Abstract
We previously demonstrated that herpes simplex virus type 1 (HSV-1) preferentially establishes latent infection in monoclonal antibody (MAb) A5-positive ganglionic neurons and that a 2.8-kb portion of the HSV-1 genome, corresponding to the 5′ end of the LAT (latency-associated transcript) coding region, is responsible for this phenotype (38, 65). In the current study we carried out further genetic mapping of this latency phenotype and investigated some of the mechanisms that might be responsible. Studies with the chimeric virus HSV-1 17syn+/LAT2, an HSV-1 virus engineered to express HSV-2 LAT, demonstrated that this virus exhibited an HSV-2 latency phenotype, preferentially establishing latency in MAb KH10-positive neurons. This result is complementary to that previously described for the chimeric virus HSV-2 333/LAT1 and indicate that the HSV-1 latency phenotype can be changed to that of HSV-2 by substitution of a 2.8-kb piece of complementary viral DNA. Sequential studies in which we evaluated the pattern of HSV-1 latent infection of the mouse trigeminal ganglion following ocular inoculation with viruses with deletions of functional thymidine kinase, glycoprotein E, ICP0, and US9 protein demonstrate that preferential establishment of HSV-1 latent infection in A5-positive neurons is not a consequence of (i) differential access of HSV-1 to A5-positive neurons,(ii) differential cell-to-cell spread of HSV-1 to A5-positive neurons, (iii) differential “round-trip” spread of HSV-1 to A5-positive neurons, or (iv) expression of ICP0. Additional mapping studies with the HSV-1 LAT deletion viruses dLAT371, 17ΔSty, and 17Δ348 indicate that most of the LAT 5′ exon is not required for HSV-1 to preferentially establish latent infection in A5-positive neurons.
Primary infection with herpes simplex virus (HSV) is characterized by local viral replication at the site of inoculation as well as retrograde axonal transport of the virus to regional sensory ganglia where a latent infection may be established. Sensory ganglia are comprised of a heterogeneous population of neurons, and work in our laboratory has demonstrated that different subpopulations of murine ganglionic neurons have different outcomes of infection with HSV (38, 39, 65). Of the subpopulations of ganglionic neurons that we have studied, those neurons recognized by monoclonal antibodies (MAbs) A5 (specific for a population of neurons expressing Galβ1-4GlcNAc-R epitopes) and KH10 (specific for a different population of ganglionic neurons expressing Galα1-3Galβ1-4NAc-R epitopes) have the most distinct susceptibility phenotypes (19, 22, 65). Although all neuronal subpopulations appear to be capable of supporting a productive infection with either HSV type 1 (HSV-1) or HSV-2, as assayed by in situ hybridization for the latency-associated transcripts (LAT), HSV-1 preferentially establishes a latent infection in A5-positive neurons whereas HSV-2 preferentially establishes a latent infection in KH10-positive neurons (38), a pattern that is observed following both ocular and footpad inoculation.
In the mouse trigeminal ganglion (TG) MAbs A5 and KH10 recognize functionally distinct neuronal populations. Most A5-positive neurons are immunoreactive for neuropeptides and the high affinity nerve growth factor receptor (and terminate in lamina I and IIa of the dorsal horn of the spinal cord), whereas KH10-positive neurons colabel with the lectin BSL-IB4 (Bandeiraea simplicifolia isolectin B4), identifying them as a population of small-diameter, peripherin-positive, glial cell line-derived neurotrophic factor-responsive, but vanilloid receptor-negative neurons that terminate largely in lamina IIb of the dorsal horn of the spinal cord (3, 19, 38, 42, 52, 67). These observations highlight the importance of studying the interaction of HSV with different sensory neuronal subtypes in order to gain a complete understanding of viral latency and reactivation.
Latent infection with HSV is characterized by limited viral transcription except for the LAT, which can accumulate to high copy numbers in the nuclei of latently infected cells (54). The LAT code from the long repeat region of the viral genome antisense to the 3′ domain of the ICP0 gene. Studies from multiple laboratories suggest that the LAT region of the viral genome plays an important role in both the establishment and reactivation of latent infection (reviewed in reference 29). The mechanisms responsible may involve functions that promote neuronal survival (1, 24, 45, 60), interfere with interferon expression (44), inhibit expression of ICP0 or ICP4 (62), inhibit transactivation by ICP0 (21), reduce productive cycle gene expression (23, 36), or stimulate expression of heat shock proteins (2).
Recent work from our laboratory suggests that the LAT coding region also plays a key role in directing which subpopulations of ganglionic neurons become latently infected. In these studies we assayed the LAT-positive sites of latent viral infection in mice inoculated with a chimeric HSV-2 virus engineered to express 2.8-kb of the HSV-1 LAT (the promoter and most of exon 1) instead of native LAT (HSV-2 333/LAT1). Our results clearly indicated that, unlike HSV-2, HSV-2 333/LAT1 preferentially established latent infection in A5-positive neurons, similar to wild-type HSV-1 (38). Since HSV-2 333/LAT1 also displays an HSV-1 recurrence phenotype in guinea pig genital and rabbit eye models (66), it is possible that this region of LAT exerts its effects on viral reactivation by directing the neuronal populations in which latent infection is established.
The studies summarized above strongly suggest that different neuronal subtypes have different degrees of permissiveness for productive and latent infection with HSV-1 and HSV-2. These studies further suggest that a function coded by the LAT region of the viral genome plays an important role in this process. The current study was designed to begin to investigate the mechanisms by which HSV LAT sequences modulate preferential establishment of latent infection in neuronal subpopulations of the mouse TG as well as to further map the location of the LAT sequences responsible. Initial studies focused on determining viral strain specificity of the HSV-1 and HSV-2 latency phenotypes as well as the latency phenotype of HSV-1 17syn+/LAT2, a chimeric HSV-1 virus engineered to express 2.8-kb of the HSV-2 LAT instead of native LAT. Since the 2.8-kb piece of the LAT regions exchanged in both HSV-1 17syn+/LAT2 and HSV-2 333/LAT1 overlap the 5′ end of the ICP0 coding regions, we also tested whether expression of the HSV-1 immediate-early protein ICP0 was necessary for the preferential establishment of HSV-1 latent infection in A5-positive neurons. We also studied whether mechanisms other than differential neuronal permissiveness for productive viral infection could account for the preferential establishment of HSV-1 latent infection in A5-positive neurons. Specific mechanisms tested included the following: (i) differential viral access to neuronal subpopulations, (ii) viral “round-trip” spread (when viral progeny travel by anterograde axonal transport either to the brainstem or out to the periphery and back again), and (iii) viral cell-to-cell spread. Finally, using LAT deletion mutants, we further mapped that portion of the viral genome responsible for the preferential establishment of HSV-1 latent infection in A5-positive neurons.
MATERIALS AND METHODS
Viruses.
Laboratory strains of wild-type HSV-1 included KOS, 17syn+, McKrae, and F. Laboratory strains of wild-type HSV-2 included MS and HG52. Construction of the LAT chimeric virus HSV-1 17syn+/LAT2 and its rescuant were previously described (26). In brief, a NotI-XhoI fragment of HSV-2 strain MS, containing the LAT promoter, the 5′ end of primary LAT, and most of the LAT intron, was recombined into a homologous site of HSV-1 strain 17syn+. The rescuant (HSV-1 17syn+/LAT2R) was created by homologous recombination of the chimeric virus with a fragment of wild-type HSV-1 strain 17syn+. The ICP0 null mutant n212 (carrying a nonsense mutation at codon 212) (11) was a generous gift of P. A. Schaffer. The thymidine kinase deletion mutants dlsptk (14) and tkLTRZ1 (where tk is thymidine kinase) (15) were generous gifts of D. M. Coen. The US9 deletion mutant US9− HSV (49) and the glycoprotein E (gE) deletion mutant F-gEβ (50) were generous gifts of D. C. Johnson. The LAT StyI-StyI deletion mutants dLAT371 (47) and 17ΔSty (37) were generous gifts of S. L. Wechsler and N. W. Fraser, respectively. The 5′ LAT deletion mutant 17Δ348 was a generous gift of D. C. Bloom (7). The low-passage-number clinical isolates of HSV-1 (AD1 and ST1), from patients cared for at the Proctor Medical Group at the University of California at San Francisco, were typed using a Syva Microtrak HSV1/HSV2 Direct Specimen Identification/Typing Test. The low-passage-number clinical isolate HSV-2 (S) was previously described (9). All viruses were propagated in rabbit skin cells as previously described (65) except for n212, which was propagated in L7 cells, and titers were determined in L7 cells. Viral titers were determined by standard viral plaque assay.
Animals and inoculations.
Six-week-old female Swiss Webster mice (Simonsen Laboratories, Gilroy, CA) were anesthetized by intraperitoneal injection with sodium pentobarbital, followed by topical administration of 0.5% proparacaine hydrochloride. Following corneal scarification eyes were inoculated with 10 μl of viral stock (KOS, 5 × 107 PFU/ml; McKrae, 1 × 108 PFU/ml; 17syn+, 4 × 107 PFU/ml; F, 1 × 106 PFU/ml; AD1, 5 × 107 PFU/ml; ST1, 2 × 108 PFU/ml; 333, 2 × 108 PFU/ml; MS, 1 × 108 PFU/ml; HG52, 5 × 106 PFU/ml; S, 1 × 106 PFU/ml; dlsptk, 8 × 107 PFU/ml; tkLTRZ1, 9 × 107 PFU/ml; n212, 5 × 107 PFU/ml; US9− HSV, 1 × 106 PFU/ml; F-gEβ, 1 × 107 PFU/ml; 17ΔSty, 1 × 108 PFU/ml; 17Δ348, 1 × 108 PFU/ml; dLAT371, 1 × 106 PFU/ml; HSV-1 17syn+/LAT2, 1 × 108 PFU/ml; HSV-1 17syn+/LAT2R, 1 × 108 PFU/ml). Forty hours after inoculation, 1.2 mg/ml acyclovir was added to the drinking water (except for mice infected with KOS-based viruses). Treatment with acyclovir was necessary in order to reduce animal mortality. As described previously, treatment with acyclovir in this manner allows virus to replicate in the TG while limiting viral spread to the brain (34, 38). However, for some viral strains used in this study (HSV-1 strains F, US9− HSV, and dLAT371 and HSV-2 strains HG52 and S), administering acyclovir in the water did not significantly reduce mortality, necessitating inoculation of mice with lower titers of these viruses. At 21 days postinoculation, mice were euthanized by carbon dioxide inhalation and thoracotomy. Cardiac perfusion with 0.1 M phosphate-buffered saline (pH 7.4) was performed immediately, followed by perfusion with 1% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Dissected TG were immersion fixed in 1% paraformaldehyde in 0.1 M phosphate buffer for 30 min at 4°C, followed by equilibration in 30% sucrose in 0.1 M phosphate-buffered saline at 4°C. Fixed ganglia were embedded in Tissue-Tek O.C.T. compound (Sakura Finetechnical, Tokyo, Japan) in sets of 8 to 10 ganglia per block and frozen in liquid nitrogen. Serial sections (7 μm) were collected as four alternate sets onto Superfrost/Plus microscope slides (Fisher Scientific, Pittsburgh, PA) and stored at −80°C. In this way we generated four equivalent sets of 28-μm-step sections through each block of tissue. These studies were approved by the University of California at San Francisco Committee on Animal Research.
Measurement of viral titers from the ocular surface and TG.
Tear film samples were collected with a cotton-tipped applicator, and the tip was transferred into 0.4 ml of minimal essential medium containing 5% fetal bovine serum, and titers were determined immediately on 96-well microtiter plates. TG were collected at 1 to 5 days after ocular inoculation (5 ganglia per time point) and homogenized in 0.5 ml of minimal essential medium containing 5% fetal bovine serum, frozen for 1 to 2 days at −80°C, and titers were then determined. Ganglionic viral titers were determined by a plaque assay in six-well plates under medium containing 0.5% methylcellulose. Titers of KOS were determined on Vero cell monolayers, and titers of n212 virus were determined on L7 cells.
Preparation of plasmid Yz-2LAT containing a partial fragment of HSV-2 LAT intron sequence.
To prepare an HSV-2 LAT intron-specific probe, a 451-bp fragment (nucleotides [nt] 120394 to 120844) of HSV-2 strain 333 genomic DNA was PCR amplified using the following primers: 5′-CCG CGA GCC AAC CCG TAT CCT TTT T-3′ and 5′-ACA ACA CGG CCC ACC ACG ACA CAA C-3′. Amplified products were electrophoresed in 1.5% agarose, purified using a QIAEX II Gel Extraction Kit (Qiagen GmbH, Hilden, Germany), ligated into pCR4-TOPO (Invitrogen, Carlsbad, CA), and transformed into TOP10F competent cells (Invitrogen). Colonies were selected by ampicillin, and plasmids were extracted by QIAprep Miniprep (Qiagen). The sequence of the final plasmid (pYz-2LAT) was confirmed by DNA sequencing.
Preparation of DIG-labeled RNA probe for fluorescent in situ hybridization (FISH).
The plasmid pATD19, which contains a 347-bp fragment of HSV-1 genomic DNA (nt 119629 to 119975), served as the template for an HSV-1 LAT-specific probe (40). The plasmid pYz-2LAT served as a template for an HSV-2 LAT-specific probe. To synthesize digoxigenin (DIG)-labeled RNA probes, linearized plasmids were incubated with DIG RNA Labeling Mix (Roche) and T3 polymerase for 3 h at 37°C. After treatment with DNase I, probes were purified by using G-50 Sephadex columns for biotinylated RNA purification (Roche) and dissolved into 50% formamide in nuclease-free water. Probe concentrations were checked on nylon membranes against DIG-labeled control RNA as described by the manufacturer (Roche).
Combined staining by FISH and IF.
Frozen tissue sections were defrosted, and combined staining by FISH and immunofluorescence (IF) was carried out as previously described (38). Every fourth tissue section taken from a minimum of two blocks of tissue (each containing 8 to 10 TG) was evaluated for each virus that was studied. Sections that had poor tissue quality were excluded from evaluation as were sections that had poor FISH or IF signal-to-noise ratios. By taking this strict approach, we stained tissue sections representative of all experimental ganglia but evaluated only those tissue sections in which unambiguous scoring of LAT-positive neurons (A5- or KH10-positive) could be carried out. Despite these precautions, 2 to 3% of LAT-positive neurons in these sections had ambiguous staining patterns and were excluded from the data set. To determine the percentage of LAT-positive neurons that colabeled with MAbs A5 or KH10, we evaluated a minimum of 200 LAT-positive neurons per MAb but frequently evaluated more. Ongoing internal audits in our lab indicate that results do not change when we evaluate a greater number of LAT-positive neurons. If pilot studies revealed apparent differences in the latent distribution of LAT-positive neurons in mice infected with an experimental virus compared to its wild-type control, scoring was repeated in a masked fashion. In the current study this specifically applied to tissue sections evaluated from mice infected with HSV-1 17syn+/LAT2, dlsptk, and tkLTRZ1 (and their respective controls).
RESULTS
Preferential establishment of latent HSV infections is not virus strain specific.
We previously demonstrated that, as assayed by in situ hybridization for LAT, both KOS and 17syn+ strains of HSV-1 preferentially establish latent infection of murine sensory ganglia in A5-positive neurons and that HSV-2 strain 333 preferentially establishes latent infection in KH10-positive neurons (38). In order to exclude the possibility that these viral phenotypes were unique to the KOS and 17syn+ strains of HSV-1 and the 333 strain of HSV-2, we evaluated the latent phenotype of additional laboratory virus strains (F and McKrae strains of HSV-1 and MS and HG52 strains of HSV-2) and low-passage-number human isolates (AD1 and ST1). Data from these studies are summarized in Table 1, where the results are expressed as the proportion of LAT-positive TG neurons that are also A5 or KH10 positive.
TABLE 1.
Distribution of latently infected neurons in murine TG following ocular inoculation with different strains of HSV-1 and HSV-2
| Strain (type) | % of LAT-positive neurons colabeled as indicated (no. of dual-labeled neurons/no. of LAT-positive neurons tested)a
|
|
|---|---|---|
| A5-positive | KH10-positive | |
| KOS (HSV-1) | 41.1 (184/448) | 2.7 (9/334) |
| 17 syn+ (HSV-1) | 39.0 (133/341) | 3.1 (11/353) |
| F (HSV-1) | 37.5 (281/748) | 7.7 (14/205) |
| McKrae (HSV-1) | 38.9 (106/272) | 2.8 (7/249) |
| AD1 (HSV-1 clinical isolate) | 48.1 (113/235) | 3.2 (9/278) |
| ST1 (HSV-1 clinical isolate) | 43.7 (184/421) | 2.3 (7/303) |
| MS (HSV-2) | 4.4 (18/409) | 52.1 (182/349) |
| HG52 (HSV-2) | 2.8 (8/289) | 43.5 (90/207) |
| 333 (HSV-2)b | 3.8 (18/468) | 40.8 (161/394) |
| S (HSV-2 clinical isolate) | 6.0 (14/231) | 52.4 (108/206) |
| Overall ganglionic compositionc | 11.5 (241/2,090) | 13.6 (301/2,209) |
Twenty-one days after ocular inoculation, sections of latently infected TG were assayed by combined FISH and IF.
Previously published data (38) presented for the purposes of comparison only.
For reference, the overall distribution of A5- and KH10-positive neurons (LAT-positive and LAT-negative) in ganglia latently infected with HSV-1 KOS is given.
Following ocular inoculation, as assayed by in situ hybridization for LAT, the F and McKrae strains of HSV-1 preferentially established latent infection in A5-positive neurons of the murine TG, with a similar neuronal distribution to that observed following infection with KOS and 17syn+. Very similar results were observed following inoculation with two low-passage-number HSV-1 clinical isolates (AD1 and ST1). In contrast, the MS and HG52 strains of HSV-2 and a low-passage-number clinical isolate of HSV-2 (strain S) preferentially established latent infection in KH10-positive neurons, a pattern very similar to that previously observed with the 333 strain of HSV-2 and very different from that observed following infection with HSV-1. These data indicate that the preferential establishment of HSV-1 latent infection in A5-positive neurons and HSV-2 latent infection in KH10-positive neurons is not restricted to the KOS and 17syn+ strains of HSV-1 and the 333 strain of HSV-2.
Treatment with acyclovir was necessary in order to keep mice alive in all experiments except those in which KOS-based viruses were used. As described previously, the treatment protocol that we used allows virus to replicate in the TG while limiting viral spread to the brain (34, 38). This was evidenced by an abundance of productively infected cells in immunostained tissue sections at 84 h after ocular inoculation (data not shown) as well as a lower mortality rate of these mice. However, for some viral strains (HSV-1 strains F, US9− HSV, and dLAT371 and HSV-2 strains HG52 and S), our treatment protocol did little to reduce mortality unless we also reduced the concentration of the viral inoculum by 0.5 to 1.5 logs compared to that used for KOS. Note that the distribution of LAT-positive neurons in latently infected ganglia following ocular inoculation with either HSV-1 or HSV-2 at these lower doses was very similar to that observed with less virulent viral strains. These results suggested that preferential establishment of HSV latent infection is independent of the size of the viral inoculum. To test this issue directly, we studied the distribution of LAT-positive neurons in latently infected TG following ocular inoculation with the HSV-1 KOS strain at a concentration of 1 × 105 PFU/ml. The results were almost identical to those presented in Table 1 (where the viral inoculum was almost 1,000-fold greater); 42.6% (93/218) of the LAT-positive neurons colabeled with MAb A5, and 4.6% (12/263) of the LAT-positive neurons colabeled with MAb KH10.
A chimeric HSV-1 virus that expresses the HSV-2 LAT intron establishes latent infection preferentially in KH10-positive neurons.
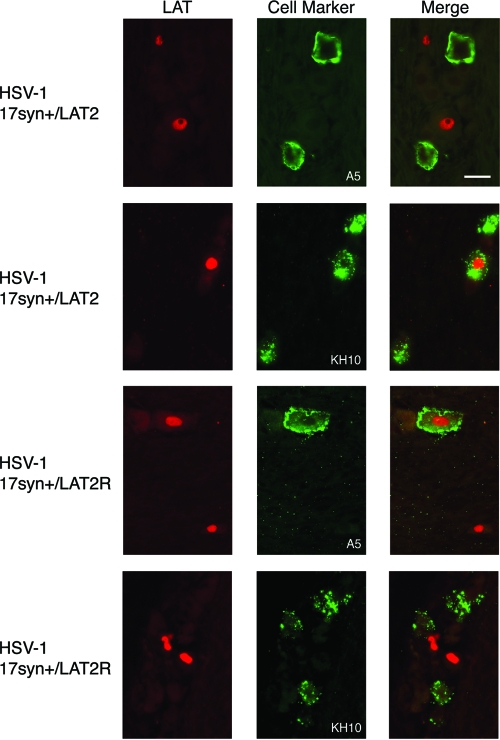
We previously demonstrated that, as assayed by in situ hybridization for LAT, a chimeric HSV-2 virus engineered to express HSV-1 LAT instead of native LAT (HSV-2 333/LAT1) preferentially established latent infection in A5-positive neurons, similar to HSV-1. Given this observation, we hypothesized that a chimeric HSV-1 virus engineered to express the HSV-2 LAT instead of native LAT (HSV-1 17syn+/LAT2) (26) would preferentially establish latent infection in KH10-positive neurons, similar to HSV-2. To test this hypothesis, the corneas of mice were inoculated with HSV-1 17syn+/LAT2 or the rescuant virus 17+syn/LAT2R. As summarized in Table 2 and illustrated in Fig. 1, in situ hybridization for LAT indicated that 17syn+/LAT2 established latent infection with the HSV-2 phenotype; 42.7% of the LAT-positive neurons in TG were KH10 positive, while only 9% expressed the A5 marker. In contrast, the viral rescuant HSV-1 17+syn/LAT2R established latent infection with an HSV-1 phenotype, with a preference for A5-positive neurons. Therefore, replacement of a 2.8-kb piece of the HSV-1 LAT coding region with a similar portion of the HSV-2 LAT genome significantly altered the latent distribution of HSV-1. These data strongly suggest that the different patterns of latent infection observed with HSV-1 and HSV-2 are due to a viral function associated with a 2.8-kb portion of the LAT coding region, the same conclusion that we reached after evaluating latent infection with HSV-2 333/LAT1, a chimeric HSV-2 virus engineered to express 2.8-kb of the HSV-1 LAT instead of native LAT.
TABLE 2.
Distribution of latently infected neurons in murine TG following ocular inoculation with HSV-1 17syn+/LAT2
| Virusa | % of LAT-positive neurons colabeled as indicated (no. of dual-labeled neurons/ no. of LAT-positive neurons tested)b
|
|
|---|---|---|
| A5-positive | KH10-positive | |
| HSV-1 17syn+/LAT2 | 9.0 (36/402) | 42.7 (132/309) |
| HSV-1 17syn+/LAT2R | 25.4 (72/283) | 1.8 (4/218) |
| 17syn+ (HSV-1) | 39.0 (133/341) | 3.1 (11/353) |
| MS (HSV-2) | 4.4 (18/409) | 52.1 (182/349) |
For purposes of comparison, data from Table 1 on the latent distribution of 17syn+ (HSV-1) and MS (HSV-2) are included.
Twenty-one days after ocular inoculation of mice with either HSV-1 17syn+/LAT2 (an HSV-1 chimera engineered to express HSV-2 LAT) or its viral rescuant (HSV-1 17syn+/LAT2R), TG were removed and assayed by combined FISH and IF analysis for LAT and neuronal markers.
FIG. 1.
Identification of TG neurons latently infected with 17syn+/LAT2 or 17syn+/LAT2R. Sections of murine TG were assayed by both in situ hybridization for LAT (red-labeled nuclei) and IF staining with MAbs A5 and KH10 (green-labeled cell bodies) in order to identify the neuronal subpopulations latently infected with either a chimeric virus or its rescuant. HSV-2-specific probes were used to detect LAT expression in 17syn+/LAT2-infected tissue, and HSV-1-specific probes were used to detect LAT expression in 17syn+/LAT2R-infected tissue. In these representative images, LAT expression of 17syn+/LAT2 is colocalized with a KH10-positive neuron but not with A5-positive neurons. In contrast, LAT expression of 17syn+/LAT2R is colocalized with an A5-positive neuron but not with KH10-positive neurons. Bar, 20 μm.
Preferential establishment of HSV-1 latent infection in A5-positive neurons depends upon viral replication in neurons but is not a consequence of differential access of the virus to these neurons.
We previously noted an effect of acyclovir on the proportion of HSV-1 KOS latently infected trigeminal neurons that were A5 positive and thus hypothesized that viral replication may play a role in this phenomenon. To further explore this finding and to determine whether preferential establishment of HSV-1 latent infection in A5-positive neurons was due to differential access of the virus to A5-positive neurons, we evaluated the latent distribution of two different thymidine kinase deletion mutants, dlsptk (14) and tkLTRZ1 (15), in murine TG after ocular inoculation. These viral deletion mutants replicate well in corneal epithelial cells and are transported in a retrograde fashion to neuronal cell bodies in the TG, where a latent infection is established. However, since neurons do not express thymidine kinase, productive viral infection does not occur in ganglia infected with these deletion mutants (14, 58, 59). We therefore reasoned that if HSV-1 has equal access to A5-positive and KH10-positive ganglionic neurons, latent infection with a thymidine kinase deletion mutant should distribute equally among these neuronal subtypes, a very different outcome than occurs with wild-type virus.
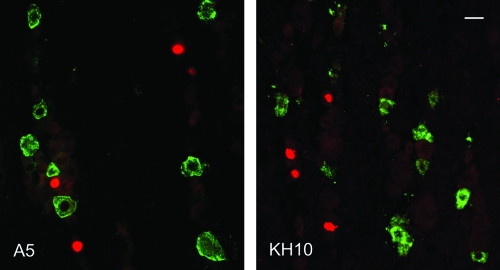
As summarized in Table 3 and illustrated in Fig. 2, the pattern of viral latent infection of the TG with either dlsptk or tkLTRZ1, as assayed by in situ hybridization for LAT, was very different from that with wild-type HSV-1. Unlike wild-type HSV-1, the thymidine kinase-deficient viruses did not preferentially establish latency in A5-positive neurons. Instead, they established latency somewhat equally among neuronal subtypes in a pattern that closely reflected the relative distribution of A5-positive and KH10-positive neurons in the ganglion. We conclude that the preferential distribution of HSV-1 latent infection in A5-positive neurons is not due to differential access of the virus to these neurons. These data also provide further support for the hypothesis that preferential establishment of latency in A5-positive neurons is not an artifact of differential LAT expression among neuronal subtypes (38). These data further imply that preferential establishment of latency in A5-positive neurons depends upon viral replication within neurons that are not A5-positive, without which there can be no loss of neuronal populations (e.g., KH10-positive) as a consequence of productive viral infection. Although we did not study viral rescuants of dlsptk and tkLTRZ1, the nearly identical latent phenotypes of these two different thymidine kinase deletion viruses strongly suggests that our results were a consequence of mutations of the thymidine kinase gene and not other viral genes.
TABLE 3.
Distribution of latently infected neurons in murine TG following ocular inoculation with HSV-1 thymidine kinase mutants
| Virus straina | % of LAT-positive neurons colabeled as indicated (no. of dual-labeled neurons/no. of LAT-positive neurons tested)b
|
|
|---|---|---|
| A5-positive | KH10-positive | |
| KOS | 41.1 (184/448) | 2.7 (9/334) |
| dlsptk | 11.4 (76/667) | 7.7 (31/402) |
| tkLTRZ1 | 10.7 (50/469) | 10.4 (37/356) |
| Overall ganglionic compositionc | 10.9 (219/2,002) | 14.4 (298/2,070) |
For purposes of comparison, data from Table 1 on the latent distribution of HSV-1 KOS (the parental strain of both dlsptk and tkLTRZ1) are included in this table.
Twenty-one days after ocular inoculation with the thymidine kinase deletion viruses dlsptk or tkLTRZ1, latently infected TG were sectioned and assayed by combined FISH and IF analysis for HSV LAT and neuronal cell markers.
For reference, the data on the overall distribution of A5- and KH10-positive neurons (LAT-positive and LAT-negative) in ganglia latently infected with dlsptk are shown.
FIG. 2.
Identification of TG neurons latently infected with tkLTRZ1. Sections of murine TG were assayed by both in situ hybridization for LAT (red-labeled nuclei) and IF staining with MAbs A5 (left) and KH10 (right) to identify the neuronal subpopulations (green-labeled cell bodies) latently infected with tkLTRZ1. In these representative images, LAT expression does not colocalize with either A5- or KH10-positive neurons. Bar, 20 μm.
Preferential establishment of HSV-1 latent infection in A5-positive neurons is not a consequence of round-trip or cell-to-cell spread.
In the course of acute ganglionic infection with HSV-1, some productively infected neurons can give rise to infectious viral progeny that travel by anterograde axonal transport either to the brainstem or out to the periphery and back again (via retrograde axonal transport) to the TG, a process referred to as round-trip spread (8, 32, 49, 53, 55, 57). In order to determine whether the preferential establishment of HSV-1 latent infection in A5-positive neurons is due to round-trip spread, we evaluated the distribution of latent HSV-1 infection in the mouse TG following ocular inoculation with US9− HSV, a US9 deletion mutant (49). This viral mutant replicates well in both epithelial cells and neurons and undergoes efficient retrograde axonal transport but does not undergo round-trip spread due to a deficiency in anterograde axonal transport (49). As summarized in Table 4, the distribution of latently infected neurons in TG latently infected with US9− HSV, as assayed by in situ hybridization for LAT, was nearly identical to that observed with the wild-type parental virus (F strain). We conclude that round-trip spread via anterograde axonal transport does not appear to be involved in the preferential establishment of latent HSV-1 infection in A5-positive neurons.
TABLE 4.
Distribution of latently infected neurons in murine TG following ocular inoculation with HSV-1 US9 and F-gEβ
| Virus strain | % of LAT-positive neurons colabeled as indicated (no. of dual-labeled neurons/ no. of LAT-positive neurons tested)a
|
|
|---|---|---|
| A5-positive | KH10-positive | |
| F | 37.5 (281/748) | 7.7 (14/205) |
| US9− HSV | 39.9 (120/301) | 3.2 (8/250) |
| F-gEβ | 40.0 (134/333) | 4.4 (14/308) |
Twenty-one days after ocular inoculation of mice with either US9− HSV or F-gEβ (a gE deletion virus), TG were removed and assayed by combined FISH and IF analysis for LAT and neuronal markers. For purposes of comparison, data from Table 1 on the latent distribution of HSV-1 F (the parental strain of both US9− HSV and F-gEβ) are included.
In order to determine whether cell-to-cell spread is involved in preferential establishment of latent infection in A5-positive neurons, we next evaluated the distribution of latent HSV-1 infection in the mouse TG following ocular inoculation with F-gEβ, an HSV-1 gE null mutant with markedly reduced cell-to-cell spread in the cornea, retina, and central nervous system (17, 18, 50). As summarized in Table 4, the distribution of latently infected neurons in TG latently infected with F-gEβ was nearly identical to that observed with the wild-type parental virus (F strain). Therefore, cell-to-cell spread, be it in the cornea or in the ganglion, does not appear to be involved in the preferential establishment of latent HSV-1 infection in A5-positive neurons.
ICP0 does not play a role in the HSV-1 preferential establishment of latency in A5-positive neurons.
As described previously, the HSV-1 17+syn/LAT2 chimeric virus was constructed so that a 2.8-kb portion of the HSV-2 LAT was substituted for a similar piece of HSV-1. This region included ∼600 bp of the LAT promoter, as well as regions coding for the first LAT exon and the entire stable LAT intron. Since the ICP0 genes in both HSV-1 and HSV-2 are encoded in the antisense direction to LAT and overlap the 3′ end of the sequences coding for the stable LAT intron, it is possible that the viral latency phenotype observed with the HSV-1 17+syn/LAT2 chimeric virus was a result of disruption of the HSV-1 ICP0 gene.
To examine this possibility, we assayed the pattern of latent infection following ocular infection with the HSV-1 (KOS) ICP0 null mutant, n212, which has a nonsense mutation at codon 212 of the ICP0 gene (10). Although n212 replicates poorly in some cell lines when inoculated at low multiplicities of infection, following ocular inoculation this virus replicates both on the ocular surface and in the TG of mice, albeit at a lower rate than wild-type virus (11). In our hands, n212 replicated well on the ocular surface of mice 24 h postinoculation (mean, 3.2 × 104 PFU/eye; n = 20 eyes) but at a level that was about 1 log less than that observed with wild-type KOS (mean, 2.9 × 105 PFU/eye; n = 20 eyes). We also found infectious virus in the TG of mice infected with n212 but with a peak infectious load that was about 1 log less than that observed following ocular inoculation with KOS (Fig. 3).
FIG. 3.
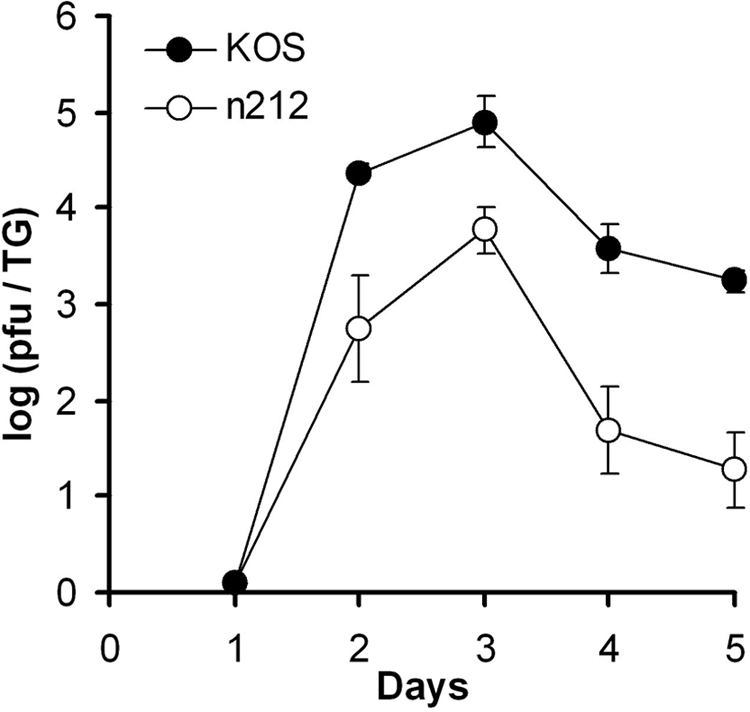
Viral titers of TG acutely infected with KOS and n212. For each time point after ocular inoculation, TG from five mice were assayed for infectious virus by standard plaque assay. Data are presented as mean and standard error of the mean per mouse.
Twenty-one days after ocular inoculation of mice with n212, we examined latently infected TG by combined in situ hybridization and immunostaining. As assayed by an in situ hybridization assay for LAT, n212 preferentially established latent infections in A5-positive neurons versus KH10-positive neurons. The percentages of LAT-positive neurons that colabeled with MAbs A5 and KH10 were 44.9% (106/236) and 3.9% (11/279), respectively, which is similar to the results observed with wild-type KOS HSV-1 (41.1% [184/448] and 2.7% [9/334], respectively). This result indicates that ICP0 does not play a role in the preferential establishment of latent HSV-1 infection in A5-positive neurons. This finding also implies that interactions between LAT and ICP0 are unlikely to be responsible for the preferential establishment of latent HSV-1 infection in A5-positive neurons.
A 476-bp region of LAT exon 1 does not play a role in the HSV-1 preferential establishment of latency in A5-positive neurons.
Since a number of well-described viral functions appear to map to the first exon of the HSV-1 LAT coding region, we hypothesized that this region of the viral genome might also play a functional role in determining the preference of HSV-1 latent infection for A5-positive neurons. To test this hypothesis, we assayed the pattern of latent infection by in situ hybridization for LAT in murine TG 21 days after ocular inoculation with either dLAT371, a McKrae-based HSV-1 with a 371-bp (StyI-StyI) deletion of LAT exon 1 (47), or 17ΔSty, a virus based on HSV-1 17syn+ with the same deletion (37). As summarized in Table 5, both deletion viruses preferentially established latent infections in A5-positive neurons, which is similar to the results observed with wild-type KOS HSV-1. To further test this hypothesis, we also assayed the pattern of latent infection in murine TG 21 days after ocular inoculation with 17Δ348, a virus containing a 348-bp deletion in LAT exon 1 starting 128 bp downstream of the deletion start site in the StyI-StyI deletion mutants (nt 119007 to 119355) (7). This deletion virus also preferentially established latent infections in A5-positive positive neurons, which is similar to results observed with wild-type KOS HSV-1 (Table 5). Taken together, these results indicate that a 476-bp region of LAT exon 1, located from 79 bp to 554 bp downstream of the LAT transcription start site, does not play a functional role in the preferential establishment of latent HSV-1 infection in A5-positive neurons.
TABLE 5.
Distribution of latently infected neurons in TG following ocular inoculation with the HSV-1 LAT mutants dLAT371, 17ΔSty, and 17Δ348
| Virus | % of LAT-positive neurons colabeled as indicated (no. of dual-labeled neurons/ no. of LAT-positive neurons tested)a
|
|
|---|---|---|
| A5-positive | KH10-positive | |
| dLAT371 | 41.1 (16/282) | 0.9 (2/224) |
| 17ΔSty | 50.5 (110/218) | 1 (2/205) |
| 17Δ348 | 58.6 (281/479) | 2.0 (6/305) |
Twenty-one days after inoculation of the ocular surface with either 17Δsty, dLAT371, or 17Δ348, TG were removed and assayed by combined FISH and IF analysis for LAT and neuronal markers.
DISCUSSION
In previous studies we demonstrated that, as assayed by in situ hybridization for LAT, HSV-1 and HSV-2 preferentially establish latent infections in different neuronal subpopulations (A5-positive or KH10-positive cells) in mouse sensory ganglia (38). We also demonstrated that a chimeric virus, HSV-2 333/LAT1, which is an HSV-2 virus engineered to express HSV-1 LAT instead of the native LAT (66), preferentially establishes latent infections in A5-positive neurons similar to wild-type HSV-1. In the current study, we found (i) that low-passage-number human isolates of HSV-1 and HSV-2 demonstrate the same latent phenotype as laboratory isolates of HSV and (ii) that the chimeric virus HSV-1 17syn+/LAT2, which is an HSV-1 virus engineered to express HSV-2 LAT (complementary to HSV-2 333/LAT1) preferentially establishes latent infections in KH10-positive neurons, as does wild-type HSV-2. These data confirm and extend our earlier work that HSV-1 and HSV-2 preferentially establish latent infections in different neuronal subpopulations and that the different latency phenotypes of HSV-1 and HSV-2 are determined by specific sequences in the LAT region of the viral genomes, the same sequences responsible for site-specific reactivation of HSV-1 and HSV-2 (26, 66).
A number of lines of evidence indicate that our results are not simply a consequence of differential LAT accumulation in latently infected A5- and KH10-positive neurons. First, in the latently infected neurons in which LAT can be detected, there is no differential accumulation of LAT among A5- and KH10-positive neurons (38). Second, in mice expressing either the HSV-1 or HSV-2 LAT transgene, there is no differential accumulation of LAT among A5- and KH10-positive neurons (38, 63). Third, the ratio of LAT-expressing A5- and KH10-positive neurons is independent of the sensitivity of the assay used to measure LAT (65). Fourth, following ocular inoculation with thymidine kinase deletion viruses, where latency is the only possible outcome of infection, a similar proportion of LAT-positive neurons were A5 positive and KH10 positive. And, fifth, the distribution of latent infection among A5- and KH10-positive neurons following ocular inoculation with a chimeric HSV-2 virus engineered with the HSV-1 LAT promoter in the place of the native HSV-2 LAT promoter is nearly identical to that observed with HSV-2 (A. Bertke, A. Patel, Y. Imai, K. Apakupakul, T. P. Margolis, and P. R. Krause, submitted for publication).
The mechanism by which the species-specific LAT regions influence differential establishment of latent infection in A5- and KH10-positive neurons remains unknown. However, sequential studies in which we evaluated the pattern of latent infection following infection with viruses with deletions of functional thymidine kinase, US9, and gE clearly demonstrate that preferential establishment of HSV-1 latent infection in A5 neurons is not a consequence of (i) differential access of HSV-1 to A5-positive neurons, (ii) differential cell-to-cell spread of HSV-1 to A5-positive neurons, or (iii) differential round-trip spread of HSV-1 to A5-positive neurons. Studies with viruses with a deletion of functional thymidine kinase further indicate that preferential establishment of latency in A5-positive neurons is dependent on the virus's ability to replicate in neurons, a function that must occur at the site of latency, the TG. These findings imply that any LAT-related role in the spread of HSV-1 and HSV-2 in the guinea pig peripheral nervous system (5) is not a factor in the differential establishment of latent infection in A5-positive neurons in the mouse TG.
In the course of investigating possible mechanisms responsible for differential establishment of latent HSV infection in A5- and KH10-positive neurons, it became clear that we needed to explore a possible role for ICP0 in this process. Not only does ICP0 play a key role in regulating viral gene expression (10), disrupting host cell proteins (20), and inhibiting the interferon response (25, 33), but the chimeric virus HSV-1 17syn+/LAT2, which has an establishment phenotype similar to that of HSV-2, also contains an alteration of the 3′ end of the HSV-1 ICP0 coding region. However, our studies with the HSV-1 ICP0 null virus, n212, clearly indicate that ICP0 plays no role in the differential establishment of HSV-1 latent infection among A5- and KH10-positive neurons. This is an important observation since it focuses attention away from the many processes that ICP0 regulates in considering the mechanisms by which differential patterns of latent infection are established.
It is important to highlight that n212 replicates on both the ocular surface and in the TG of mice, just at a reduced rate compared to wild-type virus (11). Peak viral titers of n212 in the TG were only about 1 log less than that observed with wild-type virus. And this difference may be accounted for by reduced replication of n212 on the ocular surface. In tissue sections of n212-infected TG, we also observed abundant HSV antigen-positive neurons. These observations are strikingly different from what has been described for thymidine kinase deletion viruses that fail to replicate in neurons of the TG (14) and likely explain the different results observed following infection with n212 compared to dlsptk and tkLTRZ1.
Since further mapping of the portion of the LAT genome responsible for differential establishment of latent infection might help to determine the mechanisms responsible, we also tested the pattern of latent infection of HSV-1 LAT deletion mutants dLAT371, 17ΔSty, and 17Δ348. All three of these viruses have deletions within the 5′ exon of LAT, the same region of the genome responsible for a number of latency-related viral functions, including establishment of latency (43, 48, 60), reactivation from latency (6, 7, 16, 27, 28, 47, 51), and prevention of cell death by apoptotic mechanisms (1, 46, 60). The present study implies that these findings cannot be explained solely by the HSV-1 preference for A5-positive neurons because these mutant viruses retained the A5-positive phenotype. However, results of studies with these three viral deletion mutants suggest that a very large part of the LAT 5′ exon (nt 118879 to 119355, i.e., from the upstream StyI site to the downstream of the 348-bp region in 17Δ348) is not responsible for differential establishment of HSV-1 latent infection among A5-positive and KH10-positive neurons. These data confirm and extend our earlier observations with KOS 62, a LAT deletion virus (with a 1.6-kb SacII-HpaI deletion) that expressed β-galactosidase in lieu of the stable LAT intron and whose neuronal latency phenotype matched that of wild-type KOS (65). Although the deletions in 17Δ348 and the ΔStyI viruses are not as comprehensive as those in KOS 62, studies with these additional deletion LAT viruses were critical to complete for two reasons. First, studies with KOS 62 relied on LacZ expression rather than the expression of the stable LAT intron. Second, in contrast to KOS 62, the phenotype of 17Δ348 and the ΔStyI-StyI deletion mutants have been well characterized both in vitro and in vivo. Taken together, these studies indicate that a viral function that dictates a preference for latent infection of A5-positive neurons maps to either the LAT promoter, the 70 bp immediately downstream of the LAT TATA box, or the last 947 bp of the LAT intron. This region is distinct from the downstream region of exon 1, which in rabbit studies has been implicated as playing a role in viral reactivation from latency. It is thus possible that the region responsible for the reactivation phenotype of the chimeric viruses HSV-1 17syn+/LAT2 and HSV-2 333/LAT1 is distinct from the region responsible for differential establishment of latency among neuronal subpopulations. Since studies of additional chimeric viruses by our group indicate that the neuron latency preference function maps specifically to the 5′ LAT exon (A. Bertke et al., submitted), ongoing studies are focusing on how the first 70 bp of this region regulates the neuronal pattern of latent HSV infection.
In theory, one mechanism by which LAT could regulate the differential establishment of latency is through differential regulation of HSV-1 and HSV-2 immediate-early proteins other than ICP0. These proteins are critical to the regulation of productive infection, and studies with viruses with deletions of 1.8 kb of the 5′ LAT coding region indicate that a viral function that reduces immediate-early gene expression during both productive and latent HSV-1 infection maps to this region of the viral genome (12, 23). The mechanism responsible for LAT regulation of lytic cycle gene transcription is not completely clear, but there is growing evidence that the region of HSV-1 DNA immediately downstream of the LAT promoter (alternatively called the LAT enhancer, the LTE region, and the rcr) is a cis-acting regulatory region for expression of surrounding viral genes (4, 31, 35), functioning, perhaps, by promoting assembly of heterochromatin on nearby lytic gene promoters (64).
It is also possible that viral encoded microRNAs (miRNAs) play a role in the differential establishment of latent HSV infection. Although no miRNAs have been mapped to the substituted sequences in either HSV-1 17syn+/LAT2 or HSV-2 333/LAT1, promoter sequences replaced in these viruses are required for efficient expression (in vitro and in vivo) of miR-1, an HSV-2 LAT-encoded miRNA. miR-1 maps antisense to the HSV-2 ICP34.5 gene and specifically reduces expression of ICP34.5 (56). Similar anti-ICP34.5 miRNAs have also been described in HSV-1 (56, 62). A LAT-encoded miRNA that maps antisense to the ICP0 exon 3 and inhibits ICP0 expression has also been described in HSV-1 (62). As noted above, our data with the HSV-1 ICP0 null virus, n212, argue against a role for miRNA-mediated inhibition of ICP0 expression in the differential establishment of HSV-1 latent infection. However, it remains possible that differential establishment of latent HSV infection is a consequence of LAT promoter-driven expression of miRNAs that inhibit expression of ICP34.5, a protein that is critical for productive viral gene expression in vivo and for neurovirulence (13).
We recognize that LAT accumulation is heterogeneous among ganglionic neurons and that some latently infected neurons are likely to accumulate LAT at levels that are below the sensitivity of detection by in situ hybridization. Through the use of in situ PCR, Mehta et al. (41) estimated that the number of neurons containing HSV-1 DNA in latently infected tissue is three times that at which LAT could be detected by in situ hybridization. Could the distribution of latent infection among A5 and KH10 neurons be different in neurons with levels of LAT that are below the limit of detection by our assay? We think that this is unlikely since it would require the biology governing HSV latent infection to be different at LAT levels that are below the arbitrary sensitivity of our in situ hybridization assay. This issue aside, we have chosen to assay for LAT in our studies because it is the most reliable marker of latently infected neurons. In situ PCR is a highly capricious assay, and it is unclear whether viral DNA detected by this method represents the complete viral genome in a reactivation-competent form (essential for the definition of viral latency). While it is not known if viral reactivation is restricted to neurons in which LAT expression can be detected by in situ hybridization, the altered recurrence phenotypes of viruses with impaired capacity to produce LAT indicate that it is a biologically relevant marker of HSV latency (27, 29, 30, 32, 45, 61).
In summary, we found that HSV species-specific LAT plays a role in the establishment of latent infection in different neuronal subpopulations. We have ruled out a number of potential mechanisms, but the precise mechanism remains to be elucidated. However, given the preference that HSV-1 had for establishment of latent infection in A5-positive neurons, there must be one or more cellular factors that play a role in this process.
Acknowledgments
We are deeply grateful to D. Johnson, D. Coen, D. Bloom, N. Fraser, P. Schaffer, and S. Wechsler for their generous supply of viruses used in the course of our studies. MAbs A5, developed by B. Fenderson, and KH10, developed by T. M. Jessell and J. Dodd, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa, IA 52242.
This work was supported by NIH EY10008 and EY02162, Research to Prevent Blindness (New York, NY), the Ralph and Sophie Heintz laboratory fund, and the Littlefield 2000 Trust.
Footnotes
Published ahead of print on 3 June 2009.
REFERENCES
- 1.Ahmed, M., M. Lock, C. G. Miller, and N. W. Fraser. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J. Virol. 76717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atanasiu, D., J. R. Kent, J. J. Gartner, and N. W. Fraser. 2006. The stable 2-kb LAT intron of herpes simplex stimulates the expression of heat shock proteins and protects cells from stress. Virology 35026-33. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, D. L., G. J. Michael, N. Ramachandran, J. B. Munson, S. Averill, Q. Yan, S. B. McMahon, and J. V. Priestly. 1998. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 183059-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthomme, H., J. Lokensgard, L. Yang, T. Margolis, and L. T. Feldman. 2000. Evidence for a bidirectional element located downstream from the herpes simplex virus type 1 latency-associated promoter that increases its activity during latency. J. Virol. 743613-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertke, A., A. Patel, and P. R. Krause. 2007. Herpes simplex virus latency-associated transcript sequence downstream of the promoter influences type-specific reactivation and viral neurotropism. J. Virol. 816605-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block, T. M., S. Deshmane, J. Masonis, J. Maggioncalda, T. Valyi-Nagi, and N. W. Fraser. 1993. An HSV LAT null mutant reactivates slowly from latent infection and makes small plaques on CV-1 monolayers. Virology 192618-630. [DOI] [PubMed] [Google Scholar]
- 7.Bloom, D. C., J. M. Hill, G. Devi-Rao, E. K. Wagner, L. T. Feldman, and J. G. Stevens. 1996. A 348-base-pair region in the latency-associated transcript facilitates herpes simplex virus type 1 reactivation. J. Virol. 702449-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blyth, W. A., D. A. Harbour, and T. J. Hill. 1984. Pathogenesis of zosteriform spread of herpes simplex virus in the mouse. J. Gen. Virol. 651477-1486. [DOI] [PubMed] [Google Scholar]
- 9.Bourne, N., L. R. Stanberry, B. Connelly, J. F. Kurawadwala, S. E. Straus, and P. R. Krause. 1994. Quantity of latency associated transcript produced by herpes simplex virus is not predictive of the frequency of experimental recurrent genital herpes. J. Infect. Dis. 1691084-1087. [DOI] [PubMed] [Google Scholar]
- 10.Cai, W., and P. A. Schaffer. 1992. Herpes simplex virus type 1ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 662904-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai, W., T. L. Astor, L. M. Liptak, C. Cho, D. M. Coen, and P. A. Schaffer. 1993. The herpes simplex virus type 1 regulatory protein ICP0 enhances viral replication during acute infection and reactivation from latency. J. Virol. 677501-7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, S.-H., M. F. Kramer, P. A. Schaffer, and D. M. Coen. 1997. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 715878-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou, J., and B. Roizman. 1992. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 893266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coen, D. M., M. Kosz-Vnenchak, J. G. Jacobson, D. A. Leib, C. L. Bogard, P. A. Schaffer, K. L. Tyler, and D. M. Knipe. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 864736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davar, G., M. F. Kramer, D. Garber, A. L. Roca, J. K. Andersen, W. Bebrin, D. M. Coen, M. Kosz-Vnenchak, D. M. Knipe, X. O. Breakefield, et al. 1994. Comparative efficacy of expression of genes delivered to mouse sensory neurons with herpes virus vectors. J. Comp. Neurol. 3393-11. [DOI] [PubMed] [Google Scholar]
- 16.Devi-Rao, G. B., D. C. Bloom, J. G. Stevens, and E. K. Wagner. 1994. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J. Virol. 681271-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dingwell, K. S., C. R. Brunetti, R. L. Hendricks, Q. Tang, M. Tang, A. J. Rainbow, and D. C. Johnson. 1994. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dingwell, K. S., L. C. Doehring, and D. C. Johnson. 1995. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 697087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodd, J., and T. M. Jessell. 1985. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J. Neurosci. 53278-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett, R. D., A. Orr, and C. M. Preston. 1998. A viral activator of gene expression functions via the ubiquitin-proteosome pathway. EMBO J. 177161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrell, M. J., A. T. Dobson, and L. T. Feldman. 1991. Herpes simplex virus latency-associated transcript is a stable intron. Proc. Natl. Acad. Sci. USA 88790-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenderson, B. A., A. C. Hahnel, and E. M. Eddy. 1983. Immunohistochemical localization of two monoclonal antibody-defined carbohydrate antigens during early murine embryogenesis. Dev. Biol. 100318-327. [DOI] [PubMed] [Google Scholar]
- 23.Garber, D. A., P. A. Schaffer, and D. M. Knipe. 1997. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex type 1. J. Virol. 715885-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamza, M. A., D. M. Higgins, L. T. Feldman, and W. T. Ruyechan. 2007. The latency-associated transcript of herpes simplex virus type 1 promotes survival and stimulates axonal regeneration in sympathetic and trigeminal neurons. J. Neurovirol. 1356-66. [DOI] [PubMed] [Google Scholar]
- 25.Harle, P., B. Sainz, Jr., D. J. J. Carr, and W. P. Halford. 2002. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-α/β. Virology 293295-304. [DOI] [PubMed] [Google Scholar]
- 26.Hill, J. M., A. Patel, P. Bhattacharjee, and P. R. Krause. 2003. An HSV-1 chimeric containing HSV-2 latency associated transcript (LAT) sequences has significantly reduced adrenergic reactivation in the rabbit eye model. Curr. Eye. Res. 2003 26219-224. [DOI] [PubMed] [Google Scholar]
- 27.Hill, J. M., F. Sedarati, R. T. Javier, E. K. Wagner, and J. G. Stevens. 1990. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology 174117-125. [DOI] [PubMed] [Google Scholar]
- 28.Jarman, R. G., J. M. Loutsch, G. B. Devi-Rao, M. E. Marquart, M. P. Banaszak, X. Zheng, J. M. Hill, E. K. Wagner, and D. C. Bloom. 2002. The region of the HSV-1 latency-associated transcript required for epinephrine-induced reactivation in the rabbit does not include the 2.0-kb intron. Virology 29259-69. [DOI] [PubMed] [Google Scholar]
- 29.Kent, J. R., W. Kang, C. G. Miller, and N. W. Fraser. 2003. Herpes simplex virus latency-associated transcript gene function. J. Neurovirol. 9285-290. [DOI] [PubMed] [Google Scholar]
- 30.Krause, P. R., L. R. Stanberry, N. Bourne. B. Connelly, J. F. Kurawadwala, A. Patel, and S. E. Straus. 1995. Expression of the herpes simplex virus type 2 latency associated transcript enhances spontaneous reactivation of genital herpes in latently infected guinea pigs. J. Exp. Med. 181297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubat, N. J., A. L. Amelio, N. V. Giordani, and D. C. Bloom. 2004. The herpes simplex virus type 1 latency-associated transcript (LAT) enhancer/rcr is hyperacetylated during latency independently of LAT transcription. J. Virol. 7812508-12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leib, D. A., C. L. Bogard, M. Kosz-Vnenchak, K. A. Hicks, D. M. Coen, D. M. Knipe, and P. A. Shaffer. 1989. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J. Virol. 632893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild type and mutant herpes simplex virus in vivo. J. Exp. Med. 189663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lekstrom-Himes, J. A., K. Wang, L. Pesnicak, P. R. Krause, and S. E. Straus. 1998. The comparative biology of latent herpes simplex virus type 1 and type 2 infections: latency-associated transcript promoter activity and expression in vitro and in infected mice. J. Neurovirol. 427-37. [DOI] [PubMed] [Google Scholar]
- 35.Lokensgard, J. R., H. Berthomme, and L. T. Feldman. 1997. The latency-associated promoter of herpes simplex virus type 1 requires a region downstream of the transcription start site for long-term expression during latency. J. Virol. 716714-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mador, N., D. Goldenberg, O. Cohen, A. Panet, and I. Steiner. 1998. Herpes simplex virus type 1 latency-associated transcripts suppress viral replication and reduce immediate-early gene mRNA levels in a neuronal cell line. J. Virol. 725067-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maggioncalda, J., A. Mehta, N. W. Fraser, and T. M. Block. 1994. Analysis of a herpes simplex virus type 1 LAT mutant with a deletion between the putative promoter and the 5′ end of the 2.0-kilobase transcript. J. Virol. 687816-7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margolis, T. P., Y. Imai, L. Yang, V. Vallas, and P. R. Krause. 2007. Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: role of latency-associated transcripts. J. Virol. 811872-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Margolis, T. P., C. R. Dawson, and J. H. LaVail. 1992. Herpes simplex viral infection of the mouse trigeminal ganglion. Immunohistochemical analysis of cell populations. Investig. Ophthalmol. Vis. Sci. 33259-267. [PubMed] [Google Scholar]
- 40.Margolis, T. P., F. Sedarati, A. T. Dobson, L. T. Feldman, and J. G. Stevens. 1992. Pathways of viral gene transcription during acute neuronal infection with HSV-1. Virology 189150-160. [DOI] [PubMed] [Google Scholar]
- 41.Mehta, A., J. Maggioncalda, O. Bagasra, S. Thikkavarapu, P. Saikumari, T. Valyi-Nagy, N. W. Fraser, and T. M. Block. 1995. In situ DNA PCR and RNA hybridization detection of herpes simplex virus sequences in trigeminal ganglia of latently infected mice. Virology 206633-640. [DOI] [PubMed] [Google Scholar]
- 42.Moliver, D. C., D. E. Wright, M. L. Leitner, M. S. Parsadanian, K. Doster, D. Wen, Q. Yan, and W. D. Snider. 1997. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19849-861. [DOI] [PubMed] [Google Scholar]
- 43.O'Neil, J. E., J. M. Loutsch, J. S. Aguilar, J. M. Hill, E. K. Wagner, and D. C. Bloom. 2004. Wide variations in herpes simplex virus type 1 inoculum dose and latency-associated transcript expression phenotype do not alter the establishment of latency in the rabbit eye model. J. Virol. 785038-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng, W., G. Henderson, M. Inman, L. BenMohamed, G. C. Perng, S. L. Wechsler, and C. Jones. 2005. The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal ganglia of acutely infected mice. J. Virol. 796162-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perng, G. C., E. C. Dunkel, P. A. Geary, S. M. Slaina, H. Ghiashi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 688045-8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perng, G. C., C. Jones, J. Ciacci-Zanella, M. Stone, G. Henderson, A. Yukht, S. M. Slanina, F. M. Hofman, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 2871500-1503. [DOI] [PubMed] [Google Scholar]
- 47.Perng, G. C., S. M. Slanina, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1996. A 371-nucleotide region between the herpes simplex virus type 1 (HSV-1) LAT promoter and the 2-kilobase LAT is not essential for efficient spontaneous reactivation of latent HSV-1. J. Virol. 702014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perng, G. C., S. M. Slanina, A. Yukht, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 2000. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J. Virol. 741885-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polcicova, K., P. S. Biswas, K. Banerjee, T. W. Wisner, B. T. Rouse, and D. C. Johnson. 2005. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc. Natl. Acad. Sci. USA 10211462-11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polcicova, K., K. Goldsmith, B. L. Rainish, T. W. Wisner, and D. C. Johnson. 2005. The extracellular domain of herpes simplex virus gE is indispensable for efficient cell-to-cell spread: evidence for gE/gI receptors. J. Virol. 7911990-12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawtell, N. M., and R. L. Thompson. 1992. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J. Virol. 662157-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silverman, J. D., and L. Kruger. 1990. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J. Neurocytol. 19789-801. [DOI] [PubMed] [Google Scholar]
- 53.Simmons, A., and A. A. Nash. 1984. Zosteriform spread of herpes simplex virus as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J. Virol. 52816-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevens, J. G., E. K. Wagner, G. B. Devi-Rao, M. L. Cook, and L. T. Feldman. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 2351056-1059. [DOI] [PubMed] [Google Scholar]
- 55.Summers, B. C., T. P. Margolis, and D. A. Leib. 2001. Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread. J. Virol. 755069-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang, S., A. S. Brtke, A. Patel, K. Wang, J. I. Cohen, and P. R. Krause. 2008. An acutely and latently expressed herpes simplex virus 2 viral microRNA inhibits expression of ICP34.5, a viral neurovirulence factor. Proc. Natl. Acad. Sci. USA 10510931-10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teague, O., and E. W. Goodpasture. 1923. Experimental herpes zoster. J. Med. Res. 24185-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tenser, R. B., and M. E. Dunstan. 1979. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology 99417-422. [DOI] [PubMed] [Google Scholar]
- 59.Tenser, R. B., R. L. Miller, and F. Rapp. 1979. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science 205915-917. [DOI] [PubMed] [Google Scholar]
- 60.Thompson, R. L., and N. M. Sawtell. 2001. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J. Virol. 756660-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trousdale, M. D., I. Steiner, J. G. Spivack, S. L. Deshmane, S. M. Brown, A. R. MacLean, J. H. Subak-Sharpe, and N. W. Fraser. 1991. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J. Virol. 656989-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Umbach, J. L., M. F. Kramer, I. Jurak, H. W. Karnowski, D. M. Coen, and B. R. Cullen. 2008. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 454780-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, K., G. Mahalingam, Y. Imai, L. Pesnicak, T. Margolis, S. E. Straus, and J. I. Cohen. 2009. Cell type specific accumulation of the major latency-associated transcript (LAT) of herpes simplex virus type 2 in LAT transgenic mice. Virology doi: 10.1016/j.virol.2008.12.035. [DOI] [PMC free article] [PubMed]
- 64.Wang, Q.-Y., C. Zhou, K. E. Johnson, R. C. Colgrove, D. M. Coen, and D. M. Knipe. 2005. Herpesviral latency-associated transcript gene promotes assembly of heterochromatin on viral lytic-gene promoters in latent infection. Proc. Natl. Acad. Sci. USA 10216055-16059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang, L., C. C. Voytek, and T. P. Margolis. 2000. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J. Virol. 74209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshikawa, T., J. Hill, L. R. Stanberry, N. Bourne, J. F. Kurawadwala, and P. R. Krause. 1996. The characteristic site-specific reactivation phenotypes of HSV-1 and HSV-2 depend upon the latency-associated transcript region. J. Exp. Med. 184659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zwick, M., B. M. Davis, C. J. Woodbury, J. N. Burkett, H. R. Koerber, J. F. Simpson, and K. M. Albers. 2002. Glial cell line derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J. Neurosci. 224057-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]