Abstract
The avian paramyxovirus Newcastle disease virus (NDV) selectively replicates in tumor cells and is known to stimulate T-cell-, macrophage-, and NK cell-mediated responses. The mechanisms of NK cell activation by NDV are poorly understood so far. We studied the expression of ligand structures for activating NK cell receptors on NDV-infected tumor cells. Upon infection with the nonlytic NDV strain Ulster and the lytic strain MTH-68/H, human carcinoma and melanoma cells showed enhanced expression of ligands for the natural cytotoxicity receptors NKp44 and NKp46, but not NKp30. Ligands for the activating receptor NKG2D were partially downregulated. Soluble NKp44-Fc and NKp46-Fc, but not NKp30-Fc, chimeric proteins bound specifically to NDV-infected tumor cells and to NDV particle-coated plates. Hemagglutinin-neuraminidase (HN) of the virus serves as a ligand structure for NKp44 and NKp46, as indicated by the blockade of binding to NDV-infected cells and viral particles in the presence of anti-HN antibodies and by binding to cells transfected with HN cDNA. Consistent with the recognition of sialic acid moieties by the viral lectin HN, the binding of NKp44-Fc and NKp46-Fc was lost after desialylation. NKp44- and NKp46-CD3ζ lacZ-inducible reporter cells were activated by NDV-infected cells. NDV-infected tumor cells stimulated NK cells to produce increased amounts of the effector lymphokines gamma interferon and tumor necrosis factor alpha. Primary NK cells and the NK line NK-92 lysed NDV-infected tumor cells with enhanced efficiency, an effect that was eliminated by the treatment of target cells with the neuraminidase inhibitor Neu5Ac2en. These results suggest that direct activation of NK cells contributes to the antitumor effects of NDV.
Virulent strains of Newcastle disease virus (NDV) infect domestic poultry and other birds, causing a rapidly spreading viral disease that affects the alimentary and respiratory tracts as well as the central nervous system (55). In humans, however, NDV is well tolerated (17, 18). Other than mild fever for a day, only a few adverse effects have been reported. NDV, also known as avian paramyxovirus 1, is an enveloped virus containing a negative-sense, single-stranded RNA genome which codes for six proteins in the order (from 3′ to 5′) of nucleoprotein, phosphoprotein, matrix protein, fusion (F) protein, hemagglutinin-neuraminidase (HN), and large polymerase protein (19). There are many different strains of NDV, classified as either lytic or nonlytic for different types of cells. Lytic and nonlytic NDV strains both replicate much more efficiently in human cancer cells than they do in most normal human cells (43). Viruses of both strain types have been investigated as potential anticancer agents (30, 49, 52). The NDV strains that have been evaluated most widely for the treatment of cancer are 73-T, MTH-68, and Ulster (1, 7, 11, 17, 18, 53, 54, 56, 71).
Initial binding of NDV to a host cell takes place through the interaction of HN molecules in the virus coat with sialic acid-containing molecules on the cell surface (31). NDV neuraminidase has strict specificity for the hydrolysis of the NeuAc-α2,3-Gal linkage, with no hydrolysis of the NeuAc-α2,6-Gal linkage (41).
NDV infection of tumor cells not only improves T-cell responses (53, 58, 68), but has also been reported to vigorously stimulate innate immune responses. In the course of NDV infection, large amounts of alpha interferon (IFN-α) are released (68) and in turn activate dendritic cells and NK cells and polarize, in concert with interleukin-12 (IL-12), toward a Th1 T-cell response (33, 44, 47). In addition, NDV induces antitumor cytotoxicity in murine macrophages which produce increased amounts of tumor necrosis factor alpha (TNF-α) and nitric oxide (51, 60) and in human monocytes through the induction of TRAIL (64). Little is known about the NDV-mediated activation of NK cells. The coincubation of peripheral blood mononuclear cells with NDV was shown previously to stimulate NK-mediated cytotoxicity (70). Enhanced cytotoxicity correlates with the induction of IFN-α (70). It is not known, however, whether NDV-infected cells can directly activate NK cells and, if so, which molecular interactions are involved.
The cytolytic activity of NK cells against virus-infected or tumor cells is regulated by the engagement of activating or inhibitory NK cell surface receptors, the actions of cytokines, and cross talk with other immune cells (32, 39). Most inhibitory receptors recognize particular major histocompatibility complex (MHC) class I alleles and thereby ensure the tolerance of NK cells against self antigens (38). Activating receptors on human NK cells include CD16; NKG2D; the natural cytotoxicity receptors (NCR) NKp30, NKp44, and NKp46; as well as NKp80; DNAM-1; and various stimulatory coreceptors (32).
NCR are important activating receptors for the antitumor and antiviral activities of NK cells (5, 32, 37). Heparan sulfate has been discussed previously as a cellular ligand for NKp46, NKp44, and NKp30 (9, 26, 27), and nuclear factor BAT3, which can be released from tumor cells under stress conditions, has been described as a cellular ligand for NKp30 (42). Ligands for NKp30 and NKp44 can be detected on the surfaces and in the intracellular compartments of several kinds of tumor cells (10). Moreover, a number of pathogen-derived NCR ligands have been reported. The hemagglutinin protein of influenza virus and the HN of Sendai virus can bind to NKp46 and NKp44 and activate NK cells (3, 24, 34). The pp65 protein of human cytomegalovirus has been shown to bind NKp30 and inhibit its function (4). Human immunodeficiency virus, vaccinia virus, and herpes simplex virus have also been shown to upregulate the expression of cellular NCR ligands in infected cells (13, 14, 62). The Plasmodium falciparum erythrocyte membrane protein 1 is involved in the NCR-mediated NK cell attack against infected erythrocytes (36). Furthermore, NKp46 recognizes cells infected with mycobacteria (22, 61), and NKp44 was recently reported to directly bind to the surfaces of mycobacteria and other bacteria (21).
In this study, we investigated the expression of ligand structures for NCR and NKG2D on NDV-infected cells. We demonstrate that NDV HN proteins which are strongly expressed on NDV-infected tumor cells function as activating ligand structures for NKp44 and NKp46 but that cellular ligands for NKG2D are partially downregulated during NDV infection.
MATERIALS AND METHODS
Cell lines.
HeLa cervix carcinoma, PANC-1 pancreatic adenocarcinoma, and A549 lung carcinoma cells were obtained from the American Type Culture Collection. T98G human glioblastoma cells were kindly provided by W. Roth, Deutsches Krebsforschungszentrum, Heidelberg. The cell lines Ma-Mel-8a and Ma-Mel-8b, which were recently established from two melanoma metastases from the same patient at the University Medical Center of Mannheim, have been described previously (8). Cell lines were cultured in RPMI 1640 (Invitrogen, Karlsruhe, Germany) supplemented with 2 mM glutamine and 10% fetal calf serum (FCS). A human NK tumor cell line that has been transfected with human IL-2 cDNA and grows independently of IL-2, NK-92CI (57), was grown in α medium supplemented with 2 g/liter NaHCO3, 12.5% FCS, 12.5% horse serum, penicillin-streptomycin, 1% l-glutamine, and 50 μM 2-mercaptoethanol. NK-92CI cells are hereinafter referred to as NK-92 cells.
Transfections.
Vectors containing cDNA clones for NDV Beaudette C HN (p36/7-HN) and NDV Ulster F0 protein (p36/7-F) have been described previously (68). The cDNAs were subcloned into the expression vector pcDNA3.1(+) (Invitrogen) to generate pcDNA3.1(+)/HN and pcDNA3.1(+)/F0, respectively. HeLa cells (2.5 × 105) cultured in six-well plates were transfected with 4 μg of pcDNA3.1(+)/HN, pcDNA3.1(+)/F0, pcDNA3.1(+)/HN+pcDNA3.1(+)/F0, or the empty pcDNA3.1(+) vector and 10 μl of Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Two days later, cells were selected with Geneticin (1 mg/ml in Dulbecco's phosphate buffered saline [D-PBS]; Sigma-Aldrich, Taufkirchen, Germany) and, after being sorted for high-level HN or F0 expression, maintained in 0.5 mg/ml Geneticin.
Antibodies.
The NDV HN-specific monoclonal antibody (MAb) HN.B (immunoglobulin G2a [IgG2a]) and the NDV F-specific MAb Icii (IgG1) were produced from hybridoma cell lines kindly provided by R. Iorio (Department of Molecular Genetics and Microbiology, University of Massachusetts Medical School, Worcester). The polyclonal rabbit anti-NDV Ulster antiserum 82.3 has been described previously (69). Polyclonal rabbit anti-vaccinia virus antibodies were purchased from Quartett Immunodiagnostika, Berlin, Germany. Antibodies recognizing MICA (M673), MICB (M360), ULBP1 (M295), ULBP2 (M310), ULBP3 (M550), and ULBP4 (M475) were kindly provided by D. Cosman (AMGEN, Seattle, WA). For blocking experiments, we used control IgG1 MOPC21 (Sigma), anti-NKp44 (clone p44-8, produced in our laboratory), and anti-NKp46 (clone 9E2; BioLegend, Eching, Germany). The HLA-A-, HLA-B-, and HLA-C-reactive MAb W6/32 has been described previously (40). Phycoerythrin (PE)-conjugated goat anti-mouse Ig and goat anti-human IgG (anti-hIgG) Fc fragments were from BD Pharmingen (Heidelberg, Germany) and Dianova (Hamburg, Germany), respectively.
NCR-Fc fusion proteins.
NCR-Fc fusion proteins were produced in HEK 293T cells as described previously (27, 29). By using the calcium phosphate transfection method, 293T cells were transiently transfected with NKp46-hIgG1 Fc, NKp44-hIgG1 Fc, and NKp30-hIgG1 Fc cDNAs (a kind gift of Ofer Mandelboim, Hebrew University, Jerusalem, Israel) subcloned into the expression vector pMT2+mcs. NCR-Fc chimeric proteins were purified using protein A Sepharose CL-4B (GE Healthcare Life Sciences, Freiburg, Germany). The integrity of purified NCR-Ig was confirmed on Western blots by using peroxidase-conjugated goat anti-hIgG Fc and enhanced chemiluminescence as described previously (29).
For desialylation, 5-μg aliquots of NCR-Fc fusion proteins were incubated with 5 μl of Clostridium perfringens neuraminidase attached to beaded agarose (Sigma-Aldrich) in phosphate-buffered saline (PBS) for 1.5 h at 37°C. The integrity of the proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. The activity of immobilized neuraminidase was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of neuraminidase-treated fetuin, a highly sialylated protein.
Preparation of NDV virions.
The avirulent, nonlytic NDV strain Ulster 2C was propagated in 10-day-old embryonated chicken eggs from stocks of virus and purified as described previously (48, 50). The virus was cryopreserved at −70°C, and the titer was determined by the hemagglutination test in which 1 hemagglutination unit (HU) is defined as the lowest virus concentration leading to visible sheep erythrocyte agglutination. NDV MTH-68/H was kindly provided by G. Noss (Malsch, Germany). The virus was propagated in embryonated chicken eggs, harvested from the allantoic fluid, and purified by ultracentrifugation as described previously (2).
Virus infections.
Tumor cells (HeLa, A549, PANC-1, T98G, Ma-Mel-8a, and Ma-Mel-8b) were washed twice in FCS-free medium and resuspended in PBS (106 cells/ml). Then, 100 HU/ml of NDV Ulster or 25 HU/ml of NDV MTH-68/H was added, and the cells were incubated for 1 h at 37°C in a CO2 incubator. Infected cells were washed twice and incubated for an additional 18 to 20 h before staining or use in functional assays.
Flow cytometry.
For cell surface immunofluorescence stainings, 0.5 × 106 to 1 × 106 cells were washed once in ice-cold fluorescence-activated cell sorter (FACS) buffer (D-PBS-2% FCS) and then incubated with a saturating amount of the primary mouse MAb for 45 min on ice. After two washes, cells were incubated with PE-labeled goat anti-mouse Ig for 30 min on ice. Complexes of NCR-Fc fusion proteins (1 to 2 μg per staining) and PE-labeled goat anti-hIgG Fc (Dianova; 1:100 in FACS buffer) were allowed to form for 30 min before being added to cells for 60 min on ice. Cells were washed twice and resuspended in 200 μl of FACS buffer with 0.05% propidium iodide (Sigma-Aldrich). Cytofluorometric analyses were done using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, Heidelberg, Germany). For all FACS stainings, representative examples from at least three repeats with similar results are shown.
Human primary NK cells.
Human primary NK cells were isolated from peripheral blood lymphocytes by using the NK cell negative isolation kit (Invitrogen). Between 95 and 99% of NK cells were CD3 negative and CD56 positive. Cells were grown in Iscove's modified Dulbecco's medium (Invitrogen) with 10% human serum, penicillin-streptomycin, and 100 IU/ml IL-2 (NIH Cytokine Repository, Bethesda, MD).
Chromium release assay.
Target cells (0.5 × 106) in 100 μl of assay medium (Iscove's modified Dulbecco's medium with 10% FCS and 1% penicillin-streptomycin) were labeled with 100 μCi (3.7 MBq) of 51Cr (Hartmann & Braun, Frankfurt, Germany) for 1 h at 37°C. Cells were washed twice and resuspended in assay medium at 5 × 104 cells/ml. Effector cells were resuspended in assay medium and mixed at different effector-to-target cell ratios with 5,000 labeled target cells/well in a 96-well V-bottom plate. Rabbit anti-NDV and anti-vaccinia virus antisera were added to the assay mixture at 0.3 μl/well. MAbs against HN, NKp44, or NKp46 or the irrelevant control antibody MOPC21 was added to the 96-well plate at 1 μg/200 μl of assay mixture where indicated. The soluble NKp44-Fc and NKp46-Fc fusion proteins were added together at ∼0.5 μg/200 μl each where indicated. For the preactivation of primary NK cells or NK-92 cells with NDV Ulster, viral particles were UV inactivated at 254 nm and 2 mW/cm2 by using a UV transilluminator (Bachofer, Reutlingen, Germany) for 10 min and added to effector cells (250 HU of NDV/106 effector cells for 30 min at 37°C), after which the mixtures were washed twice in assay medium. The HN inhibitor N-acetyl-2,3-dehydro-2-deoxyneuraminic acid (Neu5Ac2en [25 mM stock in PBS; Calbiochem/Merck, Darmstadt, Germany]) was added to target cells at 100 μM for 1 h during the labeling period, and the cells were then washed twice. Maximum release was determined by the incubation of target cells in 1% Triton X-100 solution. Spontaneous 51Cr release was measured by incubating target cells in the absence of effector cells. All samples were prepared in triplicate. Plates were incubated for 4 h at 37°C. Supernatant was harvested, and 51Cr release was measured in a γ-counter. The percentage of cytotoxicity was calculated according to the following formula: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. The ratio between the amounts of maximum and spontaneous release was at least 3 in all experiments. Representative examples from two to three similar experiments are shown.
ELISA.
MicroTest III enzyme-linked immunosorbent assay (ELISA) plates (BD Biosciences, Heidelberg, Germany) were coated overnight with viral particles (40 HU) in 0.05 M NaH2CO3-Na2HCO3 buffer (pH 9.6) and blocked using 3% skim milk powder (Merck) in PBS-0.05% Tween 20 (Sigma-Aldrich) (PBS-T) for 2 h at room temperature. NKp44-Fc, NKp46-Fc, and NKp30-Fc (1 μg/100 μl in PBS-T with 1% bovine serum albumin) or purified MAb HN.B or Icii (1 μg/well) was added for 1 h at room temperature, and the plates were washed three times with PBS-T. Peroxidase-conjugated goat anti-hIgG Fc or goat anti-mouse IgG Fc in PBS-T (1:2,000 with 1% bovine serum albumin) was added for 1 h at room temperature, after which the plates were again washed three times with PBS-T. For antibody inhibition of NCR-Fc binding, purified anti-HN or anti-F MAbs were added to virus-coated wells at 2 μg/well. Next, a peroxidase substrate solution (o-phenylenediamine [Sigma-Aldrich] at 1 mg/ml in 0.1 M KH2PO4 buffer [pH 6.0]) was added for 20 min at room temperature in the dark. The substrate reaction was stopped with 50 μl of 4 N H2SO4, and results were read with a Titertek Multiscan Plus MKII ELISA photometer (MP Biomedicals, Heidelberg, Germany) at 492 nm.
IFN-γ and TNF-α release assays.
HeLa and T98G cells in petri dishes were infected with NDV strain Ulster (100 HU/106 cells) or MTH-68 (25 HU/106 cells) for 20 h. Subsequently, infected cells were inactivated by UV irradiation for 5 min to prevent viral replication and spreading (48). A total of 105 primary NK cells were incubated together with 105 NDV-infected or uninfected tumor cells for 24 h at 37°C in a 96-well plate in the presence of 100 IU/ml IL-2. In the absence of NK cells, infected tumor cells survived UV irradiation for 24 h before beginning to undergo apoptosis. For the control of background cytokine release, NK cells or NDV-infected tumor cells were cultured alone. All samples were prepared in triplicate. Supernatants were harvested, and TNF-α and IFN-γ were quantified by sandwich ELISAs using the Quantikine human IFN-γ and Quantikine human TNF-α kits according to the instructions of the manufacturer (R&D Systems).
CD3ζ reporter cells and lacZ assays.
To generate chimeric proteins consisting of the ectodomains of NKp46, NKp44, and NKp30 and the mouse CD3ζ transmembrane and cytoplasmic domains, the IgG1 Fc part in pBluescript II KS(+) plasmids encoding the NCR-IgG1 Fc fusion proteins (3) was replaced by a BamHI-SmaI fragment containing residues 28 to 144 of mouse CD3ζ cDNA from plasmid pJA146 (kindly provided by B. Schraven, University of Magdeburg, Germany). The NKp46-CD3ζ, NKp44-CD3ζ, and NKp30-CD3ζ chimeras were subcloned into the expression vector pcDNA3.1(−) (Invitrogen). BWZ.36 mouse lymphoma cells (a gift of N. Shastri, University of California, Berkeley) expressing the lacZ gene under the control of the NFAT promoter have been described previously (46). BWZ.36 cells were transfected by electroporation with a GenePulser (Bio-Rad Laboratories, Munic, Germany) using 220 V and 960 μF. Cells were selected with Geneticin (1.5 mg/ml in D-PBS; Sigma-Aldrich, Taufkirchen, Germany) and, after two rounds of sorting for NCR-CD3ζ expression, maintained in a mixture of 0.5 mg/ml Geneticin and 0.25 mg/ml hygromycin.
The lacZ reporter assay was performed essentially as described previously (59). Briefly, NDV-infected HeLa cells transfected with HN cDNA or left untreated were added to a 96-well round-bottom plate (5 × 104 cells/well). Adherent tumor cells were cocultured with 105 NCR-CD3ζ reporter cells for 18 h at 37°C. A mixture of 5 ng/ml phorbol 12-myristate 13-acetate (Sigma-Aldrich) and 0.5 μg/ml ionomycin (Merck/Calbiochem) was used as the control for maximal lacZ inducibility in reporter cells. Anti-NCR antibodies (1 μg/well) were coated in 0.1 M carbonate buffer overnight and subjected to two washes in D-PBS. The stimulation of NCR-CD3ζ cells was evaluated by the addition of lacZ substrate buffer (9 mM MgCl2, 0.15 mM chlorophenyl red galactopyranoside [CPRG], 100 mM 2-mercaptoethanol, 0.125% Nonidet P-40 in PBS [pH 7.5]) for 4 h at 37°C. The cleaved CPRG was measured in an ELISA reader as the absorbance at 595 nm with 630 nm as the reference wavelength. The data are shown as means and standard errors of the means (SEM) of triplicate values from representative examples of three similar experiments.
RESULTS
NDV-infected tumor cells upregulate NKp44 and NKp46 ligands.
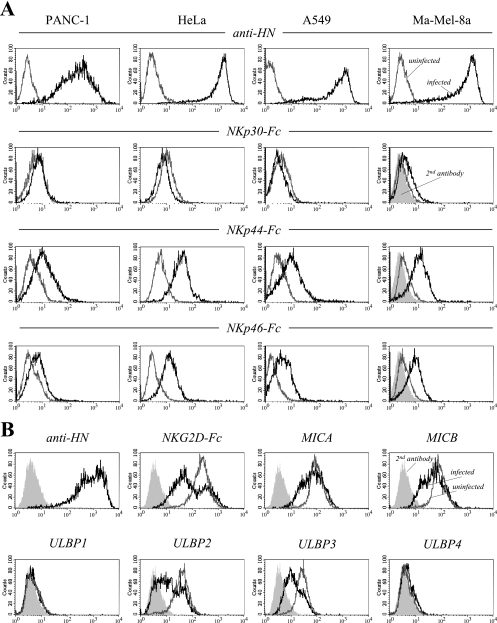
NDV has been reported previously to enhance the cytolytic activity of NK cells (70); however, the mechanisms of NK activation by NDV are largely unresolved. First, we investigated whether NDV infection of tumor targets results in the direct activation of NK cells through the induction of NK-activating ligands. The established human carcinoma lines PANC-1, HeLa, and A549 and the recently isolated melanoma cell line Ma-Mel-8a were infected with the nonlytic NDV strain Ulster for 20 h. The cells were subsequently stained with soluble chimeric NKp46-Fc, NKp44-Fc, and NKp30-Fc proteins in complexes with PE-conjugated anti-hIgG Fc secondary antibodies. NDV infection resulted in increased surface expression of ligand structures for NKp44 and, to a lesser extent, for NKp46. In contrast, staining with NKp30-Fc was not significantly altered after NDV infection (Fig. 1A). Infection efficiencies were monitored using the HN-specific MAb HN.B for surface staining. Furthermore, we analyzed the effect of NDV infection on the expression of ligands for another major NK-activating receptor, NKG2D. While soluble NKG2D-Fc receptors strongly stained uninfected HeLa cells, the binding of the NKG2D-Fc fusion protein was clearly downregulated in a major subpopulation of NDV Ulster-infected cells (Fig. 1B). A smaller fraction of cells showed unaltered expression of NKG2D ligands, suggesting differential ligand modulation patterns over the course of infection. Cell surface expression levels of the NKG2D ligands MICA, MICB, ULBP2, and ULBP3 were found to be reduced to various degrees (Fig. 1B). ULPB1 and ULBP4 were expressed only weakly by or were absent from uninfected HeLa cells, and expression was not significantly altered after NDV infection.
FIG. 1.
Soluble NKp44 and NKp46 receptors bind to various NDV-infected tumor cell lines. (A) PANC-1 pancreatic carcinoma, HeLa cervical carcinoma, A549 lung carcinoma, and Ma-Mel-8a melanoma cells were infected for 20 h with the nonlytic NDV strain Ulster (100 HU/106 cells) (black lines) or left uninfected (gray lines). Cells were stained with NKp30-, NKp44-, and NKp46-IgG1 Fc fusion proteins in complexes with goat anti-hIgG-PE secondary antibodies as indicated. Results for Ma-Mel-8a cells stained with a secondary antibody alone are represented by filled curves. To monitor infection efficiencies, tumor cells were stained with anti-NDV MAb HN.B, recognizing HN (top panels, black lines). For a control, uninfected cells were stained with HN.B (top panels, gray lines). (B) HeLa cells were infected for 18 h with the NDV strain Ulster (black lines) or left uninfected (gray lines). Cells were stained with the NKG2D-Fc fusion protein or with MAbs recognizing NDV HN, to control for infection, or the NKG2D ligands MICA, MICB, and ULBP1 to ULBP4, as indicated. Results for secondary antibody controls are represented by filled curves.
The NDV HN protein serves as an NKp44 and NKp46 ligand.
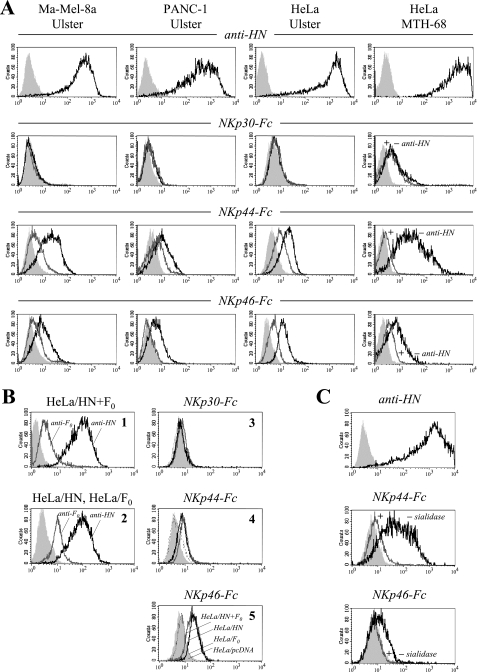
Since tumors of different histological origins (carcinomas, a melanoma, and a glioblastoma) showed NDV-induced NKp44 and NKp46 ligand upregulation, it was likely that a viral protein rather than a virus-induced cellular factor was responsible for this effect. To study the involvement of the NDV envelope proteins (HN and F), we first preincubated NDV Ulster-infected Ma-Mel-8a, PANC-1, and HeLa cells with the monoclonal anti-HN antibody HN.B. This treatment was found to significantly reduce the subsequent stainings with NKp44-Fc and NKp46-Fc, whereas the stainings with NKp30-Fc remained unaltered (Fig. 2A). A blockade of NKp44 and NKp46 binding by anti-HN was also observed for HeLa cells infected with NDV strain MTH-68 (Fig. 2A). In contrast, preincubation of NDV-infected cells with anti-F MAb Icii had no inhibitory effect on soluble NCR binding (data not shown). To obtain virus-independent evidence for NDV HN as a ligand for NKp44 and NKp46, we transfected HeLa cells with cDNA plasmids coding for NDV-derived HN or F0 protein. For HeLa cells transfected with the HN expression vector alone (HeLa/HN cells) and HeLa cells transfected with both the HN and F0 expression vectors (HeLa/HN+F0 cells), but not for HeLa cells transfected with the F0 expression vector alone (HeLa/F0 cells), we observed the induction of NKp44 and NKp46 binding (Fig. 2B). Transfection with the HN or F0 vector did not change the efficiency of NKp30 binding.
FIG. 2.
Anti-NDV HN antibody blocks NKp44 and NKp46 binding. (A) Staining of Ma-Mel-8a, PANC-1, and HeLa cells infected for 20 h with NDV Ulster (100 HU/106 cells), as well as Ma-Mel-8a cells infected for 20 h with NDV MTH-68 (25 HU/106 cells), with NKp30-Fc, NKp44-Fc, or NKp46-Fc fusion proteins without preincubation (black lines) or after preincubation with MAb HN.B (gray lines). Results for secondary antibody controls are represented by filled curves. To monitor infection efficiencies, tumor cells were stained with anti-HN MAb HN.B (top panels, black lines) and secondary antibodies for control (top panels, filled curves). (B) HeLa cells were stably transfected with either NDV HN or F0 cDNA individually or cotransfected with both cDNAs. Panel 1 shows HN expression (black line) and F0 expression (gray line) in HeLa/HN+ F0 cells as detected using MAbs HN.B and Icii, respectively. Panel 2 shows HN expression in HeLa/HN (black line) and F0 expression in HeLa/F0 (gray line) single transfectants. Results for the secondary antibody controls are shown with filled curves. For panels 3 to 5, HeLa/HN+F0 (black lines), HeLa/HN (gray lines), and HeLa/F0 (broken lines) transfectants and HeLa cells carrying the pcDNA3.1(+) vector control (filled curves) were stained with NKp30-Fc, NKp44-Fc, and NKp46-Fc as indicated. (C) HeLa cells infected for 20 h with NDV Ulster (200 HU/106 cells) were stained with NKp44-Fc or NKp46-Fc desialylated with immobilized neuraminidase (gray lines) or untreated NCR fusion proteins as controls (black lines). Filled curves depict results for secondary antibody controls. The infection efficiency was monitored with MAb HN.B (top panel, black line) and a secondary antibody control (top panel, filled curve). +, with; −, without.
The HN receptor of NDV has been reported to recognize sialic acid moieties on cell surface glycans (14, 41). We therefore asked whether sialic acid attached to the NKp44 and NKp46 glycoproteins might be relevant for the interaction with NDV-infected cells. NKp46-Fc and NKp44-Fc were desialylated using immobilized neuraminidase. After desialylation, NKp44-Fc and NKp46-Fc completely lost their enhanced binding to NDV Ulster-infected HeLa cells (Fig. 2C). This result suggests that the sialic acid lectin function of HN is involved in a major way in the binding of NKp44 and NKp46.
Binding of NKp44 and NKp46 to NDV particles.
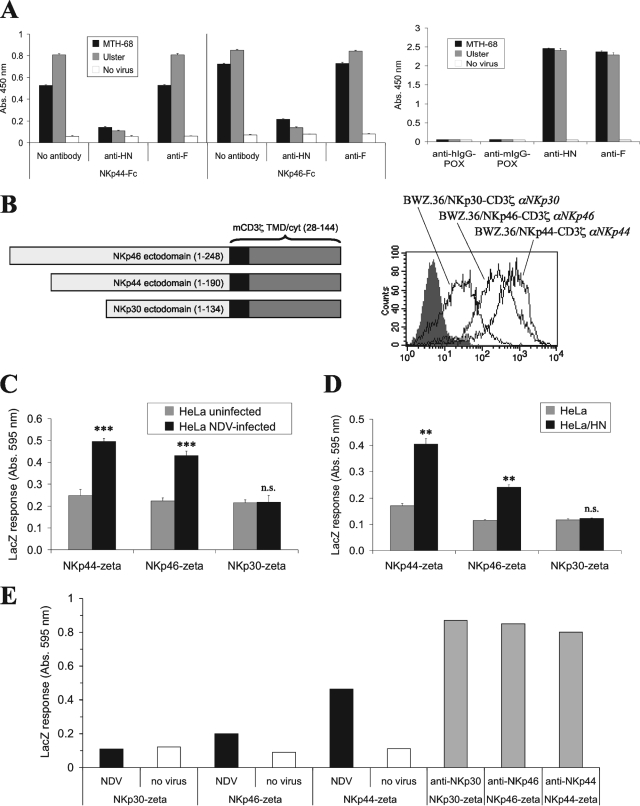
The blocking effect of the anti-HN MAb on NCR binding strongly suggests that the viral HN serves as a ligand for NKp44 and NKp46 on infected cells. To investigate the possibility of the direct binding of NCR to the viral envelope, we performed an enzyme-linked immunoassay after the coating of ELISA plates with NDV particles. As shown in Fig. 3A, NKp44-Fc and NKp46-Fc bound to Ulster- and MTH-68-coated ELISA plates. This binding could be efficiently blocked by anti-HN but not by anti-F MAbs. NKp30-Ig showed weak binding to NDV particles, and this binding could not be blocked by anti-HN or anti-F antibodies (data not shown).
FIG. 3.
NKp44-Fc and NKp46-Fc bind to NDV particle-coated plates. (A) Standard ELISA plates were coated with purified NDV (strain Ulster and MTH-68) particles, and the particles were allowed to react with NKp44-Fc and NKp46-Fc fusion proteins, followed by peroxidase-labeled anti-hIgG secondary antibody (anti-hIgG-POX). The assay results were developed using the peroxidase substrate o-phenylenediamine dihydrochloride and evaluated in an ELISA reader as the absorption (Abs.) at 450 nm. The binding of soluble NCR can be blocked by preincubation of the virus-coated plate with the anti-HN MAb HN.B but not the anti-F MAb Icii. Plate coating was checked by using anti-HN or anti-F0 MAb, followed by peroxidase-labeled anti-mouse IgG secondary antibody (anti-mIgG-POX). Wells without virus show low levels of nonspecific binding of NCR-Fc, anti-HN, anti-F, and secondary antibodies. Means and SEM for triplicate samples are shown. (B to F) Activation of NKp44-CD3ζ and NKp46-CD3ζ reporter cells by NDV-infected cells. (B, left) Schematic diagram showing NCR-CD3ζ reporter proteins. The ectodomains of NKp46, NKp44, and NKp30 were fused to the transmembrane domain (TMD) and cytoplasmic domain (cyt) of mouse CD3ζ (mCD3ζ) by using cDNA constructs. Residues of the respective proteins contained in the chimeras are indicated. (Right) BWZ.36 mouse thymoma cells harboring the lacZ gene under the control of the NFAT promoter were transfected with the cDNA constructs. Stainings with MAbs specific for NKp46 (αNKp46), NKp44 (αNKp44), and NKp30 (αNKp30) demonstrated surface expression of the indicated NCR-CD3ζ fusion proteins. (C) The indicated NCR-CD3ζ reporter lines were cocultured with uninfected HeLa cells or NDV Ulster-infected HeLa cells for 18 h, after which the lacZ response was measured with the colorigenic substrate CPRG in an ELISA reader. Absorption at 595 nm, expressed as means of triplicate values ± SEM, is shown. ***, P < 0.001; n.s., not significant. (D) Untransfected HeLa cells as well as HeLa cells transfected with HN cDNA were analyzed as described in the legend to panel C. Reporter cell stimulation by NDV-infected cells transfected with HN cDNA (HeLa/HN) and that by untreated HeLa cells were compared by Student's t test. **, P < 0.01; n.s., not significant. (E) The indicated NCR-CD3ζ reporter cells were cultured for 20 h in wells of an ELISA plate treated with coating buffer (no virus) for a control or coated with purified NDV Ulster particles. Other wells were coated with NKp30, NKp44, and NKp46 antibodies (1 μg/well) as indicated to monitor reporter cell activation following antibody-mediated NCR cross-linking.
Stimulation of NCR-CD3ζ reporter cells by NDV and transfection with HN.
Since NK cells possess various activating and inhibitory receptors which can be simultaneously triggered by virus-infected cells, we sought to prove the ligation of NKp44 and NKp46 by NDV HN through an NK-independent assay. The ectodomains of NKp30, NKp44, and NKp46 were fused to the transmembrane and cytoplasmic domains of the murine CD3ζ chain, and BWZ.36 reporter cells that express the lacZ gene under the control of the NFAT promoter (46) were transfected with the respective cDNAs (Fig. 3B). Coincubation of NDV Ulster-infected HeLa cells and HeLa/HN transfectants elicited enhanced lacZ production in NKp44-CD3ζ and NKp46-CD3ζ, but not in NKp30-CD3ζ, reporter cells (Fig. 3C and D). Furthermore, NDV Ulster particle-coated plates were able to induce lacZ responses in NKp44-CD3ζ and NKp46-CD3ζ cells (Fig. 3E). These findings provide further evidence not only that HN mediates the ligation of NKp44 and NKp46, but also that functional interaction results in cellular signal transduction.
Increased lymphokine secretion by NDV-activated NK cells.
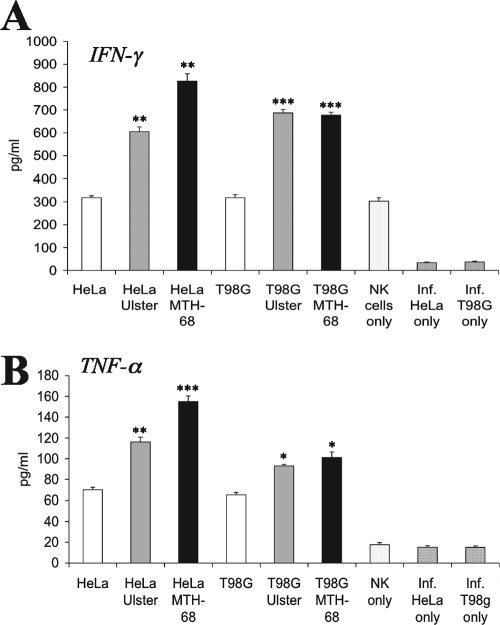
Little is known about the activation of NK cells in the course of NDV infection. We analyzed whether NDV-infected tumor cells can directly stimulate primary NK cells to secrete the NK effector cytokines TNF-α and IFN-γ. HeLa carcinoma and T98G glioblastoma cells were infected with NDV strain Ulster or MTH-68 for 20 h, UV irradiated, and cocultured with IL-2-prestimulated NK cells for 24 h. As shown in Fig. 4A, NK cells cultured in the presence of NDV-infected tumor cells exhibited significantly enhanced secretion of IFN-γ compared with NK cells cultured in the presence of uninfected HeLa or T98G cells or in the absence of stimulator cells. Coincubation of NK cells with uninfected tumor cells induced some TNF-α secretion, which could be further increased by NDV infection of tumor cells (Fig. 4B). Infected, UV-irradiated tumor cells cultured without NK cells did not release TNF-α or IFN-γ.
FIG. 4.
NK cells produce enhanced amounts of IFN-γ and TNF-α upon coincubation with NDV-infected cells. HeLa or T98G cells were infected with NDV Ulster or MTH-68 and cultured with primary NK cells in the presence of IL-2. For comparison, uninfected HeLa and T98G cells were cocultured with NK cells, or NK cells and infected (Inf.) tumor cells were cultured alone. IFN-γ and TNF-α secreted into the supernatants were quantified using respective lymphokine immunoassays. Means of triplicate values ± SEM are shown. Lymphokine production elicited by NDV-infected cells was statistically compared with lymphokine secretion in the presence of uninfected cells by Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Enhanced NK-mediated lysis of tumor cells infected with NDV or transfected with HN cDNA.
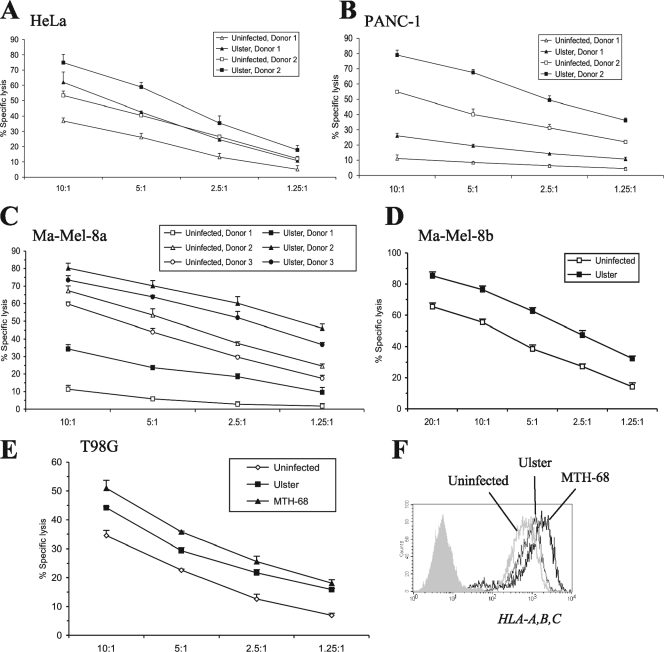
Next, we studied whether NDV infection is able to enhance the NK-mediated lysis of tumor targets in chromium release assays. Using primary, IL-2-activated NK cells from different donors, we consistently observed enhanced lysis of various tumor cell lines (HeLa, T98G, PANC-1, Ma-Mel-8a, and Ma-Mel-8b) infected with NDV Ulster (Fig. 5A to E) or MTH-68 (Fig. 5E). Interestingly, this enhancement of lysis susceptibility occurred despite the induction of potentially NK-inhibitory MHC class I molecules on the surfaces of infected target cells (Fig. 5F).
FIG. 5.
(A to D) NDV-infected tumor cells show enhanced susceptibility to NK cell lysis. HeLa, PANC-1, Ma-Mel-8a, and Ma-Mel-8b tumor cell lines were infected with NDV Ulster (100 HU/106 cells; 20 h) or left uninfected and were then subjected to a 4-h chromium release assay using primary, IL-2-activated NK cells from one to three different donors as effectors. The mean percentages of specific lysis ± SEM for triplicate samples at different effector/target cell ratios are indicated. (E) T98G cells were infected with NDV Ulster (100 HU/106 cells; 20 h) or MTH-68 (25 HU/106 cells; 12 h) or left uninfected and were then subjected to a 4-h chromium release assay using primary IL-2-activated NK cells. The anti-F protein MAb Icii was present during infection with MTH-68 to prevent syncytium formation. (F) Results from cytofluorometric staining of uninfected HeLa cells and NDV Ulster- and NDV MTH-68-infected HeLa cells with the pan-HLA-A-, HLA-B-, HLA-C-reactive antibody W6/32.
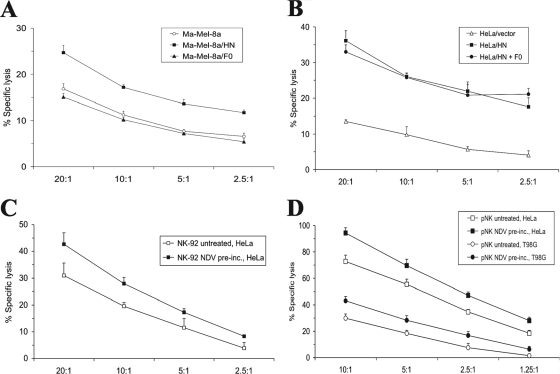
The increased binding of soluble NKp44 and NKp46 receptors to HeLa cells transfected with NDV HN or both HN and F0 (Fig. 2B) suggested that HN molecules may be able to directly activate NK cells and thereby enhance target cell lysis. To test this possibility, we used such transfectants as targets in NK lysis assays. As shown in Fig. 6A, Ma-Mel-8a cells transiently transfected with pcDNA3.1(+)/HN were more efficiently lysed than Ma-Mel-8a cells that were untransfected or transfected with pcDNA3.1(+)/F0. Likewise, HeLa cells expressing NDV HN showed a higher level of lysis than control transfectants bearing an empty vector (Fig. 6B). Productive infection with lytic NDV strains will result in the release of infectious NDV particles. To test whether NDV virions are able to activate NK cells, we preincubated NK-92 effector cells with UV-inactivated, purified NDV Ulster and then subjected the cells to extensive washing. As shown in Fig. 6C and D, the treatment of NK-92 or primary NK cells with inactivated NDV particles resulted in enhanced lytic activity against HeLa targets.
FIG. 6.
Tumor cells transfected with HN cDNA show enhanced susceptibility to NK cell lysis. (A) Ma-Mel-8a cells transiently transfected with NDV HN or F0 cDNA or an empty vector were used in a chromium release assay with the NK cell line NK-92 as effectors. (B) HeLa cells were stably transfected with pcDNA3.1(+)/HN, doubly transfected with pcDNA3.1(+)/HN and pcDNA3.1(+)/F0, or transfected with an empty vector for a control. Transfectants were subjected to a 4-h chromium release assay using NK-92 cells as effectors. (C) Results of a chromium release assay using HeLa target cells together with NK-92 effector cells. NK-92 cells were either preincubated (pre-inc.) with UV-inactivated NDV particles or left untreated. P < 0.02 by paired Student's t test. (D) Results of a chromium release assay using HeLa or T98G target cells and primary, IL-2-prestimulated NK cells (pNK) as effectors. Primary NK cells were either preincubated with UV-inactivated NDV particles or left untreated. P < 0.02 by paired Student's t test for both HeLa and T98G targets.
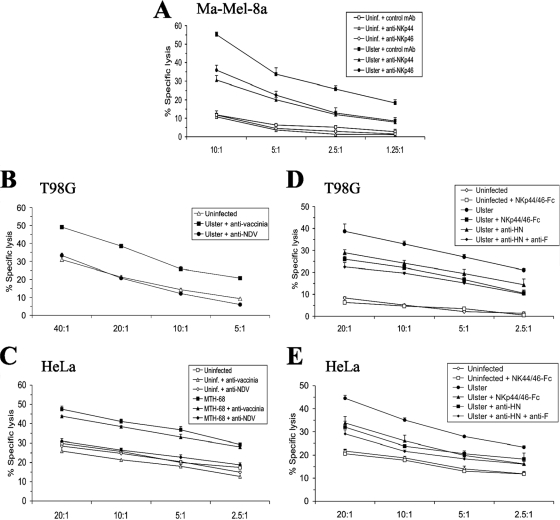
If NDV envelope proteins directly or indirectly trigger NK cells through NKp44 and NKp46, one would expect that the enhanced lysis of NDV-infected cells can be blocked by the respective antibodies. Indeed, the NDV Ulster-induced enhancement of lysis of Ma-Mel-8a targets by NK-92 effectors could be partially inhibited by MAbs recognizing NKp44 or NKp46 (Fig. 7A). The NK cell line NK-92 does not express Fc receptors which could mediate redirected lysis of infected targets (29). Furthermore, the augmented lysis of Ulster-infected T98G cells (Fig. 7B) and MTH-68-infected HeLa cells (Fig. 7C) by NK-92 effectors could be abolished by polyclonal anti-NDV antibodies. Polyclonal anti-vaccinia virus antibodies added to the lysis assay mixture used for control had no inhibitory effect (Fig. 7B and C). The inclusion of the anti-HN MAb HN.B or a mixture of NKp44-Fc and NKp46-Fc in the lysis assay mixture resulted in partially reduced killing of infected targets but had no influence on the lysis of uninfected targets (Fig. 7D and E; also data not shown). The addition of the anti-F MAb Icii together with the anti-HN MAb HN.B resulted in a slightly stronger inhibitory effect than the addition of HN.B alone (Fig. 7D and E). The results of these blocking experiments confirm the relevance of the interaction of NKp44/NKp46 with viral proteins for the observed NK cell stimulation by NDV.
FIG. 7.
(A, B, D, and E) NDV-induced enhancement of lysis is blocked by anti-NCR and anti-NDV antibodies. Ma-Mel-8a, T98G, and HeLa cells were either infected with NDV Ulster (100 HU/106 cells; 20 h) or left uninfected (uninf.). (C) Alternatively, HeLa cells were infected with NDV MTH-68 (25 HU/106 cells; 16 h) in the presence of anti-F protein MAb Icii to prevent syncytium formation or left uninfected. Fc receptor-deficient NK-92 effector cells were used throughout. For panel A, antibodies against NKp44 or NKp46 or the control antibody MOPC21 was included in the assay mixture as indicated. For panels B and C, polyclonal rabbit anti-NDV antibodies were present in a standard chromium release assay mixture with NDV-infected cells. As controls, rabbit anti-vaccinia virus polyclonal antibodies were used or no antibody was added. For panels D and E, the anti-HN MAb HN.B, HN.B plus the anti-F antibody Icii, or a combination of soluble NKp44-Fc and NKp46-Fc receptors was included in the assay mixture with infected or uninfected targets as indicated.
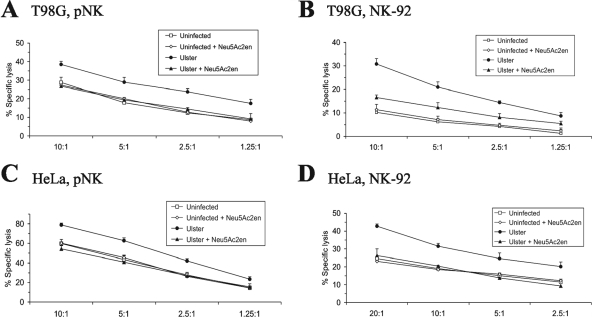
The desialylation of NKp44-Fc and NKp46-Fc chimeric proteins inhibited their binding to NDV-infected cells (Fig. 2C). To analyze whether a potential lectin function of HN would also be of relevance for the enhanced NK-mediated lysis of NDV-infected targets, we utilized the neuraminidase inhibitor Neu5Ac2en, which has been demonstrated previously to bind to the active site of crystallized NDV HN (16). Preincubation of NDV Ulster-infected T98G or HeLa cells with Neu5Ac2en substantially reduced or completely cancelled the enhanced lysis of infected targets by both primary NK cells and NK-92 effectors (Fig. 8).
FIG. 8.
The neuraminidase inhibitor Neu5Ac2en blocks NDV-mediated enhancement of NK lysis. T98G and HeLa target cells were infected with NDV Ulster (100 HU/106 cells; 20 h) or left uninfected and were then subjected to a 4-h chromium release assay using primary IL-2-activated NK cells (pNK) or NK-92 effectors. Target cells were preincubated with 100 μM neuraminidase inhibitor Neu5Ac2en during the 1-h chromium labeling period as indicated.
DISCUSSION
Infection with NDV has been described previously to cause increased cytolytic activity of the NK cell fraction in peripheral blood lymphocytes, correlating with virus-induced IFN-α production (70). In the present study, we have demonstrated that NK cells exert considerably enhanced cytolytic activity in vitro against various human tumor cell lines infected with NDV. Both the nonlytic NDV strain Ulster and the lytic strain MTH-68 caused this direct NK-triggering effect. Furthermore, we have shown that the incubation of NK cells with inactivated NDV particles was able to enhance the cytotoxic activity of the NK cells against uninfected targets. In addition to the previously reported IFN-α/β-mediated induction of the death receptor ligand TRAIL on NK cells (47), the direct activation of NK cells by NDV may thus contribute to the known oncolytic properties of certain NDV strains in vivo (53).
We have shown here that NDV-infected tumor cells induce NK cells to secrete increased amounts of IFN-γ and TNF-α. TNF-α can contribute to the antitumor cytotoxicity of NDV-activated macrophages (51). IFN-γ released by NK cells promotes Th1 cell polarization and subsequent cytotoxic-T-lymphocyte induction (35), thereby linking innate and adaptive immune responses to viral infection and also contributing to a variety of direct and indirect antitumor effector functions (28, 63).
The blockade of NKp44-Fc and NKp46-Fc binding to NDV-infected cells as well as to NDV particles by anti-HN, but not anti-F, antibodies suggests that HN is the NDV-encoded ligand for NKp44 and NKp46. This possibility was further corroborated by the finding that HeLa cells transfected with HN or HN and F0, but not F0 alone, showed enhanced binding of soluble NKp44 and NKp46 receptors. As the F protein may be closely associated with HN on the surfaces of infected target cells, the small additive effect of the anti-F antibody if used together with the anti-HN antibody in the NK lysis assay could be explained by steric hindrance of NKp44/NKp46 access to HN molecules. As the F protein does not have a known sialic acid binding function (31), it is unlikely to serve as a ligand structure itself for NKp44/NKp46. Taken together, the features of HN of the avian paramyxovirus NDV (genus Rubulavirus) closely resemble those of the HN molecule of the paramyxovirus Sendai virus (genus Respirovirus) and of the hemagglutinin of the orthomyxovirus influenza virus in terms of NCR binding (3, 34).
The NDV HN molecule recognizes terminal sialic acid residues attached to galactose by α2,3 linkages (41). Since the desialylation of NKp44 and NKp46 chimeric molecules abrogated their enhanced binding to NDV-infected cells, the sialic acid receptor function of HN appears to be essential, at least for the initial interaction with NKp44 and NKp46. This is fully confirmed by our finding that preincubation of target cells with the inhibitor Neu5Ac2en eliminated the NDV-mediated enhancement of tumor cell lysis by both NK-92 and primary NK cells. Using the neuraminidase inhibitor Neu5Ac2en, which has been shown to occupy the active sites of crystallized HN dimers (16, 67), we cannot decide whether an intact neuraminidase function needs to be present in order to bind NKp44/NKp46 and activate NK cells or whether the sialic acid lectin function, which may also involve a second sialic binding site at the HN dimer interface (67), is sufficient. The investigation of HN mutants that selectively affect the lectin or neuraminidase function may be instrumental in clarifying this point.
We cannot rule out the possibility that the binding of NKp44 and NKp46 to HN facilitates the subsequent binding to an unknown cellular receptor. In any case, sialic acid moieties displayed on NKp44 and NKp46 glycoproteins should have particular spatial arrangements, since terminal sialic acids are ubiquitously expressed on N- and O-linked oligosaccharides. For instance, NKp30, which is also sialylated (27), failed to show enhanced binding to NDV-infected cells. It has been speculated that NCR oligomerization on the surfaces of NK cells may enable multivalent interactions with trimeric influenza virus hemagglutinin complexes (34). In the same line, NCR oligomerization may also be necessary for binding to paramyxoviral HN molecules that form homodimers (16). Since the soluble NKp44 and NKp46 ectodomains, which are dimerized through the IgG Fc portion and cross-linked by anti-Ig secondary antibodies, were well able to bind to HN-expressing cells, we conclude that the oligomerization of NCR on the plasma membranes of NK cells or association with additional molecules is not an absolute prerequisite for ligand interaction.
As shown here by stainings with NKp44-Fc and NKp46-Fc, as well as by the stimulation of NKp44-CD3ζ and NKp46-CD3ζ reporter cells, NKp44 and NKp46 are individually able to interact with NDV HN molecules expressed in the course of viral infection or as a result of cell transfection. While NKp44 and NKp46 transduce NK-activating signals through different adapter molecules, namely, DAP12 and CD3ζ/FcɛRIγ (37), respectively, the engagement of either of these NCR was reported previously to result in functional cross talk, leading to phosphorylation of the adapter molecule associated with the other NCR (6). Thus, independent interaction of HN with either NKp44 or NKp46 on the same NK cell may have synergistic effects for NK cell activation. This pattern may explain why we consistently observed enhanced lysis of NDV-infected cells by primary NK cells from several donors, despite MHC class I upregulation and partial downmodulation of NKG2D ligands on NDV-infected targets.
Unexpectedly, NDV infection of HeLa cells resulted in the downmodulation of ligand structures for another important NK-triggering receptor, NKG2D. In general, these ligands have been described to be upregulated by genotoxic stress, transformation, and viral infection (12, 15, 23). In this study, NKG2D ligands were collectively shown to be partially downmodulated using a soluble NKG2D-Fc fusion protein. More specifically, we detected the reduction of surface expression of the NKG2D ligands MICA, MICB, ULBP2, and ULBP3 to various degrees (Fig. 1B). NDV-induced reduction of NKG2D-Fc and anti-ULBP2 staining, compared to the staining of uninfected HeLa cells, was detectable for only a subpopulation of infected cells. This bimodal reduction correlated with a subtle double peak in the staining for HN on NDV-infected cells, suggesting that not all infected cells expressed HN with the same kinetics. It will be interesting to study by double-staining approaches whether high HN expression levels are linked with low NKG2D ligand levels and whether this partial loss of NKG2D ligands is also observed for other types of NDV-infected tumor cells. Presently, we do not know the mechanism(s) of NKG2D ligand downregulation by NDV, but it may possibly represent a novel viral escape mechanism. It is conceivable that this occurs through active intracellular retention by a paramyxoviral gene product, similar to the endoplasmic reticulum/cis-Golgi retention of MICB and ULBP1/ULBP2 by the human cytomegaloviral gene product UL16 (20, 65, 66). Alternatively, NDV infection may induce increased shedding of NKG2D ligands from the tumor cell surface (25, 45) or transcriptional repression. Since we observed net activation rather than inhibition of NK cell cytotoxicity in the presence of NDV-infected cells, we conclude that a potential lack of NK cell activation through cellular NKG2D ligands is overcompensated for by the triggering of NKp44 and NKp46 through viral HN.
In summary, we have provided evidence that the two NCR NKp44 and NKp46 interact with the HN of the avian paramyxovirus NDV, leading to enhanced secretion of effector lymphokines by NK cells and increased susceptibility of infected tumor cells to lysis. These features are likely to contribute to the well-known oncolytic effects of NDV in vivo.
Acknowledgments
This work was supported by research grant no. 108483 from the Deutsche Krebshilfe.
We thank Ofer Mandelboim, Jerusalem, for plasmids coding for NCR-Fc chimeric proteins and Sonja Textor, Heidelberg, for supplying us with NKG2D ligand antibodies. We are grateful to Brigitta Messmer for excellent technical assistance.
Footnotes
Published ahead of print on 10 June 2009.
REFERENCES
- 1.Ahlert, T., W. Sauerbrei, G. Bastert, S. Ruhland, B. Bartik, N. Simiantonaki, J. Schumacher, B. Häcker, M. Schumacher, and V. Schirrmacher. 1997. Tumor-cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J. Clin. Oncol. 151354-1366. [DOI] [PubMed] [Google Scholar]
- 2.Apostolidis, L., V. Schirrmacher, and P. Fournier. 2007. Host mediated anti-tumor effect of oncolytic Newcastle disease virus after locoregional application. Int. J. Oncol. 311009-1019. [PubMed] [Google Scholar]
- 3.Arnon, T. I., M. Lev, G. Katz, Y. Chernobrov, A. Porgador, and O. Mandelboim. 2001. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur. J. Immunol. 312680-2689. [DOI] [PubMed] [Google Scholar]
- 4.Arnon, T. I., H. Achdout, O. Levi, G. Markel, N. Saleh, G. Katz, R. Gazit, T. Gonen-Gross, J. Hanna, E. Nahari, A. Porgador, A. Honigman, B. Plachter, D. Mevorach, D. G. Wolf, and O. Mandelboim. 2005. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat. Immunol. 6515-523. [DOI] [PubMed] [Google Scholar]
- 5.Arnon, T. I., G. Markel, and O. Mandelboim. 2006. Tumor and viral recognition by natural killer cells receptors. Semin. Cancer Biol. 16348-358. [DOI] [PubMed] [Google Scholar]
- 6.Augugliari, R., S. Parolini, R. Castriconi, E. Marcenaro, C. Cantoni, M. Nanni, L. Moretta, A. Moretta, and C. Bottino. 2003. Selective cross-talk among natural cytotoxicity receptors in human natural killer cells. Eur. J. Immunol. 331235-1241. [DOI] [PubMed] [Google Scholar]
- 7.Batliwalla, F. M., B. A. Bateman, D. Serrano, D. Murray, S. Macphail, V. C. Maino, J. C. Ansel, P. K. Gregersen, and C. A. Armstrong. 1998. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol. Med. 4783-794. [PMC free article] [PubMed] [Google Scholar]
- 8.Bloethner, S., K. Hemminki, R. K. Thirumaran, B. Chen, J. Mueller-Berghaus, S. Ugurel, D. Schadendorf, and R. Kumar. 2006. Differences in global gene expression in melanoma cell lines with and without homozygous deletion of the CDKN2A locus genes. Melanoma Res. 16297-307. [DOI] [PubMed] [Google Scholar]
- 9.Bloushtain, N., U. Qimron, A. Bar-Ilan, O. Hershkovitz, R. Gazit, E. Fima, M. Korc, I. Vlodavsky, N. V. Bovin, and A. Porgador. 2004. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J. Immunol. 1732392-2401. [DOI] [PubMed] [Google Scholar]
- 10.Byrd, A., S. C. Hoffmann, M. Jarahian, F. Momburg, and C. Watzl. 2007. Expression analysis of the ligands for the natural killer cell receptors NKp30 and NKp44. PLoS ONE 2e1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassel, W. A., and D. R. Murray. 1992. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med. Oncol. Tumor Pharmacother. 9169-171. [DOI] [PubMed] [Google Scholar]
- 12.Cerwenka, A., and L. L. Lanier. 2003. NKG2D ligands: unconventional MHC class I-like molecules exploited by viruses and cancer. Tissue Antigens 61335-343. [DOI] [PubMed] [Google Scholar]
- 13.Chisholm, S. E., and H. T. Reyburn. 2006. Recognition of vaccinia virus-infected cells by human natural killer cells depends on natural cytotoxicity receptors. J. Virol. 802225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chisholm, S. E., K. Howard, M. V. Gomez, and H. T. Reyburn. 2007. Expression of ICP0 is sufficient to trigger natural killer cell recognition of herpes simplex virusinfected cells by natural cytotoxicity receptors. J. Infect. Dis. 1951160-1168. [DOI] [PubMed] [Google Scholar]
- 15.Coudert, J. D., and W. Held. 2006. The role of the NKG2D receptor for tumor immunity. Semin. Cancer Biol. 16333-343. [DOI] [PubMed] [Google Scholar]
- 16.Crennell, S., T. Takimoto, A. Portner, and G. Taylor. 2000. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat. Struct. Biol. 71068-1074. [DOI] [PubMed] [Google Scholar]
- 17.Csatary, L. K., R. W. Moss, J. Beuth, B. Töröcsik, J. Szeberenyi, and T. Bakacs. 1999. Beneficial treatment of patients with advanced cancer using a Newcastle disease virus vaccine (MTH-68/H). Anticancer Res. 19635-638. [PubMed] [Google Scholar]
- 18.Csatary, L. K., G. Gosztonyi, J. Szeberenyi, Z. Fabian, V. Liszka, B. Bodey, and C. M. Csatary. 2004. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J. Neurooncol. 6783-93. [DOI] [PubMed] [Google Scholar]
- 19.De Leeuw, O., and B. Peeters. 1999. Complete nucleotide sequence of Newcastle disease virus: evidence for the existence of a new genus within the subfamily Paramyxovirinae. J. Gen. Virol. 80131-136. [DOI] [PubMed] [Google Scholar]
- 20.Dunn, C., N. J. Chalupny, C. L. Sutherland, S. Dosch, P. V. Sivakumar, D. C. Johnson, and D. Cosman. 2003. Human cytomegalovirus glycoprotein UL16 causes intracellular sequestration of NKG2D ligands, protecting against NK cell cytotoxicity. J. Exp. Med. 1971427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esin, S., G. Batoni, C. Counoupas, A. Stringaro, F. L. Brancatisano, M. Colone, G. Maisetta, W. Florio, G. Arancia, and M. Campa. 2008. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect. Immun. 761719-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg, A., P. F. Barnes, A. Porgador, S. Roy, S. Wu, J. S. Nanda, D. E. Griffith, W. M. Girard, N. Rawal, S. Shetty, and R. Vankayalapati. 2006. Vimentin expressed on Mycobacterium tuberculosis-infected human monocytes is involved in binding to the NKp46 receptor. J. Immunol. 1776192-6198. [DOI] [PubMed] [Google Scholar]
- 23.Gasser, S., and D. H. Raulet. 2006. Activation and self-tolerance of natural killer cells. Immunol. Rev. 214130-142. [DOI] [PubMed] [Google Scholar]
- 24.Gazit, R., R. Gruda, M. Elboim, T. I. Arnon, G. Katz, H. Achdout, J. Hanna, U. Qimron, G. Landau, E. Greenbaum, Z. Zakay-Rones, A. Porgador, and O. Mandelboim. 2006. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 7517-523. [DOI] [PubMed] [Google Scholar]
- 25.Groh, V., J. Wu, C. Yee, and T. Spies. 2002. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419734-738. [DOI] [PubMed] [Google Scholar]
- 26.Hershkovitz, O., S. Jivov, N. Bloushtain, A. Zilka, G. Landau, A. Bar-Ilan, R. Lichtenstein, K. Campbell, T. Kuppevelt, and A. Porgador. 2007. Characterization of the recognition of tumor cells by the natural cytotoxicity receptor, NKp44. Biochemistry 467426-7436. [DOI] [PubMed] [Google Scholar]
- 27.Hershkovitz, O., M. Jarahian, A. Zilka, A. Bar-Ilan, G. Landau, S. Jivov, Y. Tekoah, R. Glicklis, J. T. Gallagher, S. C. Hoffmann, H. Zer, O. Mandelboim, C. Watzl, F. Momburg, and A. Porgador. 2008. Altered glycosylation of recombinant NKp30 hampers binding to heparan sulfate: a lesson for the use of recombinant immuno-receptors as an immunological tool. Glycobiology 1828-41. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda, H., L. J. Old, and R. D. Schreiber. 2002. The roles of IFNγ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 1395-109. [DOI] [PubMed] [Google Scholar]
- 29.Jarahian, M., C. Watzl, Y. Issa, P. Altevogt, and F. Momburg. 2007. Blockade of natural killer cell-mediated lysis by NCAM140 expressed on tumor cells. Int. J. Cancer. 1202625-2634. [DOI] [PubMed] [Google Scholar]
- 30.Kirn, D., R. L. Martuza, and J. Zwiebel. 2001. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat. Med. 7783-789. [DOI] [PubMed] [Google Scholar]
- 31.Lamb, R. A., and D. Kolakofsky. 2001. Paramyxoviridae: the viruses and their replication, p. 1305-1340. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 32.Lanier, L. L. 2005. NK cell recognition. Annu. Rev. Immunol. 23225-274. [DOI] [PubMed] [Google Scholar]
- 33.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14461-470. [DOI] [PubMed] [Google Scholar]
- 34.Mandelboim, O., N. Lieberman, M. Lev, L. Paul, T. I. Arnon, Y. Bushkin, D. M. Davis, J. L. Strominger, J. W. Yewdell, and A. Porgador. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 4091055-1060. [DOI] [PubMed] [Google Scholar]
- 35.Martín-Fontecha, A., L. L. Thomsen, S. Brett, C. Gerard, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat. Immunol. 51260-1265. [DOI] [PubMed] [Google Scholar]
- 36.Mavoungou, E., J. Held, L. Mewono, and P. G. Kremsner. 2007. A Duffy binding-like domain is involved in the NKp30-mediated recognition of Plasmodium falciparum-parasitized erythrocytes by natural killer cells. J. Infect. Dis. 1951521-1531. [DOI] [PubMed] [Google Scholar]
- 37.Moretta, A., C. Bottino, M. Vitale, D. Pende, C. Cantoni, M. C. Mingari, R. Biassoni, and L. Moretta. 2001. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19197-223. [DOI] [PubMed] [Google Scholar]
- 38.Moretta, L., and A. Moretta. 2004. Killer immunoglobulin-like receptors. Curr. Opin. Immunol. 16626-633. [DOI] [PubMed] [Google Scholar]
- 39.Newman, K. C., and E. M. Riley. 2007. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat. Rev. Immunol. 7279-291. [DOI] [PubMed] [Google Scholar]
- 40.Parham, P., C. J. Barnstable, and W. F. Bodmer. 1979. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C antigens. J. Immunol. 123342-349. [PubMed] [Google Scholar]
- 41.Paulson, J., J. Weinstein, L. Dorland, H. van Halbeek, and J. Vliegenthart. 1982. Newcastle disease virus contains a linkage-specific glycoprotein sialidase. Application to the localization of sialic acid residues in N-linked oligosaccharides of α1-acid glycoprotein. J. Biol. Chem. 25712734-12738. [PubMed] [Google Scholar]
- 42.Pogge von Strandmann, E., V. R. Simhadri, B. von Tresckow, S. Sasse, K. S. Reiners, H. P. Hansen, A. Rothe, B. Böll, V. L. Simhadri, P. Borchmann, P. J. McKinnon, M. Hallek, and A. Engert. 2007. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 27965-974. [DOI] [PubMed] [Google Scholar]
- 43.Reichard, K. W., R. M. Lorence, C. J. Cascino, M. E. Peeples, R. J. Walter, M. B. Fernando, H. M. Reyes, and J. A. Greager. 1992. Newcastle disease virus selectively kills human tumor cells. J. Surg. Res. 52448-453. [DOI] [PubMed] [Google Scholar]
- 44.Rogge, L., L. Barberis-Maino, M. Biffi, N. Passini, D. H. Presky, U. Gubler, and F. Sinigaglia. 1997. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 185825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salih, H. R., H. G. Rammensee, and A. Steinle. 2002. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J. Immunol. 1694098-4102. [DOI] [PubMed] [Google Scholar]
- 46.Sanderson, S., and N. Shastri. 1994. LacZ inducible, antigen/MHC-specific T cell hybrids. Int. Immunol. 6369-376. [DOI] [PubMed] [Google Scholar]
- 47.Sato, K., S. Hida, H. Takayanagi, T. Yokochi, N. Kayagaki, K. Takeda, H. Yagita, K. Okumura, N. Tanaka, T. Taniguchi, and K. Ogasawara. 2001. Antiviral response by natural killer cells through TRAIL gene induction by IFN-α/β. Eur. J. Immunol. 313138-3146. [DOI] [PubMed] [Google Scholar]
- 48.Schirrmacher, V., C. Haas, R. Bonifer, and C. Ertel. 1997. Virus potentiation of tumor vaccine T-cell stimulatory capacity requires cell surface binding but not infection. Clin. Cancer Res. 31135-1148. [PubMed] [Google Scholar]
- 49.Schirrmacher, V., T. Ahlert, T. Pröbstle, H. H. Steiner, C. Herold-Mende, R. Gerhards, E. Hagmüller, and H. H. Steiner. 1998. Immunization with virus-modified tumor cells. Semin. Oncol. 25677-696. [PubMed] [Google Scholar]
- 50.Schirrmacher, V., C. Haas, R. Bonifer, T. Ahlert, R. Gerhards, and C. Ertel. 1999. Human tumor cell modification by virus infection: an efficient and safe way to produce cancer vaccine with pleiotropic immune stimulatory properties when using Newcastle disease virus. Gene Ther. 663-73. [DOI] [PubMed] [Google Scholar]
- 51.Schirrmacher, V., L. Bai, V. Umansky, L. Yu, Y. Xing, and Z. Qian. 2000. Newcastle disease virus activates macrophages for antitumor activity. Int. J. Oncol. 16363-373. [PubMed] [Google Scholar]
- 52.Schirrmacher, V., A. Griesbach, and T. Ahlert. 2001. Antitumor effects of Newcastle Disease Virus in vivo: local versus systemic effects. Int. J. Oncol. 18945-952. [DOI] [PubMed] [Google Scholar]
- 53.Schirrmacher, V. 2005. Clinical trials of antitumor vaccination with an autologous tumor cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory. Cancer Immunol. Immunother. 54587-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulze, T., W. Kemmner, J. Weitz, K. D. Wernecke, V. Schirrmacher, and P. M. Schlag. 2009. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol. Immunother. 5861-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seal, B. S., D. J. King, and H. S. Sellers. 2000. The avian response to Newcastle disease virus. Dev. Comp. Immunol. 24257-268. [DOI] [PubMed] [Google Scholar]
- 56.Steiner, H. H., M. M. Bonsanto, P. Beckhove, M. Brysch, K. Geletneky, R. Ahmadi, R. Schuele-Freyer, P. Kremer, G. Ranaie, D. Matejic, H. Bauer, M. Kiessling, S. Kunze, V. Schirrmacher, and C. Herold-Mende. 2004. Antitumor vaccination of patients with glioblastoma multiforme: a pilot study to assess feasibility, safety, and clinical benefit. J. Clin. Oncol. 224272-4281. [DOI] [PubMed] [Google Scholar]
- 57.Tam, Y. K., G. Maki, B. Miyagawa, B. Henneman, T. Tonn, and H. G. Klingemann. 1999. Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum. Gene Ther. 101359-1373. [DOI] [PubMed] [Google Scholar]
- 58.Termeer, C. C., V. Schirrmacher, E. B. Bröcker, and J. C. Becker. 2000. Newcastle disease virus infection induces B7-1/B7-2-independent T-cell costimulatory activity in human melanoma cells. Cancer Gene Ther. 7316-323. [DOI] [PubMed] [Google Scholar]
- 59.Tiwari, N., N. Garbi, T. Reinheckel, G. Moldenhauer, G. J. Hämmerling, and F. Momburg. 2007. A transporter associated with antigen-processing independent vacuolar pathway for the MHC class I-mediated presentation of endogenous transmembrane proteins. J. Immunol. 1787932-7942. [DOI] [PubMed] [Google Scholar]
- 60.Umansky, V., V. A. Shatrov, V. Lehmann, and V. Schirrmacher. 1996. Induction of nitric oxide synthesis in macrophages by Newcastle disease virus is associated with activation of nuclear factor κB. Int. Immunol. 8491-498. [DOI] [PubMed] [Google Scholar]
- 61.Vankayalapati, R., B. Wizel, S. E. Weis, H. Safi, D. L. Lakey, O. Mandelboim, B. Samten, A. Porgador, and P. F. Barnes. 2002. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J. Immunol. 1683451-3457. [DOI] [PubMed] [Google Scholar]
- 62.Vieillard, V., J. L. Strominger, and P. Debre. 2005. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc. Natl. Acad. Sci. USA 10210981-10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wallace, M. E., and M. J. Smyth. 2005. The role of natural killer cells in tumor control—effectors and regulators of adaptive immunity. Springer Semin. Immunopathol. 2749-64. [DOI] [PubMed] [Google Scholar]
- 64.Washburn, B., M. A. Weigand, A. Grosse-Wilde, M. Janke, H. Stahl, E. Rieser, M. R. Sprick, V. Schirrmacher, and H. Walczak. 2003. TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle disease virus. J. Immunol. 1701814-1821. [DOI] [PubMed] [Google Scholar]
- 65.Welte, S. A., C. Sinzger, S. Z. Lutz, H. Singh-Jasuja, K. L. Sampaio, U. Eknigk, H. G. Rammensee, and A. Steinle. 2003. Selective intracellular retention of virally induced NKG2D ligands by the human cytomegalovirus UL16 glycoprotein. Eur. J. Immunol. 33194-203. [DOI] [PubMed] [Google Scholar]
- 66.Wu, J., N. J. Chalupny, T. J. Manley, S. R. Riddell, D. Cosman, and T. Spies. 2003. Intracellular retention of the MHC class I-related chain B ligand of NKG2D by the human cytomegalovirus UL16 glycoprotein. J. Immunol. 1704196-4200. [DOI] [PubMed] [Google Scholar]
- 67.Zaitsev, V., M. von Itzstein, D. Groves, M. Kiefel, T. Takimoto, A. Portner, and G. Taylor. 2004. Second sialic acid binding site in Newcastle disease virus hemagglutinin-neuraminidase: implications for fusion. J. Virol. 783733-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng, J., P. Fournier, and V. Schirrmacher. 2002. Induction of interferon-α and tumor necrosis factor-related apoptosis-inducing ligand in human blood mononuclear cells by hemagglutinin-neuraminidase but not F protein of Newcastle disease virus. Virology 29719-30. [DOI] [PubMed] [Google Scholar]
- 69.Zeng, J., P. Fournier, and V. Schirrmacher. 2004. High cell surface expression of Newcastle disease virus proteins via replicon vectors demonstrates syncytia forming activity of F and fusion promotion activity of HN molecules. Int. J. Oncol. 25293-302. [PubMed] [Google Scholar]
- 70.Zorn, U., I. Dallmann, J. Grosse, H. Kirchner, H. Poliwoda, and J. Atzpodien. 1994. Induction of cytokines and cytotoxicity against tumor cells by Newcastle disease virus. Cancer Biother. 9225-235. [DOI] [PubMed] [Google Scholar]
- 71.Zorn, U., S. Duensing, F. Langkopf, G. Anastassiou, H. Kirchner, M. Hadam, J. Knüver-Hopf, and J. Atzpodien. 1997. Active specific immunotherapy of renal cell carcinoma: cellular and humoral immune responses. Cancer Biother. Radiopharm. 12157-165. [DOI] [PubMed] [Google Scholar]