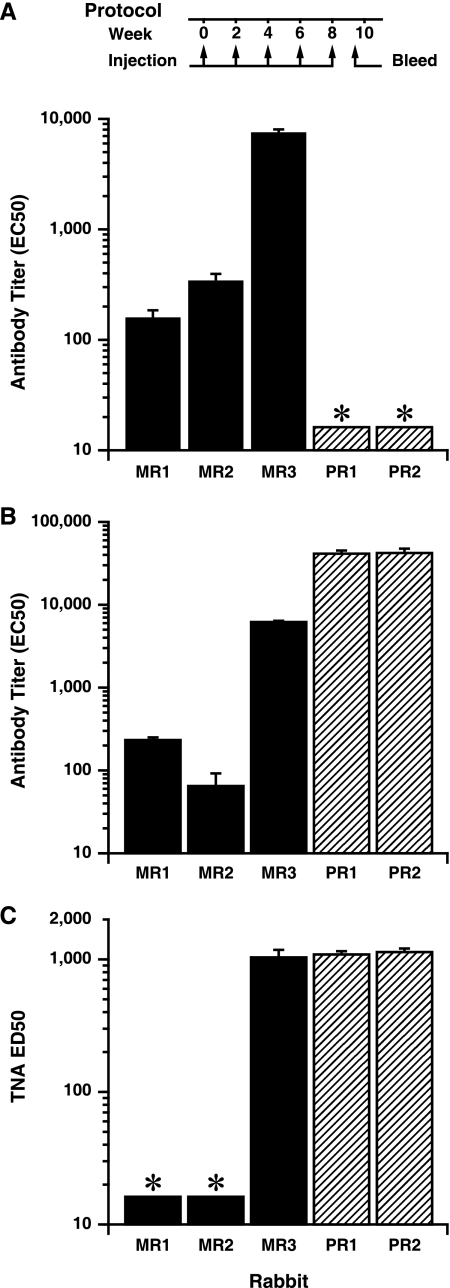
To evaluate whether antibodies could be elicited with specificity toward sequences within the 2β2-2β3 loop sequence of PA, we synthesized a MAP peptide displaying four copies per molecule of aa 305 to 319 of PA and assessed its immunogenicity in rabbits, the species used most frequently to evaluate the protective efficacy of new anthrax vaccines. Three rabbits were immunized with the MAP peptide in an emulsion with CFA and boosted four times at 2-week intervals in an emulsion with IFA. As positive controls, two rabbits were immunized with full-length PA by using the same adjuvant regimen. Rabbits were bled 10 days after the final immunization, and sera were evaluated by ELISA for induction of antibodies immunoreactive with the 305-319 peptide and with PA. One of the three rabbits immunized with the MAP peptide developed a significant antibody response which bound both the peptide sequence and PA (Fig. 1A and B, respectively). As expected, both rabbits immunized with full-length PA developed high-titer PA-specific antibody responses (Fig. 1B).
FIG. 1.
Antibody and toxin-neutralizing responses in sera of rabbits immunized with the MAP peptide or with full-length PA. Shown are antibody responses to the immobilized 305-319 peptide (A) or to PA (B) and TNA activity (C) for sera of rabbits immunized five times at 2-week intervals with either the MAP peptide (MR1 to MR3) or PA (PR1 and PR2) as described in Materials and Methods and as depicted graphically in the timeline. Serum responses are from test bleeds obtained 10 days after the fifth immunization. For analysis of peptide reactivity, the immobilized antigen was a recombinant protein displaying two copies of the peptide sequence comprising aa 299 to 327 of PA. Antibody and TNA titers were determined as described in Materials and Methods and are expressed as reciprocals of the EC50 and ED50, respectively. The lower limit of assay detection for the ELISA and TNA is 16, and sample data below this level are indicated with an asterisk (*). Error bars represent standard errors of the means.
We next evaluated whether antibodies from the serum of the MAP responder rabbit had activity in the TNA. This assay evaluates the functional ability of antibody to neutralize LeTx in vitro, and TNA titers have been shown to correlate well with protection in inhalation spore challenges (19, 26, 27, 46). The MAP-immune serum exhibited neutralization of LeTx at levels equivalent to those in the sera of the two positive-control rabbits, even though the PA-specific antibody titer in the serum of this MAP-immune rabbit was less than 15% of those for the control PA-immune rabbits (Fig. 1C). These data suggested that antibodies to the 2β2-2β3 LND possess neutralizing potency exceeding that of PA antiserum.
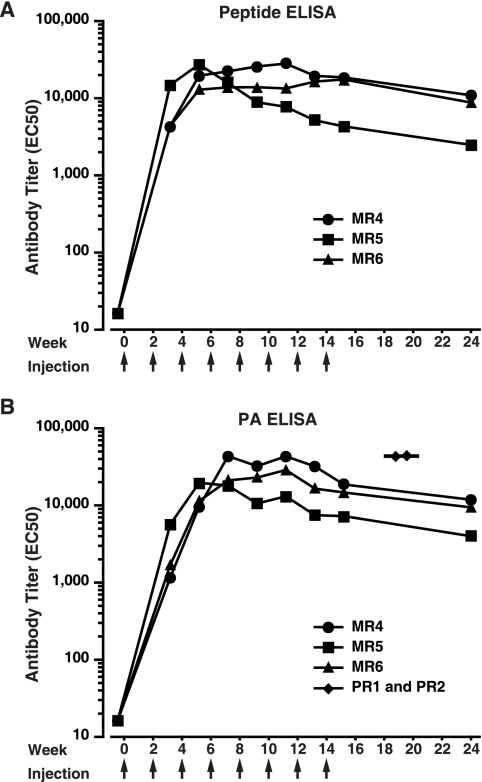
Having demonstrated that antibody to the LND mediated significant neutralization, we expanded our study to evaluate an additional group of three rabbits. With the exception of a slight dose modification and additional booster immunizations, the second group of rabbits were immunized with the MAP peptide in a fashion identical to that for the first group. As shown in Fig. 2A, all three rabbits from the second cohort developed significant antipeptide titers as early as after the second injection (week 2). Near-peak titers were maintained in rabbits MR4 and MR6 through the eighth injection (week 14), while rabbit MR5 had antipeptide titers which peaked early and then declined more rapidly. When assessed 2.5 months after the last injection, significant antipeptide titers were observed in all three rabbits. Importantly, the antibodies were also found to be immunoreactive with immobilized PA (Fig. 2B). As with the antipeptide titers, peak anti-PA titers appeared promptly and were maintained through injection 8 in rabbits MR4 and MR6. Thereafter, anti-PA titers dropped slightly but remained significant at the time of the terminal bleed, 2.5 months after the final booster immunization, with reciprocal EC50 titers of 11,857, 9,447, and 3,990.
FIG. 2.
Antibody responses in sera of rabbits immunized with the MAP peptide. Sera obtained from MAP peptide-immune rabbits 10 days after the indicated immunizations were tested by ELISA for reactivity with immobilized peptide sequence (A) or with PA (B). For analysis of peptide reactivity, the immobilized antigen was a recombinant protein displaying two copies of the peptide sequence comprising aa 299 to 327 of PA. Antibody titers were determined as described in Materials and Methods and are expressed as the reciprocal of the EC50. As controls, immunoreactivity with immobilized PA from the antisera of the two PA-immune rabbits is shown in panel B (diamonds), with the horizontal line representing the GMT. Responses from the control rabbits are from antisera obtained at approximately week 10.
Neutralization analysis of the expanded cohort showed that all three MAP-immune rabbit antisera exhibited high-titer neutralization of LeTx in vitro (Fig. 3). At their peak levels, rabbit MR4 demonstrated neutralization titers that were over 450% of the mean neutralization titers for the two positive-control PA-immune rabbits, with the other two MAP-immune rabbits, MR5 and MR6, demonstrating peak neutralization titers which were 41% and 67% of the positive-control neutralization titer, respectively. Unlike the kinetics of antibody induction, neutralization peaked later, occurring between the fifth and sixth immunizations (week 8 and week 10, respectively) for rabbits MR5 and MR6 and at the final bleed for rabbit MR4, 2.5 months after the final immunization. At this final time point, sera from the three rabbits demonstrated neutralization levels that were 452%, 34%, and 12% of the levels for two PA-immune control rabbits (Fig. 3).
FIG. 3.
Analysis of the MAP-peptide antisera in the TNA. Sera obtained from the second group of MAP peptide-immune rabbits 10 days after the indicated immunizations were tested in the TNA as described in Materials and Methods. The left y axis corresponds to the reciprocal of the ED50 neutralization titers, and the right y axis denotes the ED50 titers normalized to the geometric mean ED50 neutralization titers in the sera of the two PA-immune control rabbits obtained 10 days after their fifth immunization (diamonds). There was no detectable neutralization in the preimmune sera from any of the rabbits.
Assessment of antibody avidity in MAP and PA-immune rabbit sera.
In vitro and in vivo neutralization of virus and toxin has been shown to correlate well with the presence of highly avid antigen-specific antibodies (4, 32). Observing that the levels of neutralization evident in the sera of the MAP-immune rabbits were quite high relative to the levels of antibody (i.e., potent), we characterized the progression of PA-specific avidity maturation in the sera of the second group of MAP-immune rabbits by using an NH4SCN chaotrope elution ELISA (3, 31, 41). In this ELISA, resistance to disruption of the antigen-antibody interaction in the presence of a fixed concentration of chaotrope is directly correlated with antibody avidity (12).
The development of antibody avidity was found to trail the development of antibody titer, peaking considerably later, as has been noted in other studies evaluating avidity maturation in human and murine models (22, 41). Avidity rose over time, peaking in rabbits MR4 and MR5 at the time of the terminal bleed, 2.5 months after the final immunization, and in rabbit MR6, after the final immunization (Fig. 4). Antiserum from rabbit MR3, which, like rabbit MR4, had neutralization levels equivalent to or greater than those observed in the PA controls, also demonstrated highly avid antibody, as did the polyclonal antisera from the two PA-immune control rabbits.
FIG. 4.
MAP-immune antisera contain highly avid antibody. Shown are the avidity maturation data from the second cohort of MAP-immune rabbits (MR4 to MR6) and individual avidity data from the MAP-immune rabbit (MR3) and the two positive-control PA-immune rabbits (PR1 and PR2) as determined through use of a chaotrope ELISA as described in Materials and Methods. The avidity index was determined from sera obtained 10 days after the indicated immunizations for the second group of MAP peptide-immune rabbits or after the fifth immunization for rabbits MR3, PR1, and PR2.
The neutralizing activity in the sera of MAP-immune rabbits is completely inhibitable with peptide.
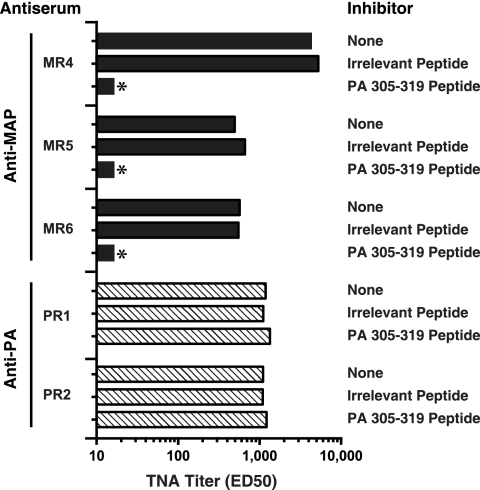
To assess whether the neutralizing antibodies in the sera of the MAP-immune rabbits were specific for, and inhibitable by, a linear sequence within the 2β2-2β3 loop of PA, antisera from the second group of MAP-immune rabbits and sera from the PA-immune controls were preincubated with the 305-319 peptide prior to assessment in the TNA. All neutralizing activity in the sera of the MAP-immune rabbits was completely inhibited when sera were preincubated with the 305-319 peptide (Fig. 5). This inhibition was specific, as no inhibition was detected when the antisera were incubated with an irrelevant peptide. As shown, there was no detectable inhibition in the positive-control sera from the rabbits immunized with PA, suggesting that the LND neutralizing specificity was not present at meaningful levels in the PA antisera.
FIG. 5.
Effect of preincubation with the 305-319 peptide or with an irrelevant peptide on the neutralization titers in the MAP and PA antisera. Antisera from the three MAP-immune rabbits obtained after the sixth immunization and from the PA-immune rabbits after the fifth immunization were incubated with 20 μM of the indicated peptides for 30 min prior to assessment in the TNA. TNA titers are expressed as the reciprocal of the ED50 and were determined as described in Materials and Methods. The lower limit of assay detection for the TNA is 16; samples below this limit are indicated with an asterisk (*).
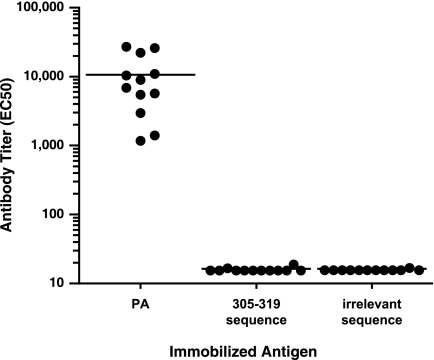
PA-immune rabbits do not develop significant antibody to the LND.
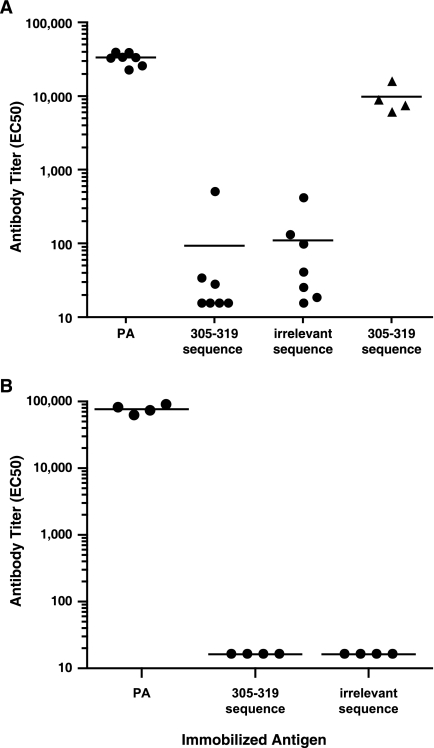
The absence of any detectable effect on neutralization observed when the 305-319 peptide was coincubated with the control PA antiserum, combined with the finding by ELISA that the PA antiserum lacks any immunoreactivity with the immobilized 305-319 peptide (Fig. 1A), led us to more definitively assess whether PA-immune rabbits develop antibodies specific for the LND. We therefore immunized seven rabbits with PA in Freund's adjuvant and boosted the rabbits 2 weeks later with PA in IFA. Rabbit antiserum was obtained 10 days after the booster immunization and was analyzed by ELISA for immunoreactivity with PA and with the 305-319 peptide. All rabbits developed robust anti-PA responses, with a reciprocal geometric mean titer (GMT) of 32,696 (Fig. 6A). In contrast, the reactivity of the PA antisera with the 305-319 peptide was not significantly different from their reactivity with an irrelevant peptide sequence. As a control, sera from the four MAP-responder rabbits were shown to react strongly to the immobilized 305-319 peptide. Antisera from the 7 PA-immune rabbits were also found to lack significant reactivity with a longer peptide sequence from domain 2 of PA which spanned aa 299 to 327 (not shown). The results demonstrated that there is no significant antibody reactivity with the LND in rabbits immunized with PA in Freund's adjuvant.
FIG. 6.
Immunoreactivity with PA and with the 305-319 peptide from sera of rabbits immunized with PA. Seven rabbits received priming immunizations with PA in CFA and then were boosted 2 weeks later with PA in IFA (A), and four rabbits received priming immunizations with PA in Alhydrogel and were boosted twice at 2-week intervals (B). Ten days after the final booster immunization (day 24 for rabbits in panel A and day 34 for rabbits in panel B), rabbits were bled and individual rabbit sera (circles) were analyzed for immunoreactivity with either PA, the 305-319 peptide, or an irrelevant peptide sequence. For analysis of peptide reactivity, a recombinant protein displaying two copies per molecule of aa 305 to 319 of PA or an irrelevant peptide sequence was immobilized on the ELISA plates as described in Materials and Methods. As a positive control, the immunoreactivity of the sera from four MAP peptide-immune rabbits (triangles) is also shown in panel A. There was no significant difference in GMT observed in the sera of the rabbits immunized with PA in Freund's (A) upon testing with either the immobilized 305-319 peptide or the irrelevant peptide sequence (P = 0.8513; Student's t test). The lower limit of assay detection for the ELISA is 16; samples below this limit are plotted at 16. Horizontal lines represent GMTs.
To further evaluate whether these results were specific only for the combination of PA and Freund's adjuvant, perhaps reflecting an interaction of the oil-in-water formulation on the presentation of PA, we immunized an additional cohort of four rabbits three times at 2-week intervals with PA in Alhydrogel, the adjuvant used in AVA for humans. Rabbits were bled 10 days after the final immunization, and antisera were evaluated by ELISA. Like the rabbits immunized with PA in Freund's, all rabbits developed high-titer responses reactive with immobilized PA, with a GMT of 76,782; however, there was no reactivity with the 305-319 peptide by ELISA (Fig. 6B).
Finally, to more formally exclude the possibility that adjuvant formulations per se are responsible for the absence of LND-specific antibodies in PA-immune rabbits, we evaluated antiserum from an additional series of 12 rabbits from a separate study, which had been inoculated either with rAAV vectors expressing PA63 or with vectors expressing both PA63 and LF (30). Though antisera from all the rabbits had significant anti-PA titers, none of the rabbits demonstrated any reactivity to the LND peptide sequence by ELISA (Fig. 7).
FIG. 7.
Immunoreactivity with PA and with the 305-319 peptide in sera from rabbits inoculated one time with rAAV vectors expressing PA63. Twelve rabbits received a single inoculation with rAAV vectors expressing PA63. Of these 12 rabbits, 6 also received rAAV expressing LF. Eight weeks after being inoculated, all rabbits were bled and sera were analyzed by ELISA for immunoreactivity with PA, with the peptide sequence comprising aa 305 to 319, or with an irrelevant peptide sequence. For analysis of peptide reactivity, a recombinant protein displaying two copies per molecule of aa 305 to 319 of PA or an irrelevant peptide sequence was immobilized on the ELISA plates as described in Materials and Methods. The lower limit of assay detection for the ELISA is 16; sample data below this limit are plotted at 16. Horizontal lines represent GMTs.
DISCUSSION
The diversity and relative abundance of neutralizing antibodies present in AVA vaccinee serum cannot be known with certainty. Nevertheless, current data from isolation of MAbs in PA-immune mice and humans suggest a neutralizing antibody repertoire of limited specificity (2, 7, 28, 29, 44). Assays for evaluating the PA-specific neutralizing repertoire directly in the sera of AVA-immune primates have been explored, but a reliance on predefined MAb specificities constrains this approach (17, 45). A limited neutralizing repertoire could represent a theoretical but real threat to the efficacy of vaccines that rely on the induction of PA-specific antibody (48, 53).
In reviewing the available crystal structure, we were attracted to the 2β2-2β3 loop of PA as a target for an epitope-specific vaccine for anthrax. Unresolved in the crystal structure of monomeric PA (1ACC and 1T6B), the 2β2-2β3 loop was first revealed with the crystallization and solution of heptameric PA and CMG2 (23, 36). The loop has been shown to be critical for translocation of LF and EF into the cytosol and contains the chymotrypsin cleavage site, which must be intact for LeTx cytotoxicity (33, 34, 55). It follows that antibodies specific for this site may interfere with critical molecular interactions.
We evaluated whether a MAP peptide displaying four copies per molecule of the 305-319 peptide of the 2β2-2β3 loop of PA could be used to stimulate epitope-specific humoral immunity in rabbits. Overall, four out of six rabbits immunized with the MAP peptide developed high-titer, high-avidity antibodies, which were immunoreactive with immobilized peptide and PA, and which exhibited high levels of LeTx neutralization. Significant durability of neutralization was also observed, as reflected in the increase in neutralization titers for rabbits MR4 and MR6 between their eighth and final injection and their terminal bleeds 2.5 months later. We considered the potential role of Freund's adjuvant, a water-in-oil emulsion, in explaining this finding; however, we have not seen this effect in rabbits immunized with PA in Freund's adjuvant, where, more typically, we have observed significant diminution in LeTx neutralization by 4 to 6 weeks after the final immunization, a pattern also seen with rabbits immunized with PA formulated with other adjuvants (26, 27). The evidence, instead, suggests that the durability more likely results from the use of the MAP peptide, a form of immunogen which has been shown by others to be capable of eliciting highly durable antibody responses (59).
The neutralization titers among the MAP-immune responder rabbits compared favorably with the titers observed in the positive-control rabbits immunized with PA in Freund's. One MAP-immune rabbit, in particular, had neutralization titers that were greater than 450% of the mean neutralization titers observed in the sera of the control PA-immune rabbits, indicating that antibody to this neutralizing determinant can exhibit significant potency. This rabbit also demonstrated the highest titers of antibody to immobilized PA among the MAP-immune responder rabbits (Fig. 2B). Among the three other responder rabbits, the peak levels of neutralization were 100%, 67%, and 41% of those for the PA-immune control rabbits. Though neutralization is considered the best in vitro surrogate for vaccine efficacy in aerosol spore challenges in rabbits, there is overlap in the ranges of neutralization titers which are associated with survivors and nonsurvivors (19, 26, 27, 38, 46). Nevertheless, PA-specific responses with neutralization titers exceeding 750 to 1,000 appear predictive of protection. Employing such criteria, three out of four MAP-immune responder rabbits would be predicted to survive a B. anthracis spore challenge if this challenge was performed after their fifth immunization. More importantly, the data suggest that effective targeting of this epitope, whether by use of a totally synthetic MAP peptide as outlined here, or by use of an alternative immunogen, could lead to meaningful and potentially protective levels of epitope-specific immunity against anthrax. One alternative immunogen was recently reported by Yin and colleagues, who inserted sequences from the 2β2-2β3 loop into the major immunodominant region of hepatitis B core protein and expressed the recombinant fusion as virus-like particles (60). Sera from guinea pigs immunized with the virus-like particles demonstrated LeTx-neutralizing activity and were partially protected from a subcutaneous challenge with B. anthracis.
The LND specificity appeared to be absent from the rabbit anti-PA antibody responses, and evidence from other studies suggests that the specificity is lacking in the PA-immune response of guinea pigs and primates as well. With regard to rabbits, the sera of the two PA-immune control rabbits had no detectable peptide-specific titers by ELISA (Fig. 1A) and their TNA titers were unaffected by preincubation of their antisera with the LND peptide, whereas the same treatment of antisera from the MAP-immune rabbits led to complete abrogation of LeTx neutralization (Fig. 5). The absence of LND-specific antibody in rabbit PA antisera was corroborated in two subsequent studies by groups of rabbits immunized with PA in Freund's or Alhydrogel adjuvants. The study examining PA with Alhydrogel, the only adjuvant approved for human use, highlights that the absence of the LND specificity is not dependent on the choice of adjuvant used for immunization. Further corroboration of this was demonstrated through evaluation of sera from 12 rabbits which had been inoculated with rAAV vectors expressing PA63 (30). Though the antisera from these rabbits had high levels of PA-specific antibody, none of the rabbits demonstrated any meaningful reactivity to the LND peptide sequence. The results from the rAAV/PA63-inoculated rabbits also suggest that the form of PA used to induce immunity (PA63 versus PA83 and expressed versus exogenous) does not appear to play a role in determining whether rabbits with immunity to PA develop LND-specific antibody. Together, the data provide strong evidence that rabbits immunized with PA do not develop significant levels of antibody specific for the LND.
The work of others suggests that guinea pigs immunized with PA also lack meaningful levels of LND-specific antibody. As described previously, the LND straddles the chymotrypsin cleavage site at 313FFD315 in domain 2 of PA, and both our data and data from others evaluating MAbs to the LND suggest that this region is a critical component of the neutralizing epitope (16). Rhie et al. found no significant difference in the neutralization titers among guinea pigs immunized with either intact PA or chymotrypsin-cleaved PA, suggesting that the LND neutralizing specificity was not meaningfully represented within the repertoire of neutralizing antibodies in guinea pig PA antiserum (47).
Finally, human vaccinee antisera also appear to contain minimal LND-specific antibody. Gubbins et al. utilized a competitive enzyme-linked assay to evaluate whether AVR801 (51), a human standard AVA-immune reference serum, and other individual AVA-immune human sera could effectively compete with an LND-specific MAb (F20G75) for binding to PA. They found that only very high concentrations of AVA serum (half-maximal inhibition at approximately neat dilutions) could inhibit the binding of the MAb to PA (17). In contrast, AVA vaccinee serum demonstrates 100-fold more activity in inhibiting the binding of the 14B7 and 2D3 MAbs to PA, highlighting the significant presence in AVA serum of neutralizing specificities for the ATR and LF binding regions of PA, respectively (45).
Collectively, these results suggest that the 2β2-2β3 loop region encompassing the LND may behave as if immunologically cryptic. Though clearly antigenic in immobilized PA, as demonstrated through immunoreactivity by ELISA, and in solution, as reflected in its availability for protease cleavage by chymotrypsin, the 2β2-2β3 loop region encompassing the LND appears to be poorly immunogenic in PA.
Cryptic B-cell epitopes have been described for other B-cell targets, notably the V3 loop region of human immunodeficiency virus type 1 and the circumsporozoite protein of Plasmodium falciparum. Recently, two independent groups identified antibodies with specificity for a conserved site within the tail section of the hemagglutinin protein of influenza virus which possess the capacity for neutralizing diverse strains of influenza virus (13, 56). This specificity, however, does not appear to be significantly induced through immunization with influenza vaccines or through natural infection, where only limited cross-protection is observed. While it is postulated that crypticity may have evolved to promote immune evasion in the aforementioned examples (5, 43), this explanation is unlikely to be applicable to the crypticity associated with the LND, since the fulminant course of anthrax precludes any significant immunological selection. The crypticity associated with the LND, alternatively, may be a reflection of complex protein structural factors which render the LND immunologically inert. Regardless of the mechanism explaining this immunological phenomenon, it appears likely that the addition of the LND specificity to the repertoire of neutralizing antibody specificities generated in PA antisera could result in more-efficacious and more-broad-based immunity to anthrax.
The addition of the LND specificity to the current neutralizing specificities generated in primate PA antisera could also improve the breadth of the neutralizing repertoire in ways important for countering potential bioterrorist threats. Analysis of mouse and human MAbs suggests that the ATR binding region in domain 4 is a dominant target for neutralizing antibody induced through immunization with PA (7, 28, 29, 44). Experimental mutations in domain 4, however, have been shown to abrogate the effectiveness of 14B7, a well-studied and efficacious MAb with specificity for the ATR interface, while having little effect on the formation and toxicity of LeTx (48). This demonstration of evasion of an important neutralizing specificity, which is a constituent specificity of a potentially limited neutralizing repertoire, may suggest a vulnerability for PA-based vaccination strategies. These findings have prompted efforts to develop novel therapeutics designed to address such antibody-resistant forms of LeTx (53). The LND neutralizing specificity targets the 2β2-2β3 loop region, which is critical for LeTx function, and the primacy of the loop sequences in enabling LeTx cytotoxicity might hinder or even preclude the malicious reengineering of PA to evade the LND specificity. Other potential applications for a totally synthetic, epitope-specific anthrax vaccine targeting the LND might be for use in individuals who respond poorly to vaccination with PA-based vaccines and possibly in postexposure scenarios, where a role for vaccination is being studied, but where a reluctance to give vaccines containing whole PA might be warranted (6, 18).
Finally, though the current MAP peptide targeting the LND elicited neutralizing responses in the majority of rabbits, two MAP-immune rabbits were nonresponders, presumably due to a lack of T-cell help. Our data from mice show that the 305-319 peptide is haptenic, or devoid of intrinsic helper T-cell epitopes (not shown). Since the MAP construct lacks exogenous helper T-cell sequences, the helper T-cell stimulation necessary for the induction of antibody to the 305-319 peptide appears principally derived from neodeterminant sequences within the MAP construct. It is likely, therefore, that the immunity toward the LND could be significantly enhanced, and major histocompatibility complex-restricted nonresponsiveness minimized, by incorporation of potent helper T-cell epitopes into future versions of MAP immunogens targeting the LND.
Acknowledgments
This work was supported by award number U01-AI56580 from the National Institute of Allergy and Infectious Diseases. This work was also supported in part with resources and the use of facilities at the Veterans Administration Healthcare System, Ann Arbor, MI.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the Department of Veteran's Affairs.
We thank Stephen Little, Arthur Friedlander, Freyja Lynn, Marty Crumrine, and Lanling Zou for their helpful discussions.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 1 June 2009.
REFERENCES
- 1.Abboud, N., and A. Casadevall. 2008. Immunogenicity of Bacillus anthracis protective antigen domains and efficacy of elicited antibody responses depend on host genetic background. Clin. Vaccine Immunol. 151115-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht, M. T., H. Li, E. D. Williamson, C. S. LeButt, H. C. Flick-Smith, C. P. Quinn, H. Westra, D. Galloway, A. Mateczun, S. Goldman, H. Groen, and L. W. Baillie. 2007. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect. Immun. 755425-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 1771614-1621. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, M. F., U. Kalinke, A. Althage, G. Freer, C. Burkhart, H. Roost, M. Aguet, H. Hengartner, and R. M. Zinkernagel. 1997. The role of antibody concentration and avidity in antiviral protection. Science 2762024-2027. [DOI] [PubMed] [Google Scholar]
- 5.Bou-Habib, D. C., G. Roderiquez, T. Oravecz, P. W. Berman, P. Lusso, and M. A. Norcross. 1994. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J. Virol. 686006-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brookmeyer, R., E. Johnson, and R. Bollinger. 2004. Public health vaccination policies for containing an anthrax outbreak. Nature 432901-904. [DOI] [PubMed] [Google Scholar]
- 7.Brossier, F., M. Levy, A. Landier, P. Lafaye, and M. Mock. 2004. Functional analysis of Bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect. Immun. 726313-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chichester, J. A., K. Musiychuk, P. de la Rosa, A. Horsey, N. Stevenson, N. Ugulava, S. Rabindran, G. A. Palmer, V. Mett, and V. Yusibov. 2007. Immunogenicity of a subunit vaccine against Bacillus anthracis. Vaccine 253111-3114. [DOI] [PubMed] [Google Scholar]
- 9.Collier, R. J., and J. A. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 1945-70. [DOI] [PubMed] [Google Scholar]
- 10.Demicheli, V., D. Rivetti, J. J. Deeks, T. Jefferson, and M. Pratt. 1998. The effectiveness and safety of vaccines against human anthrax: a systematic review. Vaccine 16880-884. [DOI] [PubMed] [Google Scholar]
- 11.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280734-737. [DOI] [PubMed] [Google Scholar]
- 12.Edgington, T. S. 1971. Dissociation of antibody from erythrocyte surfaces by chaotropic ions. J. Immunol. 106673-680. [PubMed] [Google Scholar]
- 13.Ekiert, D. C., G. Bhabha, M. A. Elsliger, R. H. Friesen, M. Jongeneelen, M. Throsby, J. Goudsmit, and I. A. Wilson. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fellows, P. F., M. K. Linscott, B. E. Ivins, M. L. Pitt, C. A. Rossi, P. H. Gibbs, and A. M. Friedlander. 2001. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine 193241-3247. [DOI] [PubMed] [Google Scholar]
- 15.Galloway, D., A. Liner, J. Legutki, A. Mateczun, R. Barnewall, and J. Estep. 2004. Genetic immunization against anthrax. Vaccine 221604-1608. [DOI] [PubMed] [Google Scholar]
- 16.Gubbins, M. J., J. D. Berry, C. R. Corbett, J. Mogridge, X. Y. Yuan, L. Schmidt, B. Nicolas, A. Kabani, and R. S. Tsang. 2006. Production and characterization of neutralizing monoclonal antibodies that recognize an epitope in domain 2 of Bacillus anthracis protective antigen. FEMS Immunol. Med. Microbiol. 47436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubbins, M. J., L. Schmidt, R. S. Tsang, J. D. Berry, A. Kabani, and D. I. Stewart. 2007. Development of a competitive enzyme linked immunosorbent assay to identify epitope specific antibodies in recipients of the U.S. licensed anthrax vaccine. J. Immunoassay Immunochem. 28213-225. [DOI] [PubMed] [Google Scholar]
- 18.Hanson, J. F., S. C. Taft, and A. A. Weiss. 2006. Neutralizing antibodies and persistence of immunity following anthrax vaccination. Clin. Vaccine Immunol. 13208-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hering, D., W. Thompson, J. Hewetson, S. Little, S. Norris, and J. Pace-Templeton. 2004. Validation of the anthrax lethal toxin neutralization assay. Biologicals 3217-27. [DOI] [PubMed] [Google Scholar]
- 20.Ivins, B. E., M. L. Pitt, P. F. Fellows, J. W. Farchaus, G. E. Benner, D. M. Waag, S. F. Little, G. W. Anderson, Jr., P. H. Gibbs, and A. M. Friedlander. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 161141-1148. [DOI] [PubMed] [Google Scholar]
- 21.Joellenbeck, J. M., L. L. Zwanziger, J. S. Durch, and B. L. Strom (ed.). 2002. The anthrax vaccine: is it safe? Does it work? National Academy Press, Washington, DC. [PubMed]
- 22.Kim, Y. T., and G. W. Siskind. 1978. Studies on the control of antibody synthesis. XII. Genetic influences on antibody affinity. Immunology 34669-678. [PMC free article] [PubMed] [Google Scholar]
- 23.Lacy, D. B., D. J. Wigelsworth, R. A. Melnyk, S. C. Harrison, and R. J. Collier. 2004. Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc. Natl. Acad. Sci. USA 10113147-13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 793162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little, S. F., B. E. Ivins, P. F. Fellows, and A. M. Friedlander. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 655171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little, S. F., B. E. Ivins, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22422-430. [DOI] [PubMed] [Google Scholar]
- 27.Little, S. F., B. E. Ivins, W. M. Webster, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2006. Duration of protection of rabbits after vaccination with Bacillus anthracis recombinant protective antigen vaccine. Vaccine 242530-2536. [DOI] [PubMed] [Google Scholar]
- 28.Little, S. F., S. H. Leppla, and E. Cora. 1988. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect. Immun. 561807-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little, S. F., J. M. Novak, J. R. Lowe, S. H. Leppla, Y. Singh, K. R. Klimpel, B. C. Lidgerding, and A. M. Friedlander. 1996. Characterization of lethal factor binding and cell receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology 142707-715. [DOI] [PubMed] [Google Scholar]
- 30.Liu, T. H., J. Oscherwitz, B. Schnepp, J. Jacobs, F. Yu, K. B. Cease, and P. R. Johnson. 2009. Genetic vaccines for anthrax based on recombinant adeno-associated virus vectors. Mol. Ther. 17373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcus, H., R. Danieli, E. Epstein, B. Velan, A. Shafferman, and S. Reuveny. 2004. Contribution of immunological memory to protective immunity conferred by a Bacillus anthracis protective antigen-based vaccine. Infect. Immun. 723471-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maynard, J. A., C. B. Maassen, S. H. Leppla, K. Brasky, J. L. Patterson, B. L. Iverson, and G. Georgiou. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20597-601. [DOI] [PubMed] [Google Scholar]
- 33.Miller, C. J., J. L. Elliott, and R. J. Collier. 1999. Anthrax protective antigen: prepore-to-pore conversion. Biochemistry 3810432-10441. [DOI] [PubMed] [Google Scholar]
- 34.Novak, J. M., M. P. Stein, S. F. Little, S. H. Leppla, and A. M. Friedlander. 1992. Functional characterization of protease-treated Bacillus anthracis protective antigen. J. Biol. Chem. 26717186-17193. [PubMed] [Google Scholar]
- 35.Oscherwitz, J., F. C. Hankenson, F. Yu, and K. B. Cease. 2006. Low-dose intraperitoneal Freund's adjuvant: toxicity and immunogenicity in mice using an immunogen targeting amyloid-beta peptide. Vaccine 243018-3025. [DOI] [PubMed] [Google Scholar]
- 36.Petosa, C., R. J. Collier, K. R. Klimpel, S. H. Leppla, and R. C. Liddington. 1997. Crystal structure of the anthrax toxin protective antigen. Nature 385833-838. [DOI] [PubMed] [Google Scholar]
- 37.Pezard, C., M. Weber, J. C. Sirard, P. Berche, and M. Mock. 1995. Protective immunity induced by Bacillus anthracis toxin-deficient strains. Infect. Immun. 631369-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 194768-4773. [DOI] [PubMed] [Google Scholar]
- 39.Pittman, P. R., G. Kim-Ahn, D. Y. Pifat, K. Coonan, P. Gibbs, S. Little, J. G. Pace-Templeton, R. Myers, G. W. Parker, and A. M. Friedlander. 2002. Anthrax vaccine: immunogenicity and safety of a dose-reduction, route-change comparison study in humans. Vaccine 201412-1420. [DOI] [PubMed] [Google Scholar]
- 40.Price, B. M., A. L. Liner, S. Park, S. H. Leppla, A. Mateczun, and D. R. Galloway. 2001. Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect. Immun. 694509-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 8683-87. [DOI] [PubMed] [Google Scholar]
- 42.Ralph, P., and I. Nakoinz. 1977. Antibody-dependent killing of erythrocyte and tumor targets by macrophage-related cell lines: enhancement by PPD and LPS. J. Immunol. 119950-954. [PubMed] [Google Scholar]
- 43.Rathore, D., R. Nagarkatti, D. Jani, R. Chattopadhyay, P. de la Vega, S. Kumar, and T. F. McCutchan. 2005. An immunologically cryptic epitope of Plasmodium falciparum circumsporozoite protein facilitates liver cell recognition and induces protective antibodies that block liver cell invasion. J. Biol. Chem. 28020524-20529. [DOI] [PubMed] [Google Scholar]
- 44.Reason, D., J. Liberato, J. Sun, W. Keitel, and J. Zhou. 2009. Frequency and domain specificity of toxin-neutralizing paratopes in the human antibody response to anthrax vaccine adsorbed. Infect. Immun. 772030-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed, D. S., J. Smoll, P. Gibbs, and S. F. Little. 2002. Mapping of antibody responses to the protective antigen of Bacillus anthracis by flow cytometric analysis. Cytometry 491-7. [DOI] [PubMed] [Google Scholar]
- 46.Reuveny, S., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 692888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhie, G. E., Y. M. Park, J. S. Han, J. Y. Yu, W. K. Seong, and H. B. Oh. 2005. Efficacy of non-toxic deletion mutants of protective antigen from Bacillus anthracis. FEMS Immunol. Med. Microbiol. 45341-347. [DOI] [PubMed] [Google Scholar]
- 48.Rosovitz, M. J., P. Schuck, M. Varughese, A. P. Chopra, V. Mehra, Y. Singh, L. M. McGinnis, and S. H. Leppla. 2003. Alanine-scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J. Biol. Chem. 27830936-30944. [DOI] [PubMed] [Google Scholar]
- 49.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 745463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schenk, D., R. Barbour, W. Dunn, G. Gordon, H. Grajeda, T. Guido, K. Hu, J. Huang, K. Johnson-Wood, K. Khan, D. Kholodenko, M. Lee, Z. Liao, I. Lieberburg, R. Motter, L. Mutter, F. Soriano, G. Shopp, N. Vasquez, C. Vandevert, S. Walker, M. Wogulis, T. Yednock, D. Games, and P. Seubert. 1999. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400173-177. [DOI] [PubMed] [Google Scholar]
- 51.Semenova, V. A., E. Steward-Clark, K. L. Stamey, T. H. Taylor, Jr., D. S. Schmidt, S. K. Martin, N. Marano, and C. P. Quinn. 2004. Mass value assignment of total and subclass immunoglobulin G in a human standard anthrax reference serum. Clin. Diagn. Lab. Immunol. 11919-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sever, J. L., A. I. Brenner, A. D. Gale, J. M. Lyle, L. H. Moulton, and D. J. West. 2002. Safety of anthrax vaccine: a review by the Anthrax Vaccine Expert Committee (AVEC) of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS). Pharmacoepidemiol. Drug Saf. 11189-202. [DOI] [PubMed] [Google Scholar]
- 53.Sharma, S., D. Thomas, J. Marlett, M. Manchester, and J. A. Young. 2009. Efficient neutralization of antibody-resistant forms of anthrax toxin by a soluble receptor decoy inhibitor. Antimicrob. Agents Chemother. 531210-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh, Y., H. Khanna, A. P. Chopra, and V. Mehra. 2001. A dominant negative mutant of Bacillus anthracis protective antigen inhibits anthrax toxin action in vivo. J. Biol. Chem. 27622090-22094. [DOI] [PubMed] [Google Scholar]
- 55.Singh, Y., K. R. Klimpel, N. Arora, M. Sharma, and S. H. Leppla. 1994. The chymotrypsin-sensitive site, FFD315, in anthrax toxin protective antigen is required for translocation of lethal factor. J. Biol. Chem. 26929039-29046. [PubMed] [Google Scholar]
- 56.Sui, J., W. C. Hwang, S. Perez, G. Wei, D. Aird, L. M. Chen, E. Santelli, B. Stec, G. Cadwell, M. Ali, H. Wan, A. Murakami, A. Yammanuru, T. Han, N. J. Cox, L. A. Bankston, R. O. Donis, R. C. Liddington, and W. A. Marasco. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taft, S. C., and A. A. Weiss. 2008. Neutralizing activity of vaccine-induced antibodies to two Bacillus anthracis toxin components, lethal factor and edema factor. Clin. Vaccine Immunol. 1571-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turnbull, P. C., S. H. Leppla, M. G. Broster, C. P. Quinn, and J. Melling. 1988. Antibodies to anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Med. Microbiol. Immunol. 177293-303. [DOI] [PubMed] [Google Scholar]
- 59.Wang, C. Y., D. J. Looney, M. L. Li, A. M. Walfield, J. Ye, B. Hosein, J. P. Tam, and F. Wong-Staal. 1991. Long-term high-titer neutralizing activity induced by octameric synthetic HIV-1 antigen. Science 254285-288. [DOI] [PubMed] [Google Scholar]
- 60.Yin, Y., J. Zhang, D. Dong, S. Liu, Q. Guo, X. Song, G. Li, L. Fu, J. Xu, and W. Chen. 2008. Chimeric hepatitis B virus core particles carrying an epitope of anthrax protective antigen induce protective immunity against Bacillus anthracis. Vaccine 265814-5821. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, J., J. Xu, G. Li, D. Dong, X. Song, Q. Guo, J. Zhao, L. Fu, and W. Chen. 2006. The 2beta2-2beta3 loop of anthrax protective antigen contains a dominant neutralizing epitope. Biochem. Biophys. Res. Commun. 3411164-1171. [DOI] [PubMed] [Google Scholar]