Abstract
Gene conversion, defined as the nonreciprocal transfer of DNA, is one result of homologous recombination. Three steps in recombination could give rise to gene conversion: (i) DNA synthesis for repair of the degraded segment, (ii) Holliday junction migration, leading to heteroduplex formation, and (iii) repair of mismatches in the heteroduplex. There are at least three proteins (RuvAB, RecG, and RadA) that participate in the second step. Their roles have been studied for homologous recombination, but evidence of their relative role in gene conversion is lacking. In this work, we showed the effect on gene conversion of mutations in ruvB, recG, and radA in Rhizobium etli, either alone or in combination, using a cointegration strategy previously developed in our laboratory. The results indicate that the RuvAB system is highly efficient for gene conversion, since its absence provokes smaller gene conversion segments than those in the wild type as well as a shift in the preferred position of conversion tracts. The RecG system possesses a dual role for gene conversion. Inactivation of recG leads to longer gene conversion tracts than those in the wild type, indicating that its activity may hinder heteroduplex extension. However, under circumstances where it is the only migration activity present (as in the ruvB radA double mutant), conversion segments can still be seen, indicating that RecG can also promote gene conversion. RadA is the least efficient system in R. etli but is still needed for the production of detectable gene conversion tracts.
DNA may be the target of several intracellular and extracellular injuries that can either modify or break it. Many of these may cause, either directly or indirectly, single- or double-strand breaks, leading to replication fork collapses. Independent of their origin, breaks provoke the activation of several pathways that can repair the damage; homologous recombination is the most important of these because of its ability to repair without a loss of information. Besides its role in repair, homologous recombination helps the diversification of the genome through the acquisition of foreign DNA sequences. Paradoxically, recombination also participates in the maintenance of identity among multigenic families, a process known as concerted evolution (38).
Concerted evolution can be generated through gene conversion, an outcome of homologous recombination, which is defined as the nonreciprocal transfer of DNA between two or more homologous sequences. In bacteria, this nonreciprocal transfer of information usually entails sizable gene segments (400 to 600 bp are frequent) and occurs at frequencies higher than the mutation frequency (1, 38, 39). The mechanism of gene conversion has as a consequence the spread of sequence polymorphisms present in one of the two recombining homologs; these polymorphisms can be either maintained or eliminated in both sequences, thus giving rise to identity between homologs. The occurrence of gene conversion in bacteria has been either demonstrated experimentally (3, 12, 21, 31; see reference 38 for a review) or inferred from the conservation pattern among repeated genes through phylogenetic analysis (5, 9, 14, 20, 24, 32, 34, 46; see reference 38 for a review).
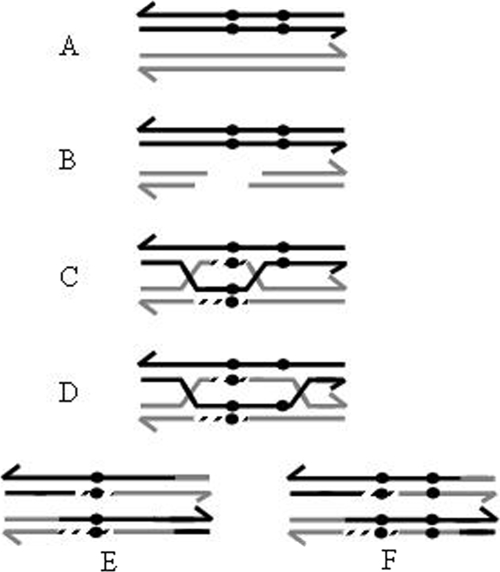
The most accepted model that explains homologous recombination and gene conversion is the double-strand-break repair (DSBR) model (47). This model, characterized for the presence of double Holliday junctions (HJs) (Fig. 1), is flexible enough to explain gene conversion and its association with crossovers. In the DSBR model (Fig. 1), the length of gene conversion segments can be modulated by three separate events, namely (i) resynthesis of the degraded segment, using information from the uncut homolog (Fig. 1C); (ii) the extent of migration of the HJs (Fig. 1D); and (iii) mismatch repair in the resulting heteroduplex regions (Fig. 1E and F). Consistent with these predictions, inactivation of bacterial systems participating in the generation of the degraded segment, such as the RecBCD or AddAB system (1, 3), or in charge of mismatch repair, such as MutS (1, 39), provokes a reduction in the frequency and/or extent of gene conversion in bacteria. Oddly enough, the extent of migration of the HJs on gene conversion has been a poorly studied factor.
FIG. 1.
Gene conversion under the DSBR model. (A) Two homologous sequences are shown, differing by sequence polymorphisms (black circles). (B) A double-strand break in the recipient homolog is processed (by RecBCD or AddAB) to a gap, generating 3′ tails. (C) After homolog invasion, DNA synthesis (discontinuous lines) fills the gap, and upon ligation, two HJs are formed. At the gap-filling step, gene conversion has occurred because the uncut sequence was the template for gap resynthesis (note the black circles in the cut homolog). (D) HJ migration (by RuvAB, RecG, or RadA) leads to heteroduplex formation. Mismatch repair in the heteroduplex region (mediated by MutS) dictates, depending on the orientation, whether further gene conversion occurs. (E) After HJ resolution, a crossover event with gene conversion to both the gain (black circles in both homologs) and loss of the polymorphisms is generated. (F) Another possible outcome is a crossover event with gene conversion to the gain of both polymorphisms. Only the resolution of HJs leading to crossovers is shown.
Movement of the HJs may be a crucial factor to determine the extent of gene conversion, because long heteroduplex regions can be processed afterwards by the mismatch repair system, generating extensive tracts of gene conversion. At least three systems (RuvAB, RecG, and RadA) participate in the migration of HJs in Escherichia coli. RuvA binds to HJs either as a homotetramer (16) or a double homotetramer (35), maintaining the HJs in a planar form; RuvA is also needed for the binding of the RuvB helicase to DNA (33). RuvB forms a hexameric ring responsible for the migration of the HJs away from the initiation site through ATP hydrolysis.
The RecG helicase binds to HJs as a monomer; the so-called “wedge” domain in this protein is responsible for both strand separation and processivity (6). RecG was demonstrated in vitro to drive branch migration in the opposite direction of that of RecA (52); consequently, RecG may undo preformed HJs (an antirecombinogenic activity), but it also has recombinogenic activity (30). Although the third protein, RadA, has not been studied in vitro, mutations in the gene displayed genetic synthetic effects with both ruvA and recG mutations (4, 28), indicating that RadA participates in the migration of HJs.
Single mutations in ruvB, recG, or radA reduce homologous recombination to about the same extent in Escherichia coli (4, 22), an unexpected result given the differing in vitro activities for RuvB and RecG. The effect of these mutations on gene conversion has been studied only, to our knowledge, in the case of gonococcal pilin variation (a specialized gene conversion system), where mutations in either ruvB or recG equally reduce the frequency of gene conversion (40). As interesting as these data are, there are some doubts as to whether these phenotypes are applicable for all bacteria. For instance, in several bacteria, such as Helicobacter pylori (18, 19), Acinetobacter baylyi (15), and Rhizobium etli (27, 28), inactivation of ruvB reduces recombination markedly, but inactivation of recG enhances recombination. The role of RadA outside of E. coli has been studied only in Bacillus subtilis (7), where it affects chromosome segregation, and in R. etli, where radA mutants were only weakly affected in recombination (28). These data open up the possibility of differing contributions of RuvB, RecG, and RadA to gene conversion in bacteria other than E. coli.
One interesting system in this regard is in Rhizobium etli, a symbiotic nitrogen-fixing alphaproteobacterium. R. etli CFN42 has a multipartite genome of 6.53 Mb, harboring approximately 200 reiterated DNA families. More than 133 of these families are comprised of identical repeats longer than 100 bp (13), long enough to be substrates for homologous recombination (43). Sequence identity among members of repeated families in R. etli (at least for the nitrogenase multigene family) is maintained by multiple recombination events, including gene conversion (36). Phylogenetic analysis of the nitrogenase multigene family members in several R. etli isolates is fully consistent with the operation of gene conversion as a homogenizing mechanism (E. Sepúlveda, M. Castellanos, and D. Romero, unpublished results).
To gain insight into the mechanism of gene conversion in R. etli, we have studied the anatomies of tracts undergoing gene conversion in this organism (39). Our results revealed that (i) crossover events were almost invariably accompanied by a gene conversion event occurring nearby; (ii) gene conversion tract lengths ranged in size from 150 bp up to 800 bp; (iii) gene conversion events displayed a strong bias, favoring the preservation of incoming sequences; and (iv) the MutS mismatch repair system plays an important role in determining the length of gene conversion segments (39).
The differential roles of RuvB, RecG, and RadA in homologous recombination in this organism were described recently (28). Based on the effects of single and multiple mutations on recombination frequency, we proposed that RuvAB is the main system for migration of HJs, with RadA playing an ancillary role. RecG, in contrast, appears to inhibit recombination, perhaps due to HJ regression. Gene conversion would be an ideal system to test these proposals because it allows us to explore the length of converted segments, a factor highly related to HJ migration. In particular, we predict that in comparison to the wild type, (i) inactivation of radA should not affect gene conversion tract length, (ii) null mutations in recG should lead to longer gene conversion tracts, and (iii) absence of ruvB would instigate a marked reduction in the length of gene conversion segments, or even their disappearance. Evidence reported here fully confirms these predictions.
MATERIALS AND METHODS
Bacterial strains and media.
All Rhizobium etli strains were grown in peptone-yeast extract medium (39) at 30°C. E. coli strains were grown in Luria-Bertani (LB) medium at 37°C. Antibiotics were used in the following concentrations (in micrograms per milliliter): kanamycin (Km), 15; nalidixic acid (Nal), 20; spectinomycin (Sp), 100; and tetracycline (Tc), 5.
Molecular analysis of transconjugants.
Escherichia coli S17-1 (F− pro-82 thi-1 endA1 hsdR17 supE44 recA13; chromosomally integrated RP-4-2 [Tc::Mu Km::Tn7]) (45) was used as a host for conjugative transfer of pJGus28 (39). The integrative plasmid pJGus28 harbors a kanamycin resistance gene, allowing convenient selection for its presence, and a derivative of the nifH gene modified to contain eight unique restriction sites (restriction fragment length polymorphisms [RFLPs]) spaced nearly every 100 bp (39). Biparental matings between Escherichia coli S17-1 harboring pJGus28 and the desired R. etli strains were set up on solid peptone-yeast extract medium lacking antibiotics (Fig. 2 and see below); transconjugants were selected by their resistance to nalidixic acid (a naturally occurring trait in all R. etli strains) and kanamycin (selecting for the integration of pJGus28). To avoid the analysis of siblings, 10 different conjugation experiments were set up for each R. etli recipient strain, retaining not more than five single colonies from each experiment. Genomic DNA was isolated from 50 transconjugants from every recipient strain, and each one was analyzed by PCR using a Techgene thermal cycler and a conventional Taq DNA polymerase, with a regime of 31 cycles with denaturation at 94°C for 1 min, annealing at 45°C (primers 1 and 2) (Fig. 2B) or 48°C (primers 3 and 4) (Fig. 2B) for 1 min, and extension at 72°C for variable times ranging from 1 to 1.5 min. To amplify both nifH products in the cointegrates, specific primers were employed (primers 1 and 2 for the upper part of the cointegrate; primers 3 and 4 for the lower part) (39) (Fig. 2). The resulting PCR products were purified by using Centri-Sep spin columns (Applied Biosystems) before digestion with appropriate restriction enzymes. Restriction enzymes were purchased from diverse companies and used according to the recommendations of the suppliers. Restricted products were separated on 1% agarose gels and visualized after staining with ethidium bromide.
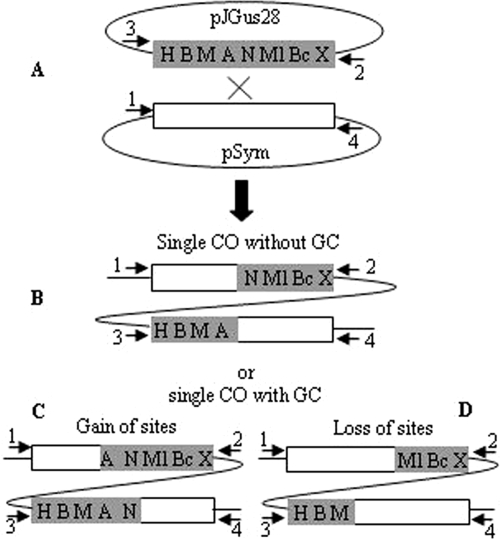
FIG. 2.
Experimental strategy for analyzing gene conversion. (A) The modified nifH gene is represented by a gray rectangle, and capital letters indicate different restriction sites as follows: H, HindIII; B, BamHI; M, MaeIII; A, ApaLI; N, NarI; Ml, MluI; Bc, BclI; X, XbaI. The only wild-type nifH gene of the symbiotic plasmid of R. etli is depicted as a white rectangle. Upon recombination of pJGus28 and pSym (a cross between both nifH genes), we could expect a reciprocal recombination event (markers are just redistributed) (B), a nonreciprocal recombination event to a gain of sites (note the central markers A and N) (C), or a loss of sites (note that the central markers A and N have been lost in both parts of the cointegrate) (D). Arrows in all panels represent the specific primers used for amplifying each nifH gene in the cointegrate molecule.
Construction of R. etli mutants.
To direct the integration of pJGus28 into a single nifH region, we took advantage of a deletion derivative from pSym (strain CFNX55) that lacks two of the three nifH genes from strain CFN42 (37). This strain was used as the wild-type strain. Strain CFNX55 was modified afterwards by allelic exchange with the ruvB::loxPSp or radA::loxPSp allele (28). To that end, plasmid pJMS3 (ruvB::loxPSp) or pJMS15 (radA::loxPSp) was mobilized separately from E. coli to R. etli by biparental matings; double recombinants were identified by their Nalr Spr Kms phenotype, giving rise to strains CFNX728 (ruvB::loxPSp Δsym) and CFNX730 (radA::loxPSp Δsym).
For some mutant derivatives, deletion of the symbiotic region of R. etli was achieved by recombination enhancement by replication (RER) (50). In this system, activation of a supernumerary replication origin on pSym leads to the high-frequency generation of a deletion on pSym identical to the one in strain CFNX55 (39, 50). The recG mutant was generated by exchange with the recG::loxPSp allele (pJMS11) on the CE3 strain, and after double recombination, the symbiotic region was removed by RER, leaving a single nifH gene (CFNX729). To introduce further mutations, the loxPSp cassette of strain CFNX729 (recG::loxPSp Δsym) was first excised by using the Cre recombinase expressed from plasmid pJMS8 (28). Losses of the Sp marker, as well as of pJMS8, were selected by screening single-colony isolates for a Sps Tcs phenotype. After that first step, strain recG::loxP Δsym was conjugated with pJMS15 (radA::loxPSp allele). Double recombinants were selected by their Nalr Spr Kms phenotype. This strain was called CFNX734 (recG::loxP radA::loxPSp Δsym).
To construct ruvB recG and ruvB radA double mutants, the ruvB::loxPSp allele (pJMS3) (27, 28) was introduced first by gene replacement in strain CE3. The Sp marker from the loxPSp allele was excised by pop-out recombination mediated by the Cre recombinase as described above, and then either the recG::loxPSp (pJMS11) or the radA::loxPSp (pJMS15) allele was introduced in the ruvB background, selecting for double recombinants. In the case of ruvB::loxP radA::loxPSp, the subsequent excision of the loxPSp marker was obtained as for the ruvB allele. Once double mutants were obtained, deletions of the symbiotic region were generated either by RER (strain CFNX732; ruvB::loxP recG::loxPSp Δsym) or spontaneously (strain CFNX733; ruvB::loxP radA::loxP Δsym). The triple mutant (strain CFNX735; ruvB::loxP recG::loxP radA:loxPSp) was obtained by introducing the radA::loxPSp allele (pJMS15) in the ruvB recG background by the same strategy as described previously (28).
Statistical analyses.
All the statistical analyses were done with Excel software (Microsoft Corp., Redmond, WA). Nonparametrical binomial tests were applied to those data divided in two excluding categories (i.e., cointegrates associated or not with gene conversion and gene conversion gain or loss) employing the absolute numbers for each category. The goal of these tests was to evaluate if proportions of the two categories under consideration were equal or not. Chi-square tests for seven independent samples were used for comparisons between strains; when the P values were significantly different at the 5% level, chi-square tests for paired samples were done, comparing the wild-type strain with every mutant strain.
RESULTS
Experimental design.
To evaluate the characteristics of gene conversion in each of the recombination mutants, we employed a strategy developed previously in our laboratory (39) (Fig. 2). This strategy is based on the use of a Rhizobium etli nifH gene derivative, differing from the wild-type gene in that it harbors eight unique RFLPs, spaced approximately every 100 bp along the gene. These RFLPs serve as convenient landmarks to evaluate the occurrence and extension of gene conversion events.
This modified nifH gene was cloned into a mobilizable plasmid that is able to replicate in Escherichia coli but does not replicate in R. etli. Upon introduction of this plasmid in R. etli derivatives possessing only a single copy of a wild-type nifH gene on a deleted pSym (264 kb), single-crossover recombination generates cointegrates between both plasmids, separated by nifH gene copies (Fig. 2A).
Both nifH sequences of the cointegrate molecule were amplified separately by using specific primer pairs for the nonhomologous regions flanking each nitrogenase gene. The PCR products were subjected to digestions with the eight different enzymes that recognize the introduced RFLPs. Of course, simple crossover recombination without gene conversion leads to a mere redistribution of the RFLP markers (Fig. 2B). In contrast, crossovers associated with gene conversion were easily identified by digestion of both nifH gene segments flanking the cointegrate with the same restriction enzyme(s) (gene convertants toward a gain of markers) (Fig. 2C) or by lack of restriction on both sides of the cointegrate (gene convertants toward a loss of markers) (Fig. 2D). Application of this strategy to a set of 50 independent cointegrates allowed the evaluation of occurrence, extension, and localization of gene conversion events in the wild-type strain (39).
In this work, we aimed to evaluate the differences in gene conversion architecture between the wild-type strain and seven mutant strains affected in HJ migration activity. To ensure that the observed changes were not due to statistical variability, we decided to analyze a new set of 50 cointegrates from the wild-type strain. In the course of this analysis, we discovered that a significant fraction of the convertants in our previous work was wrongly identified as having a conversion of the BamHI RFLP. This error was due to the unnoticed presence of a BamHI site on the plasmid sequence, which generated digestion patterns reminiscent of bona fide gene convertants for this RFLP. This error was corrected both in the previous data and in the new data set. Comparison of gene conversion parameters in both data sets by chi-square tests revealed the absence of significant differences, as expected (data not shown). For this reason, all subsequent statistical analyses (comparison of the wild type versus mutants) were done by pooling together data from both wild-type data sets, representing 96 cointegrates (see Fig. S1 in the supplemental material).
To evaluate the effect on the gene conversion of mutations in genes affecting HJ migration, 50 independent cointegrates were generated in each of six different mutants (the ruvB, recG, radA, ruvB recG, ruvB radA, and recG radA mutants) as described in Materials and Methods. It was not possible to analyze gene conversion in the ruvB recG radA triple mutant because its low recombination activity (28) precluded the isolation of cointegrates. The low recombination frequency observed in this triple mutant supports the view that RuvAB, RecG, and RadA are the main systems for HJ migration in R. etli. Detailed data on gene conversion for each mutant are shown (see Fig. S1 in the supplemental material).
Association of gene conversion to crossover events and gene conversion skew in mutants does not change with respect to that of the wild type.
Previously (39), we have reported a strong association between cointegration and gene conversion in the R. etli wild-type strain (i.e., over 80% of the cointegrates have an associated gene conversion event). Here we found that in all the HJ migration mutants, over 70% of the cointegrates analyzed harbor a gene conversion event (Table 1). For all the strains, including the wild type, a nonparametrical binomial test revealed that the proportion of cointegrates with gene conversion was significantly larger than the proportion of cointegrates without gene conversion (Table 1). To ascertain if there were significant changes among the different strains in the number of cointegrates with a gene conversion versus the number of cointegrates lacking a gene conversion, a chi-square test for seven independent samples was applied. The calculated P value for this comparison was 0.1892; therefore, there were not significant differences among the strains. Thus, mutations in ruvB, recG, and radA, either alone or in combination, do not affect the association between gene conversion and homologous recombination.
TABLE 1.
High association of cointegration with gene conversion in all the HJ migration mutants
| Strain (relevant genotype) | No. of cointegrates with:
|
P valuea | |
|---|---|---|---|
| Gene conversion | No gene conversion | ||
| CFNX55 (wild type) | 84 | 12 | 0 |
| CFNX728 (ruvB::loxPSp) | 37 | 13 | 8 × 10−11 |
| CFNX729 (recG::loxPSp) | 43 | 7 | 0 |
| CFNX730 (radA::loxPSp) | 40 | 10 | 0 |
| CFNX732 (ruvB::loxP recG::loxPSp) | 36 | 14 | 9 × 10−9 |
| CFNX733 (ruvB::loxP radA::loxP) | 38 | 12 | 2 × 10−13 |
| CFNX734 (recG::loxP radA::loxPSp) | 42 | 8 | 0 |
Probability that the observed distribution agrees with the null expectation (cointegrates with gene conversion being equal to cointegrates without gene conversion), evaluated by a nonparametrical binomial test. All P values are significant at the 5% level.
We have reported previously (39) that gene conversion in R. etli is highly skewed toward the acquisition of markers located in the incoming plasmid (i.e., a gain of RFLPs in this case). To explain this skew, we have proposed an alternative, based on the DSBR model, in which the modified nifH gene in the incoming plasmid frequently functions as a template for repairing the degraded segment. In this way, we proposed that breaks happen preferentially in the resident wild-type nifH gene because it is inside R. etli all the time (39). This alternative has turned out to be more convincing than explanations based on the conventional Holliday model or its modifications, such as the Aviemore (Meselson-Radding) model (39). According to this proposal, the reason for this skewed distribution is events at the beginning of recombination and they should not be affected by modifications in later events (such as HJ migration). Supporting this proposal, all the HJ migration mutants tested were still strongly biased toward the gain of RFLPs (Table 2). This bias was highly significant in every strain, as evaluated by a nonparametrical binomial test (Table 2). As expected, a chi-square test for seven independent samples revealed that there were no significant differences among strains in this parameter (a calculated P value of 0.1036).
TABLE 2.
Gene conversion is biased toward gain of markers in all the HJ migration mutants
| Strain (relevant genotype) | No. of gene conversion tracts with:
|
P valuea | |
|---|---|---|---|
| Gain of markers | Loss of markers | ||
| CFNX55 (wild type) | 85 | 25 | 9 × 10−9 |
| CFNX728 (ruvB::loxPSp) | 28 | 9 | 0.0015 |
| CFNX729 (recG::loxPSp) | 43 | 4 | 1 × 10−8 |
| CFNX730 (radA::loxPSp) | 36 | 9 | 5 × 10−5 |
| CFNX732 (ruvB::loxP recG::loxPSp) | 29 | 11 | 0.0035 |
| CFNX733 (ruvB::loxP radA::loxP) | 29 | 15 | 0.025 |
| CFNX734 (recG::loxP radA::loxPSp) | 40 | 8 | 3 × 10−6 |
Probability that the observed distribution agrees with the null expectation (gene conversion toward gain of markers being equal to gene conversion toward loss of markers), evaluated by a nonparametrical binomial test. All P values are significant at the 5% level.
RadA is an inefficient system for gene conversion in R. etli.
For evaluation of the effect of HJ migration systems on gene conversion, it can be anticipated, based on current models, that the most sensitive parameter would be the length of gene conversion segments. This is due to the fact that conversion length will be affected by the extent of heteroduplex migration. Thus, evaluation of this parameter will reveal the relative efficiency of each of the HJ migration systems.
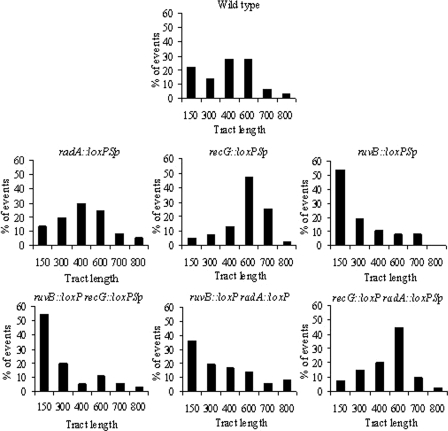
As shown in Fig. 3, inactivation of radA does not have a strong effect on gene conversion tract length. The length of gene conversion segments in this strain shows a unimodal distribution centered at 400 bp, similar to that in the wild-type strain. Comparisons of the length distribution in the wild type versus the distribution in the radA mutant revealed that the differences were not significant (chi-square test; a P value of 0.87). Thus, these results suggest that RadA is a relatively inefficient system for gene conversion in an otherwise wild-type strain.
FIG. 3.
Lengths of gene conversion tracts are modified in strains with mutations affecting HJ migration. Each panel shows the distribution of lengths of gene conversion tracts (excluding discontinuous events) in the indicated strains, derived from Fig. S1 in the supplemental material. To facilitate comparisons between the strains, data are presented as percentages, although the statistical analysis presented in the text was done using the actual values. Lengths of gene conversion tracts are shown in base pairs. Numbers of gene conversion tracts analyzed for each strain were as follows: wild type, 65; radA::loxPSp, 37; recG::loxPSp, 40; ruvB::loxPSp, 37; ruvB::loxP recG::loxPSp, 35; ruvB::loxP radA::loxP, 36; and recG::loxP radA::loxPSp, 40.
To support this interpretation, analysis of the length of gene conversion segments in a ruvB recG mutant was instructive. In this mutant, two of the three main HJ migration systems were removed, allowing us to evaluate the participation of RadA on gene conversion in a more direct way. In this mutant (Fig. 3), the gene conversion tract length was severely affected, displaying segments with a modal length of just 150 bp. This difference was highly significant compared to the wild-type strain (chi-square test; a P value of 0.006). Therefore, these results confirm the participation of RadA in gene conversion, although its efficiency is very low.
RecG plays a dual role in gene conversion.
Inactivation of recG has a striking effect on the length of gene conversion segments. The modal length of gene conversion tracts (Fig. 3) was longer in the recG mutant (600 bp) than in the wild-type strain (400 bp). In fact, in the recG mutant, about 75% of the segments were longer than 600 bp, compared to less than 40% for the wild-type strain. These differences were highly significant as evaluated by a chi-square test (P = 0.003). The observed increase in gene conversion tract length in the recG mutant is fully consistent with the proposed antirecombinogenic role of RecG in R. etli (28), as has been demonstrated in vitro in E. coli (52).
To evaluate if RecG in R. etli is also able to migrate HJ away from the recombination start point (recombinogenic role), the gene conversion tract length was analyzed for the ruvB radA mutant strain. As shown in Fig. 3, although this double mutant showed a distribution of conversion lengths with a mode of 150 bp, the overall distribution was not significantly different from the distribution found in the wild-type strain (chi-square test; a P value of 0.241). Even though many of the tracts in this mutant fell in the 150-bp size class, 45% of the segments were longer than 400 bp, similar to the case in the wild-type strain (65%, Fig. 3). Thus, these results indicate that RecG in R. etli plays a dual role, having both recombinogenic and antirecombinogenic activities.
RuvB is the most efficient HJ migration system, and its absence causes a shift in the distribution of most converted sites.
As shown in Fig. 3, inactivation of the RuvB protein causes a notable reduction in gene conversion lengths. The modal length for gene conversion segments (150 bp) was, in fact, the shortest of those observed among the single-HJ migration mutants. Comparison of the length distribution for this mutant with the wild-type distribution revealed highly significant differences (chi-square test; a P value of 0.005). Simultaneous inactivation of the RecG and RadA systems allowed us to analyze the activity of the RuvB system alone. In this double mutant, the observed modal length was 600 bp (Fig. 3); although the differences in the length distribution in this double mutant were not significant from the distribution in the wild-type strain (chi-square test; a P value of 0.27), it is clear that this mutant displays the longest tract length mode among the double mutants. These results support the interpretation that the RuvB protein is the most efficient system for gene conversion in R. etli.
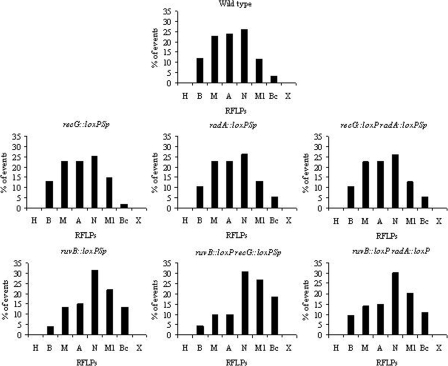
Our approach allows us to explore the location of the most highly converted sites. As shown in Fig. 4, the most highly converted RFLPs in the wild-type strain showed a symmetrical distribution, centered in the ApaLI site. This was expected because the interval spanning the ApaLI and NarI sites (200 bp) is the longest segment in the nifH gene lacking RFLPs. One consequence of this distribution is that the MluI and BclI RFLPs were converted in a minority of the cases. This distribution was maintained in the recG, radA, and recG radA mutants (chi-square test; P values of 0.83, 0.54, and 0.28, respectively). Notably, in the set of mutations where the RuvB system is inactive (ruvB, ruvB recG, ruvB radA) (Fig. 4), the distribution of the most converted sites was shifted toward sites located to the right of the ApaLI RFLP. Instead of a symmetrical distribution centered on the ApaLI site, these mutants showed a highly skewed distribution, favoring conversion of the NarI RFLP. Moreover, the MluI and BclI RFLPs were also significantly converted in these strains, being converted at a frequency twice as high as the one observed in the other strains (Fig. 4). These differences were highly significant, as evaluated by a chi-square test (P ≤ 0.001). Possible reasons for this skewed distribution, based on the preferred role of RuvB in gene conversion, will be presented in Discussion.
FIG. 4.
Distribution of converted sites in continuous gene conversion tracts in the wild-type and HJ mutant strains. For each panel, the percentage of events in which the corresponding site appears converted (either for gain or for loss) in the indicated strain is shown. Data are derived from Fig. S1 in the supplemental material (excluding discontinuous events). For clarity, data are presented as percentages, although the statistical analysis presented in the text was done using the actual values. Capital letters on the x axis represent each RFLP introduced along the nifH gene, oriented from the 5′ end to the 3′ end of nifH. H, HindIII; B, BamHI; M, MaeIII; A, ApaLI; N, NarI; Ml, MluI; Bc, BclI; X, XbaI.
DISCUSSION
For the interpretation of the data reported in this paper, it is important to stress that RuvAB, RecG, and RadA appear to be the main systems responsible for HJ migration in R. etli (28). The main evidence in favor of this interpretation is the very low recombination frequency in a ruvB recG radA triple mutant, which is as low as the one observed in a recA mutant (28). It has been reported that topoisomerase III may also promote HJ migration and resolution in E. coli; this resolution pathway, however, can produce only gene conversion not associated with crossovers (23). As mentioned in Results, all the gene convertants analyzed here require the presence of a prior crossover; therefore, the contribution of a topoisomerase III pathway is judged to be negligible under our conditions.
The main conclusions obtained here, namely that (i) inactivation of radA does not affect gene conversion tract length, (ii) null mutations in recG lead to longer gene conversion tracts, and (iii) the absence of ruvB instigates a marked reduction in the length of gene conversion segments, fully agree with previous proposals that RuvAB is the main system for HJ migration while RecG promotes HJ regression (but also migration away from the recombination start site) and that RadA has a minor role in HJ migration. These conclusions, based on the analysis of single-gene mutations, are also fully consistent with the results found with multiple-gene mutations.
In this regard, we have explored here four different scenarios after formation of HJs. In both the wild-type and radA genetic backgrounds, RuvAB and RecG must be acting, competing with HJs, and originating gene conversion tracts of about 400 bp on average. The second scenario is that in which either RuvAB (in the recG radA mutant) or RuvAB-RadA (in the recG mutant) is active. In these cases, migration of HJs by RuvAB proceeds without interference by RecG, leading to the production of long gene conversion tracts; tract length is further extended by a small but significant contribution by RadA. The third scenario is where RecG (in the ruvB radA mutant) or RecG-RadA (in the ruvB mutant) is present; in these cases, upon elimination of the main HJ migration activity (RuvAB), HJ migration should be relatively restricted as a result of the relative contribution of the recombinogenic and antirecombinogenic activities of RecG, leading to the generation of short gene conversion tracts. Interestingly, the fact that the range of tract lengths was wider for cases in which RecG was the only active system than for the situations where both RecG and RadA were functioning suggests the intriguing possibility that RadA may potentiate the antirecombinogenic activity of RecG. The last scenario is where RadA is the only system migrating HJs (in the ruvB recG mutant) and thereby producing very short gene conversion tracts.
Based on published data on the in vitro activities of at least some of these systems in E. coli as well as their relative abundance in the cell, it is possible to make a rough approximation of how big the antirecombinogenic effect of RecG should be in vivo. The E. coli RuvAB enzyme shows in vitro preferential migration of HJs away from the initiation site (48), a high level of processivity (98 ± 3 bp s−1) (2), and high intracellular abundance (200 protein copies per cell or, considering the multimerization state, roughly 35 active copies per cell) (44, 51). RecG, in contrast, exhibits an in vitro preference in E. coli for regression of HJ intermediates, although it also promotes its migration away from the initiation site (30, 52). This fact, coupled with RecG's lower level of processivity (26 bp s−1) (29) compared to that of RuvAB and low intracellular abundance (less than 10 copies per cell) (6), militates in favor of an inhibitory role of this system in gene conversion.
Assuming, for simplicity, that RecG's effects are mostly inhibitory, the relative effect on HJ migration in a wild-type strain can be shown as follows: [(effect of the RuvAB system) − (effect of the RecG system) × 100]. Since the processivity of RecG is roughly one quarter of that seen for RuvAB and its abundance is 3.5 times lower, the length of gene conversion segments in a wild-type strain should be just 7% shorter than the length that would be observed in a recG mutant. The predicted modest effect of recG elimination in E. coli agrees with published data where recG mutants displayed weak reductions in recombination frequency (22) except in systems such as adaptive mutagenesis, where inactivation of RecG provokes a marked increase in the frequency of this interesting phenomenon (11, 17). This behavior contrasts with the 50% increase in the modal gene conversion tract length observed in R. etli (from 400 bp to 600 bp) upon recG inactivation. These differences in the in vivo behavior of R. etli versus that of E. coli lead us to suggest that RecG parameters should differ between these species in abundance, processivity, or even preference for HJ regression. These possible differences should be explained by our proposal that RadA potentiates the antirecombinogenic activity of RecG. Experimental support for these proposals, including an exploration of how general they can be to explain gene conversion in bacteria, should have to wait for the in vivo quantification, purification, and kinetic characterization of RuvAB, RecG, and RadA in R. etli.
A relatively unexpected feature of our data is the shift in the position of gene conversion tracts toward the 3′ end of the nifH gene whenever RuvB is absent. Although this shift might be explained by invoking a differential association of HJ proteins with repair systems, our previous data militate against such an explanation (39). In fact, an analysis of preferred converted markers in a mutS derivative failed to reveal such a shift (39). However, two non-mutually exclusive alternatives can be postulated, namely (i) preferential HJ migration toward the 3′ end of the gene by RecG and RadA and (ii) a preferential association of the RuvC HJ endonuclease with RuvAB coupled with the association of RecG and RadA with a different HJ resolvase.
Regarding the first alternative, it has been reported that RuvAB (49) and RecG (52, 53) are helicases with opposing strand polarities on RecA-coated substrates. These opposing polarities impose different migration abilities, which may translate into different positions for gene conversion tracts. It was previously proposed that the RuvAB system extends 3′ end invasions, while the RecG system unwinds this intermediate, and that the reverse happens with a 5′ end invasion intermediate (17). Both 3′ and 5′ invading ends occur in the DSBR model (Fig. 1). Invasions employing a 3′ end are commonly regarded as preferred alternatives for gene conversion because this kind of invasion may give rise to gene convertants either through limited DNA synthesis or by heteroduplex extension. According to the previous proposal (17), extension in this circumstance occurs efficiently by the RuvAB system, aided by its high copy number and significant processivity. Invasions using the 5′ end, in contrast, may generate only gene convertants through extension, which occurs in this case by using the less-efficient RecG or RadA system. According to this proposal (Fig. 5), in the wild-type situation, gene convertants may be generated either in the 5′ half or the 3′ half of the gene by the combined action of these two helicases (and perhaps through the action of RadA). However, in the absence of the RuvAB system, convertants should be generated by the action of RecG and RadA, with a preferential location toward the 3′ half of the gene.
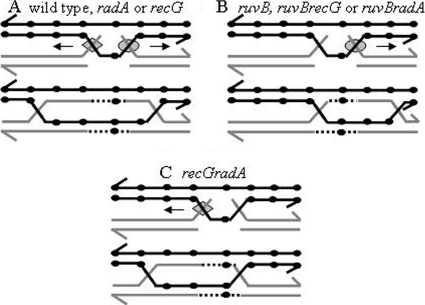
FIG. 5.
Proposal to explain shifting of the most converted sites in HJ mutants. In all panels, two recombining homologs are shown, as well as 3′ ends (half-arrowheads), RFLPs (small black circles), RuvAB (gray diamonds), RecG or RadA (gray circles), and the direction of movement of HJ migration proteins (arrows). (A) HJ migration and gene conversion in the wild type and recG or radA mutant strain. At the top of panel A, 3′ end invasions are processed by RuvAB, while 5′ end invasions are processed by RecG or RadA. These intermediates migrate preferentially in the direction indicated by the small arrows. Given the high processivity of RuvAB, migration toward the 5′ end spans a larger extension than migrations toward the 3′ end. The product of both migration events encompasses a sector located in the middle of the gene. (B) HJ migration and gene conversion in the absence of ruvB (the ruvB, ruvB recG, or ruvB radA mutant strain). Under these circumstances, 3′ end invasions are not processed, leaving only the processing of 5′ invasions by RecG or RadA. Preferential movement of these intermediates toward the 3′ end (top) instigates gene conversion events shifted toward the 3′ end of the gene (bottom). (C) HJ migration and gene conversion in the absence of both recG and radA. In this case, processing of 3′ end invasions by RuvAB provokes the movement of HJs (and hence gene conversion) mostly toward the 5′ end, although migration covering the 3′ end is yet possible, given the high processivity of this complex.
A second alternative is to postulate the presence of preferred resolution sites depending on the HJ migration system used. There is clear evidence, both in vitro and in vivo (10, 42), that the RuvAB complex associates with the HJ-specific endonuclease RuvC. RecG-loaded HJs, in contrast, are thought to be resolved by another unrelated endonuclease, called RusA (26). E. coli RuvC depicts clear preferences for sites with the sequence A/TTTG/C (41); RusA, in contrast, shows somewhat less-stringent sequence requirements, favoring cleavage at the 5′ end of a CC dinucleotide at the point of crossover of the HJ (8, 25). Interestingly, potential RuvC resolution sites are twice as frequent (eight versus four) in the 5′ half than in the 3′ half of the nifH gene. This suggests that RuvAB migrating complexes have an increased probability of being resolved on the 5′ half of nifH. Since there are no clear rusA orthologs in the R. etli genome, RecG migrating complexes should be resolved by another RusA analogous activity in this organism. Identification of this putative endonuclease and elucidation of its participation in gene conversion constitute an interesting avenue for future research.
Acknowledgments
We are indebted to Verónica Rohen for insightful advice with the statistical analysis. We gratefully acknowledge Paul Gaytán and Eugenio López (Unidad de Síntesis de Oligonucleótidos, Instituto de Biotecnología, Universidad Nacional Autónoma de México) for help in oligonucleotide synthesis, Jaime Martínez-Salazar and César Rodríguez for help in strain construction, and Javier Rivera and Laura Cervantes for skillful technical assistance.
Partial financial support was provided by grant no. 31753 from the Consejo Nacional de Ciencia y Tecnología (México). M.C. was supported during the Ph.D. program (Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México) by a scholarship from Consejo Nacional de Ciencia y Tecnología (México).
Footnotes
Published ahead of print on 5 June 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abdulkarim, F., and D. Hughes. 1996. Homologous recombination between the tuf genes of Salmonella typhimurium. J. Mol. Biol. 260506-522. [DOI] [PubMed] [Google Scholar]
- 2.Amit, R., O. Gileadi, and J. Stavans. 2004. Direct observation of RuvAB-catalyzed branch migration of single Holliday junctions. Proc. Natl. Acad. Sci. USA 10111605-11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amundsen, S. K., J. Fero, L. M. Hansen, G. A. Cromie, J. V. Solnick, G. R. Smith, and N. R. Salama. 2008. Helicobacter pylori AddAB helicase-nuclease and RecA promote recombination-related DNA repair and survival during stomach colonization. Mol. Microbiol. 69994-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beam, C. E., C. J. Saveson, and S. T. Lovett. 2002. Role for radA/sms in recombination intermediate processing in Escherichia coli. J. Bacteriol. 1846836-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergholz, T. M., C. L. Tarr, L. M. Christensen, D. J. Betting, and T. S. Whittam. 2007. Recent gene conversions between duplicated glutamate decarboxylase genes (gadA and gadB) in pathogenic Escherichia coli. Mol. Biol. Evol. 242323-2333. [DOI] [PubMed] [Google Scholar]
- 6.Briggs, G. S., A. A. Mahdi, Q. Wen, and R. G. Lloyd. 2005. DNA binding by the substrate specificity (wedge) domain of RecG helicase suggests a role in processivity. J. Biol. Chem. 28013921-13927. [DOI] [PubMed] [Google Scholar]
- 7.Carrasco, B., M. C. Cozar, R. Lurz, J. C. Alonso, and S. Ayora. 2004. Genetic recombination in Bacillus subtilis 168: contribution of Holliday junction processing functions in chromosome segregation. J. Bacteriol. 1865557-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, S. N., L. Harris, E. L. Bolt, M. C. Whitby, and R. G. Lloyd. 1997. Sequence-specificity and biochemical characterization of the RusA Holliday junction resolvase of Escherichia coli. J. Biol. Chem. 27214873-14882. [DOI] [PubMed] [Google Scholar]
- 9.Cho, N. H., H. R. Kim, J. H. Lee, S. Y. Kim, J. Kim, S. Cha, S. Y. Kim, A. C. Darby, H. H. Fuxelius, J. Yin, J. H. Kim, J. Kim, S. J. Lee, Y. S. Koh, W. J. Jang, K. H. Park, S. G. Andersson, M. S. Choi, and I. S. Kim. 2007. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc. Natl. Acad. Sci. USA 1047981-7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eggleston, A. K., A. H. Mitchell, and S. C. West. 1997. In vitro reconstitution of the late steps of genetic recombination in E. coli. Cell 89607-617. [DOI] [PubMed] [Google Scholar]
- 11.Foster, P., J. M. Trimarchi, and R. A. Maurer. 1996. Two enzymes, both of which process recombination intermediates, have opposite effects on adaptive mutation in Escherichia coli. Genetics 14225-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry, D. R., and D. J. Holmes. 2008. Selection for high-level telithromycin resistance in Staphylococcus aureus yields mutants resulting from an rplB-to-rplV gene conversion-like event. Antimicrob. Agents Chemother. 521156-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González, V., R. I. Santamaría, P. Bustos, I. Hernández-González, A. Medrano-Soto, G. Moreno-Hagelsieb, S. C. Janga, M. A. Ramírez, V. Jiménez-Jacinto, J. Collado-Vides, and G. Dávila. 2006. The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc. Natl. Acad. Sci. USA 1033834-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Escalona, N., J. Romero, and R. T. Espejo. 2005. Polymorphism and gene conversion of the 16S rRNA genes in the multiple rRNA operons of Vibrio parahaemolyticus. FEMS Microbiol. Lett. 246213-219. [DOI] [PubMed] [Google Scholar]
- 15.Gore, J. M., F. A. Ran, and L. N. Ornston. 2006. Deletion mutations caused by DNA strand slippage in Acinetobacter baylyi. Appl. Environ. Microbiol. 725239-5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hargreaves, D., D. W. Rice, S. E. Sedelnikova, P. J. Artymiuk, R. G. Lloyd, and J. B. Rafferty. 1998. Crystal structure of E. coli RuvA with bound DNA Holliday junction at 6 Å resolution. Nat. Struct. Biol. 5441-446. [DOI] [PubMed] [Google Scholar]
- 17.Harris, R. S., K. J. Rosst, and S. M. Rosenberg. 1996. Opposing roles of the Holliday junction processing systems of Escherichia coli in recombination-dependent adaptive mutation. Genetics 142681-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang, J., and M. J. Blaser. 2008. Repair and antirepair DNA helicases in Helicobacter pylori. J. Bacteriol. 1904218-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang, J., D. Tavakoli, A. Tschumi, A. R. Aras, and M. J. Blaser. 2004. Effect of host species on recG phenotypes in Helicobacter pylori and Escherichia coli. J. Bacteriol. 1867704-7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondrashov, F. A., T. A. Gurbich, and P. K. Vlasov. 2007. Selection for functional uniformity of tuf duplicates in γ-proteobacteria. Trends Genet. 23215-218. [DOI] [PubMed] [Google Scholar]
- 21.Lindroos, H., O. Vinnere, A. Mira, D. Repsilber, K. Näslund, and S. G. Andersson. 2006. Genome rearrangements, deletions, and amplifications in the natural population of Bartonella henselae. J. Bacteriol. 1887426-7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd, R. G., and C. Buckman. 1991. Genetic analysis of the recG locus of Escherichia coli K-12 and of its role in recombination and DNA repair. J. Bacteriol. 1731004-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez, C. R., S. Yang, R. W. Deibler, S. A. Ray, J. M. Pennington, R. J. DiGate, P. J. Hastings, S. M. Rosenberg, and E. L. Zechiedrich. 2005. A role for topoisomerase III in a recombination pathway alternative to RuvABC. Mol. Microbiol. 5880-101. [DOI] [PubMed] [Google Scholar]
- 24.Ma, L., J. S. Jensen, L. Myers, J. Burnett, M. Welch, Q. Jia, and D. H. Martin. 2007. Mycoplasma genitalium: an efficient strategy to generate genetic variation from a minimal genome. Mol. Microbiol. 66220-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macmaster, R., S. Sedelnikova, P. J. Baker, E. L. Bolt, R. G. Lloyd, and J. B. Rafferty. 2006. RusA Holliday junction resolvase: DNA complex structure: insights into selectivity and specificity. Nucleic Acids Res. 345577-5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahdi, A. A., G. J. Sharples, T. N. Mandal, and R. G. Lloyd. 1996. Holliday junction resolvases encoded by homologous rusA genes in Escherichia coli K-12 and phage 82. J. Mol. Biol. 257561-573. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Salazar, J. M., and D. Romero. 2000. Role of the ruvB gene in homologous and homeologous recombination in Rhizobium etli. Gene 243125-131. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Salazar, J. M., J. Zuñiga-Castillo, and D. Romero. 2009. Differential roles of proteins involved in migration of Holliday junctions on recombination and tolerance to DNA damaging agents in Rhizobium etli. Gene 43226-32. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Senac, M. M., and M. R. Webb. 2005. Mechanism of translocation and kinetics of DNA unwinding by the helicase RecG. Biochemistry 4416967-16976. [DOI] [PubMed] [Google Scholar]
- 30.Meddows, T. R., A. P. Savory, and R. G. Lloyd. 2004. RecG helicase promotes DNA double-strand break repair. Mol. Microbiol. 52119-132. [DOI] [PubMed] [Google Scholar]
- 31.Miller, K., A. J. O'Neill, M. H. Wilcox, E. Ingham, and I. Chopra. 2008. Delayed development of linezolid resistance in Staphylococcus aureus following exposure to low levels of antimicrobial agents. Antimicrob. Agents Chemother. 521940-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris, R. T., and G. Drouin. 2007. Ectopic gene conversions in bacterial genomes. Genome 50975-984. [DOI] [PubMed] [Google Scholar]
- 33.Müller, B., I. R. Tsaneva, and S. C. West. 1993. Branch migration of Holliday junctions promoted by the Escherichia coli RuvA and RuvB proteins. I. Comparison of RuvAB- and RuvB-mediated reactions. J. Biol. Chem. 26817179-17184. [PubMed] [Google Scholar]
- 34.Nystedt, B., A. C. Frank, M. Thollesson, and S. G. Andersson. 2008. Diversifying selection and concerted evolution of a type IV secretion system in Bartonella. Mol. Biol. Evol. 25287-300. [DOI] [PubMed] [Google Scholar]
- 35.Privezentzev, C. V., A. Keeley, B. Sigala, and I. R. Tsaneva. 2005. The role of RuvA octamerization for RuvAB function in vitro and in vivo. J. Biol. Chem. 2803365-3375. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez, C., and D. Romero. 1998. Multiple recombination events maintain sequence identity among members of the nitrogenase multigene family in Rhizobium etli. Genetics 149785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero, D., S. Brom, J. Martínez-Salazar, M. L. Girard, R. Palacios, and G. Dávila. 1991. Amplification and deletion of a nod-nif region in the symbiotic plasmid of Rhizobium phaseoli. J. Bacteriol. 1732435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santoyo, G., and D. Romero. 2005. Gene conversion and concerted evolution in bacterial genomes. FEMS Microbiol. Rev. 29169-183. [DOI] [PubMed] [Google Scholar]
- 39.Santoyo, G., J. M. Martínez-Salazar, C. Rodríguez, and D. Romero. 2005. Gene conversion tracts associated with crossovers in Rhizobium etli. J. Bacteriol. 1874116-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sechman, E. V., K. A. Kline, and H. S. Seifert. 2006. Loss of both Holliday junction processing pathways is synthetically lethal in the presence of gonococcal pilin antigenic variation. Mol. Microbiol. 61185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah, R., R. Cosstick, and S. C. West. 1997. The RuvC protein dimer resolves Holliday junctions by a dual incision mechanism that involves base-specific contacts. EMBO J. 161464-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharples, G. J., F. E. Benson, G. T. Illin, and R. G. Lloyd. 1990. Molecular and functional analysis of the ruv region of Escherichia coli K-12 reveals three genes involved in DNA repair and recombination. Mol. Gen. Genet. 221219-226. [DOI] [PubMed] [Google Scholar]
- 43.Shen, P., and H. V. Huang. 1986. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics 112441-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shurvinton, C. E., and R. G. Lloyd. 1982. Damage to DNA induces expression of the ruv gene of Escherichia coli. Mol. Gen. Genet. 185352-355. [DOI] [PubMed] [Google Scholar]
- 45.Simon, R. 1984. High frequency mobilization of gram-negative bacterial replicons by the in vivo constructed Tn5-Mob transposon. Mol. Gen. Genet. 196413-416. [DOI] [PubMed] [Google Scholar]
- 46.Stewart, F. J., and C. M. Cavanaugh. 2007. Intragenomic variation and evolution of the internal transcribed spacer of the rRNA operon in bacteria. J. Mol. Evol. 6544-67. [DOI] [PubMed] [Google Scholar]
- 47.Szostak, J. W., T. L. Orr-Weaver, R. J. Rothstein, and F. W. Stahl. 1983. The double-strand-break repair model for recombination. Cell 3325-35. [DOI] [PubMed] [Google Scholar]
- 48.Tsaneva, I. R., B. Müller, and S. C. West. 1992. ATP-dependent branch migration of Holliday junctions promoted by the RuvA and RuvB proteins of E. coli. Cell 691171-1180. [DOI] [PubMed] [Google Scholar]
- 49.Tsaneva, I. R., B. Müller, and S. C. West. 1993. RuvA and RuvB proteins of Escherichia coli exhibit DNA helicase activity in vitro. Proc. Natl. Acad. Sci. USA 901315-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valencia-Morales, E., and D. Romero. 2000. Recombination enhancement by replication (RER) in Rhizobium etli. Genetics 154971-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.West, S. C. 1997. Processing of recombination intermediates by the Ruv-ABC proteins. Annu. Rev. Genet. 31213-244. [DOI] [PubMed] [Google Scholar]
- 52.Whitby, M. C., L. Ryder, and R. G. Lloyd. 1993. Reverse branch migration of Holliday junctions by RecG protein: a new mechanism for resolution of intermediates in recombination and DNA repair. Cell 75341-350. [DOI] [PubMed] [Google Scholar]
- 53.Whitby, M. C., S. D. Vincent, and R. G. Lloyd. 1994. Branch migration of Holliday junctions: identification of RecG protein as a junction specific DNA helicase. EMBO J. 135220-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]