Abstract
The PA5471 gene required for induction of the MexXY multidrug efflux system in response to ribosome-targeting antimicrobials was itself shown to be inducible by ribosome-targeting antimicrobials (Y. Morita, M. L. Sobel, and K. Poole, J. Bacteriol. 188:1847-1855, 2006). Using a lacZ transcriptional reporter, drug inducibility of PA5471 was shown to require the entirety of the 367-bp PA5472-PA5471 intergenic region. A constitutive promoter activity was, however, localized to the first 75 bp of this region, within which a single PA5471 transcription initiation site was mapped. That 3′ sequences of the intergenic region blocked PA5471 expression and made it antibiotic dependent was suggestive of an attenuation mechanism of control. A 13-amino-acid leader peptide (LP)-encoding open reading frame preceded by a Shine-Dalgarno sequence was identified ca. 250 bp upstream of the PA5471 coding sequence, and its expression and translation were confirmed using a lacZ translational reporter. Alteration of the initiation codon (M1T) or introduction of translational stop signals at codons 3 (Q3Am) and 8 (C8Op) of this LP sequence (PA5471.1) yielded high-level constitutive expression of PA5471, suggesting that interference with LP translation was linked to PA5471 gene expression. Consistent with this, a Q3K mutation in the LP sequence maintained the drug inducibility of PA5471 expression. Introduction of the LP Q3Am mutation into the chromosome of Pseudomonas aeruginosa yielded stronger expression of PA5471 than did antibiotic (chloramphenicol) exposure of wild-type P. aeruginosa, in agreement with lacZ transcriptional fusion data. Still, the Q3Am mutation yielded modest expression of mexXY, less than that seen for antibiotic-treated wild-type P. aeruginosa. These data suggest that PA5471 is not sufficient for MexXY recruitment in response to antibiotic exposure and that additional antibiotic-dependent effects are needed.
Multidrug efflux systems of the three-component resistance-nodulation-division (RND) family contribute significantly to intrinsic and acquired resistance to antimicrobials in a number of gram-negative bacteria (43, 44). Despite their significance as determinants of antibiotic resistance, however, RND-type multidrug exporters also, in many instances, accommodate biocides (41, 44), organic solvents (48), detergents (43) including bile salts (11, 27, 46, 58), toxic fatty acids/lipids (17), and in some instances plant-derived antimicrobials (phytoalexins and isoflavanoids) (7, 8, 38), metabolic inhibitors (53), quorum-sensing effector molecules (10, 14, 24) and, possibly, virulence factors (18, 22), in addition to antibiotics. Clearly, RND pumps can and do function as other than antibiotic exporters.
Pseudomonas aeruginosa expresses several RND-type multidrug efflux systems, of which four, MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY-OprM, are reported to be significant determinants of multidrug resistance in lab and clinical isolates (40, 42). A clear indication, however, that antimicrobial export may not be the intended function of many of these comes from the observation that, while these pumps accommodate many of the same antimicrobials, each appears to be independently regulated by linked regulatory genes (42) but not (with the exception of MexXY [36]) in response to antibiotics.
MexXY-OprM is somewhat unique in P. aeruginosa in that the mexXY operon is induced upon exposure to many of the antibiotics that this efflux system exports (31). Still, only those agents known to target the ribosome promote mexXY expression (21, 31), and this is compromised by so-called ribosome protection mechanisms (21), suggesting that the MexXY efflux system is recruited in response to ribosome disruption and not antibiotics per se. Possibly, antibiotic-induced ribosome perturbation stimulates MexXY-OprM production in order to counter or alleviate some stress or adverse effect resultant from such perturbation. Transcriptomic and proteomic studies certainly confirm that agents that interfere with prokaryotic translation impact expression of a myriad of genes (1, 3, 15, 28, 37, 47, 49, 54) including, in some instances, genes associated with stress responses (28, 37, 47, 49). Recently, a gene, PA5471, encoding a conserved hypothetical protein has been shown to be induced by the same ribosome-disrupting antimicrobials as mexXY and to be required for antibiotic-inducible mexXY expression (36). Indeed, PA5471 expression in the absence of antibiotic exposure was able to promote mexXY expression, arguing that antibiotic induction of mexXY was mediated by PA5471 (36). To gain some understanding of the antibiotic inducibility of PA5471 and, ultimately, mexXY and to provide a link to ribosome disruption, we investigated possible mechanisms of antibiotic-inducible PA5471 expression. We report here the identification of a leader peptide (LP)-encoding sequence (PA5471.1) upstream of PA5471 whose translation provides a sensor of ribosome function and, so, is able to mediate PA5471 expression in response to ribosome-disrupting antimicrobials.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacterial cells were cultured in or on Luria broth and Luria agar (8) with antibiotics as necessary at 37°C. Plasmids pEX18Tc, mini-CTX-lacZ, and their derivatives were maintained or selected in Escherichia coli with 2.5 to 10 μg/ml of tetracycline. Plasmid pSPORT1 and its derivatives were maintained or selected in E. coli with 100 μg/ml of ampicillin. Plasmid pRK415 and its derivatives were maintained or selected with 10 (in E. coli) or 50 (in P. aeruginosa) μg/ml of tetracycline. Plasmid pUCP19 and its derivatives were maintained or selected using 100 μg/ml ampicillin (in E. coli) and 400 (on plates) or 2,000 (in broth culture) μg/ml carbenicillin (in P. aeruginosa). Plasmid pPZ30 and its derivatives were maintained or selected in E. coli with 100 μg/ml of ampicillin and in P. aeruginosa with 200 μg/ml of carbenicillin. Plasmid pFLP2 was maintained or selected using 100 μg/ml ampicillin (in E. coli) and 200 μg/ml carbenicillin (in P. aeruginosa). Plasmid pCR-Blunt II TOPO was maintained or selected in E. coli using 50 μg/ml kanamycin. Phagemid pBC KS(+) was maintained or selected in E. coli using 30 μg/ml chloramphenicol.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH5α | φ80ΔlacZΔM15 endA1 recA1 hsdR17(rK− mK+) supE44 thi-1 gyrA96 relA1 F− Δ(lacZYA-argF)U169 | 2 |
| S17-1 | thi pro hsdR recA Tra+ | 55 |
| P. aeruginosa strains | ||
| K767 | PAO1 prototroph | 30 |
| K2798 | K767 attB::promoterless lacZ | This study |
| K2790 | K767 attB::PA5471(1-367)-lacZb | This study |
| K2808 | K767 attB::PA5471(1-63)-lacZ | This study |
| K2809 | K767 attB::PA5471(1-75)-lacZ | This study |
| K2792 | K767 attB::PA5471(1-113)-lacZ | This study |
| K2794 | K767 attB::PA5471(1-252)-lacZ | This study |
| K2795 | K767 attB::PA5471(1-299)-lacZ | This study |
| K2796 | K767 attB::PA5471(21-367)-lacZ | This study |
| K2799 | K767 attB::PA5471(1-367; PA5471.1M1T)-lacZc | This study |
| K2800 | K767 attB::PA5471(1-367; PA5471.1Q3Am)-lacZ | This study |
| K2801 | K767 attB::PA5471(1-367; PA5471.1Q3K)-lacZ | This study |
| K2802 | K767 attB::PA5471(1-367; PA5471.1C8Op)-lacZ | This study |
| K2806 | K767 attB::PA5471(1-252; PA5471.1Q3Am)-lacZ | This study |
| K2811 | K767 attB::PA5471(1-155)-lacZ | This study |
| K2812 | K767 attB::PA5471(1-155; PA5471.1Q3Am)-lacZ | This study |
| K2813 | K767 attB::PA5471(90-367)-lacZ | This study |
| K2814 | K767 attB::PA5471(90-367; PA5471.1Q3Am)-lacZ | This study |
| K2817 | K767 PA5471.1Q3Amc | This study |
| K2818 | K767 PA5471.1Q3Kc | This study |
| Plasmids | ||
| pPZ30 | E. coli-P. aeruginosa shuttle vector; used to construct translational lacZ fusions; Apr/Cbr | 52 |
| pYM080 | pPZ30::PA5471.1WT-lacZd | This study |
| pYM082 | pPZ30::PA5471.1M1T-lacZ | This study |
| pYM083 | pPZ30::PA5471.1Q3Am-lacZ | This study |
| pYM084 | pPZ30::PA5471.1C8Op-lacZ | This study |
| pYM081 | pPZ30::PA5471.1TAA-lacZe | This study |
| pSport1 | Cloning vector; plac MCS lacI+ Apr | Invitrogen |
| pYM039 | pSportI::PA5471(1-367)f | This study |
| pYM040 | pSportI::PA5471(1-367; PA5471.1M1T) | This study |
| pYM041 | pSportI::PA5471(1-367; PA5471.1Q3Am) | This study |
| pYM042 | pSportI::PA5471(1-367; PA5471.1Q3K) | This study |
| pYM043 | pSportI::PA5471(1-367; PA5471.1C8Op) | This study |
| mini-CTX-lacZ | Integration vector with promoterless lacZ; oriT+ Tcr | 5 |
| pYM052 | mini-CTX::PA5471(1-367)-lacZg | This study |
| pYM054 | mini-CTX::PA5471(1-63)-lacZ | This study |
| pYM074 | mini-CTX::PA5471(1-75)-lacZ | This study |
| pYM055 | mini-CTX::PA5471(1-113)-lacZ | This study |
| pYM058 | mini-CTX::PA5471(1-252)-lacZ | This study |
| pYM059 | mini-CTX::PA5471(1-299)-lacZ | This study |
| pYM060 | mini-CTX::PA5471(21-367)-lacZ | This study |
| pYM064 | mini-CTX::PA5471(1-367; PA5471.1M1T)-lacZ | This study |
| pYM065 | mini-CTX::PA5471(1-367; PA5471.1Q3Am)-lacZ | This study |
| pYM066 | mini-CTX::PA5471(1-367; PA5471.1Q3K)-lacZ | This study |
| pYM067 | mini-CTX::PA5471(1-367; PA5471.1C8Op)-lacZ | This study |
| pYM071 | mini-CTX::PA5471(1-252; PA5471.1Q3Am)-lacZ | This study |
| pYM076 | mini-CTX::PA5471(1-155)-lacZ | This study |
| pYM077 | mini-CTX::PA5471(1-155; PA5471.1Q3Am)-lacZ | This study |
| pYM078 | mini-CTX::PA5471(90-367)-lacZ | This study |
| pYM079 | mini-CTX::PA5471(90-367; PA5471.1Q3Am)-lacZ | This study |
| pBC KS(+) | Phagemid cloning vector; Cmr | Stratagene |
| pCR-Blunt II TOPO | PCR cloning vector; Kmr | Invitrogen |
| pFLP2 | Source of Flp recombinase; Apr/Cbr | 5 |
| pEX18Tc | Gene replacement vector; sacB Tcr | 19 |
| pYM086 | pEX18Tc::PA5471.1Q3Amh | This study |
| pYM087 | pEX18Tc::PA5471.1Q3K | This study |
| pRK415 | P. aeruginosa-E. coli shuttle cloning vector; Tcr | 23 |
| pCG005 | pRK415::PA5471.1 | This study |
| pUCP19 | P. aeruginosa-E. coli shuttle cloning vector; Apr/Cbr | 51 |
| pCG006 | pUCP19::PA5471.1 | This study |
Apr, ampicillin resistance; Cbr, carbenicillin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance; MCS, multiple cloning site.
Chromosomal insertions (at the phage D113 attB site) of transcriptional PA5471-lacZ fusions carrying different portions of the PA5471 upstream region fused to lacZ. Portions of the PA5471 upstream sequence included in each fusion are indicated in parentheses, where 1 is the first base pair after the PA5472 stop codon and 367 is the last base pair prior to the PA5471 start codon. Mutations in the LP-encoding PA5471.1 sequence, when present, are also indicated. Am, amber nonsense mutation; Op, opal nonsense mutation.
Derivatives of P. aeruginosa K767 carrying the indicated mutations in PA5471.1.
In-frame translational fusion of PA5471.1 to lacZ on plasmid pPZ30. The sequence inserted into pPZ30 begins with bp 1 of the PA5471 upstream sequence and ends after the final amino acid-encoding codon of PA5471.1. Mutations present within PA5471.1 are indicated in subscript. WT, wild-type PA5471.1.
Fusion of PA5471.1 to lacZ on plasmid pPZ30. The sequence inserted into pPZ30 begins with bp 1 of the PA5471 upstream sequence and ends after the TAA termination codon of PA5471.1.
Plasmid pSportI derivative carrying the entire PA5471 upstream sequence (i.e., from the end of PA5472 to the beginning of PA5471) with PA5471.1 mutations as indicated.
Plasmid mini-CTX derivative carrying transcriptional PA5471-lacZ fusions with different portions of the PA5471 upstream region fused to lacZ. Portions of the PA5471 upstream sequence included in each fusion are indicated in parentheses, where 1 is the first base pair after the PA5472 stop codon and 367 is the last base pair prior to the PA5471 start codon. Mutations in PA5471.1, when present, are also indicated.
Plasmid pEX18Tc derivatives carrying the PA5471 upstream region with the indicated PA5471.1 mutations.
DNA manipulation.
Standard protocols were used for restriction endonuclease digestions, ligations, transformation, plasmid isolation, and agarose gel electrophoresis, as described by Sambrook and Russell (50). Plasmid DNA was also prepared from E. coli or P. aeruginosa using a QIAprep Spin miniprep kit or QIAfilter plasmid midikit (Qiagen Inc., Mississauga, Ontario, Canada) according to a protocol provided by the manufacturer. Genomic DNA of P. aeruginosa was extracted following the protocol of Barcak et al. (4). DNA fragments or PCR products used for cloning were extracted from agarose gels using a QIAquick gel extraction kit (Qiagen) and purified using the Wizard SV gel and PCR clean-up system (Promega Corp., Madison, WI). Competent P. aeruginosa (12) and E. coli (50) cells were prepared as described elsewhere.
PA5471.1-LacZ translational fusions.
The PA5471 upstream region beginning 15 bp into PA5472 and extending to the 13th and last amino acid-encoding codon of the LP sequence (i.e., PA5471.1) was amplified using PCR and cloned initially into mini-CTX-lacZ. This region was subsequently excised using EcoRI and BamHI for cloning into pPZ30 to yield pYM080, in which the 13-amino-acid PA5471.1-encoded LP is fused in frame to LacZ. A sequence extending to immediately after the PA5471.1 TAA stop codon was similarly amplified and cloned into pPZ30. Translational PA5471.1-lacZ fusions with M1T-, Q3Am-, and C8Op-containing PA5471.1 sequences were generated via PCR amplification of mutation-containing PA5471.1 sequences off of their respective pSportI derivatives (see below), purification of PCR products using the Wizard SV gel and PCR clean-up system (Promega), and cloning first into pBC KS(+) (Stratagene) for sequencing and finally into pPZ30. All amplifications were carried out with Vent DNA polymerase (New England Biolabs) using primer pairs 5471-F and LP-R with the exception of the TAA stop codon fusion (where primers 5471-F and LPTAA-R were used) (see the supplemental material for primer sequences). PCR mixtures were formulated as described previously for amplification of mexZ (36) and were heated to 94°C for 3 min prior to 40 cycles of 94°C for 30 s, 58.8°C for 30 s, and 72°C for 20 s, with a final 5-min incubation at 72°C.
Cloning PA5471.1.
The PA5471.1 open reading frame (ORF) was amplified from P. aeruginosa K2790 chromosomal DNA via PCR using primers 5471.1-F and 5471.1-R (see the supplemental material) in a reaction mixture formulated as described before (9). Amplification was achieved by heating at 94°C for 3 min followed by 30 cycles of 94°C for 45 s, 59.2°C for 45 s, and 72°C for 30 s, followed by 72°C for 10 min. The PA5471.1-carrying PCR product was purified, digested (with HindIII and BamHI), and cloned into pRK415 (to yield pCG005) and pUCP19 (to yield pCG006). Inserts were sequenced to ensure the absence of PCR-generated mutations in the amplified PA5471.1.
Chromosomal PA5471-lacZ transcriptional fusions.
Transcriptional fusions of the PA5471 upstream region and lacZ were engineered in plasmid mini-CTX-lacZ and then introduced into the P. aeruginosa PAO1 strain K767 chromosome at the phage D113 attB site using established protocols (5, 20). The entirety of the PA5472-PA5471 intergenic region was initially recovered on a 414-bp PCR fragment that extended from 15 bp into the upstream PA5472 gene to 32 bp into the PA5471 coding region. Amplification was achieved using strain K767 chromosomal DNA and primers 5471-F and 5471-R in a PCR mixture formulated as above for construction of the LacZ translational fusions. The reaction mixture was subjected to an initial 3-min denaturation step at 94°C, followed by 35 cycles of 30 s at 94°C, 30 s at 70°C, and 20 s at 72°C, before finishing with a 5-min elongation at 72°C. PCR products were subsequently purified, digested with EcoRI and BamHI, and cloned into plasmid pSportI (Invitrogen) to yield pYM039. Nucleotide sequencing confirmed the absence of PCR-generated mutations in the cloned DNA, which was excised with EcoRI and BamHI and cloned upstream of the promoterless lacZ gene of the mini-CTX-lacZ vector to yield pYM052. Restriction digestion of or PCR involving plasmids pYM039 and/or pYM052 was used to generate fragments carrying different regions of the PA5471 upstream sequence that were also fused to lacZ in mini-CTX-lacZ (see the supplemental material). To introduce a Q3Am mutation into the PA5471.1-encoded LP sequence on some of these, PCR was carried out using the Q3Am mutation-containing pSportI vector pYM041 as template and the resultant PCR product was again cloned into mini-CTX-lacZ. Plasmid mini-CTX-lacZ and its derivatives were mobilized into P. aeruginosa from E. coli S17-1 as described before (9) using, however, 6- or 18-hour incubations, and transconjugants carrying chromosomal inserts of mini-CTX-lacZ and its derivatives were recovered on L-agar containing tetracycline (75 μg/ml) and chloramphenicol (5 μg/ml; to counterselect E. coli S17-1). The plasmid backbone was then cured from the chromosome of each transconjugant using the pFLP2-encoded Flp recombinase (19), to leave only the PA5471-lacZ fusions in the chromosome. Plasmid pFLP2 was introduced into P. aeruginosa via electroporation, and pFLP2-containing carbenicillin-resistant colonies were subsequently streaked onto L-agar containing 10% (wt/vol) sucrose to select for the loss of plasmid pFLP2 (following excision of the mini-CTX backbone from the chromosome). Sucrose-resistant colonies were patched on L-agar plates containing tetracycline (25 μg/ml) or carbenicillin (100 μg/ml) to confirm the loss of the mini-CTX backbone (tetracycline sensitive) and the pFLP2 plasmid (carbenicillin sensitive).
Site-directed mutagenesis.
Amino acid substitution (M1T or Q3K) and nonsense (Q3Am or C8Op) mutations were introduced in the LP-encoding PA5471.1 open reading frame of the PA5471 insert in plasmid pYM039 using a protocol provided with the QuickChange site-directed mutagenesis kit (Stratagene) with modifications. A 50-μl mixture consisting of 50 ng of plasmid DNA, 0.3 μM of each mutagenic primer pair (for M1T, LPM1T-F and LPM1T-R; for Q3Am, LPQ3Am-F and LPQ3Am-R; for Q3K, LPQ3K-F and LPQ3K-R; for C8Op, LPC8Op-F and LPC8Op-R [see the supplemental material for primer sequences]), 0.2 mM each deoxynucleoside triphosphate, 1 mM MgSO4, 2.5 U of KOD Hot Start DNA polymerase (EMD Biosciences, Inc., Madison, WI), 1× KOD buffer, and 4.0% (vol/vol) dimethyl sulfoxide was heated to 94°C for 2 min followed by 18 cycles of 0.5 min at 94°C, 1 min at 60°C, and 5 min at 68°C. The resultant DNA products were purified as above, digested overnight with 10 U DpnI (New England Biolabs; to eliminate template plasmid), and used to transform E. coli DH5α. Plasmids were recovered from individual transformants and the PA5471 insert sequenced to identify those bearing the desired mutation. The PA5471 inserts carrying the various PA5471.1 mutations were excised from pSportI using EcoRI and BamHI and cloned into plasmid mini-CTX-lacZ to yield PA5471-lacZ transcriptional fusions in which PA5471.1 had been mutated. The aforementioned approach was also used to introduce the Q3Am mutation into the PA5471.1-encoded LP sequence of a PA54711-252 insert in pSportI (see the supplemental material), which was then cloned into mini-CTX-lacZ. A PA547190-367-lacZ fusion-containing mini-CTX derivative carrying a Q3Am mutation in the leader peptide region, pYM079, was constructed exactly as described for construction of the mutation-free PA547190-367-lacZ fusion (see the supplemental material), except that the Q3Am mutation-containing vector pYM041 was used as template.
Construction of P. aeruginosa PA5471.1 mutants.
To introduce the PA5471.1 Q3Am and Q3K mutations into the chromosome of P. aeruginosa PAO1 strain K767, Q3Am and Q3K mutation-containing PA5471.1 sequences were cloned into the gene replacement vector pEX18Tc for delivery to P. aeruginosa according to an established protocol (9, 36). The Q3 mutation-containing sequences were recovered from the relevant pSportI derivatives (see above) via excision with EcoRI and BamHI, and the resultant pEX18Tc derivatives were mobilized into strain K767 from E. coli S17-1 as described before (9) with P. aeruginosa transconjugants carrying chromosomal inserts of the pEX18Tc derivatives selected on tetracycline (75 μg/ml) and chloramphenicol (5 μg/ml; to counterselect the donor E. coli). Transconjugants were subsequently streaked onto L-agar containing sucrose (10% [wt/vol]), and sucrose-resistant colonies were then screened for the presence of the Q3Am or Q3K mutation following amplification of the PA5471.1-containing PA5471 upstream region using primers 5471-F and 5471-R (conditions and parameters as above), digestion with EcoRI and BamHI, and cloning into pSportI for sequencing.
β-Galactosidase assay.
Overnight cultures of P. aeruginosa strains harboring chromosomal insertions of mini-CTX-lacZ or its derivatives were diluted 1:49 into fresh L-broth with or without chloramphenicol (4 μg/ml) and grown to log phase before being assayed for β-galactosidase activity as described previously (34).
RT-PCR.
Total bacterial RNA was isolated from log-phase P. aeruginosa L-broth cultures with or without chloramphenicol (8 μg/ml) or tetracycline (4 μg/ml) using the Qiagen RNeasy minikit and RNase-free DNase (Qiagen) and a protocol provided by the manufacturer. The reverse transcription (RT)-PCR was performed with ca. 500 ng RNA using the Qiagen One-Step RT-PCR kit according to a protocol provided by the manufacturer. Primers and reaction conditions for amplification of rpoD, PA5471, and mexX have been described previously (36). In some experiments primer pairs targeted to different regions of the P5471 upstream region were employed: RTLP-F and LP-R (encompasses the PA5471.1 sequence), LP-F and PA5471215-R (extends from upstream of PA5471.1 to bp 215 of the PA5472-PA5471 intergenic region), and LP-F and PA5471-R2 (extends from upstream of PA5471.1 into the PA5471 coding sequence) (see the supplemental material for primer sequences). Reaction conditions were again as described previously (36) except that the PCR portion of the reactions involved various numbers of cycles (see figure legends for details) of 0.5 min sequentially at 94°C, 60°C (56.5°C for reactions involving primers pair LP-F and LP-R), and 72°C.
Mapping the PA5471 transcription start site.
The transcription start site for the PA5471 gene was determined using the 5′ rapid amplification of cDNA ends (RACE) protocol and a 5′/3′ RACE kit (Roche Diagnostics, Laval, Quebec, Canada) as described previously (13). Total RNA for RACE was prepared from P. aeruginosa strain K2817 (carries the Q3Am mutation in the PA5471.1-encoded LP sequence) and PA5471-specific primers SP1 (in the initial reverse transcription reaction), SP2 (in the first PCR), and SP3 (in the final PCR) (see the supplemental material for primer sequences). The amplified product was purified using a High Pure PCR product purification kit (Roche Diagnostics) and cloned into the pCR-Blunt II TOPO vector (Invitrogen), and plasmids were recovered from six independent kanamycin-resistant transformants for sequencing.
mRNA folding.
The mRNA corresponding to the PA5471 upstream (i.e., leader) region was folded using the program mfold (32, 60).
RESULTS
Mapping the antibiotic-inducible PA5471 promoter region.
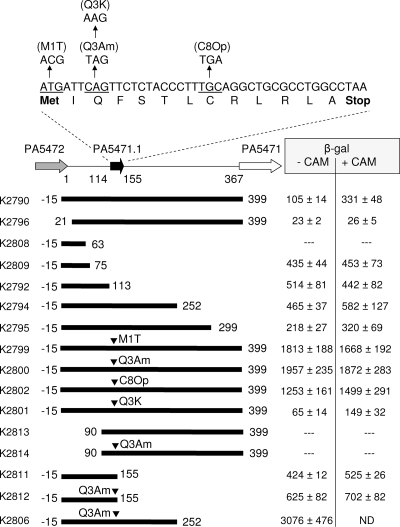
In an attempt to localize the PA5471 promoter and identify sequences needed for antibiotic inducibility of this gene, various portions of the PA5472-PA5471 367-bp intergenic region were fused upstream of the promoterless lacZ gene of plasmid mini-CTX-lacZ and introduced into the chromosome of wild-type P. aeruginosa PAO1 strain K767. As seen in Fig. 1, the entire intergenic region yielded a weak promoter activity that was increased threefold upon exposure to sub-MIC levels of chloramphenicol (4 μg/ml), an antibiotic shown previously to induce PA5471 (and mexXY) expression. Fusions carrying the first 75 to 250 bp of the intergenic region (strains K2809, K2792, and K2794) produced a constitutive promoter activity that was not affected by antibiotic exposure (Fig. 1), indicating that the 3′ end of the intergenic region carried sequences that dampened PA5471 expression and made it antibiotic dependent. Fusions that carried less than the first 75 bp of the intergenic region (i.e., bp 1 to 63 of K2808 [Fig. 1]) or lacked the first 20 bp of this region (K2796 [Fig. 1]) yielded little or no activity, indicating that the PA5471 promoter resided within the first 75 bp of the intergenic region. While sigma 70-like −10/−35 regions were identifiable within the first 75 bp of this region (Fig. 2B), attempts to map a PA5471 transcription initiation site here using RACE and antibiotic-treated K767 were unsuccessful.
FIG. 1.
Localization of the antibiotic-regulated PA5471 promoter activity and impact of mutations in the leader peptide-encoding PA5471.1 sequence on PA5471 expression. The PA5471 upstream region including PA5471.1 (bp 114 to 155) are highlighted with numbers representing positions relative to the first base pair after PA5472 (1) and the last base pair before the PA5471 coding region (367). Portions of the PA5471 upstream sequence fused to lacZ of miniCTX-lacZ and inserted into the chromosome of P. aeruginosa K767 are highlighted below the schematic with fusion-containing strain designations (see Table 1) given on the left. Fusions carrying mutations in PA5471.1 (sequence provided above the schematic) are also highlighted. The β-galactosidase activity of P. aeruginosa carrying the indicated fusions is shown without (-CAM) or with (+CAM) prior chloramphenicol exposure and is the mean ± standard deviation of a minimum of three experiments carried out in triplicate. Values have been adjusted for background activity measured using strain K2798 carrying a chromosomal promoterless lacZ insert. −, no activity detected; ND, not determined.
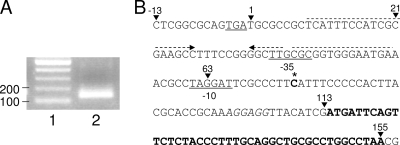
FIG. 2.
Mapping the PA5471 transcriptional start site. (A) The 5′ RACE product (lane 2) from P. aeruginosa strain K2817 (PA5471.1Q3Am) obtained with the 5′ RACE kit-provided 5′-end primer (20-mer) and PA5471-specific primer annealing within PA5471. Lane 1, 100-bp ladder. (B) PA5471 upstream sequence highlighting the RACE-determined transcription start site (bolded and with an asterisk), the PA5472 termination codon (underlined), putative −10/−35 sites (underlined), and the LP-encoding PA5471.1 sequence (bolded). Endpoints of lacZ transcriptional fusions described in Fig. 1 are identified by arrowheads and numbered as per Fig. 1. An interrupted inverted repeat is identified by dashed arrows above the DNA sequence.
Translation of an upstream leader peptide sequence is linked to PA5471 transcription.
The observation that a PA5471 promoter activity mapped to the 5′ end of the PA5472-PA5471 intergenic region was attenuated and made antibiotic dependent by downstream sequences was reminiscent of previously described transcriptional attenuation mechanisms (25, 57). With such mechanisms, gene expression is controlled by the translation status of an upstream LP, which in turn impacts leader mRNA secondary structure and, so, the formation of a transcription terminator upstream of the gene being regulated. Typically, translation of the LP leads to terminator formation and no downstream gene expression, while impeded LP translation/ribosome stalling on the LP sequence obviates terminator formation and promotes downstream gene expression (59). In searching the PA5471 upstream region, a putative 13-amino-acid-encoding ORF preceded by a strong Shine-Dalgarno sequence (AAGGAGG) was identified beginning 114 bp downstream of PA5472 (Fig. 1), which places it after the above-defined PA5471 promoter region. An in-frame fusion of the LP region (after codon 13) to lacZ on plasmid pPZ30 yielded substantial β-galactosidase activity (2,784 ± 699 Miller units), activity that was lost if the fusion junction was immediately after the TAA stop codon, consistent with the LP region being translated. The LP ORF is hereafter referred to as PA5471.1 and is annotated as such in the P. aeruginosa PAO1 genome sequence (www.pseudomonas.com).
One way that PA5471.1 translation could link chloramphenicol (as an example of a ribosome-targeting antibiotic) exposure to PA5471 expression is that antibiotic-induced stalling/slowing of the ribosome on PA5471.1 during translation of newly synthesized mRNA provides for transcription of the downstream PA5471 coding region, transcription that does not occur in the absence of antibiotic, and so, no ribosome stalling. To assess this, amber (TAG) and Opal (TGA) nonsense mutations were introduced at codon 3 (Q3Am) or 8 (C8Op), respectively, of PA5471.1 on the lacZ transcriptional fusion containing the entire PA5472-PA5471 intergenic region (strains K2800 and K2802 [Fig. 1]) to promote “stalling” of the ribosome on the PA5471.1 sequence during its translation, and the impact on PA5471 expression was measured. These mutations were also introduced into the PA5471.1-LacZ translational fusion plasmid and shown to block PA5471.1 translation as evidenced by the lack of β-galactosidase activity (data not shown). As seen in Fig. 1, these mutations yielded a very high level of expression of PA5471 (12- to 20-fold higher than wild-type basal levels) that was unaffected by chloramphenicol exposure. The elevated PA5471 expression in strain K2800 was unaffected by the introduction of the cloned PA5471.1 gene (on high-copy-number, pUCP19, and medium-copy-number, pRK415, vectors [data not shown]), ruling out PA5471.1 as playing a negative regulatory role in PA5471 expression that is simply lost in the Q3Am mutant.
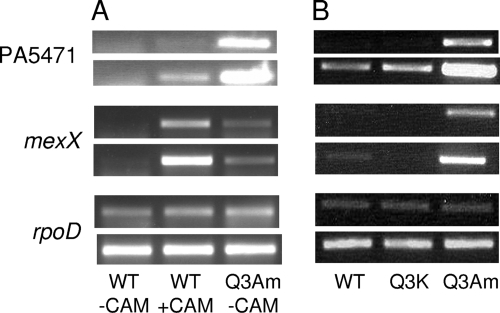
In contrast to the results obtained with the Q3Am mutation, a Q3K mutation in PA5471.1 (strain K2801) did not enhance PA5471 expression—although intrinsic activity was lowered, it remained antibiotic (chloramphenicol) inducible (Fig. 1)—consistent with premature PA5471.1 translation termination (and attendant ribosome stalling) being key to the observed marked increase in PA5471 expression in fusion strains K2800 and K2802. Interestingly, a PA5471.1 M1T mutation in the aforementioned lacZ transcriptional fusion, which effectively prevented any PA5471.1 translation, also yielded high-level expression of PA5471 that was chloramphenicol unresponsive (Fig. 1, K2799). In agreement with the above results, a mutant P. aeruginosa harboring a PA5471.1Q3Am mutation (K2817) but not a PA5471.1Q3K mutation (K2818) showed strong expression of PA5471 as measured using RT-PCR (Fig. 3B). As with the lacZ transcriptional fusions, PA5471 expression promoted by the Q3Am mutation in strain K2817 was markedly higher than for chloramphenicol-treated wild-type P. aeruginosa K767 (Fig. 3A).
FIG. 3.
Impact of the PA5471.1Q3Am mutation on expression of PA5471 and mexX. Expression, based on RT-PCR, of PA5471, mexX, and rpoD was assessed in P. aeruginosa K767 (wild type; WT) and a derivative, K2817, carrying a Q3Am mutation in PA5471.1 grown to log phase in the absence (-CAM) or presence (+CAM) of chloramphenicol (4 μg/ml) (A) or P. aeruginosa K767 (WT), K2817 (PA5471.1Q3am), and a derivative, K2818, carrying a Q3K mutation in PA5471.1 grown without chloramphenicol (B). The rpoD reaction served as an internal control that ensured equal amounts of RNA were employed in all of the RT-PCRs shown. The PCR portion of the reactions was carried out for 18 (top panel) or 20 (bottom panel) cycles (PA5471), 38 (top panel) or 40 (bottom panel) cycles (mexX), and 19 (top panel) or 21 (bottom panel) cycles (rpoD). Data are representative of two to three replicates.
The PA5471.1 Q3Am mutation in a PA5471-lacZ transcriptional fusion strain carrying intergenic sequences 1 to 155 (i.e., to just after the LP sequence) did not show the same high level of constitutive expression (Fig. 1, compare K2811 and K2812), indicating that the amber mutation did not enhance the activity of the upstream PA5471 promoter. Similarly, the Q3Am mutation in a PA5471-lacZ transcriptional fusion carrying intergenic sequences 90 to 367 (i.e., beginning after the previously defined PA5471 promoter) did not yield any β-galactosidase activity (Fig. 1, K2814), indicating that the mutation did not activate a downstream promoter more proximal to the PA5471 coding region. These data are, therefore, consistent with a model whereby interference with PA5471.1 translation promotes downstream PA5471 expression from a promoter upstream of PA5471.1 (i.e., an attenuation mechanism of control).
Possible influence of a PA5471.1 nonsense mutation and ribosome-targeting antimicrobials on PA5471 mRNA stability.
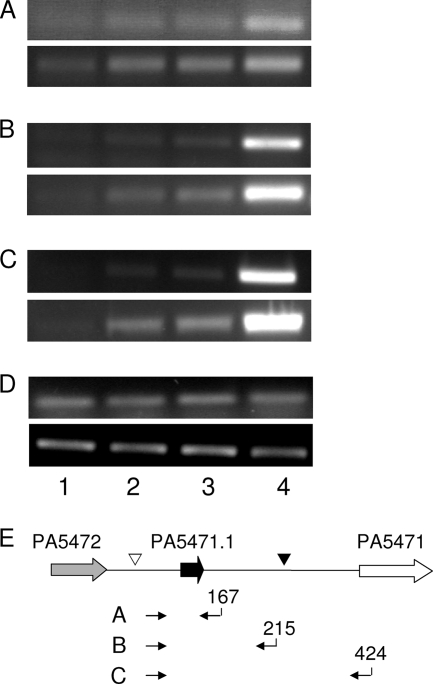
The observation that neither chloramphenicol exposure nor a PA5471.1 Q3Am mutation enhanced the activity of a PA5471-lacZ transcriptional fusion carrying intergenic sequences 1 to 155 (i.e., upstream of a putative attenuator [Fig. 1, K2792 and K2812]) while both enhanced the activity of PA5472-lacZ fusions carrying the complete intergenic region (i.e., including the putative attenuator region [Fig. 1, K2790 and K2800]) was consistent with a model whereby a PA5471 promoter activity was constant under all conditions but extension beyond the attenuator region and into PA5471 was dependent upon drug treatment or a PA5471.1 nonsense mutation. As such, the total level of PA5471 message-carrying sequences upstream of the putative attenuator (i.e., those that end at the attenuator and those that extend beyond it into the PA5471 coding region) should remain constant regardless of antimicrobial presence or a PA5471.1 nonsense mutation, while message-carrying sequences downstream of the attenuator should increase with antimicrobial exposure or a PA5471.1 nonsense mutation. To examine this, RT-PCR was carried out using a constant 5′ primer (which anneals immediately upstream of PA5471.1) and various 3′ primers that anneal both upstream and downstream of the attenuator region (Fig. 4E), and message-carrying attenuator upstream (i.e., those that truncate at the attenuator and those that extend into PA5471) and downstream (those that extend into PA5471 only) sequences were assessed in response to ribosome-targeting antimicrobials or a PA5471.1 Q3Am mutation. Surprisingly, all primer pairs yielded increased product in the presence of antimicrobials (Fig. 4A, B, and C, lanes 2 and 3) or PA5471.1 Q3Am mutation (Fig. 4A, B, and C, lane 4), including those that targeted the PA5471.1 region only (Fig. 4A). The simplest explanation for these results is that the extended message that forms in the presence of antimicrobials or a PA5471.1 nonsense mutation is more stable than the truncated message expected to form in their absence.
FIG. 4.
Influence of ribosome-targeting antimicrobials and PA5471.1Q3Am mutation on production of PA5471 message amplifiable with attenuator-upstream and attenuator-downstream primers. RNA extracted from P. aeruginosa K767 (wild type; lanes 1 to 3) and K2817 (PA5471.1Q3Am; lane 4) grown without antimicrobial (lanes 1 and 4) or with chloramphenicol (lane 2) or tetracycline (lane 3) was subjected to RT-PCR using primers directed at the PA5471 upstream region (A to C) or rpoD (D). (E) The position of the primer pairs used in panels A through C on the PA5471 upstream region is shown with each pair labeled at left and its corresponding panel designation and the position of the 3′ primer of each pair relative to first base pair of the PA5472-PA5471 intergenic region (as in Fig. 1) indicated. The PA5471 transcription initiation site is highlighted (▿) as is the site at which a putative terminator structure (see Fig. 5) begins (▾). The PCR portion of the reactions was carried out for 18 (top panel) or 20 (bottom panel) cycles in all instances except panel A, where 23 (top panel) and 25 (bottom panel) cycles were used. Data are representative of two to three replicates.
Mapping the PA5471 transcription initiation site.
The earlier difficulty in mapping the PA5471 transcription initiation site using RACE may have resulted from the comparatively low level of PA5471 expression promoted by chloramphenicol in wild-type P. aeruginosa K767 (Fig. 3A). Using RNA isolated from the PA5471.1Q3Am mutant, K2817, which showed a markedly higher level of PA5471 expression (Fig. 3A), a strong RACE product was recovered (Fig. 2A) and a transcription initiation site readily and unambiguously identified. Six independent clones were sequenced, all yielding the same cytosine residue at bp 75 of the PA5472-PA5471 intergenic region as the initiation site (Fig. 2B). Moreover, the initiation site was appropriately placed downstream of the putative −10 and −35 sites identified previously. The fusion junction of the PA54711-63-lacZ fusion occurs within the putative −10 region, providing an explanation for its lack of promoter activity (Fig. 1, K2808), while the PA54711-75-lacZ junction occurs immediately after the transcription start site, consistent with it showing constitutive promoter activity (Fig. 1, K2809). Intriguingly, the virtually inactive fusion encompassing bp 21 to 367 of the intergenic region (Fig. 1, K2796) extends to 17 bp upstream of the putative −35 site and 55 bp upstream of the transcription initiation site, suggesting that additional regulatory elements that act upstream of the PA5471 promoter may be involved in PA5471 transcription. A lengthy interrupted inverted repeat that could be involved in regulator binding was identified upstream of the putative PA5471 −35 site (Fig. 2). Beginning at bp 9 it would be truncated in the PA547121-367-lacZ fusion present in K2796.
PA5471 is not sufficient for antibiotic-inducible mexXY expression.
Previous work from this lab demonstrated that the cloned PA5471 gene was able to promote mexXY expression and MexXY-mediated antibiotic resistance (36). Still, despite the markedly higher level of PA5471 expression seen in the PA5471.1 Q3Am mutant K2817 compared to chloramphenicol-treated wild-type strain K767 (Fig. 3A), mexXY expression was actually higher in chloramphenicol-treated K767 than in untreated K2817 (Fig. 3A). This suggested that while PA5471 is involved in MexXY recruitment in response to antibiotic-promoted ribosome disruption its production is not sufficient for optimal mexXY induction, and additional antibiotic-inducible factors are also necessary.
DISCUSSION
The mexXY multidrug efflux operon is inducible by antimicrobials that target the ribosome (21), dependent upon the PA5471 gene that is itself inducible by such antibiotics and whose expression promotes mexXY expression independent of antimicrobials (36). The current study demonstrates that P. aeruginosa senses antibiotic-mediated ribosome disruption and links it to PA5471 gene expression by monitoring the translation of a leader peptide region (PA5471.1) found upstream of the PA5471 coding sequence on PA5471 mRNA. This is reminiscent of erythromycin and tetracycline induction of the erm (macrolide resistance) and tet (tetracycline resistance) genes, respectively, in Bacillus subtilis. Expression of the ermB (35) and ermC (33) genes is controlled by macrolides via the use of leader peptide sequences whose translation is linked to secondary structure formation in mRNA that is or is not compatible with subsequent erm translation (termed translational attenuation) (33, 35). Translational attenuation has also been reported for the tet efflux gene of plasmid pBC16 (29) and probably the tetA(L) efflux gene (56), both in Bacillus subtilis. As with PA5471, the introduction of nonsense mutations at certain positions within these erm (26, 35) and tet (29, 56) LP sequences, which is intended to mimic ribosome stalling, promotes high-level constitutive production of the efflux proteins that is not influenced by antibiotic.
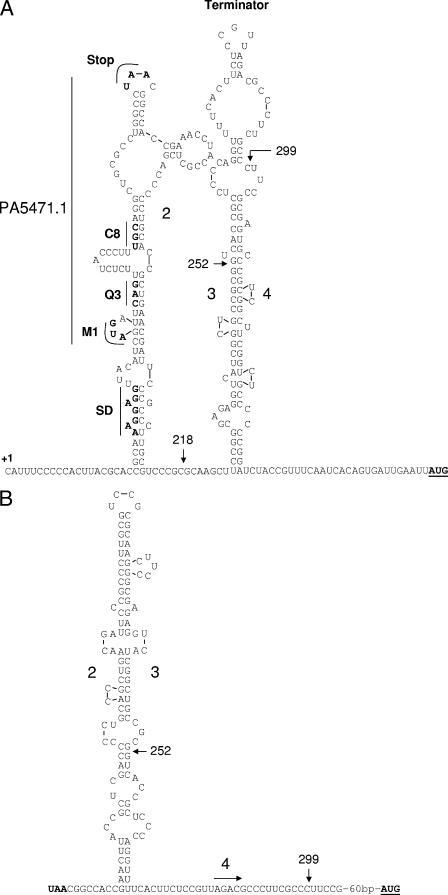
While the LP-based control mechanisms appear to be similar for these erm/tet determinants and PA5471, PA5471 is, however, regulated at the level of transcription. While transcriptional (versus translational) attenuation has most commonly been described for regulation of amino acid biosynthetic operons (16, 59) it has also been reported for tet [tet(M) of transposon Tn916] (57) and erm (emrK of Bacillus licheniformis) (25) resistance genes. In these instances, transcription terminates prior to the tet/erm genes in the absence of drug while transcription extends into the tet/erm coding regions in the presence of drug. In both cases, mutually exclusive secondary structures are proposed whose formation is dictated by the translation status of an upstream LP [14 amino acids for ermK; 28 amino acids for tet(M)]. Intriguingly, the PA5471 LP-encoding sequence (PA5471.1) occurs in a region upstream of PA5471 that is predicted to form a stem-loop structure (PA5471.1-2), with PA5471.1 forming the first half of the stem (Fig. 5A). Sequences downstream of this stem-loop are predicted to form a second stem-loop (3-4) that ends very near the translation start site for PA5471 and is followed by a uracil-rich sequence that is characteristic of a transcription terminator (Fig. 5A). One possibility, then, is that this structure forms in the transcribed leader mRNA when translation of PA5471.1 is not compromised, leading to transcription termination prior to the PA5471 coding region. In contrast, antibiotic-induced ribosome stalling on the PA5471.1 sequence would preclude formation of the PA5471.1-1 stem-loop, freeing 2 to pair with 3 (forming what might be called the antiterminator), which is then unavailable for pairing with 4 to form the transcription terminator (Fig. 5B) and so transcription proceeds into the PA5471 coding region. Consistent with this model, the introduction of nonsense mutations but not a missense mutation into PA5471.1 promoted high-level constitutive expression of PA5471. Moreover, the Q3K missense mutation in PA5471.1 results from a change in the same base as the Q3Am nonsense mutation (C→A for Q3K; C→T for Q3Am) (Fig. 1) and still retains the drug inducibility of PA5471 expression, indicating that the impact of the nonsense mutations on PA5471 expression cannot be explained on the basis of disruption of key base pair interactions important for PA5471.1-2 stem formation. Significantly, the natural stop codon for PA5471.1 occurs on the predicted loop at the end of the PA5471.1-2 stem such that any ribosome stalling that might occur during termination of PA5471.1 translation would not impact formation of this stem-loop and, so, not impact formation of the putative terminator.
FIG. 5.
Proposed secondary structures for PA5471 leader region mRNA. (A) Putative uninduced or “off” state of the leader mRNA. The leader region begins with +1 of the message and ends at the PA5471 translation initiation site and forms two stem-loop structures with the leader peptide-encoding PA5471.1 sequence pairing with segment 2 to form the first stem-loop and segment 3 pairing with segment 4 to form the second stem-loop, which is predicted to be a transcription terminator. The PA5471.1 Shine-Dalgarno (SD) sequence is highlighted in bold, as are the PA5471.1 translation stop codon and residues M1, Q3, and C8, which are sites of mutation in this study and are referred to elsewhere. End points of leader region subclones fused to lacZ as described in Fig. 1 are indicated with arrows. The indicated stem-loop structures were predicted with the assistance of mfold (60) and demonstrated the most favorable free energy (ΔG of −31.9 and −32.9 kcal/mol, respectively) of any individual stem-loop structures defined for the PA5471 leader region. (B) Putative induced or on state of the leader mRNA. The leader region shown here begins with the PA5471.1 translation termination codon and ends at the PA5471 translation initiation site and forms a single stem-loop structure owing to segment 2 pairing with segment 3, precluding formation of the segment 3-4 stem-loop terminator. The beginning of the segment 4 sequence is highlighted as are the end points of leader region subclones fused to lacZ as described in Fig. 1. The indicated stem-loop structure was predicted with the assistance of mfold (60) and demonstrated the most favorable free energy (ΔG, −30.1 kcal/mol) of any individual stem-loop structures defined for the PA5471 leader region lacking the LP-encoding PA5471.1 sequence.
Interestingly, elimination of PA5471.1 translation via an M1T mutation also enhanced PA5471 expression, reminiscent of the effects of eliminating the pBC16 tet LP ribosome binding site (i.e., no translation of the tet gene possible) where Tet protein expression was enhanced 10-fold and was tetracycline unresponsive (29). It is unclear, however, how lack of PA5471.1 translation would provide the same signal as ribosome stalling, although in the case of the M1T mutation the ribosomes may be pausing at the ribosome binding site in the absence of a start codon.
The observation that fusion strains carrying 3′-truncated fragments of the PA5471 upstream region lacking a putative attenuator (Fig. 1, K2809, K2792, and K2794) yielded less β-galactosidase activity than full-length upstream fragments containing the attenuator sequences but carrying a nonsense mutation (Q3Am) in PA5471.1 (Fig. 1, K2800) was puzzling given the expectation that an attenuator-minus sequence would, in theory, provide for maximal PA5471 gene expression. One possibility was that 3′-truncated sequences that end before the putative attenuator sequence, similar to what would be formed in vivo in wild-type cells in the absence of ribosome-targeting antimicrobials, are comparatively unstable while full-length transcripts that form in the presence ribosome-targeting antimicrobials or PA5471.1 nonsense mutations (where an attenuator is expected not to form) are comparatively stable. Consistent with this was the observation that RT-PCR-amplifiable products generated with primers that target the attenuator upstream region were increased upon antimicrobial exposure or PA5471.1 mutation despite the fact that the PA5471 promoter was not impacted by antimicrobial exposure or mutation (i.e., the total amount of message-carrying attenuator upstream sequences was expected to be constant and only the amount that extended beyond the attenuator would be increased). Enhanced stability of the mRNA of erm genes that are under translational attenuation control has also been reported in response to erythromycin (6, 35), with erythromycin-induced stalling of the ribosome on the emr leader sequences directly linked to this stabilization (6). Still, there are no prior reports of antibiotic-dependent stabilization of mRNA for resistance genes whose antibiotic inducibility involves a transcriptional attenuation mechanism. Interestingly, while the PA5471.1 Q3Am mutation failed to enhance activity of a PA54711-115-lacZ fusion, it did enhance activity of a PA54711-252-lacZ fusion (Fig. 1, K2806), comparable to that seen for a full-length PA5471-lacZ fusion. The 1-252 region lacks the 3-4 sequences necessary for forming the putative terminator stem-loop (Fig. 5A), although it retains most of the 2-3 sequences needed to form the so-called antiterminator (Fig. 5B). Possibly, it is this antiterminator structure that stabilizes the PA5471 message.
While the results of this and a previous study (36) highlight the importance of PA5471 for ribosome-targeting-antibiotic-inducible mexXY expression, it is clear that PA5471 alone is insufficient for maximal mexXY induction by antibiotics, and other antibiotic-promoted changes are needed for the full recruitment of this efflux system. One possibility is that PA5471 directly or indirectly processes a product generated by antibiotic disruption of ribosomes (e.g., truncated or aberrant peptides), which then function as mexXY inducers and, possibly, MexXY substrates (45). In the absence of antibiotic there would be relatively less PA5471 “substrate” (presumably arising from a basal error rate in translation) for production of the mexXY inducer(s). As such, enhanced PA5471 expression only, resultant, for example, from a PA5471.1 nonsense mutation, would not yield substantial mexXY inducer production. In contrast, elevated levels of PA5471 and its substrates resultant from antibiotic exposure would produce comparatively higher levels of the mexXY inducers and, so, provide for more mexXY expression. In any case, PA5471 and MexXY-OprM are clearly linked to ribosome function, and their recruitment in response to ribosome stress suggests that they play some role in helping P. aeruginosa deal with the adverse consequences of ribosome dysfunction. Work is ongoing to define PA5471's activity and to determine how it functions to promote mexXY expression, thereby providing insights into the intended function of the MexXY-OprM efflux system.
Supplementary Material
Acknowledgments
This work was supported by an operating grant from the Canadian Cystic Fibrosis Foundation.
Footnotes
Published ahead of print on 22 May 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aakra, A., H. Vebo, L. Snipen, H. Hirt, A. Aastveit, V. Kapur, G. Dunny, B. E. Murray, and I. F. Nes. 2005. Transcriptional response of Enterococcus faecalis V583 to erythromycin. Antimicrob. Agents Chemother. 492246-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Short protocols in molecular biology, 2nd ed. John Wiley & Sons, Inc., New York, NY.
- 3.Bandow, J. E., H. Brotz, L. I. Leichert, H. Labischinski, and M. Hecker. 2003. Proteomic approach to understanding antibiotic action. Antimicrob. Agents Chemother. 47948-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barcak, G. J., M. S. Chandler, R. J. Redfield, and J. F. Tomb. 1991. Genetic systems in Haemophilus influenzae. Methods Enzymol. 204321-342. [DOI] [PubMed] [Google Scholar]
- 5.Becher, A., and H. P. Schweizer. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. BioTechniques 29948-952. [DOI] [PubMed] [Google Scholar]
- 6.Bechhofer, D. H., and D. Dubnau. 1987. Induced mRNA stability in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 84498-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, D. G., J. K. Swanson, and C. Allen. 2007. Two host-induced Ralstonia solanacearum genes, acrA and dinF, encode multidrug efflux pumps and contribute to bacterial wilt virulence. Appl. Environ. Microbiol. 732777-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burse, A., H. Weingart, and M. S. Ullrich. 2004. The phytoalexin-inducible multidrug efflux pump AcrAB contributes to virulence in the fire blight pathogen, Erwinia amylovora. Mol. Plant-Microbe Interact. 1743-54. [DOI] [PubMed] [Google Scholar]
- 9.Cao, L., R. Srikumar, and K. Poole. 2004. MexAB-OprM hyperexpression in NalC type multidrug resistant Pseudomonas aeruginosa: identification and characterization of the nalC gene encoding a repressor of PA3720-PA3719. Mol. Microbiol. 531423-1436. [DOI] [PubMed] [Google Scholar]
- 10.Chan, Y. Y., H. S. Bian, T. M. Tan, M. E. Mattmann, G. D. Geske, J. Igarashi, T. Hatano, H. Suga, H. E. Blackwell, and K. L. Chua. 2007. Control of quorum sensing by a Burkholderia pseudomallei multidrug efflux pump. J. Bacteriol. 1894320-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee, A., S. Chaudhuri, G. Saha, S. Gupta, and R. Chowdhury. 2004. Effect of bile on the cell surface permeability barrier and efflux system of Vibrio cholerae. J. Bacteriol. 1866809-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuanchuen, R., C. T. Narasaki, and H. P. Schweizer. 2002. Benchtop and microcentrifuge preparation of Pseudomonas aeruginosa competent cells. BioTechniques 33760, 762, 763. [DOI] [PubMed] [Google Scholar]
- 13.Evans, K., L. Adewoye, and K. Poole. 2001. MexR repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa: identification of MexR binding sites in the mexA-mexR intergenic region. J. Bacteriol. 183807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, K., L. Passador, R. Srikumar, E. Tsang, J. Nezezon, and K. Poole. 1998. Influence of the MexAB-OprM multidrug efflux system on quorum-sensing in Pseudmonas aeruginosa. J. Bacteriol. 1805443-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evers, S., K. Di Padova, M. Meyer, H. Langen, M. Fountoulakis, W. Keck, and C. P. Gray. 2001. Mechanism-related changes in the gene transcription and protein synthesis patterns of Haemophilus influenzae after treatment with transcriptional and translational inhibitors. Proteomics 1522-544. [DOI] [PubMed] [Google Scholar]
- 16.Gollnick, P., and P. Babitzke. 2002. Transcription attenuation. Biochim. Biophys. Acta 1577240-250. [DOI] [PubMed] [Google Scholar]
- 17.Hagman, K. E., W. Pan, B. G. Spratt, J. T. Balthazar, R. C. Judd, and W. M. Shafer. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141611-622. [DOI] [PubMed] [Google Scholar]
- 18.Hirakata, Y., R. Srikumar, K. Poole, N. Gotoh, T. Suematsu, S. Kohno, S. Kamihira, R. E. Hancock, and D. P. Speert. 2002. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J. Exp. Med. 196109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 21277-86. [DOI] [PubMed] [Google Scholar]
- 20.Hoang, T. T., A. J. Kutchma, A. Becher, and H. P. Schweizer. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 4359-72. [DOI] [PubMed] [Google Scholar]
- 21.Jeannot, K., M. L. Sobel, F. El Garch, K. Poole, and P. Plesiat. 2005. Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug-ribosome interaction. J. Bacteriol. 1875341-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang, H., and D. C. Gross. 2005. Characterization of a resistance-nodulation-cell division transporter system associated with the syr-syp genomic island of Pseudomonas syringae pv. syringae. Appl. Environ. Microbiol. 715056-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70191-197. [DOI] [PubMed] [Google Scholar]
- 24.Kohler, T., C. Van Delden, L. K. Curty, M. M. Hamzehpour, and J. C. Pechere. 2001. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 1835213-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwak, J. H., E. C. Choi, and B. Weisblum. 1991. Transcriptional attenuation control of ermK, a macrolide-lincosamide-streptogramin B resistance determinant from Bacillus licheniformis. J. Bacteriol. 1734725-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon, A. R., Y. H. Min, E. J. Yoon, J. A. Kim, M. J. Shim, and E. C. Choi. 2006. ErmK leader peptide: amino acid sequence critical for induction by erythromycin. Arch. Pharm. Res. 291154-1157. [DOI] [PubMed] [Google Scholar]
- 27.Lin, J., C. Cagliero, B. Guo, Y. W. Barton, M. C. Maurel, S. Payot, and Q. Zhang. 2005. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J. Bacteriol. 1877417-7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, J. T., M. B. Connelly, C. Amolo, S. Otani, and D. S. Yaver. 2005. Global transcriptional response of Bacillus subtilis to treatment with subinhibitory concentrations of antibiotics that inhibit protein synthesis. Antimicrob. Agents Chemother. 491915-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodato, P. B., E. J. Rogers, and P. S. Lovett. 2006. A variation of the translation attenuation model can explain the inducible regulation of the pBC16 tetracycline resistance gene in Bacillus subtilis. J. Bacteriol. 1884749-4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuda, N., and S. Ohya. 1992. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 361847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Contribution of the MexX-MexY-OprM efflux system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 442242-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathews, D. H., J. Sabina, M. Zuker, and D. H. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288911-940. [DOI] [PubMed] [Google Scholar]
- 33.Mayford, M., and B. Weisblum. 1990. The ermC leader peptide: amino acid alterations leading to differential efficiency of induction by macrolide-lincosamide-streptogramin B antibiotics. J. Bacteriol. 1723772-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria, p. 72-74. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 35.Min, Y. H., A. R. Kwon, E. J. Yoon, M. J. Shim, and E. C. Choi. 2008. Translational attenuation and mRNA stabilization as mechanisms of erm(B) induction by erythromycin. Antimicrob. Agents Chemother. 521782-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita, Y., M. L. Sobel, and K. Poole. 2006. Antibiotic inducibility of the MexXY multidrug efflux system of Pseudomonas aeruginosa: involvement of the antibiotic-inducible PA5471 gene product. J. Bacteriol. 1881847-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng, W. L., K. M. Kazmierczak, G. T. Robertson, R. Gilmour, and M. E. Winkler. 2003. Transcriptional regulation and signature patterns revealed by microarray analyses of Streptococcus pneumoniae R6 challenged with sublethal concentrations of translation inhibitors. J. Bacteriol. 185359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palumbo, J. D., C. I. Kado, and D. A. Phillips. 1998. An isoflavonoid-inducible efflux pump in Agrobacterium tumefaciens is involved in competitive colonization of roots. J. Bacteriol. 1803107-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penketh, A., T. Pitt, D. Roberts, M. E. Hodson, and J. C. Batten. 1983. The relationship of phenotypic changes in Pseudomonas aeruginosa to the clinical condition of patients with cystic fibrosis. Am. Rev. Respir. Dis. 127605-608. [DOI] [PubMed] [Google Scholar]
- 40.Poole, K. 2003. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms, p. 273-298. In I. T. Paulsen and K. Lewis (ed.), Microbial multidrug efflux. Horizon Scientific Press, Norwich, United Kingdom.
- 41.Poole, K. 2004. Acquired resistance, p. 170-183. In A. P. Fraise, P. A. Lambert, and J.-Y. Maillard (ed.), Russell, Hugo & Ayliffe's principles and practice of disinfection, preservation & sterilization. Blackwell Publishing, Oxford, United Kingdom.
- 42.Poole, K. 2004. Efflux pumps, p. 635-674. In J.-L. Ramos (ed.), Pseudomonas, Vol. I. Genomics, life style and molecular architecture. Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 43.Poole, K. 2004. Efflux-mediated multiresistance in gram-negative bacteria. Clin. Microbiol. Infect. 1012-26. [DOI] [PubMed] [Google Scholar]
- 44.Poole, K. 2005. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 5620-51. [DOI] [PubMed] [Google Scholar]
- 45.Poole, K. 2008. Bacteria multidrug efflux pumps serve other functions. Microbes 3179-185. [Google Scholar]
- 46.Prouty, A. M., I. E. Brodsky, S. Falkow, and J. S. Gunn. 2004. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella typhimurium. Microbiology 150775-783. [DOI] [PubMed] [Google Scholar]
- 47.Qiu, J., D. Zhou, Y. Han, L. Zhang, Z. Tong, Y. Song, E. Dai, B. Li, J. Wang, Z. Guo, J. Zhai, Z. Du, X. Wang, and R. Yang. 2005. Global gene expression profile of Yersinia pestis induced by streptomycin. FEMS Microbiol. Lett. 243489-496. [DOI] [PubMed] [Google Scholar]
- 48.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-Gonzalez, A. Rojas, W. Teran, and A. Segura. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56743-768. [DOI] [PubMed] [Google Scholar]
- 49.Sabina, J., N. Dover, L. J. Templeton, D. R. Smulski, D. Soll, and R. A. LaRossa. 2003. Interfering with different steps of protein synthesis explored by transcriptional profiling of Escherichia coli K-12. J. Bacteriol. 1856158-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 51.Schweizer, H. P. 1991. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene 97109-121. [DOI] [PubMed] [Google Scholar]
- 52.Schweizer, H. P. 1991. Improved broad-host-range lac-based plasmid vectors for the isolation and characterization of protein fusions in Pseudomonas aeruginosa. Gene 10387-92. [DOI] [PubMed] [Google Scholar]
- 53.Schweizer, H. P. 1998. Intrinsic resistance to inhibitors of fatty acid biosynthesis in Pseudomonas aeruginosa is due to efflux: application of a novel technique for generation of unmarked chromosomal mutations for the study of efflux systems. Antimicrob. Agents Chemother. 42394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw, K. J., N. Miller, X. Liu, D. Lerner, J. Wan, A. Bittner, and B. J. Morrow. 2003. Comparison of the changes in global gene expression of Escherichia coli induced by four bactericidal agents. J. Mol. Microbiol. Biotechnol. 5105-122. [DOI] [PubMed] [Google Scholar]
- 55.Simon, R., U. Priefer, and A. Puehler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology 1784-791. [Google Scholar]
- 56.Stasinopoulos, S. J., G. A. Farr, and D. H. Bechhofer. 1998. Bacillus subtilis tetA(L) gene expression: evidence for regulation by translational reinitiation. Mol. Microbiol. 30923-932. [DOI] [PubMed] [Google Scholar]
- 57.Su, Y. A., P. He, and D. B. Clewell. 1992. Characterization of the tet(M) determinant of Tn916: evidence for regulation by transcription attenuation. Antimicrob. Agents Chemother. 36769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thanassi, D. G., L. W. Cheng, and H. Nikaido. 1997. Active efflux of bile salts in Escherichia coli. J. Bacteriol. 1792512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yanofsky, C. 2000. Transcription attenuation: once viewed as a novel regulatory strategy. J. Bacteriol. 1821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 313406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.