Abstract
In Mycobacterium tuberculosis, the sensor kinases DosT and DosS activate the transcriptional regulator DosR, resulting in the induction of the DosR regulon, which is important for anaerobic survival and perhaps latent infection. The individual and collective roles of these sensors have been postulated biochemically, but their roles in vivo have remained unclear. This work demonstrates distinct and additive roles for each sensor during anaerobic dormancy. Both sensors are necessary for wild-type levels of DosR regulon induction, and concomitantly, full induction of the regulon is required for wild-type anaerobic survival. In the anaerobic model, DosT plays an early role, responding to hypoxia. DosT then induces the regulon and with it DosS, which sustains and further induces the regulon. DosT then loses its functionality as oxygen becomes limited, and DosS alone maintains induction of the genes from that point forward. Thus, M. tuberculosis has evolved a system whereby it responds to hypoxic conditions in a stepwise fashion as it enters an anaerobic state.
Tuberculosis continues to have a serious impact on global health, with 8.8 million new cases reported in 2005 giving rise to 1.6 million deaths, or roughly 4,400 deaths per day (43). Mycobacterium tuberculosis has the capability of residing in its human host for decades without causing clinical symptoms. Despite this latency of infection, the bacilli maintain their ability to reactivate and cause disease (10, 37). An estimated one-third of the world's population is currently latently infected with M. tuberculosis, and approximately 10% of those infections will reactivate to acute disease.
Mycobacteria are strict aerobes, but M. tuberculosis encounters microaerobic environments during the course of infection. This likely occurs in mature granulomas, which are known to be avascular, inflammatory, and necrotic, conditions shown to have low levels of oxygen (1, 8, 17, 27, 42). It is commonly thought that these lesions are the site of dormant bacilli responsible for latent infection. However, other reports have detected mycobacterial DNA in visibly normal lung tissue (15), as well as adipose tissue (23), and interestingly, adipose tissue has been associated with hypoxia, as well as the presence of nitric oxide (NO) (25, 35, 44, 48). M. tuberculosis can survive for long periods of time in a nonreplicating anaerobic state in vitro as well (13). The anaerobic nonreplicating persistence model, developed by L. G. Wayne, theoretically emulates aspects of in vivo latency conditions. Through several iterations of the model (reviewed in reference 41), it was refined, and in its most contemporary form consists of sealed and slowly stirred cultures with a defined headspace of one-half of the culture volume. This permits the bacteria to slowly exhaust the supply of oxygen, allowing time to adapt to anaerobic conditions. As oxygen is consumed, culture optical density (OD) stops increasing and growth ceases (39). Slow adaptation is necessary in order for the bacilli to undergo the changes required for their long-term survival (32, 39). Oxygen deprivation is thought to parallel oxygen diminution during infection, with oxygen becoming limited as a result of necrosis and calcification of the granuloma (40). The resultant nonreplicating state is thus thought to be analogous to that of dormant bacilli during latent infection.
The DosR regulon is expressed in response to hypoxia, NO, and carbon monoxide (CO) and is thought to be important for early adaptation to these stimuli, as well as long-term survival in the host (2, 19, 20, 30, 31, 36, 46). Despite induction by other gases, the presence of oxygen itself inhibits induction of the regulon (20, 28, 29, 33). Specific growth conditions, such as stationary phase and settled cultures, also induce the DosR regulon (12, 22, 45). Under these conditions, low oxygen tension is again thought to be the stimulus. The DosR regulon is regulated by the response regulator DosR (DevR, Rv3133c) and was recently shown to be positively regulated by PhoP (Rv0757) to a basal level during aerobic growth (14). DosR is activated by two sensor histidine kinases, DosT (Rv2027c) and DosS (DevS, Rv3132c). The activation of DosR occurs through autophosphorylation of either of the sensors, followed by transfer of the phosphate to DosR (26). dosS is itself part of the DosR regulon, and thus its regulation is also controlled by DosR (36). dosT is adjacent to one of several regions containing DosR regulon genes; however, it does not itself appear to be induced by DosR (30, 36). dosT is expressed in aerobic cultures, and its expression is unaltered after 48 h of defined (0.2%) low-level oxygen (28, 30). Despite the similar sequences of the sensors, their efficiencies under similar conditions have been differentially reported. In one report, DosT was shown to be more efficient than DosS at autophosphorylation and phosphotransfer (26), although the converse was demonstrated in a second report (28).
The ability of NO and CO to induce the DosR regulon is congruent with what is known of these sensors. Both of these gases can bind to the heme irons of DosT and DosS, possibly serving to displace O2, resulting in activation of the sensor (9, 21, 33, 47). A related model indicates that NO and CO can only bind to the sensors when the iron is in its ferric form (20). After the gases are thus bound, because of their high affinity for the heme irons, they have the effect of locking the sensor in an active state. Also, both of these gases also reversibly inhibit respiration via interaction with the heme group of cytochrome oxidase (7, 11). Therefore, physiologically, the bacillus responds to inhibition of respiration by induction of the DosR regulon regardless of whether the source of the inhibition is hypoxia or respiration-blocking gases.
DosT and DosS both contain GAF (cGMP, adenylyl cyclase, FhlA) domains, which are small-molecule binding regulatory domains found throughout prokaryotes and eukaryotes. Both DosT and DosS bind heme, and in DosS, the location of heme binding within the proximal GAF domain is known. The heme moiety itself binds the divalent gases (NO, CO, O2) responsible for modulating the activity of the sensor (9, 20, 28, 29, 33).
Based on in vitro biochemical data, two recent reports proposed a model for DosT/S signaling wherein DosS is a redox sensor and DosT is a hypoxia sensor (9, 20). Aerobically, DosS is in its inactive Fe3+ form as a result of its rapid oxidation (but not direct binding) by O2 (9, 20). When DosS is reduced to Fe2+, its autokinase activity is greatly increased; thus, it uses its oxidation state as a signal for activation (9, 20). Further, it was demonstrated that flavin nucleotides are capable of reducing the heme iron of DosS (9). DosT exists aerobically in the O2-bound form, which is inactive (20, 33), and its resistance to oxidation removes the possibility of its functioning as a redox sensor (33). When the protein was deoxygenated, it had significantly increased autokinase activity; thus, it uses its oxygenation state as a signal for activation (20). Similar behavior exists in the homolog of DosT in Mycobacterium smegmatis (21).
Few studies have focused on the biological relevance of DosT and DosS and their roles in adaptation to hypoxia and anaerobic conditions. It has been shown that after 2 h under 0.2% oxygen conditions, dosT and dosS single mutants are only able to induce DosR regulon expression at 40 to 45% of normal levels, and bacteria that lack both sensors are completely unable to induce the expression of DosR-regulated genes (26). Another study reported that a dosS deletion mutant had no apparent effect on acr (a member of the DosR regulon) induction in an overnight settled culture model or after 2 h in 0.2% oxygen (30). A study that used the Wayne model indicated that the dosS mutant had a 15-fold decrease in survival by day 40 (5). The dosS mutant, at day 5 of the model, maintains protein expression levels of DosR regulon proteins, indicating that at this time point in this particular model, DosT is able by itself to induce the expression of the DosR regulon (5). These reports indicate the need for further experiments designed to examine the changing and combinatorial roles of these two sensors in the bacilli's adaptation to hypoxic conditions and eventual anaerobiosis. The literature is unclear about whether DosT and DosS function simply in an additive fashion to activate DosR or rather if they have unique individual roles for sensing changing environmental conditions.
MATERIALS AND METHODS
Strains and culture conditions.
M. tuberculosis wild-type strain H37Rv was the parent strain for mutant construction. The DosT and DosS deletion mutants were constructed as previously described (3, 4, 6), and the construct described therein was a gift from Tsungda Hsu and William R. Jacobs, Jr., at Albert Einstein College of Medicine. Primers with incorporated restriction sites for amplification of upstream and downstream regions of dosT and dosS were as follows: dosS upstream forward, TTTTTTTTCAGAAACTGCGAGGTGCGGGTGGATCGGGG; upstream reverse, TTTTTTTTCAGTTCCTGGTCCATCACCGGGTGGCCGC; downstream forward, TTTTTTTTCCATAGATTGGCTCTTCCGGCCACTCAACGA; downstream reverse, TTTTTTTTCCATCTTTTGGACCACTGCGTCGTCGCTTTG); dosT upstream forward, TTTTTTTTCCATAAATTGGGGCCGTGCAAGCCGCACTGT; upstream reverse, TTTTTTTTCCATTTCTTGGTATGTTGACACCGGAGCTGCGC; downstream forward, TTTTTTTTCACAGAGTGTTGATGGACTGTGTTCCGACCGCAAGG; downstream reverse, TTTTTTTTCACCTTGTGTGTCGCACACCAGCGATCGG.
The dosS/T mutant and the dosS/T mutant complemented with dosT constitutively expressed on a plasmid were obtained from David Sherman at the Seattle Biomedical Research Institute, and their construction was as described previously (26). dosT and dosS complements were constructed by using the integrative plasmids pMV361for dosT and pMV306 for dosS (34).
To initiate dormancy, cultures were grown in Dubos Tween albumin medium (Becton Dickinson) by using a model based on the Wayne nonreplicating persistence, or dormancy, model with a starting culture OD at 600 nm (OD600) of 0.004 (38, 39). Stir bars (12 by 4.5 mm) from VWR were used, and cultures were stirred with a rotary magnetic tumble stirrer from V & P Scientific (San Diego, CA). This model differs from the Wayne model in the larger size of the stir bars and the faster rate of stirring resulting in a more homogeneous population of bacilli that more rapidly exhausts the available oxygen to anaerobiosis. For anaerobic chamber experiments, BD BBL GasPak Plus envelopes were used to achieve anaerobic conditions. Vented culture flasks were placed in sealed GasPak containers (BD, Franklin Lakes, NJ) and stirred with a starting culture OD600 of 0.1 in Dubos Tween albumin medium (Becton Dickinson). Anaerobic indicator strips were used to ensure anaerobiosis. For oxygen levels in the GasPak containers, a culture of H37Rv was started as described above with the addition of 9 μg/ml methylene blue, and the OD600 was recorded in an anaerobic chamber to avoid contamination by atmospheric oxygen.
Cultures treated with NO were exposed to 0.1 mM NO donor diethylenetriamine/NO adduct (Sigma) for 1 h. Growth of bacterial cultures was assayed via OD determination and CFU counting as previously described (16). Experiments were repeated thrice with independently grown cultures. Statistical significance was determined by using the paired, two-tailed Student t test and represents the difference between H37Rv and the mutant strains.
Microarrays.
Oligonucleotide-based microarrays were printed in the University of Colorado Denver microarray core by using oligonucleotides purchased from Operon. Microarrays were processed as previously reported (36). Arrays were scanned with a GenePix 4000b scanner (Molecular Devices), and spot intensities were obtained by using GenePix Pro 6.0 (Molecular Devices.) Data analysis was performed as previously described (36). Microarray data are available at the NCBI Gene Expression Omnibus website (http://www.ncbi.nlm.nih.gov/geo/). RNA was extracted, and cDNA was prepared and labeled as previously described (36). Arrays were repeated in triplicate for the single-mutant growth experiments and in quadruplicate for the NO experiments. For percent induction values (Fig. 1), the average spot intensities of DosR regulon genes were calculated and the percentage of the mutant versus the wild type was determined.
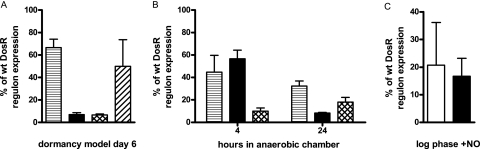
FIG. 1.
DosR regulon induction by DosT or DosS. Shown is the percent induction observed by microarray analysis of the DosR regulon genes of each mutant compared to those of the wild type (wt). (A) Anaerobic dormancy model sampled at day 6, 1 day after the onset of anaerobiosis. (B) Anaerobic GasPak chamber sampled at 4 and 24 h with single- and double-sensor mutants. Standard deviation is indicated. The ΔdosT mutant is horizontally striped, the ΔdosS mutant is black, the ΔdosS/T mutant is cross-hatched, and the ΔdosS/T mutant with constitutively plasmid-expressed dosT is diagonally striped. (C) Both DosT and DosS are able to respond to the NO stimulus provided by the NO donor diethylenetriamine/NO adduct via induction of the DosR regulon. The NO donor was added to log-phase cultures at a concentration of 0.1 mM, and they were incubated for 1 h. Shown is the percent induction observed by microarray analysis of the DosR regulon genes of each mutant compared to that of the wild type. Standard deviation is indicated. The white bar indicates data from the ΔdosT mutant strain, and the black bar indicates data from the ΔdosS mutant strain.
Real-time PCR.
Reverse transcription and quantitative real-time PCR were performed with gene-specific primers and fluorescent probes designed with the program FastPCR (18). The primer and probe sequences were as follows: Rv0239 forward, TGATTCGAACGCAAGTCCAGCTC; reverse, AGATCCGCACCATGTGCTCCA; probe, (TCGCGCACGAGCACGAAATG; dosS forward, ACCGGCAGCATCGGGTATTGC; reverse, TCGATGAGCAGCCCGATGAC; probe, (AAGGCATCGACGAGGAGACC; dosT forward, TGCGCGGTTACGACCATAGA; reverse, AGCCGGATCGGCTTTGGCT; probe, TCGACGAAGAGACCCGGCACCT. Sequence data for primer design was obtained from TubercuList (10). Real-time PCR was performed on the Roche LightCycler 480. A reverse transcriptase negative reaction was used to account for residual DNA, and copy numbers were normalized to the level of Rv0239 transcripts. These copy numbers were then used to determine the copy number per nanogram of total RNA in order to provide a sense of the transcript's overall abundance. Rv0239 was chosen because of the stability of its transcript level as determined by microarray analysis throughout a wide variety of experimental conditions (data not shown). RNA was obtained through the same extraction procedure as noted for microarray analysis. Three different biological replicates were each tested in duplicate. Specificity of primers was confirmed by assaying the dosT mutant with dosS primers and vice versa and subtracting any background from the reported values.
RESULTS
Temporal and additive roles of DosT and DosS.
We were interested in determining the conditions under which DosT and DosS individually play a role in the induction of the DosR genes. Further, we wanted to address the apparent redundancy of the sensors and their ability to compensate for one another. To address these issues, microarray analysis of bacterial cultures under various in vitro conditions was undertaken to elucidate the role of each sensor. The ability of single mutants to induce the regulon with either dosT deleted (ΔdosT) or dosS deleted (ΔdosS) was compared to that of the wild type.
The anaerobic dormancy model was examined first. In this model, the bacteria were sealed in glass tubes with stir bars and over the course of 4 to 5 days the available oxygen in the medium and headspace was consumed and the culture became anaerobic. At day 6 of the model, a predominant role of DosS was observed in the ΔdosT mutant, where it was responsible for 67% of DosR regulon induction compared to that in the wild-type strain, as opposed to DosT (in the ΔdosS mutant), which was responsible for only 7% (Fig. 1A). However, a role for DosT was apparent, as DosS alone was unable to induce the genes to wild-type levels. A specific role for DosT was further supported by growth data, where the ΔdosT mutant exhibited decreased survival (Fig. 2A); however, discovery of this role proved difficult in this model: at day 5, the DosR regulon induction was similar to that at day 6, and day 4 was too early to show gene induction (data not shown). The transition from aerobic to anaerobic conditions, during which DosT was likely important, occurred over approximately 24 h, beginning between 4.5 and 5 days in the anaerobic dormancy model. The variability in the onset of hypoxia made it difficult to determine the exact time at which the culture was passing through the hypoxic phase. Therefore, anaerobic GasPak chambers were used to provide a more precise period of hypoxia to examine the role of DosT. These chambers became anaerobic over several hours, as shown by methylene blue decolorization (Fig. 2B; see Fig. S1 in the supplemental material).
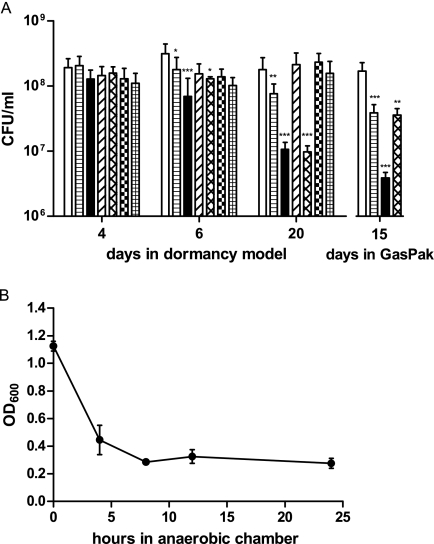
FIG. 2.
Survival defects of various strains in the anaerobic dormancy model or in an anaerobic GasPak chamber sampled for CFU counting at the days indicated. (A) In the anaerobic dormancy model, day 4 is entry into hypoxia and day 6 is 1 day after the onset of anaerobiosis (data not shown.) Statistical significance of differences: *, P < 0.01; **, P < 0.001; ***, P < 0.0001. P values were determined for differences between H37Rv and the mutant strains. Standard deviation is indicated. H37Rv is white, the ΔdosT mutant strain is horizontally striped, the ΔdosS mutant strain is black, the ΔdosS/T mutant strain with constitutively plasmid-expressed dosT is diagonally striped, the ΔdosS/T mutant strain is cross-hatched, the complemented ΔdosS mutant strain is checked, and the complemented ΔdosT mutant strain is gridded. (B) Oxygen utilization in the GasPak chamber by H37Rv, shown by surveying methylene blue decolorization via OD600 determination.
With this system, it was observed that DosT plays a significant role, with 56% of the wild-type level of induction after 4 h in the chamber, which then dropped to 8% by 24 h (Fig. 1B). DosS again exhibited a steadier role, with 45% of the wild-type level of induction at 4 h and 32% at 24 h. The low overall level of regulon expression at 24 h in the dosT mutant is presumably a result of the lack of DosT induction of the regulon early in the experiment. This would result in a lower level of DosS and DosR later and thus less overall regulon induction at 24 h.
DosT and DosS are both required for wild-type survival in the anaerobic dormancy model.
The growth behavior of dosT and dosS single mutants and a double mutant was assayed throughout different time points in the anaerobic dormancy model, as well as in the GasPak model, via CFU counting. Statistically significant intermediate phenotypes of the single mutants, compared to the wild type and the double mutant, were observed (Fig. 2A). Up to day 4, all of the strains exhibited very close bacterial numbers. At later time points, when oxygen became limited, they diverged. The wild-type (H37Rv) CFU count increased until day 6, after which it slightly droped. In contrast, survival of the double-knockout (ΔdosS/T) strain began to decrease after day 4, continued to drop until day 20, and was consistently lower than that of the wild type. The ΔdosT mutant demonstrated intermediate survival, commensurate with its level of DosR regulon expression, which was lower than that of the wild type and higher than that of the dosS mutant (ΔdosS). However, the ΔdosS mutant demonstrated a decrease in survival nearly identical to that of the ΔdosS/T mutant strain. Interestingly, complementation of the ΔdosS/T mutant with a plasmid [ΔdosS/T(pdosT)], constitutively expressing high levels of dosT, restored survival to wild-type levels. Similar CFU counts were observed in the GasPak model.
Loss of DosT function is not attributable to its downregulation.
The lack of observed signaling via DosT in the anaerobic dormancy model could be attributable to downregulation of its transcription. This possibility was tested in two ways. First, a dosS/dosT mutant complemented with dosT constitutively expressed on a plasmid [ΔdosS/T(pdosT)] was analyzed via microarray, and its growth and survival characteristics were observed. Constitutively overexpressed dosT was able to restore expression of the DosR regulon in the dosS/T mutant to levels approximately equal to those of the dosT mutant (Fig. 1A). Additionally, survival was also complemented to wild-type levels (Fig. 2A). Thus, the phenotypic data correlate with the expression data, where induction of the DosR regulon results in complementation of the growth phenotypes. Importantly, this indicates that functionally, when DosT is present at high levels during anaerobic dormancy, it is able to induce the regulon.
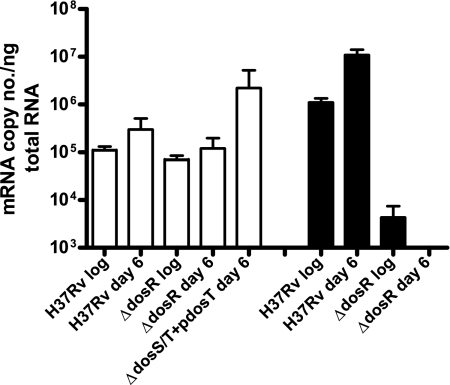
To determine the actual level of dosT expression in anaerobic dormancy, real-time PCR analysis of dosT transcripts was undertaken. dosT transcript numbers were slightly higher at day 6 than during aerobic growth, but the difference was not significant (Fig. 3). Thus, loss of transcriptional expression of dosT does not account for the inability of DosT to activate DosR later in dormancy. In addition, to ensure that expression dosT is not under the control of DosR, a dosR mutant was assayed, and transcript levels similar to those of the wild type were observed. Additionally, the ΔdosS/T(pdosT) strain was examined to show that, in fact, dosT was being constitutively expressed during dormancy from the plasmid, and levels nearly 100-fold higher than that of the wild type were observed. Levels of dosS transcript were monitored as a positive control for induction of the DosR regulon, and a roughly 1-log increase was observed between bacilli during aerobic growth and the same bacilli at day 6. Levels of dosS transcript were highly reduced in the dosR mutant, as expected.
FIG. 3.
Real-time PCR analysis of dosT and dosS mutant strains during aerobic growth and day 6 of the anaerobic dormancy model. dosT mutant strain levels remain relatively constant during anaerobiosis with or without the presence of DosR, and dosS mutant strain levels serve as a control for DosR regulon induction. Transcript levels are normalized to those of Rv0239. Standard deviation is indicated. White bars represent the transcript level of dosT and black bars represent the level of dosS in the noted strains. No significant difference in the dosT copy number exists between H37Rv aerobic growth and anaerobic dormancy at day 6 or ΔdosR mutant strain aerobic growth and anaerobic dormancy at day 6.
NO activates both DosT and DosS.
NO induces expression of the DosR regulon (24, 36). To examine the role of each individual sensor in DosR regulon induction, microarray analysis of aerobically growing ΔdosT and ΔdosS mutant bacilli stimulated with NO was performed. Both sensors were able to induce the DosR regulon genes to approximately the same level (Fig. 1C). These results support the recent findings of Shiloh et al. that indicate roles for both DosT and DosS in NO signaling (31).
DISCUSSION
This work exhibits individual, collective, and temporal roles for DosT and DosS in a widely used model system that is thought to be physiologically relevant. With the anaerobic dormancy model system, we demonstrated that DosR regulon expression is induced in a stepwise manner, with DosT only important early and DosS important late in the model, despite the fact that DosT is constitutively expressed. Additionally, survival of M. tuberculosis during anaerobic conditions was commensurate with the level of DosR regulon expression. This indicates the importance of the regulon, and specifically these two sensors working in sequence toward full regulon induction, in an adaptive response that aids the bacteria in anaerobic survival. It also provides an explanation for the presence of two sensors and indicates that they are not merely redundant or additive.
Single mutants were unable to fully induce the DosR regulon to wild-type levels, nor were they able to survive as well as wild-type M. tuberculosis. Interestingly, the drop in survival correlated with each strain's ability to induce the DosR regulon—the dosS mutant, for example, was more defective in survival than the dosT mutant and was also less able to induce the DosR regulon.
Temporally, DosT was responsible for early induction of the genes as the bacilli entered anaerobic dormancy. Real-time PCR data indicated that dosT is transcribed constitutively during aerobic growth and during anaerobic dormancy, and the presence of DosR had no effect on its expression. This is interesting, considering that dosT is located at the end of a highly induced cluster of genes all regulated by DosR. DosT is likely highly sensitive to the concentration of oxygen and may respond to fluctuations in the oxygen concentration that are insufficient to activate DosS. The feasibility of this idea is supported by various biochemical differences between the two sensors (9, 20, 21, 33). This difference in functionality between the two sensors is further underscored by the fact that DosT is not able to signal efficiently under the low-oxygen or anaerobic conditions present at later time points in the dormancy model. Thus, the window of DosT activity appears to be a narrow one under the conditions of the anaerobic dormancy model. It occurs at some point between day 4, when the DosR regulon genes are not yet induced, and day 5, when DosS already plays the predominant role (data not shown). Under the conditions in an anaerobic chamber, where hypoxia is more controlled and clearly defined temporally, the role of DosT became more apparent and could be more accurately observed. After 4 h in the chamber, at which point oxygen still exists in the culture medium (although not in the anaerobic chamber itself), DosT is responsible for half of the DosR regulon induction. This effect is clearly important, considering that at 24 h the effect of not having DosT can be observed in the dosT mutant, where induction by DosS is only 32% of that of the wild type.
A previous study by Dick and Boon with Mycobacterium bovis bacillus Calmette-Guérin (BCG) reported a much less substantial growth defect in their dosS mutant than that observed in our model (5). This study concluded that DosS plays only a “minor role” in the induction of the DosR regulon. The probable explanation for this discordance is that their phenotype is likely due to an increased role for DosT under the extended hypoxic conditions (5 days) present in their model, compared to 1 day of hypoxia in the model presented here. However, the difference could also be due, in part, to the behavior of BCG, which was used in their study, as opposed to that of M. tuberculosis.
In our model system, the early activity by DosT is likely important to kick start induction of DosR regulon genes, among them dosS. As the DosR regulon is expressed, levels of dosS become elevated, enabling further induction of the regulon. DosS is able to maintain its own transcription at this point and concomitantly maintains sustained induction of the regulon. DosT plays no role at this point, likely as a result of the high level of dosS (more than 2 logs higher than that of dosT at day 6 of the model). It is also possible that DosT is inefficient under anaerobic conditions, and far higher levels (such as those present in the constitutively expressing strain) are required to observe appreciable DosT activity. These data suggest separate but overlapping roles for the two sensors that correspond to physiologically different conditions in the host in which the DosR regulon is induced.
It is interesting that despite a constitutive level of expression, in the wild type, DosT alone is unable to induce the expression of the DosR regulon in the anaerobic dormancy model (Fig. 1A). Contrarily, when highly overexpressed in the ΔdosS/T(pdosT) strain, DosT is able to function and restore expression of the genes, indicating that the sensor is physically able to function to some degree at this time point. It is possible that a mechanism of posttranslational regulation exists which renders the sensor nonfunctional and that this system is saturated by the overabundance of protein in the constitutive strain. It is also possible than under anaerobic conditions, DosT is simply not efficient. This lack of efficiency would be the inverse of its functionality under hypoxic conditions, where its efficiency appears to be quite high, as shown by its ability to induce the DosR regulon (Fig. 1B), despite the fact that it is present at a much lower level than DosS (Fig. 3). Supportive of this idea is the higher efficiency of DosT than DosS reported for the purified protein (26).
The role of DosS/T in NO signaling was predicted via biochemical analysis to involve both sensors, as NO is a ligand for both (20). This was recently shown by microarray and reverse transcription-PCR analyses (31) and was confirmed by microarray analysis in this study. Thus, it appears that the bacterium has mechanisms in place to always be able to induce the genes when NO is present, indicating the important role of this signal. It is interesting that NO signaling through each individual sensor is approximately the same despite the different amounts of transcript present for them during aerobic growth and that this level of induction is roughly half of the previously reported wild-type level (36). This indicates that NO and CO likely play key roles in activating the DosR regulon during infection. However, the unique roles of DosT and DosS during anaerobic adaptation, but not in their response to NO, indicate that adaptation to anaerobiosis is evolutionarily conserved and thus likely important during infection.
Supplementary Material
Acknowledgments
This research project was funded by NIH grants RO1 AI061505, entitled The Mycobacterium tuberculosis Dormancy Program and awarded to M.I.V.; T32 AI052066-06, awarded to R.W.H.; T32 AI052066-05, awarded to R.L.L.; and T32 AI052066-07, awarded to I.L.B., and by U.S. Civilian Research and Development Foundation grant 14575, entitled Molecular Markers of Dormant Mycobacterium tuberculosis.
We thank Anthony Baughn, Tsungda Hsu, and William R. Jacobs, Jr., for materials and instruction in constructing the mutant strains and David R. Sherman for providing the ΔdosS/T and ΔdosS/T(pdosT) mutant strains.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 1 June 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Anonymous. 1953. Report of panel discussion on survival and revival of tubercle bacilli in healed tuberculous lesions. Am. Rev. Tuberc. 68477-495. [DOI] [PubMed] [Google Scholar]
- 2.Balázsi, G., A. P. Heath, L. Shi, and M. L. Gennaro. 2008. The temporal response of the Mycobacterium tuberculosis gene regulatory network during growth arrest. Mol. Syst. Biol. 4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardarov, S., S. Bardarov, Jr., M. S. Pavelka, Jr., V. Sambandamurthy, M. Larsen, J. Tufariello, J. Chan, G. Hatfull, and W. R. Jacobs, Jr. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 1483007-3017. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt, A., L. Kremer, A. Z. Dai, J. C. Sacchettini, and W. R. Jacobs, Jr. 2005. Conditional depletion of KasA, a key enzyme of mycolic acid biosynthesis, leads to mycobacterial cell lysis. J. Bacteriol. 1877596-7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon, C., and T. Dick. 2002. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J. Bacteriol. 1846760-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, A. K., A. Bhatt, A. Singh, E. Saparia, A. F. Evans, and G. S. Besra. 2007. Identification of the dehydratase component of the mycobacterial mycolic acid-synthesizing fatty acid synthase-II complex. Microbiology 1534166-4173. [DOI] [PubMed] [Google Scholar]
- 7.Brown, G. C. 2001. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim. Biophys. Acta 150446-57. [DOI] [PubMed] [Google Scholar]
- 8.Canetti, G. 1955. The tubercle bacillus in the pulmonary lesion of man. Springer Publishing Co., Inc., New York, NY.
- 9.Cho, H. Y., H. J. Cho, Y. M. Kim, J. I. Oh, and B. S. Kang. 2009. Structural insight into the heme-based redox sensing by DosS from Mycobacterium tuberculosis. J. Biol. Chem. 28413057-13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comstock, G. W., V. T. Livesay, and S. F. Woolpert. 1974. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am. J. Epidemiol. 99131-138. [DOI] [PubMed] [Google Scholar]
- 11.Cooper, C. E., and G. C. Brown. 2008. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 40533-539. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham, A. F., and C. L. Spreadbury. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J. Bacteriol. 180801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez, J. E., and J. D. McKinney. 2004. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinburgh) 8429-44. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalo-Asensio, J., S. Mostowy, J. Harders-Westerveen, K. Huygen, R. Hernandez-Pando, J. Thole, M. Behr, B. Gicquel, and C. Martin. 2008. PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS ONE 3e3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández-Pando, R., M. Jeyanathan, G. Mengistu, D. Aguilar, H. Orozco, M. Harboe, G. A. Rook, and G. Bjune. 2000. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 3562133-2138. [DOI] [PubMed] [Google Scholar]
- 16.Honaker, R. W., A. Stewart, S. Schittone, A. Izzo, M. R. Klein, and M. I. Voskuil. 2008. Mycobacterium bovis BCG vaccine strains lack narK2 and narX induction and exhibit altered phenotypes during dormancy. Infect. Immun. 762587-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imboden, P., and G. K. Schoolnik. 1998. Construction and characterization of a partial Mycobacterium tuberculosis cDNA library of genes expressed at reduced oxygen tension. Gene 213107-117. [DOI] [PubMed] [Google Scholar]
- 18.Kalendar, R. 2006. FastPCR, PCR primer design, DNA and protein tools, repeats and own database searches program (www.biocenter.helsinki.fi/bi/programs/fastpcr.htm). University of Helsinki, Helsinki, Finland.
- 19.Kumar, A., J. S. Deshane, D. K. Crossman, S. Bolisetty, B. S. Yan, I. Kramnik, A. Agarwal, and A. J. Steyn. 2008. Heme oxygenase-1 derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J. Biol. Chem. 28318032-18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar, A., J. C. Toledo, R. P. Patel, J. R. Lancaster, Jr., and A. J. Steyn. 2007. Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc. Natl. Acad. Sci. USA 10411568-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, J. M., H. Y. Cho, H. J. Cho, I. J. Ko, S. W. Park, H. S. Baik, J. H. Oh, C. Y. Eom, Y. M. Kim, B. S. Kang, and J. I. Oh. 2008. O2 and NO sensing mechanism through the DevSR two-component system in Mycobacterium smegmatis. J. Bacteriol. 1906795-6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim, A., M. Eleuterio, B. Hutter, B. Murugasu-Oei, and T. Dick. 1999. Oxygen depletion-induced dormancy in Mycobacterium bovis BCG. J. Bacteriol. 1812252-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neyrolles, O., R. Hernandez-Pando, F. Pietri-Rouxel, P. Fornes, L. Tailleux, J. A. Payan, E. Pivert, Y. Bordat, D. Aguilar, M. C. Prevost, C. Petit, and B. Gicquel. 2006. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS ONE 1e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohno, H., G. Zhu, V. P. Mohan, D. Chu, S. Kohno, W. R. Jacobs, Jr., and J. Chan. 2003. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell Microbiol. 5637-648. [DOI] [PubMed] [Google Scholar]
- 25.Ribiere, C., A. M. Jaubert, N. Gaudiot, D. Sabourault, M. L. Marcus, J. L. Boucher, D. Denis-Henriot, and Y. Giudicelli. 1996. White adipose tissue nitric oxide synthase: a potential source for NO production. Biochem. Biophys. Res. Commun. 222706-712. [DOI] [PubMed] [Google Scholar]
- 26.Roberts, D. M., R. P. Liao, G. Wisedchaisri, W. G. Hol, and D. R. Sherman. 2004. Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J. Biol. Chem. 27923082-23087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russel, W. F., S. H. Dressler, G. Middlebrook, and J. Denst. 1955. Implications of the phenomenon of open cavity healing for the chemotherapy of pulmonary tuberculosis. Am. Rev. Tuberc. 71441-446. [PubMed] [Google Scholar]
- 28.Saini, D. K., V. Malhotra, and J. S. Tyagi. 2004. Cross talk between DevS sensor kinase homologue, Rv2027c, and DevR response regulator of Mycobacterium tuberculosis. FEBS Lett. 56575-80. [DOI] [PubMed] [Google Scholar]
- 29.Sardiwal, S., S. L. Kendall, F. Movahedzadeh, S. C. Rison, N. G. Stoker, and S. Djordjevic. 2005. A GAF domain in the hypoxia/NO-inducible Mycobacterium tuberculosis DosS protein binds haem. J. Mol. Biol. 353929-936. [DOI] [PubMed] [Google Scholar]
- 30.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding α-crystallin. Proc. Natl. Acad. Sci. USA 987534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiloh, M. U., P. Manzanillo, and J. S. Cox. 2008. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe 3323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohaskey, C. D. 2008. Nitrate enhances the survival of Mycobacterium tuberculosis during inhibition of respiration. J. Bacteriol. 1902981-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sousa, E. H., J. R. Tuckerman, G. Gonzalez, and M. A. Gilles-Gonzalez. 2007. DosT and DevS are oxygen-switched kinases in Mycobacterium tuberculosis. Protein Sci. 161708-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351456-460. [DOI] [PubMed] [Google Scholar]
- 35.Trayhurn, P., and I. S. Wood. 2004. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 92347-355. [DOI] [PubMed] [Google Scholar]
- 36.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wayne, L. G. 1994. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur. J. Clin. Microbiol. Infect. Dis. 13908-914. [DOI] [PubMed] [Google Scholar]
- 38.Wayne, L. G. 2001. In vitro model of hypoxically induced nonreplicating persistence of Mycobacterium tuberculosis. Methods Mol. Med. 54247-269. [DOI] [PubMed] [Google Scholar]
- 39.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 642062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wayne, L. G., and K. Y. Lin. 1982. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect. Immun. 371042-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wayne, L. G., and C. D. Sohaskey. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55139-163. [DOI] [PubMed] [Google Scholar]
- 42.Wolstenholme, G. E. W., M. P. Cameron and C. M. O'Connor (ed.). 1955. CIBA Foundation symposium on experimental tuberculosis, bacillus and host, with an addendum on leprosy. Little, Brown, Boston, MA.
- 43.World Health Organization. 2007. posting date. Tuberculosis fact sheet number 104, revised March 2007. World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs104/en/.
- 44.Yan, H., E. Aziz, G. Shillabeer, A. Wong, D. Shanghavi, A. Kermouni, M. Abdel-Hafez, and D. C. Lau. 2002. Nitric oxide promotes differentiation of rat white preadipocytes in culture. J. Lipid Res. 432123-2129. [DOI] [PubMed] [Google Scholar]
- 45.Yuan, Y., D. D. Crane, and C. E. Barry, 3rd. 1996. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J. Bacteriol. 1784484-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan, Y., D. D. Crane, R. M. Simpson, Y. Q. Zhu, M. J. Hickey, D. R. Sherman, and C. E. Barry, 3rd. 1998. The 16-kDa α-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc. Natl. Acad. Sci. USA 959578-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yukl, E. T., A. Ioanoviciu, P. R. de Montellano, and P. Moenne-Loccoz. 2007. Interdomain interactions within the two-component heme-based sensor DevS from Mycobacterium tuberculosis. Biochemistry 469728-9736. [DOI] [PubMed] [Google Scholar]
- 48.Yun, Z., H. L. Maecker, R. S. Johnson, and A. J. Giaccia. 2002. Inhibition of PPARγ2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev. Cell 2331-341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.