Abstract
Helicobacter pylori requires flagellar motility and orientation to persist actively in its habitat. A particular feature of flagella in most Helicobacter species including H. pylori is a membraneous flagellar sheath. The anti-sigma factor FlgM of H. pylori is unusual, since it lacks an N-terminal domain present in other FlgM homologs, e.g., FlgM of Salmonella spp., whose regulatory function is intimately coupled to its secretion through the flagellar type III secretion system. The aim of the present study was to characterize the localization and secretion of the short H. pylori FlgM in the presence of a flagellar sheath and to elucidate its interaction with other flagellar proteins, such as the basal body protein FlhA, which was previously shown to cooperate with FlgM for regulation. H. pylori FlgM was only released into the medium in minor amounts in wild-type bacteria, where the bulk amount of the protein was retained in the cytoplasm. Some FlgM was detected in the flagellar fraction. FlgM was expressed in flhA mutants and was less soluble and differentially localized in bacterial fractions of the flhA mutant in comparison to wild-type bacteria. FlgM-green fluorescent protein and FlgM-V5 translational fusions were generated and expressed in H. pylori. FlgM displayed a predominantly polar distribution and interacted with the C-terminal domain of FlhA (FlhAC). We suggest that, in H. pylori, FlgM secretion may not be paramount for its regulatory function and that protein interactions at the flagellar basal body may determine the turnover and localization of functional FlgM.
Helicobacter pylori causes chronic infection of the human stomach mucosa in about 50% of the world population. H. pylori requires flagellar motility and taxis for persistent survival in the gastric mucus of humans (11, 23, 27, 40). The flagellar apparatus of H. pylori shows some specific properties. The bacterium may have acquired those properties as an adaptation to an inhospitable environment of low pH and high proteolytic activity (e.g., pepsin), which may be damaging to flagellar components and function. In particular, a flagellar sheath covers each flagellum and is continuous with the bacterial outer membrane and of similar lipid composition (14).
Many H. pylori flagellar components are similar to the well-characterized flagellar apparatus of Salmonella spp. (3, 30). The complex hierarchical regulation of flagellar genes in H. pylori (24, 33, 51) suggests that flagellar components in this species are also assembled in a highly ordered fashion. The anti-sigma factor FlgM and the sigma factor FliA jointly control the expression of late flagellar genes in H. pylori, in particular of the major filament protein FlaA (6, 24). Interestingly, H. pylori FlgM is truncated at its extreme N terminus in comparison to its counterpart in Salmonella. Still, H. pylori FlgM is functional as an anti-sigma factor in Salmonella (24). FlgM in H. pylori is a central component in the regulation of flagellar genes. It is important at all regulatory levels and not only for the regulation of late flagellar genes (33). In H. pylori and numerous other Helicobacter spp., the presence of a flagellar sheath is a common and conspicuous trait and raises the question of whether FlgM secretion through the flagellar central channel into the environment is possible or whether it might be hampered under these circumstances. In Salmonella, FlgM secretion is crucial for its function as an anti-sigma factor, as FlgM secretion from the bacterial cytoplasm through the flagellar type III secretion system itself is intimately coupled to its regulatory function (15, 20). An unusually short FlgM is found not only in H. pylori but also in other Campylobacterales species whose whole genome sequences have been decoded (e.g., Helicobacter mustelae, Helicobacter hepaticus, Campylobacter coli, Campylobacter jejuni, Wolinella succinogenes [12, 35, 44; P. W. O'Toole et al., Sanger Center, unpublished data]). It is assumed that the type III secretion signal of FlgM is encoded in its N-terminal amino acid sequence as in other type III secreted substrate proteins (43). Proposed evolutionary degeneration at the N terminus of FlgM in all of the Epsilonproteobacteria may therefore suggest that the type III secretion signal responsible for secretion of FlgM is not present in those bacterial species. This raises the question of whether FlgM cannot be secreted in those bacteria. Under those circumstances, secretion may not be essential for FlgM function.
Our previous data on flagellar transcriptional regulation in H. pylori flhA flgM double knockout mutants (33) indicated that inactivation of flgM resulted in a partial suppressor mutation over a previous inactivation of flhA. It is of interest that the transcriptional regulatory phenotype of wild-type bacteria was reconstituted in the double mutants. Despite the regulatory suppressor phenotype, the morphological flagellar phenotype was not reconstituted back to wild type (33). This was probably due to the total lack of FlhA protein in those mutants. Thus, the hypothesis was generated that FlhA and FlgM cooperate closely or may physically interact in order to coordinate flagellar gene regulation.
The goal of this study was therefore to establish whether FlgM is secreted from the H. pylori cell and to determine FlgM expression and localization in wild-type H. pylori and flagellar mutants with defined defects in flagellar regulation and flagellar secretion. We also hypothesized that protein-protein interactions at the flagellar basal body, in particular with FlhA, may be required for the function and localization of H. pylori FlgM.
In this study we found that FlgM is only secreted in small quantities from H. pylori wild-type cells. Furthermore, FlhA had an influence on the localization of FlgM, and interaction between FlgM and the C-terminal domain of FlhA (FlhAC) was demonstrated by several methodologies.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
H. pylori strains N6 (13) and 88-3887 (motile variant of the fully sequenced strain 26695 [21]) were used. The bacterial strains and H. pylori mutants used are listed in Table 1. Bacteria were routinely cultured on blood agar plates (Oxoid blood agar base and 5% horse blood) or in brain heart infusion broth supplemented with 2.5% yeast extract and 10% horse serum and the following antibiotics: vancomycin (10 mg/liter), polymyxin B (2,500 U/liter), trimethoprim (5 mg/liter), and amphotericin B (4 mg/liter). The selective antibiotics included ampicillin (300 mg/liter), kanamycin (20 mg/liter), and chloramphenicol (10 mg/liter) and were added to the media as required. Escherichia coli strains used for cloning were DH5α and MC1061 (38), and for overexpression of proteins, the strains ER2566 (New England Biolabs Inc.) and TG1 (38) were employed.
TABLE 1.
Strains used in this study
| Strain | Genotypea | Reference |
|---|---|---|
| H. pyloristrains | ||
| 26695 | Wild-type strain | 45 |
| 88-3887 | Motile variant of 26695 | 21 |
| N6 | Wild-type stain | 13 |
| N6flhA | N6 flhA::aphA3′-III | 39 |
| N6flgM | N6 flgM::aphA3′-III | 24 |
| N6fliA | N6 fliA::aphA3′-III | 24 |
| N6fliI | N6 fliI::aphA3′-III | This study |
| N6rpoN | N6 rpoN::aphA3′-III | 33 |
| 88-3887flgM | 88-3887 flgM::aphA3′-III | 24 |
| 88-3887flhA | 88-3887 flhA::aphA3′-III | 33 |
| 88-3887fliP | 88-3887 fliP::aphA3′-III | 21 |
| 88-3887fliI | 88-3887 fliI::aphA3′-III | This study |
| 88-3887fliF | 88-3887 fliF::aphA3′-III | This study |
| N6flhF | N6 flhF::aphA3′-III | 33 |
| N6(pCJ604) | N6 + plasmid pCJ604 | This study |
| N6flgM(pCJ604) | N6 flgM::aphA3′-III + plasmid pCJ604 (FlgM-GFP) | This study |
| N6fliA(pCJ604) | N6 fliA::aphA3′-III + plasmid pCJ604 (FlgM-GFP) | This study |
| N6flhA(pCJ604) | N6 flhA::aphA3′-III + plasmid pCJ604 (FlgM-GFP) | This study |
| N6fliI(pCJ604) | N6 fliI::aphA3′-III + plasmid pCJ604 (FlgM-GFP) | This study |
| N6flgM(pCJ607) | N6 flgM::aphA3′-III + plasmid pCJ607 (FlgM-V5) | This study |
| E. coli strains | ||
| DH5α | F−endA1 recA1 hsdR17 Δ(lacZYA-argF)U169 thi1 supE44 gyrA96 relA1 | 17 |
| ER2566 | F− λ−fhuA2[Ilon], ompT lacZ::T7 gene I gal sulA11Δ(mcrC-mrr)11::IS10 R (mcr-73::miniTn10)2 R(zgb-210)::Tn10)1 (Tets) endA1 [dcm] | New England Biolabs |
| MC1061 | F−araD139 Δ(ara-leu)7696 galE15 galK16 Δ(lac)X74 rpsL (Strr) hsdR2 (rK+ mK+) mcrA mcrB1 | 5 |
| TG1 | Expression of FlhAC | 38 |
| BTH101 | Cya-BACTH expression strain; F−cya-99 araD139 galE15 galK16 rpsL1 (Strr) hsdR2 mcrA1 mcrB1 | 25 |
Kmr (aphA3′-III), kanamycin resistance cassette (28).
Techniques of molecular cloning and protein analysis.
DNA purification, DNA manipulation, and cloning procedures were performed according to standard protocols (38). PCRs were run in Perkin-Elmer or Biometra thermocyclers using Amersham Taq polymerase. The Expand High-Fidelity kit (Roche) was used for longer fragments (>2 kb) or if high amplification accuracy for protein expression was required. Plasmids are listed in Table 2. Protein analysis was performed using denaturing sodium dodecyl sulfate (SDS)-polyacrylamide gels containing between 11% and 16% acrylamide, depending on the molecular mass of the proteins to be detected (29), and applying Western immunoblot detection according to the methods described by Towbin (46). Antibodies for immunolabeling were used as indicated in the results or in the figure legends. Protein concentrations were determined using the bichinchoninic acid assay. H. pylori was transformed with plasmids by natural transformation or electroporation. H. pylori crude flagellar preparations and bacterial lysates were obtained as previously described (23, 41).
TABLE 2.
Plasmids constructed or used in this study
| Plasmid | Vector | Descriptiona | Reference or source |
|---|---|---|---|
| pHel2 | Cmr RepEc RepHp; multicopy shuttle vector for E. coli and H. pylori | 18 | |
| pUC18 | Apr RepEc, high-copy-number cloning vector | 48 | |
| pCJ105 | pHel2 | RepEc RepHpflgM-FLAG tag fusion; flgM from H. pylori 26695 | C. Josenhans, unpublished |
| pCJ521 | pEF6-V5 | RepEc RepHp Ampr Blastr; source of V5 sequence | 41 |
| pCJ601 | pUC18 | Apr Kmr RepEcfliI::aphA3′-III; fliI from H. pylori 88-3887 | This study |
| pCJ604 | pHel2 | RepEc RepHp CmrflgM-gfpmut2 fusion; flgM from H. pylori 88-3887 | This study |
| pCJ607 | pHel2 | RepEc RepHp CmrflgM-V5 tag fusion; flgM from H. pylori 88-3887 | This study |
| pILL600 | pBR322 | Apr Kmr RepEc; source of Kmr cassette (aphA3′-III) | 28 |
| pSUS1608 | pSUS2/pUC18 | fliF-aphA3′-III | This study |
| pSUS1614 | pUC18 | flhB2-aphA3′-III | This study |
| pSUS1620 | pUC18 | flhB1-aphA3′-III | This study |
| pCJ1001 | pKNT25 (BACTH) | RepEc KmrflhAC-t25 gene fusion flhAC from strain N6 | This study |
| pCJ1004 | pUT18 (BACTH) | RepEc AprflgM-t18 gene fusion flgM from strain 26695 | This study |
| pUT18 (BACTH) | RepEc Apr | 25 | |
| pKNT25 (BACTH) | RepEc Kmr | 25 | |
| pUT18C-ZIP | pUT18C (BACTH) | RepEc Apr, leucin zipper gene | 25 |
| pKT25-ZIP | pKT25 (BACTH) | RepEc Kmr, leucin zipper gene | 25 |
| pCJ309 | pTWIN1 (NEB) | RepEc Apr, H. pylori FlgM expression plasmid | This study |
| pSUS225 | pQE30 (Qiagen) | RepEc Apr, H. pylori His6-FlhAC expression plasmid | 39 |
Apr, ampicillin resistance; Kmr, kanamycin resistance; Cmr, chloramphenicol resistance (50); Blastr, blasticidin resistance; RepEc and RepHp, replication origin for E. coli and H. pylori, respectively.
Crude fractionation of bacteria.
Bacteria, grown either on plates or in liquid culture to defined optical densities, were fractionated into supernatant, insoluble (membrane-enriched), and soluble (cytoplasmic) fractions. Also, the surface appendages containing flagella and other surface-associated material were isolated as previously described (21). Briefly, liquid culture bacteria were harvested by centrifugation (4,500 × g for 20 min at 4°C). The cell culture supernatant was removed and further purified according to a method originally developed for Salmonella by Hughes and Karlinsey (26). The supernatant was first sterile filtered through a 0.22-μm-pore-size MillexR-GV polyvinylidene difluoride membrane. The cell-free supernatant was then filtered through a Protran nitrocellulose immobilization membrane (Schleicher & Schuell, Germany), which binds proteins present in the cell-free suspension. The membrane was then bathed in 20 to 25 μl of SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer to elute the concentrated proteins from the membrane, which were loaded onto SDS-PAGE gels. For isolation of flagellar fractions, the bacterial pellets were resuspended in 0.9% NaCl and subjected to mild shearing forces by repeated (30 times) pushing of the suspension through 26-gauge syringe needles to shear off appendages from the bacterial surface. Intact bacterial cells and the sheared-off material were then separated by differential centrifugation steps (first at 9,000 × g for 30 min followed by 40,000 × g for 1 h in a Beckman Optima100 ultracentrifuge). The quality of the surface preparations was confirmed by electron microscopy and by Western immunoblotting using flagellin-specific antisera. These controls confirmed that the protocol successfully isolated surface-associated material. The separation of bacteria into insoluble (mainly membrane-associated) and soluble (predominantly cytoplasmic) fractions followed after the separation from the sheared-off material. The intact bacterial cells were resuspended again in 0.9% NaCl and lysed by sonication (Branson sonifier). After centrifugation (two times at 9,000 × g for 30 min at 4°C), the pellet was resuspended in 0.9% NaCl and contained the crude membrane fraction, whereas the supernatant represented the soluble fraction. The quality of the fractionation procedure to isolate insoluble and soluble fractions was tested by electron microscopy and by Western blot analysis for membrane proteins (using anti-HPFlhA antiserum).
RNA preparation and RT-PCR.
RNA was prepared from H. pylori bacteria harvested from liquid cultures in different growth phases using the Qiagen RNeasy kit with slight modifications as described elsewhere (24). Semiquantitative reverse transcription-PCRs (RT-PCRs) were performed on 2 μg of DNase I-treated RNA samples. Reverse transcription was performed using a random hexamer primer mix and SuperScript III reverse transcriptase (Invitrogen) at 42°C for 2 h. The cDNA was adjusted to 50 μl with double-distilled H2O. One-microliter aliquots of cDNA sample were amplified in different PCRs (including negative controls) with primers specific for the corresponding genes. H. pylori 16S rRNA-specific primers were used in each experiment to verify that comparable amounts of cDNA were used.
Construction of H. pylori flagellar mutants.
Plasmid constructs for three additional flagellar mutants (fliI, gene for flagellar ATPase [32, 36]; fliF, flagellar MS ring protein gene [30]; and flhB1 and flhB2, genes for flagellar basal body proteins cooperating with FlhA [30]) were generated in E. coli. In brief, PCR-amplified fragments of the genes were cloned into pUC18 or pILL570. Antibiotic resistance cassettes were inserted into the 3′ parts of the genes either by inverse PCR amplification of the plasmids or using natural restriction sites. Insertion of the cassettes was in the same orientation with respect to the target gene, in order to avoid polar effects. Resulting suicide plasmids (Table 2) were then introduced into H. pylori strains N6 and the motile isogenic clone 88-3887 of 26695 (21) by natural transformation as described previously (16). Transformants or allelic exchange mutants were selected on kanamycin- or chloramphenicol-containing plates. The genotypes of the mutants were verified by PCR using different combinations of oligonucleotide primers. All mutant clones were verified by PCR to carry the genomic disruptions as a result of a double-crossover and not a single-crossover event. Details of the mutant constructions and oligonucleotide primers are provided in the supplemental material. In addition, we used H. pylori mutants in flhA (39), fliP (21), flgM and fliA (24), and rpoN and flhF (33) that had been previously generated and characterized.
Construction of FlgM-GFP and FlgM-V5 transcriptional fusion plasmids for complementation in H. pylori in trans and for localization studies. (i) FlgM-GFP.
For the expression of a FlgM-green fluorescent protein (GFP) fusion in H. pylori, a plasmid was constructed starting with pCJ105 (FlgM-FLAG [C. Josenhans, unpublished data]), which contains the H. pylori flgM gene followed by a FLAG tag sequence. This tag was removed via inverse amplification (primers HPFlgM_GFP_1 and HPFlgM_GFP_2; both BglII sites). Using oligonucleotides GFP-FlgM_1 and GFP-FlgM_2 (BglII sites) the gene encoding a red-shifted and enhanced GFP variant, GFPmut2 (7), was amplified. Both amplification products were cut and ligated to yield plasmid pCJ604 (FlgM-GFP), from which a FlgM protein with a C-terminally fused GFP protein could be expressed in H. pylori. The shuttle plasmid pCJ604 was transformed into H. pylori N6 wild type and also for comparison into N6flgM, N6flhA, N6fliI, and N6fliA mutant strains by natural transformation (50), yielding chloramphenicol-resistant colonies. The rationale for using a fliI mutant was that it is expected to be impaired for separation of FlgM from its chaperone FliA (36). The transformants were characterized by PCR, RT-PCR, Western blot analysis, transmission electron microscopy, and immune fluorescence.
(ii) FlgM-V5.
The flgM gene, including its own untranslated 5′ region containing putative promoter sequences, was also C-terminally fused to a V5 tag sequence (GKPIPNPLLGLDST) with a 7-amino-acid linker (GGSSAAG [4]) and cloned into the E. coli/H. pylori shuttle plasmid pHel2 (18). Initially, the vector pEF6-V5 (Invitrogen) was amplified by inverse PCR using primers (OLpEF6_R and OLV5_F) and including the sequence encoding the 7-amino-acid linker (GGSSAAG) fused to the 5′ end of the encoded V5 tag (from plasmid pCJ521 [41]). The PCR product was digested with BglII and ligated with the BamHI-digested PCR product of the flgM gene (pCJ606). The flgM gene had been amplified before from H. pylori 26695 with primers (HPFM5 and FlgM_rev_2). By sequencing pCJ606, the in-frame fusion of the flgM gene with the V5 tag was checked. For cloning into pHel2, which was required for expression in trans in H. pylori, the FlgM-V5 gene fusion was amplified from the plasmid pCJ606 using primers (HPFM5 and OLV5_R, BamHI sites), which introduced a stop codon at the 3′ end. The amplified gene was cloned into the BamHI site of pHel2 (resulting shuttle plasmid, pCJ607; FlgM-V5). pCJ607 was then introduced into the N6flgM mutant using natural transformation. Plasmid-containing transformant clones were selected on kanamycin-chloramphenicol-containing medium and characterized. The expression of the FlgM-V5 fusion protein was assessed by Western blotting. Primer sequences used for amplification and cloning are available in the supplemental material.
Motility testing (motility plates).
Motility testing was performed in semisolid plates consisting of brucella broth supplemented with 0.3% agar and 3% fetal calf serum (FCS), both as stab tests and as single colony motility tests as described previously (23).
Recombinant expression and purification of H. pylori FlhA and FlgM proteins in E. coli.
A partial C-terminal protein domain of approximately 46.5 kDa of H. pylori FlhA (amino acids 351 to 732) was expressed as described previously using plasmid pSUS225 (39). For technical reasons it was not feasible to overexpress the complete H. pylori FlhA including its N-terminal membrane domain (39). H. pylori FlgM was expressed in the pTWIN system (New England Biolabs, Cambridge, MA) as a C-terminal FlgM-intein-chitin binding domain fusion protein. FlgM was cloned into the pTWIN1 C-term vector (according to the New England Biolabs information manual) by PCR and ligation. Plasmid pCJ309 obtained in E. coli DH5α was characterized and verified by nucleotide sequencing of the complete insert. The expression of the fusion construct in E. coli ER2566 (New England Biolabs) was induced at early exponential phase by administering 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h at 37°C. Purification of the fusion protein was performed by affinity chromatography using a chitin column as recommended by the manufacturer (New England Biolabs information manual). Binding to the column was followed by an overnight purification step involving an intein autocleavage reaction in the presence of the reducing agent dithiothreitol. Dithiothreitol causes the autocatalytic cleavage of the intein domain from the fusion protein and enables the subsequent elution of an untagged version of the overexpressed protein (H. pylori FlgM). The eluted FlgM was further purified by gel elution and repeated dialysis against phosphate-buffered saline.
Generation of antisera.
An anti-HPFlhA antiserum raised against the recombinantly expressed C-terminal domain (FlhAc) was generated in rabbits as previously described (39). An antiserum directed against the recombinantly overexpressed and gel-purified untagged FlgM (described above) was raised in rabbits (Eurogentec, Seraing, Belgium) by repeated intradermal injection.
Electron microscopy.
Copper electron microscropy (EM) grids coated with Formvar and carbon were directly placed on plate-grown bacteria to let them stick and to negatively stain them with 1% phosphotungstate (pH 7.0). EM grids were viewed in a Zeiss EM10 microscope at an acceleration voltage of 80 kV.
Immunofluorescence labeling and microscopy for intracellular detection of tagged FlgM.
Coverslips were coated with 0.3% gelatin and were placed directly on plate-grown bacteria to let them attach. Liquid grown bacteria were also used but did not become attached to the coverslips as reproducibly as plate-grown bacteria. The bacteria were fixed with 2% paraformaldehyde in 100 mM phosphate buffer for 3 h and for another hour after exchanging the fixative with fresh fixative. Nonadhering bacteria were removed by washing the coverslips four times with 0.1% glycine in phosphate-buffered saline (PBS). To permeabilize the fixed bacteria, they were incubated for 30 min with 0.1% Triton X-100. After washing three times with PBS, the coverslips were incubated for 30 min in blocking buffer (1% bovine serum albumin [BSA], 1% FCS in PBS). Due to a weak GFP signal in H. pylori cells transformed with the FlgM-GFP fusion construct, the bacteria had to be immunostained with polyclonal anti-GFP antibodies (R970-01; Invitrogen). The bacteria were incubated overnight with the anti-GFP antibodies diluted in blocking buffer (1:2,000). After washing four times with 0.1% BSA in PBS, the fixed bacteria were incubated with secondary antibody (goat anti-rabbit Alexa488; 1:5,000; Invitrogen) for 1 h. The bacteria were washed four times with 0.1% BSA in PBS and also three times with PBS. The coverslips were mounted with the cell side down on a microscope slide with mounting medium (2.5% DABCO in Mowiol) and dried overnight protected from light. The coverslips were sealed with nail polish. Fluorescence microscopy was performed using an Olympus BX40 microscope with a mercury light source and a narrow-band-pass filter combination optimized for GFP detection (excitation at 490 nm, emission at 515 nm), outfitted with a ColorviewIII digital camera (Olympus). Immunofluorescent labeling for the V5-tagged FlgM was performed according to the same experimental protocol using mouse monoclonal anti-V5 antibody (dilution of 1:500; Invitrogen) and goat anti-mouse-Alexa488 (1:5,000; Invitrogen) as a secondary antibody. Labeling with anti-V5 monoclonal antibody, in contrast to anti-GFP polyclonal serum, did not detectably stain all bacteria for FlgM-V5, and this was reproduced several times. We assume that this is due to the single epitope being recognized by the antibody, which may be masked in some states of FlgM, e.g., when it is in a stable complex with FliA.
Protein coprecipitations.
Coprecipitations (pull-down assays) were performed either with highly purified H. pylori FlgM or His6-FlhAC proteins or with bacterial lysates which were sonicated in modified radioimmunoprecipitation assay (RIPA) buffer (1% Nonidet P-40, 100 mM NaCl, 25 mM Tris-HCl, pH 7.5, 10% glycerol, 20 mM imidazole, 0.1% Tween 20) and cleared by centrifugation to contain only soluble proteins. In the case of purified recombinant proteins (see above), His6-FlhAC (5 μg) and FlgM (2 μg) were mixed and modified RIPA buffer was added to a total volume of 500 μl. The samples were sonicated for 2 min at 4°C and incubated with the buffer for 1 h at 4°C with rotation. They were further incubated for 2 h at 4°C with 25 μl of TALON metal affinity resin (Clontech), which had been prewashed three times with ice-cold PBS. The samples were centrifuged at 1,006 × g for 1 min at 4°C, and the TALON resin pellets were washed three times with modified RIPA buffer (without Nonidet P-40) and two times with ice-cold PBS. The proteins were eluted from the TALON beads by incubating at 100°C for 10 min in 30 μl of 2× SDS-PAGE sample buffer. Alternatively, for pull-down from bacterial lysates, the amount of FlhAC or FlgM-V5 in the respective lysates was determined in Western blot assay and compared to purified proteins loaded in adjacent lanes. Equivalent amounts of cleared lysates, containing approximately 5 μg of His6-FlhAC and 2 μg of FlgM, were then used for the experiments. Purified FlgM (2 μg) was mixed with cleared lysate of E. coli expressing recombinant H. pylori His6-FlhAC. In this case, control experiments using the same amount of His6-FlhAc-expressing lysate only were always performed in parallel. The mixtures were brought to a volume of 500 μl with modified RIPA buffer and further handled for the pull-down assay as described above, including five subsequent wash steps before the elution.
Bacterial two-hybrid system to investigate protein-protein interactions.
The bacterial two-hybrid system BACTH, which was originally developed by Ladant, Ullmann, and Karimova (25) and is now commercially available from EuroMEdEx Inc., was used to assess the potential interactions between H. pylori FlgM and FlhAC in a bacterial native system (in E. coli). The BACTH plasmids pUT18 and pKNT25 were engineered to express FlgM and FlhAC (see Fig. S3 in the supplemental material; primer sequences are also provided in the supplemental material) as C-terminal fusion proteins to the T18 and T25 domains of Bordetella pertussis adenylate cyclase (cya). Again, only the C-terminal soluble part of FlhA was cloned into pKNT25 (FlhAC; cloning primers HP_FlhApKNT_fw and HP_FlhApKNT_rvEco; resulting plasmid pCJ1001). FlgM was inserted into pUT18, starting from the first possible start codon of the open reading frame (primers HPFM24_fw and HPFM25_rvEco; resulting plasmid, pCJ1004). pKT25-zip and pUT18C-zip plasmids (25) were used as a positive control for protein-protein interactions. All possible single and double transformants of the BACTH plasmids with and without inserts were prepared in E. coli BTH101 (Table 2). The plasmid content of the transformants was screened using Qiagen plasmid spin columns, and protein expression of the transformants was detected using polyclonal antisera against FlgM and FlhA in Western immunoblot assays. Metabolic screening of the clones after transformation was performed on Luria-Bertani plates supplemented with IPTG (0.2 mM) and 5-bromo-4-chloro-3-indolyl-β-d-galacatopyranoside (X-Gal; 40 μg/ml) at 37°C as described in the BACTH manual (EuroMedEx). Quantitative measurements of β-galactosidase activities in the transformants were performed according to the method developed by Miller as described previously (24).
RESULTS
H. pylori FlgM is a predominantly cytoplasmic protein and is only secreted in minor amounts into H. pylori flagella.
In H. pylori, a flagellar sheath envelopes the whole filament and is continuous with the bacterial outer membrane. Under these circumstances, it is not easy to envisage how FlgM could work as a secreted regulator. Our hypothesis was that H. pylori FlgM might undergo gain and loss of its regulatory anti-sigma function without the absolute requirement of secretion or might not be secreted at all.
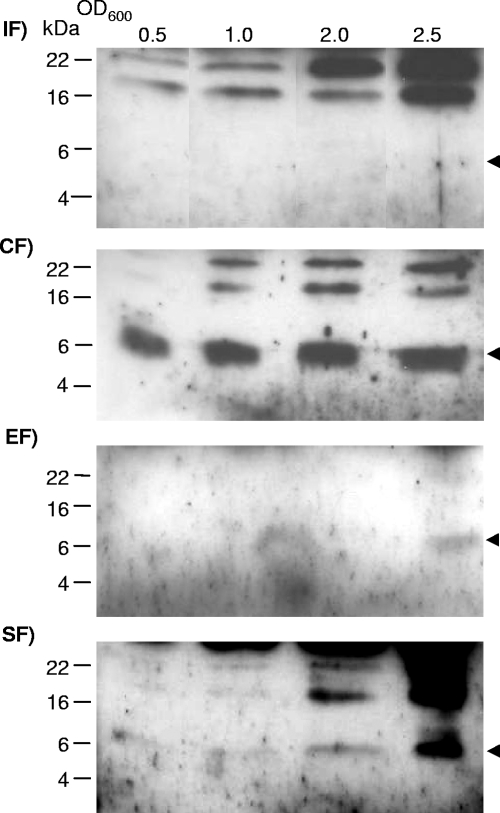
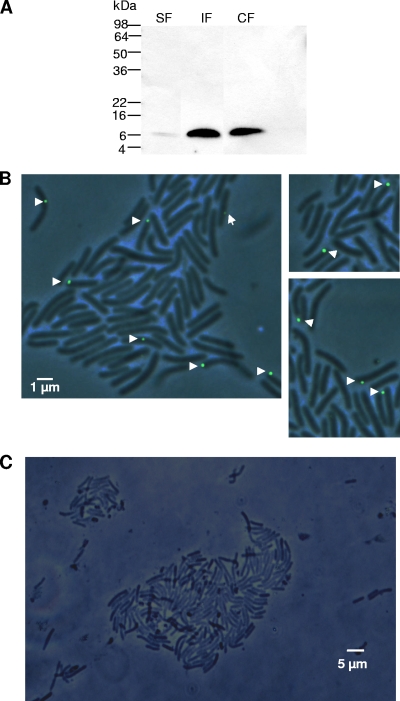
The potential for secretion of FlgM was first assayed by filter concentrating supernatants of H. pylori wild-type bacteria grown in liquid medium to different optical densities. In concentrated bacterial supernatants of wild-type H. pylori harvested from liquid culture at different time points from lag phase to late exponential phase, almost no FlgM was detected (Fig. 1). At very late time points of growth (an optical density of 600 nm [OD600] of approximately 2.5), minor amounts of FlgM were detected in the cell supernatants (Fig. 1). We then attempted to determine the subcellular localization of FlgM in H. pylori wild type further by crude fractionation, separating the sheared-off fraction (containing flagellar material including flagellar sheaths), soluble (expected to contain predominantly cytoplasmic and periplasmic materials), and insoluble (crude membranes) bacterial components (see Materials and Methods). In these experiments, we detected the bulk amount of FlgM in the soluble fraction at all time points during growth (Fig. 1). Osmolytic treatment of whole bacteria according to the methods described in reference 1 (before and after shearing of flagella) (data not presented), intended to release periplasmic proteins, did release some FlgM from the bacterial bodies, but only in the presence of flagella. This finding suggested that the soluble part of FlgM is predominantly cytoplasmic and to some extent flagellum bound. In concordance, minor amounts of FlgM were also detected in the isolated sheared-off flagellar preparations of wild-type bacteria, with an increase of FlgM in this fraction at the onset of stationary growth (Fig. 1).
FIG. 1.
Localization of native FlgM in cellular fractions of H. pylori wild-type bacteria. Bacteria (wild-type HP 88-3887) grown in liquid culture were harvested in different growth phases (OD600 of 0.5 to 2.5) and fractionated. Western blots after SDS-PAGE (16% acrylamide) were incubated with anti-HPFlgM antiserum (1:200), and secondary antibodies were goat anti-rabbit, peroxidase coupled (1:10,000). Ten-microliter aliquots of the respective fractions were loaded in each lane. The molecular mass of FlgM is designated by the arrows. IF, insoluble fraction; CF, soluble fraction; EF, culture supernatant (external fraction); SF, sheared fraction (flagella-containing fractions).
We therefore concluded that FlgM is predominantly a cell-bound and soluble cytoplasmic protein in H. pylori wild-type bacteria, while small amounts of FlgM can be released into and retained within the flagellar fraction, which includes the flagellar sheath.
FlgM is variably expressed in various H. pylori flagellar mutants.
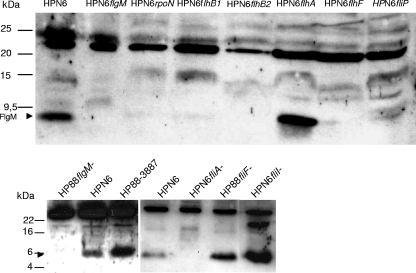
Previous results indicated that FlgM cooperates closely with FlhA to ensure the timely regulation of flagellar class II and III regulons in H. pylori (33). We therefore hypothesized that flagellar basal body proteins, in particular FlhA, are involved in the function and cytosolic localization of H. pylori FlgM. Thus, in order to determine the requirements for FlgM expression in H. pylori with regard to the presence of different flagellar proteins and regulons, we tested a panel of flagellar basal body and regulatory mutants in two different H. pylori strains, N6 and 88-3887 (flhA, flhB1, flhB2, flhF, fliA, fliF, fliI, fliP, and rpoN, which were generated and characterized in our laboratory) (21, 24, 33, 39).
Analysis of whole-cell lysates (Fig. 2) by SDS-PAGE and Western blotting against FlgM demonstrated that FlgM expression was low in flhB1, flhF, fliP, and rpoN allelic disruption mutants and almost undetectable in isogenic flhB2, fliA, and fliF disruption mutants. These results indicated that either transcript abundance, translation, or stability of H. pylori FlgM depended crucially on the flagellar basal body proteins FlhB1, FlhB2, FlhF, FliF, and FliP, as well as on the flagellar sigma factors RpoN and FliA. The latter result also corresponds with earlier work performed in Salmonella, which determined that FliA acts as a stabilizing type III secretion chaperone on FlgM (2). In contrast, the fliI mutants expressed FlgM. Likewise, in flhA mutants, which lack FlhA as a possible interaction partner for FlgM (33), FlgM expression in whole-cell lysates appeared to be similar to the wild type (Fig. 2).
FIG. 2.
FlgM expression in various H. pylori flagellar basal body mutants. FlgM expression in H. pylori wild-type strains (HPN6 and HP88) and defined isogenic mutants in flagellar regulatory and basal body genes (whole-cell lysates; see Materials and Methods for descriptions of the mutants) and flgM mutant (negative control). M, molecular mass marker. Approximately 10 μg of total cell lysate protein for the upper panel or 30 μg for the lower panel was loaded in each lane. Immunodetection was performed using anti-H. pylori FlgM antiserum (1:300).
FlgM displays a different subcellular distribution in H. pylori flhA mutants compared to wild-type bacteria.
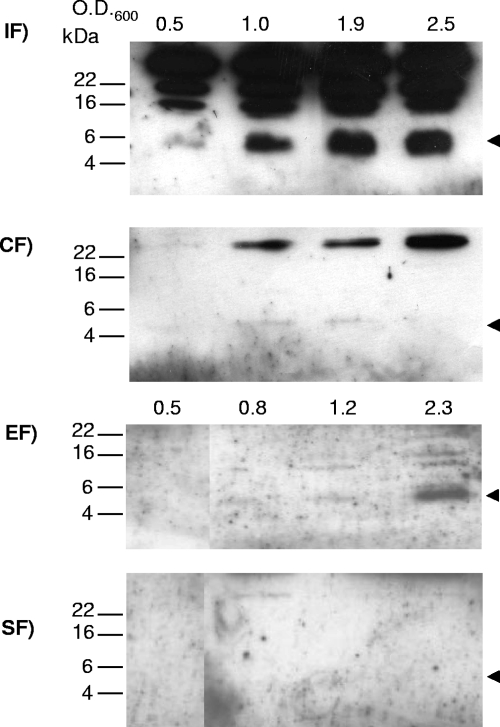
Since the flagellar basal body protein FlhA was assumed to be an important cooperative or interactive partner of FlgM in H. pylori, the next step was to investigate whether the subcellular localization of FlgM in H. pylori flhA mutant bacteria is altered in comparison to the wild type. Therefore, we cultured H. pylori flhA mutants in liquid media to different cell densities and crudely fractionated them, as with the wild-type bacteria above. We then analyzed these four fractions using Western blotting and compared the fractions to those of the wild-type bacteria.
In several experiments, less overall FlgM was detected in flhA mutants after separation of the soluble and insoluble fractions than in the crude lysate before fractionation of the same flhA mutants (data not shown). This suggested that FlhA might be involved directly or indirectly in stabilizing FlgM. In contrast to the wild-type strain, where FlgM was predominantly detected in the soluble fraction, we recovered the highest amount of FlgM within the insoluble fraction of the flhA mutant (Fig. 3). Very little FlgM was detected at all time points in the soluble fraction. Thus, apparently, the soluble and cytoplasmic localization of FlgM was decreased in the absence of FlhA. Some FlgM was also recovered in the secreted supernatant of flhA mutants in later phases of growth. No FlgM was detected in the sheared-off fraction. This was consistent with expectations, since the H. pylori flhA mutants have been reported to lack flagellar formation and also the formation of flagellar sheath structures (39).
FIG. 3.
Localization of native FlgM in fractions of H. pylori flhA mutant bacteria. Bacteria (HP88-3887 flhA) grown in liquid culture were harvested in different growth phases (OD600 of 0.5 to 2.5) and fractionated. Western blots after SDS-PAGE (16% acrylamide) were incubated with anti-FlgM antiserum (1:200), and secondary antibodies were goat anti-rabbit, peroxidase coupled (1:10,000). Ten-microliter aliquots of the respective fractions were loaded in each lane. The molecular mass of FlgM is designated by the arrows. IF, insoluble fraction; CF, soluble fraction; EF, culture supernatant (external fraction); SF, sheared fraction (surface associated, containing flagella).
Expression of tagged FlgM fusions and predominantly polar localization of FlgM-V5 in H. pylori.
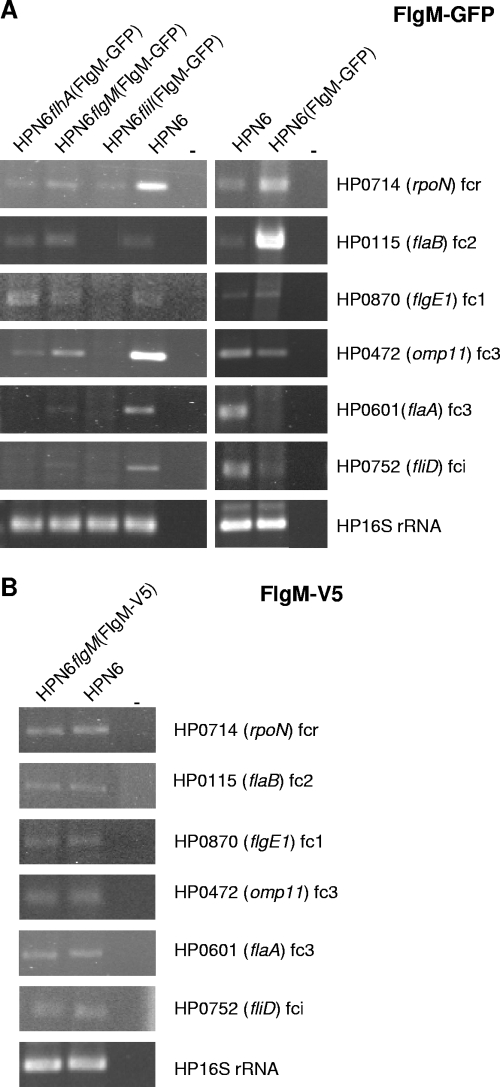
Subsequently, in order to investigate the localization of FlgM in situ with alternative methods, two different expression plasmids were constructed which produce tagged fusion proteins of FlgM at its C-terminal end (FlgM-GFP and FlgM-V5). These shuttle plasmids coding for FlgM fusions were transformed into H. pylori (strain N6) flgM mutants for the FlgM-GFP plasmid and also into wild-type N6, N6 flhA, and fliI mutants (Fig. 4). Owing to gene copy effects, genes encoded by the pHel2 derivative plasmids are approximately fivefold overexpressed in H. pylori (data not shown).
FIG. 4.
Flagellum-related transcripts in H. pylori transformants expressing tagged FlgM. Shown is the detection of selected flagellar transcripts in H. pylori (HP) N6(FlgM-GFP), HPN6flhA(FlgM-GFP), HPN6flgM(FlgM-GFP), HPN6fliI(FlgM-GFP), and HPN6flgM(FlgM-V5) transformants in comparison to HPN6 wild type. (A) FlgM-GFP-expressing strains; (B) FlgM-V5-expressing flgM(FlgM-V5). flhA and fliI flagellar basal body protein mutants with defined defects in the flagellar assembly process (described in Materials and Methods) transformed with FlgM-GFP-expressing plasmid were used as controls for transcript-level comparison with transformed flgM and wild-type bacteria. Relative transcript amounts of selected regulatory and flagellar genes of different hierarchical classes were determined using semiquantitative PCR on equivalent amounts of cDNA (RNA preparation performed from liquid cultures, all bacteria harvested at an equivalent OD600 of approximately 1.6). fcr, flagellar regulatory class; fc1, flagellar class 1; fc2, flagellar class 2; fc3, flagellar class 3; fci, flagellar intermediate class (see reference 33). In flgM mutants which are not depicted here, transcripts of class 3 genes HP0472 and flaA are highly upregulated (24). The respective gene names are shown on the right side. −, negative control. H. pylori 16S rRNA-specific primers were used to control for equivalent amounts of cDNA.
The tagged versions of FlgM were expressed in H. pylori N6flgM(FlgM-GFP) and in the other FlgM-GFP transformants (data not shown) and in N6flgM(FlgM-V5) (Fig. 5A). Both fusion proteins were functional as inhibitory anti-sigma factors, since they were able to diminish the transcript abundance for most FliA-dependent class III genes (flaA; HP0472) and one intermediate class flagellar gene (fliD) tested in the transformed strains to at least the level of wild type for FlgM-V5 (Fig. 4B) and to even lower levels for FlgM-GFP (Fig. 4A). This was in contrast to flgM mutants, which showed higher transcript levels than the wild type for class III and some intermediate genes, as previously reported (24). FlgM-GFP expression enhanced class II gene transcripts in comparison to wild type, which was not observed for FlgM-V5 (Fig. 4A). Bacteria expressing FlgM-GFP showed increased repression abilities for class III genes in comparison to FlgM-V5, suggesting that the anti-sigma factor effect of the GFP fusion is stronger than that of wild-type FlgM and FlgM-V5 (Fig. 4) or that repression by FlgM-GFP does not become appropriately relieved.
FIG. 5.
Localization of FlgM-V5 in subcellular fractions of H. pylori and in intact bacteria. (A) FlgM-V5 was detected in fractions of H. pylori N6flgM(pCJ607) using anti-V5 monoclonal antibody (1:3,000; Invitrogen; secondary antibody was goat anti-mouse peroxidase coupled, at 1:15,000). Bacteria were cultured on plates for 2 days. SF, sheared-off surface-localized fraction; IF, insoluble fraction; CF, soluble fraction. (B) FlgM was localized by immunofluorescence on fixed, permeabilized intact H. pylori N6flgM(pCJ607) bacteria expressing FlgM-V5, using anti-V5 antibody (1:500; secondary antibody was goat anti-mouse Alexa 488 at 1:5,000). (C) Fluorescence micrograph after control labeling of intact, nontransformed H. pylori N6 wild type using the same antibodies.
GFP is a 27-kDa protein tag and, to a large extent, gets folded into its barrel-like three-dimensional structure in the bacterial cytoplasm in the presence of oxygen (49). Therefore, the secretion of proteins through the narrow channel of the flagellar secretion system is largely abolished by GFP tagging (19) (unpublished data). V5 is a short epitope tag (14 amino acids) which can be used as a specific tag for fluorescence immunodetection and is not predicted to hamper type III secretion. The morphology of the transformants expressing FlgM-GFP was characterized by the lack of flagella, whereas the FlgM-V5-expressing bacteria possessed flagella (data not shown). These results suggested that FlgM-V5 permits the chronology of events that lead to flagellar assembly to take place, whereas FlgM-GFP does not.
Fractionation of bacteria expressing FlgM-GFP and FlgM-V5 was performed. Neither tagged FlgM version was secreted into the culture supernatants (data not shown), as shown for wild-type FlgM. Western blot analysis revealed that FlgM-V5 was localized both in the soluble fraction and in the insoluble fraction (Fig. 5A), while FlgM-GFP localized predominantly to the insoluble fraction (data not shown). Some FlgM-V5 was also detected in the sheared off-fraction (Fig. 5A), similar to the nontagged native FlgM (Fig. 1). Furthermore, fluorescent immunolabeling was performed for both fusion constructs in order to localize the tagged FlgM versions in intact bacteria. In fluorescence microscopy of live and fixed bacteria, GFP fluorescence of the FlgM-GFP fusion was quite low and did not allow protein localization in microscopy (22). We therefore used very specific anti-GFP and anti-V5 antibodies for immunofluorescent labeling of both fusion proteins in intact bacteria (see Materials and Methods). Control strains not expressing the fusion proteins were negative in fluorescence microscopy, whereas a clear signal could be observed with both FlgM fusions. As the regulatory and morphological phenotype of the transformed FlgM-V5 bacteria resembled wild-type bacteria, we continued the immunolocalization approach using only the FlgM-V5 fusion. For FlgM-GFP, severe changes in morphology and regulatory phenotype indicated clogging of the flagellar secretion channel and possibly other side effects, for which reason we did not continue localization studies with this strain. The FlgM-V5-specific signal in immunofluorescence microscopy was predominantly polar (Fig. 5B). Despite repeated attempts, it was not possible to obtain transformants with the FlgM-V5 plasmid in a flhA mutant. Therefore, FlgM-V5 localization in situ in this mutant could not be determined.
Interaction between H. pylori FlgM and FlhAC.
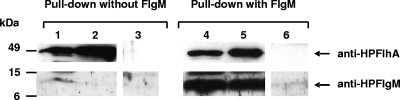
In order to gain some insight into the molecular basis of the function and subcellular localization of H. pylori FlgM in cooperation with FlhA as hypothesized earlier, we investigated the potential for interaction between FlgM and FlhA. For this purpose, the soluble C-terminal domain of H. pylori FlhA, which is supposed reach into the bacterial cytoplasm (37, 47), was overexpressed as a His6-tagged fusion protein in E. coli and then purified as previously described (39). H. pylori FlgM was also expressed and purified from E. coli (see Materials and Methods). Using both H. pylori FlgM and FlhAC as highly purified proteins after overexpression in E. coli, we observed a nonreproducible interaction of the two proteins in coprecipitation experiments (data not shown). Surface plasmon resonance analysis, using the same purified proteins in standard buffers, posed persistent technical problems and yielded no clear interaction profile. In a second biosensor setup using a Biolayer Interferometry platform, immobilized purified FlgM was screened against bacterial lysate containing H. pylori FlhA, but this method also failed to detect binding (see the supplemental material). Neither did we observe an interaction between purified denatured FlhAC and FlgM in overlay blot assays (data not shown). Thus, we hypothesized that the folding of one or both interaction partners might be hampered during or after the purification process, which requires alternative strategies. A subsequent approach simulated more-physiological conditions and included the possibility that FlgM and FlhA might cooperate indirectly, aided by other proteins. This approach included only one purified partner, nontagged FlgM, which was combined in solution with whole-cell lysate of His6-FlhAC-expressing E. coli. This combination finally yielded a reproducible interaction between FlgM and FlhAC in pull-down assays (Fig. 6). Hence, FlgM was suggested to be able to interact with FlhAC, either directly or indirectly.
FIG. 6.
Recombinantly expressed H. pylori FlhAC pulls down purified H. pylori FlgM. Results of a Western immunoblot analysis after pull down of overexpressed H. pylori His6-FlhAC contained in E. coli lysate, which was mixed with purified H. pylori FlgM are shown. Western blots were immunolabeled using anti-HPFlhA (1:2,000) and anti-HPFlgM (1:300) polyclonal antisera. Various samples of the coprecipitation procedure are depicted: lanes 1, 2, and 3 are from a control experiment containing FlhAC lysate only (“without FlgM”); lanes 4, 5, and 6 are from one representative pull-down experiment (out of three similar experiments) combining both FlhAC-containing lysate and purified FlgM (“with FlgM”). Lanes 1 and 4, starting material of FlhAC-containing lysate, either combined (lane 4) or not (control; lane 1) with purified FlgM; lanes 2 and 5, the eluted material from TALON resin after pull down. Lanes 3 and 6, the last wash steps of the TALON resin prior to elution.
As a further strategy to support our interaction results, we used a bacterial two-hybrid system (BACTH) in order to assess the interaction potential of those two molecules in a native physiological environment inside the bacterial cell. Clones expressing C-terminal fusion proteins between FlhAC (pCJ1001) or FlgM (pCJ1004) and the T25 or T18 domains of Bordetella pertussis adenylate cyclase and appropriate positive and negative controls (see Materials and Methods; see also Fig. S3 in the supplemental material) were cotransformed into the BACTH expression strain E. coli BTH101. Only bacteria cotransformed with both FlgM and FlhAC plasmids and the positive control consistently gave rise to blue colonies on IPTG-X-Gal-containing plates (see Fig. S3 in the supplemental material). We could detect expression of both fusion proteins in Western blot assays by using anti-HPFlhA and anti-HPFlgM antisera (data not shown). In β-galactosidase assays, negative controls and single FlgM-T18 transformants (in combination with empty pKNT25) did not produce activities above background. The doubly flhAC-t25- and flgM-t18-transformed bacteria consistently yielded values of at least 7% of the β-galactosidase activities obtained from a positive control (Table 3). Taken together, these findings indicate that H. pylori FlgM and the cytoplasmic domain of the flagellar basal body protein FlhA (FlhAC) are able to interact with each other.
TABLE 3.
β-Galactosidase assays of transformants for BACTH system suggest interaction of FlgM and FlhACa
| Sample (doubly transformed E. coli BH101) | β-Galactosidase activity (% of control) ina:
|
|||||
|---|---|---|---|---|---|---|
| Expt 1
|
Expt 2
|
Expt 3
|
||||
| MU | % | MU | % | MU | % | |
| pUT18/pKNT25 (negative control) | ND | ND | 0 | 0 | 0 | 0 |
| pKNT25/pCJ1004 (FlgM-only control) | −20 | 0 | −1 | 0 | ND | ND |
| pKT25-Zip/pUT18C-Zip (I) (positive control) | 7,622 | 100 | 4,147 | 100 | 1,188 | 100 |
| pKT25-Zip/pUT18C-Zip(II) | 7,178 | 94.2 | 3,881 | 93.6 | ND | ND |
| pCJ1001/pCJ1004 (I) (FlhA+ FlgM) | 508 | 6.7 | 293 | 7.1 | 547 | 46 |
| pCJ1001/pCJ1004 (II) (FlhA + FlgM) | 324 | 4.3 | 172 | 4.1 | 329 | 27.6 |
| pUT18/pCJ1001 (FlhA-only control) | ND | ND | ND | ND | 48 | 4 |
Activities in Miller units (MU) and percentage of positive control for BACTH cotransformants in E. coli BTH101 from three separate induction experiments are depicted. One or two separate cultures of each transformation (induced from mid-log growth with 0.2 mM IPTG, 37°C for 3 h) were tested for each experiment in the β-galactosidase activity assay. All presented values are mean values of duplicate measurements. ND, not determined.
DISCUSSION
Although only a few eubacterial species possess flagellar sheaths (e.g., Vibrio cholerae, Bdellovibrio bacteriovorus, Roseburia cecicola), most Helicobacter species including H. pylori do possess a flagellar sheath. This probably serves to protect the flagellar apparatus and filament from inactivation in the harsh environment within the intestinal mucus of various animals, which is rich in proteolytic activity and mucosal antibodies and has a low pH. Possession of a flagellar sheath is likely to hamper FlgM secretion, which was one initial motivation to study FlgM secretion and function in H. pylori.
The present investigations revealed that in H. pylori, FlgM is a predominantly soluble cytoplasmic protein. It is released into the flagellar compartment in only low amounts and is not detectable in cell supernatants during exponential growth. This result could be a consequence of the shortened FlgM N terminus, which may have a lower propensity of being secreted. Detection of FlgM in cell supernatants in late exponential phase may be due to bacterial lysis. It cannot be excluded at present that FlgM in H. pylori is released in larger amounts into the periplasm, although several observations suggest this is not the case. First, FlgM was found in the sheared-off flagellar fraction, presumably inside the flagellar channel or collected within the sheath surrounding the filament. Mild osmolytic treatment of bacteria posterior to flagellar shearing did not release FlgM from the bacterial cells and did not reduce the amount of FlgM found in the bacteria-bound soluble fraction. This argues against FlgM accumulating in the periplasm. In Salmonella basal body and flk mutants, but notably not in the wild type, FlgM was present in the periplasm, where it was highly unstable (1). The possibility that FlgM is released into the periplasm in H. pylori wild type and then quickly degraded merits further investigation. Still, the fact that some FlgM could be reproducibly detected in flagellar fractions in wild-type bacteria indicates that FlgM can stay stable upon its release. In a control experiment where H. pylori supernatants were coincubated with purified H. pylori FlgM, FlgM remained stable at room temperature for more than 2 h (M. Rust and C. Josenhans, unpublished data). In contrast to our results in H. pylori, FlgM in Vibrio cholerae was reported to be secreted into the medium, although the bacteria possess sheathed flagella (8). For that study, it was not discussed how FlgM secretion outside of the sheath might take place.
Summarizing from our present data, FlgM is a predominantly cell-bound protein in H. pylori and very likely also in other species of the Campylobacterales, which possess a short FlgM. Our data imply that the H. pylori FlgM may exert its regulatory functions predominantly without being secreted. This is in contrast to Salmonella flagella, where the function of FlgM is tightly connected to its secretion into the environment through the flagellar type III secretion system (20). Cytoplasmic localization of the anti-sigma factor FlgM in H. pylori can be partially explained by its interaction with the sigma factor FliA, when FliA is in its inhibited state, before late flagellar gene transcription is initiated.
However, as soon as FliA is released and becomes functional, which has to occur at later stages of flagellar assembly, a different mechanistic explanation is required for the predominantly cytoplasmic localization of FlgM in H. pylori. We hypothesized that the recycling or shuttling of FlgM between an active, FliA-bound state and an inactive noninhibitory state in H. pylori is mediated by a mechanism which differs from the loss of activity by abundant secretion. Several possibilities can be envisaged, including FlgM being degraded by proteolysis or interactions with different proteins when FlgM is not bound to FliA. Using assays to test for FlgM stability in lysed or native bacteria when protein synthesis was inhibited, we did not find any evidence for FlgM being rapidly degraded (Rust and Josenhans, unpublished). For this reason, we focused on the possibility that FlgM interacts with cytoplasmic proteins other than FliA for being functionally inactivated, and one putative candidate was FlhAC. Previously, data on flagellar regulation in H. pylori wild type and various flagellar mutants (33) suggested that FlgM may act as an intergenic suppressor of the flagellar basal body protein gene flhA and exerts its function in close cooperation with FlhA. In the present study, the intrabacterial localization of FlgM was altered in the absence of FlhA, providing further evidence for a close cooperation between these proteins. Furthermore, we gathered evidence that FlgM interacts directly or indirectly with FlhAC.
We therefore propose a model for H. pylori and closely related species in which the anti-sigma factor FlgM is predominantly retained in the cytoplasm and can be shuttled between an active FliA-bound state and an inactive state. We propose that FlgM may be bound to a different protein complex, after being transferred from FliA to the flagellar basal body. One binding partner is suggested to be the flagellar basal protein FlhA, specifically, FlhAC. Interestingly, axial export substrates of different substrate classes, including the late flagellar export substrate flagellin (FliC), interacted directly with denatured Salmonella FlhAC in a blot overlay system (31). So far, these interactions have not been confirmed in a native system. In blot overlays, we were not able to detect an interaction using overexpressed H. pylori FlhAC and purified FlgM. Optical biosensor assays, using one purified interaction partner randomly fixed on a solid support, did not reveal interaction either, further hinting at specific structural requirements for the interaction. In particular the ability of FlgM to interact may be impeded when FlgM is immobilized, since FlgM has a dynamic or disordered structure in solution which is required for its function (9, 10). Three-dimensional structural modeling of H. pylori FlhAC (data not shown) and the substantial overall secondary structure and amino acid similarity between H. pylori FliA and FlhAC (11% identity, 30% similarity) (see Fig. S2 in the supplemental material) suggest that FlgM could bind to α-helically folded FlhAC in a similar conformational arrangement as FliA (34, 42). This mode of binding would require FlhAC to be in a native conformation.
FlgM in Salmonella is released from FliA (acting as its chaperone) with the help of the flagellar ATPase FliI (32, 36), a process which would occur concomitantly with FlgM being transferred to the flagellar basal body complex containing FlhAC. While bound to FlhAC, H. pylori FlgM might be stabilized and retained in the bacterial cytoplasm, which could enable a recycling and renewed binding to FliA at an appropriate later time point. Direct (31) and indirect (32, 36) binding of axial flagellar export substrates other than FlgM to FlhA has been reported previously. It is unclear at this point whether the nature of FlgM binding to FlhAC in H. pylori is similar to the binding of other flagellar export substrates to FlhAC, which has been demonstrated using partially denatured proteins (31). Further investigations will also have to elucidate how the transfer of FlgM from FliA to the FlhA protein complex is occurring and if other binding partners are involved. It also remains to be determined whether the cytoplasmic shuttling process postulated here is sufficient for full FlgM function or whether FlgM localization within the flagellar compartment is required.
Supplementary Material
Acknowledgments
We thank Shin Ichi Aizawa and Ikuro Kawagishi for helpful comments. We are very grateful to Daniela Fischer, Verena Ryan, and Daniela Goeppel for expert technical assistance.
Work in the laboratory of C.J. was funded by the German Research Council, grant JO344/2-2, and the Pathogenomics/ERAnet HELDIVNet research networks by the German Ministry of Education and Research. J.L.M. acknowledges support by PHS grant GM080701 from the National Institutes of Health and Cottrell College Science Award CC6900 from the Research Corporation. The K.T.H. laboratory was supported by PHS grant GM056141 from the NIH.
Footnotes
Published ahead of print on 22 May 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aldridge, P., J. E. Karlinsey, E. Becker, F. F. Chevance, and K. T. Hughes. 2006. Flk prevents premature secretion of the anti-sigma factor FlgM into the periplasm. Mol. Microbiol. 60630-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldridge, P. D., J. E. Karlinsey, C. Aldridge, C. Birchall, D. Thompson, J. Yagasaki, and K. T. Hughes. 2006. The flagellar-specific transcription factor, sigma28, is the type III secretion chaperone for the flagellar-specific anti-σ28 factor FlgM. Genes Dev. 202315-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardy, S. L., S. Y. Ng, and K. F. Jarrell. 2003. Prokaryotic motility structures. Microbiology 149295-304. [DOI] [PubMed] [Google Scholar]
- 4.Cantwell, B. J., R. R. Draheim, R. B. Weart, C. Nguyen, R. C. Stewart, and M. D. Manson. 2003. CheZ phosphatase localizes to chemoreceptor patches via CheA-short. J. Bacteriol. 1852354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadaban, M., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in E. coli. J. Mol. Biol. 138179-207. [DOI] [PubMed] [Google Scholar]
- 6.Colland, F., J. C. Rain, P. Gounon, A. Labigne, P. Legrain, and H. de Reuse. 2001. Identification of the Helicobacter pylori anti-σ28 factor. Mol. Microbiol. 41477-487. [DOI] [PubMed] [Google Scholar]
- 7.Cormack, B. P., R. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 17333-38. [DOI] [PubMed] [Google Scholar]
- 8.Correa, N. E., J. R. Barker, and K. E. Klose. 2004. The Vibrio cholerae FlgM homologue is an anti-σ28 factor that is secreted through the sheathed polar flagellum. J. Bacteriol. 1864613-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daughdrill, G. W., L. J. Hanely, and F. W. Dahlquist. 1998. The C-terminal half of the anti-sigma factor FlgM contains a dynamic equilibrium solution structure favoring helical conformations. Biochemistry 371076-1082. [DOI] [PubMed] [Google Scholar]
- 10.Dedmon, M. M., C. N. Patel, G. B. Young, and G. J. Pielak. 2002. FlgM gains structure in living cells. Proc. Natl. Acad. Sci. USA 9912681-12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton, K. A., S. Suerbaum, C. Josenhans, and S. Krakowka. 1996. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes Infect. Immun. 642445-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eppinger, M., C. Baar, G. Raddatz, D. H. Huson, and S. C. Schuster. 2004. Comparative analysis of four Campylobacterales. Nat. Rev. Microbiol. 2872-885. [DOI] [PubMed] [Google Scholar]
- 13.Ferrero, R. L., V. Cussac, P. Courcoux, and A. Labigne. 1992. Construction of isogenic urease-negative mutants of Helicobacter pylori by allelic exchange. J. Bacteriol. 1744212-4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geis, G., S. Suerbaum, B. Forsthoff, H. Leying, and W. Opferkuch. 1993. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J. Med. Microbiol. 38371-377. [DOI] [PubMed] [Google Scholar]
- 15.Gillen, K. L., and K. T. Hughes. 1991. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J. Bacteriol. 1732301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas, R., T. F. Meyer, and J. P. van Putten. 1993. Aflagellated mutants of Helicobacter pylori generated by genetic transformation of naturally competent strains using transposon shuttle mutagenesis. Mol. Microbiol. 8753-760. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 18.Heuermann, D., and R. Haas. 1998. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257519-528. [DOI] [PubMed] [Google Scholar]
- 19.Hirano, T., S. Shibata, K. Ohnishi, T. Tani, and S. Aizawa. 2005. N-terminal signal region of FliK is dispensable for length control of the flagellar hook. Mol. Microbiol. 56346-360. [DOI] [PubMed] [Google Scholar]
- 20.Hughes, K. T., K. L. Gillen, M. J. Semon, and J. E. Karlinsey. 1993. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science 2621277-1280. [DOI] [PubMed] [Google Scholar]
- 21.Josenhans, C., K. A. Eaton, T. Thevenot, and S. Suerbaum. 2000. Switching of flagellar motility in Helicobacter pylori by reversible length variation of a short homopolymeric sequence repeat in fliP, a gene encoding a basal body protein. Infect. Immun. 684598-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josenhans, C., S. Friedrich, and S. Suerbaum. 1998. Green fluorescent protein as a novel marker and reporter system in Helicobacter sp. FEMS Microbiol. Lett. 161263-273. [DOI] [PubMed] [Google Scholar]
- 23.Josenhans, C., A. Labigne, and S. Suerbaum. 1995. Comparative ultrastructural and functional studies of Helicobacter pylori and Helicobacter mustelae flagellin mutants: both flagellin subunits, FlaA and FlaB, are necessary for full motility in Helicobacter species. J. Bacteriol. 1773010-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josenhans, C., E. Niehus, S. Amersbach, A. Hörster, C. Betz, B. Drescher, K. T. Hughes, and S. Suerbaum. 2002. Functional characterization of the antagonistic flagellar late regulators FliA and FlgM of Helicobacter pylori and their effects on the H. pylori transcriptome. Mol. Microbiol. 43307-322. [DOI] [PubMed] [Google Scholar]
- 25.Karimova, G., J. Pidoux, A. Ullmann, and D. Ladant. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. USA 955752-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlinsey, J. E., J. Lonner, K. L. Brown, and K. T. Hughes. 2000. Translation/secretion coupling by type III secretion systems. Cell 102487-497. [DOI] [PubMed] [Google Scholar]
- 27.Kavermann, H., B. P. Burns, K. Angermuller, S. Odenbreit, W. Fischer, K. Melchers, and R. Haas. 2003. Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J. Exp. Med. 197813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labigne-Roussel, A., P. Courcoux, and L. Tompkins. 1988. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J. Bacteriol. 1701704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 30.Macnab, R. M. 2004. Type III flagellar protein export and flagellar assembly. Biochim. Biophys. Acta 1694207-217. [DOI] [PubMed] [Google Scholar]
- 31.Minamino, T., and R. M. Macnab. 2000. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol. Microbiol. 351052-1064. [DOI] [PubMed] [Google Scholar]
- 32.Minamino, T., and K. Namba. 2008. Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature 451485-488. [DOI] [PubMed] [Google Scholar]
- 33.Niehus, E., H. Gressmann, F. Ye, R. Schlapbach, M. Dehio, C. Dehio, A. Stack, T. F. Meyer, S. Suerbaum, and C. Josenhans. 2004. Genome-wide analysis of transcriptional hierarchy and feedback regulation in the flagellar system of Helicobacter pylori. Mol. Microbiol. 52947-961. [DOI] [PubMed] [Google Scholar]
- 34.Okada, K., H. Ichihara, H. Takahashi, N. Fujita, A. Ishihama, and T. Hakoshima. 2007. Preparation and preliminary X-ray diffraction analysis of crystals of bacterial flagellar sigma factor sigma 28 in complex with the sigma 28-binding region of its antisigma factor, FlgM. Acta Crystallogr. F 63196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403665-668. [DOI] [PubMed] [Google Scholar]
- 36.Paul, K., M. Erhardt, T. Hirano, D. F. Blair, and K. T. Hughes. 2008. Energy source of flagellar type III secretion. Nature 451489-492. [DOI] [PubMed] [Google Scholar]
- 37.Saijo-Hamano, Y., K. Imada, T. Minamino, M. Kihara, R. M. Macnab, and K. Namba. 2005. Crystallization and preliminary X-ray analysis of the C-terminal cytoplasmic domain of FlhA, a membrane-protein subunit of the bacterial flagellar type III protein-export apparatus. Acta Crystallogr. F 61599-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., and D. G. Russell. 2004. Molecular cloning: a laboratory manual, 4th ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 39.Schmitz, A., C. Josenhans, and S. Suerbaum. 1997. Cloning and characterization of the Helicobacter pylori flbA gene, which codes for a membrane protein involved in coordinated expression of flagellar genes. J. Bacteriol. 179987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreiber, S., M. Konradt, C. Groll, P. Scheid, G. Hanauer, H. O. Werling, C. Josenhans, and S. Suerbaum. 2004. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc. Natl. Acad. Sci. USA 1015024-5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweinitzer, T., T. Mizote, N. Ishikawa, A. Dudnik, S. Inatsu, S. Schreiber, S. Suerbaum, S. Aizawa, and C. Josenhans. 2008. Functional characterization and mutagenesis of the proposed behavioral sensor TlpD of Helicobacter pylori. J. Bacteriol. 1903244-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorenson, M. K., S. S. Ray, and S. A. Darst. 2004. Crystal structure of the flagellar sigma/anti-sigma complex σ28/FlgM reveals an intact sigma factor in an inactive conformation. Mol. Cell 14127-138. [DOI] [PubMed] [Google Scholar]
- 43.Sory, M. P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. USA 9211998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suerbaum, S., C. Josenhans, T. Sterzenbach, B. Drescher, P. Brandt, M. Bell, M. Droege, B. Fartmann, H.-P. Fischer, Z. Ge, A. Hörster, R. Holland, K. Klein, J. König, L. Macko, G. L. Mendz, G. Nyakatura, D. B. Schauer, Z. Shen, J. Weber, M. Frosch, and J. G. Fox. 2003. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc. Natl. Acad. Sci. USA 1007901-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388539-547. [DOI] [PubMed] [Google Scholar]
- 46.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Arnam, J. S., J. L. McMurry, M. Kihara, and R. M. Macnab. 2004. Analysis of an engineered Salmonella flagellar fusion protein, FliR-FlhB. J. Bacteriol. 1862495-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19259-268. [DOI] [PubMed] [Google Scholar]
- 49.Wachter, R. M. 2007. Chromogenic cross-link formation in green fluorescent protein. Acc. Chem. Res. 40120-127. [DOI] [PubMed] [Google Scholar]
- 50.Wang, Y., and D. E. Taylor. 1990. Natural transformation in Campylobacter species. J. Bacteriol. 172949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye, F., T. Brauer, E. Niehus, K. Drlica, C. Josenhans, and S. Suerbaum. 2007. Flagellar and global gene regulation in Helicobacter pylori modulated by changes in DNA supercoiling. Int. J. Med. Microbiol. 29765-81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.