Abstract
The role of Cardif-dependent signaling in controlling dengue virus (DENV) infection and regulating type I interferon (IFN) production in vivo was examined in Cardif-deficient mice. DENV RNA levels were significantly elevated in both the serum and lymphoid tissues of Cardif−/− mice at early times compared to those in wild-type animals. Type I IFN production was delayed in these locales of Cardif−/− mice until 18 h postinfection, indicating that Cardif regulates the initial type I IFN response in lymphoid tissues. In contrast, DENV viral loads in nonlymphoid tissues were similar between Cardif−/− and wild-type mice. These results reveal that RNA helicase-mediated sensing acts as a first line of innate defense against DENV infection in vivo and functions in a tissue-dependent manner.
Dengue virus (DENV) is a positive-sense, single-stranded RNA virus that belongs to the family Flaviviridae, which includes other arthropod-borne viruses such as Japanese encephalitis, yellow fever, and West Nile viruses (12). DENV is transmitted to humans by mosquitoes and causes an estimated 50 million new cases of dengue fever and 250,000 cases of dengue hemorrhagic fever/dengue shock syndrome per year worldwide (3). Despite the prevalence of DENV, the virus-host interactions that ultimately determine the disease remain largely uncharacterized.
Several lines of evidence implicate a key role for type I interferon (IFN) in protection against DENV infection, as follows. (i) DENV-infected patients contain a high level of alpha interferon (IFN-α) in the serum (11). (ii) Human peripheral blood mononuclear cells exposed to DENV-infected monocytes secrete IFN-α and protect other human monocytes from DENV infection (10). (iii) IFN-α/β inhibits DENV infection in a variety of human and nonhuman cells (1, 2). (iv) Mice lacking receptors for both IFN-α/β and IFN-γ, but not wild-type (WT) control animals, succumb to primary DENV infection (7, 22). (v) Expression of DENV nonstructural proteins NS2A, NS2B, and NS4B inhibit IFN-β-induced activation of STAT1 in a monkey cell line (15, 16). However, little is known about the mechanisms by which the anti-DENV IFN response is initiated in vivo.
The retinoic acid-inducible gene I (RIG-I) protein (5), which is composed of a DExD/H-box RNA helicase domain fused to a caspase recruitment domain (CARD), mediates induction of type I IFN in response to RNA virus infection (28). Two other RIG-I-like receptors are currently known, MDA5 and LGP2, and these and RIG-I reside in the cell cytoplasm and interact with viral RNA via their helicase domains. Upon RNA binding, the CARD domain of RIG-I/MDA5 interacts with the CARD of Cardif/MAVS/IPS-1/VISA, ultimately leading to type I IFN production (4). DENV recognition by both RIG-I and MDA5 and downstream Cardif-dependent signaling have been recently shown in cultured fibroblasts (13), suggesting that RIG-I-like receptor signaling affects DENV replication in vivo.
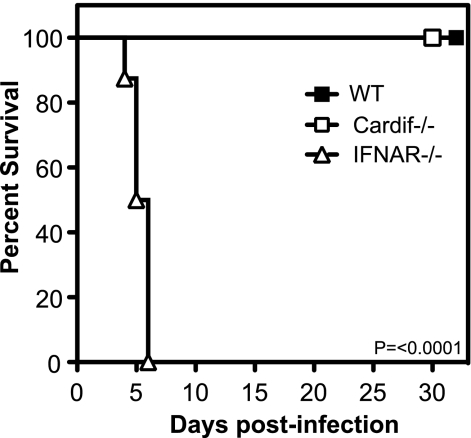
To assess the role of Cardif-dependent signaling in protection against DENV, Cardif−/− C57BL/6 mice (14) were infected intravenously (i.v.) with a triple-plaque-purified biological clone of D2S10 (S221), a DENV serotype 2 strain that causes a nonparalytic, severe disease in IFN-α/β and IFN-γ receptor-deficient 129/Sv (AG129) mice (23). At a challenge dose of 1 × 1011 genomic equivalents (GE) (∼3 × 106 PFU), WT, Cardif−/−, and type I IFN receptor (IFNAR−/−) mice remained healthy until day 30 postinfection, when the experiment was terminated (data not shown). At a challenge dose of 1 × 1012 GE (∼3 × 107 PFU), IFNAR−/− mice were very sensitive to primary DENV infection, with 0% survival at this dose (Fig. 1), confirming the importance of type I IFN in resolving DENV infection. In contrast, both WT and Cardif−/− mice exhibited no overt signs of disease and remained healthy until the experiment was terminated on day 32 postinfection. These results indicate that Cardif is nonessential for protection against DENV-induced mortality in mice.
FIG. 1.
Cardif−/− mice are resistant to DENV-induced disease. WT, Cardif−/−, and IFNAR−/− mice were infected i.v. with 1012 GE of DENV strain S221 and monitored for survival. The difference in survival between Cardif−/− and IFNAR−/− strains was statistically significant (P < 0.0001) using the log-rank test. Eight mice per group.
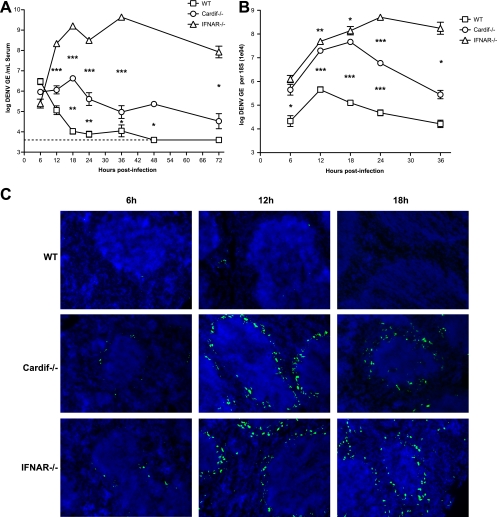
Although 129/Sv mice lacking STAT1 show no overt disease when infected with DENV, they are compromised in their ability to control early replication (24). To determine whether Cardif had a similar effect on infection, WT, Cardif−/−, and IFNAR−/− mice were infected i.v. with 1 × 1011 GE (∼3 × 106 PFU) of S221, and viral loads were examined at multiple time points after infection by quantitative reverse transcriptase PCR (qRT-PCR) (18). Viral load in the serum of WT mice steadily decreased from 6 h to 24 h postinfection until viral RNA levels were near or below the limit of detection (Fig. 2A). In contrast, DENV serum levels in Cardif−/− mice increased during the first 18 h of infection and remained 10- to 100-fold higher than the levels in WT mice from 12 h to 72 h, suggesting that viral replication was occurring in these mice. Serum viral load in IFNAR−/− mice reached a 100-fold-higher level than that in Cardif−/− mice as early as 12 h postinfection and remained at the same level throughout the first 72 h of infection. These results indicate that both Cardif-dependent and Cardif-independent pathways are controlling viremia within the first 12 h of infection. In agreement with studies of DENV-infected patients (6, 19), we have previously identified the spleen as a likely site of early DENV infection in IFN-α/β and IFN-γ receptor-deficient AG129 mice (18). Therefore, WT, Cardif−/−, and IFNAR−/− mice were infected with 1 × 1011 GE (∼3 × 106 PFU) of S221, and at various times during the first 36 h of infection, spleens were collected for isolation of total splenic RNA and quantification of viral burden by qRT-PCR (18). An increase in viral RNA was observed in all three strains between 6 h and 12 h postinfection, suggesting early replication in the spleen. Like the observations for serum, viral load in the spleen was consistently higher in both Cardif−/− and IFNAR−/− mice than in the WT animals (Fig. 2B). However, unlike the serum results, the splenic viral loads in both knockout mouse strains remained close (3-fold or less) through 18 h but diverged at 24 h when viral load reached a 100-fold-higher level in the IFNAR−/− spleen than in the Cardif−/− spleen. To confirm productive infection in the spleen, the expression of the DENV nonstructural gene 3 (NS3) protein was assessed by immunohistochemistry. NS3 is not a component of the virion and is, therefore, a marker of productive viral replication. WT, Cardif−/−, and IFNAR−/− mice were infected with 1 × 1011 GE (∼3 × 106 PFU) of S221, and spleens were harvested and frozen at 6 h, 12 h, and 18 h postinfection. Tissue sections (6 μm) were prepared, fixed in acetone and 1% paraformaldehyde, and stained with purified rabbit polyclonal anti-DENV NS3 (obtained from NITD, Singapore) and Cy5-labeled secondary antibody (SouthernBiotech). NS3 expression was detected as early as 6 h after infection in both Cardif−/− and IFNAR−/− mice, and at 12 h, NS3-positive cells appeared primarily in cells of the splenic marginal zone, proximal to the dense nuclei (counterstained with DAPI [4′,6-diamidino-2-phenylindole]) of the white pulp (Fig. 2C). In contrast, only a small number of NS3-positive cells were detected in the spleens of WT mice at 12 h postinfection, again supporting a role for Cardif-dependent signaling in controlling DENV replication in the spleen.
FIG. 2.
Productive DENV infection in Cardif−/− mice. WT, Cardif−/−, and IFNAR−/− mice were infected i.v. with 1011 GE of S221 and evaluated for viral load in the serum (A) and spleen (B) at the indicated times postinfection. (A) Serum RNA was isolated, and viral genome copies were quantitated by qRT-PCR. Each time point represents four to eight mice per group, with means ± standard errors of the means. Differences between WT and Cardif−/− mice and between Cardif−/− and IFNAR−/− mice were statistically significant using the unpaired t test at 12 h (Cardif−/−/IFNAR−/−, P = 0.0007), 18 h (P = 0.0041; P = 0.0006), 24 h (P = 0.0086; P < 0.0001), 36 h (P = 0.0380; P < 0.0001), 48 h (WT/Cardif−/−, P = 0.0184), and 72 h (Cardif−/−/IFNAR−/−, P = 0.0121). The limit of detection was 3.09 × 103 GE/ml (dotted line). (B) At the indicated times postinfection, mice were sacrificed, and total RNA was extracted from the spleens. Viral genome copies were determined by qRT-PCR from total RNA, and values shown are normalized to 18S rRNA analysis run in parallel. Each time point represents four to eight mice per group, with means ± standard errors of the means, and differences between WT and Cardif−/− mice and between Cardif−/− and IFNAR−/− mice at 6 h (WT/Cardif−/−, P = 0.0184), 12 h (P = 0.0002; P = 0.0036), 18 h (P = 0.0009; P = 0.0333), 24 h (P = 0.0003; P < 0.0001), and 36 h (Cardif−/−/IFNAR−/−, P = 0.0101) were statistically significant using the unpaired t test. (C) Spleens harvested from WT, Cardif−/−, and IFNAR−/− mice at 6 h, 12 h, and 18 h postinfection were sectioned, fixed in acetone and paraformaldehyde, and stained with rabbit polyclonal anti-NS3 antibody (green). Nuclei were counterstained using DAPI (blue). One representative animal out of three for each group is shown.
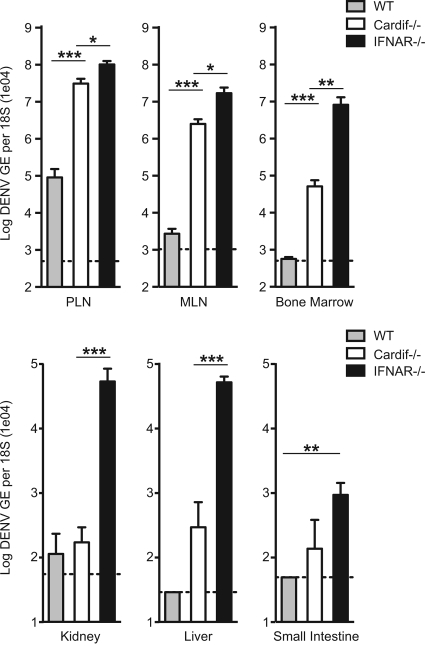
Although similar viral loads in the spleen were observed between Cardif−/− and IFNAR−/− mice, viremia was 100-fold higher in IFNAR−/− mice than in Cardif−/− mice, suggesting that viral replication was occurring in a separate location in the absence of type I IFN control and independently of Cardif-mediated restriction. To identify the other sites of viral infection, mice were infected with 1 × 1011 GE (∼3 × 106 PFU) of S221 and assessed for viral RNA levels in the peripheral lymph nodes (PLN), mesenteric lymph nodes (MLN), bone marrow, liver, kidney, and small intestine at 18 h postinfection (Fig. 3). In the lymph nodes and bone marrow, Cardif−/− mice harbored a significantly higher viral load than WT mice, whereas in other tissues, differences of less than 10-fold were found between WT and Cardif−/− mice. IFNAR−/− mice displayed DENV replication levels <10-fold above Cardif−/− levels in the lymph nodes, similar to what was observed in the spleen, but they carried >100-fold-more viral RNA than Cardif−/− mice in the bone marrow, kidney, and liver. Taken together, these data suggest that Cardif is the major pathway responsible for controlling DENV replication in the spleen and lymph nodes, whereas Cardif-independent mechanisms limit DENV infection in the other tissues examined.
FIG. 3.
Productive DENV infection in Cardif−/− mice. WT, Cardif−/−, and IFNAR−/− mice were infected i.v. with 1011 GE of S221 and evaluated for viral load in the PLN, MLN, bone marrow, liver, kidney, and small intestine at 18 h postinfection. Viral genome copies were determined by qRT-PCR from total RNA, and values shown are normalized to 18s rRNA analysis run in parallel. Each time point represents four mice per group, with means ± standard errors of the means, and differences between WT and Cardif−/− mice and between Cardif−/− and IFNAR−/− mice in the PLN (P = 0.0006; P = 0.0168), MLN (P < 0.0001; P = 0.0101), and bone marrow (P = 0.0008; P = 0.0015) were statistically significant. Differences between Cardif−/− and IFNAR−/− mice in the kidney (P = 0.0002) and liver (P = 0.0003) and between WT and IFNAR−/− mice in the small intestine (P = 0.0091) were also statistically significant.
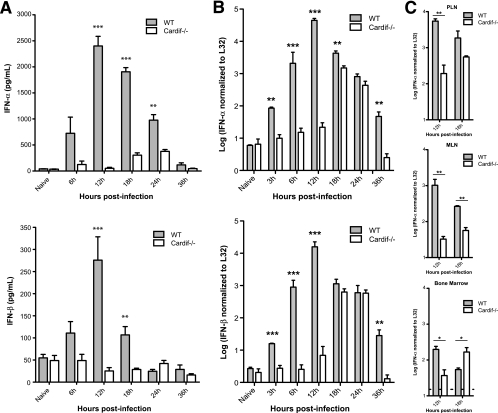
Next, we examined whether Cardif-dependent signaling regulated systemic type I IFN production during DENV infection. WT and Cardif−/− mice were infected with 1 × 1011 GE (∼3 × 106 PFU) of S221, and levels of serum IFN-α and IFN-β were measured by enzyme-linked immunosorbent assay (PBL Technologies). Both IFN-α and IFN-β levels were elevated in the serum of WT mice as soon as 6 h after infection, peaked at 12 h, and declined steadily between 18 and 36 h (Fig. 4A). IFN-α levels in infected WT mice remained significantly above the level in uninfected mice at 24 h, although DENV levels in the serum were at or below the limit of detection. In contrast, levels of serum IFN-α and IFN-β in Cardif−/− mice remained similar to those in uninfected animals during the first 12 h of infection, demonstrating that Cardif signaling is required for the initial type I IFN in circulation during DENV infection in vivo. A significant increase in serum IFN-α levels was observed in Cardif−/− mice at 18 and 24 h postinfection (P = 0.0039 and P = 0.0008, respectively), although the levels were still markedly below the levels in WT mice. Since the spleen is a likely source of type I IFN in the serum, the kinetics of IFN-α/β induction in the spleens of WT and Cardif−/− mice was investigated. Animals were infected with 1 × 1011 GE (∼3 × 106 PFU) of S221, total RNA from the spleen was recovered, and splenic IFN-α and IFN-β mRNA levels were determined via quantitative PCR, as described previously (21). Consistent with observations for serum, IFN-α and IFN-β transcript levels in the spleen in Cardif−/− mice were similar to those in uninfected WT mice but increased 5- to 30-fold over those seen in infected WT mice at 6 h and peaked at ∼12 h (Fig. 4B). Like the kinetics observed in the serum, both IFN-α and IFN-β mRNA levels in Cardif−/− mice remained comparable to those in naïve mice until 18 h postinfection, at which time they increased by more than 200-fold. In addition to the findings for the spleen, IFN-α transcripts in the PLN and MLN were 30-fold lower in Cardif−/− mice at 12 h than in WT mice and increased only slightly by 18 h postinfection (Fig. 4C). Taken together, these results demonstrate that Cardif is essential for the initial type I IFN induction in the serum, spleen, and lymph nodes and suggest the presence of a secondary, Cardif-independent pathway that regulates type I IFN in response to DENV spread within lymphoid tissues. A likely candidate for this alternative pathway of type I IFN induction is the Toll-like receptor (TLR) pathway. In fact, this closely parallels what we have observed for cytomegalovirus infection, where the primary type I IFN response is derived from the splenic stroma via a TLR-independent pathway (21), followed by TLR-dependent control in response to the first round of cytomegalovirus spread in this organ (9, 26). DENV activates TLR7-mediated responses in plasmacytoid dendritic cells (pDC) in vitro (25, 27), and a “blunted” pDC response has been associated with severe dengue disease in humans (17). Based on these observations, pDC production of type I IFN via TLR7 signaling may be responsible for the later, Cardif-independent control of DENV infection in mice. In summary, our data demonstrate a crucial role for Cardif signaling in induction of the initial type I IFN response to DENV infection and in control of DENV replication in vivo.
FIG. 4.
Delayed type I IFN responses to DENV infection in Cardif−/− mice. (A) Sera of WT and Cardif−/− mice that were infected i.v. with 1011 GE of S221 were collected and analyzed for IFN-α and IFN-β by enzyme-linked immunosorbent assay. Each time point represents three to eight mice per group, with means ± standard errors of the means. Differences in IFN levels between WT and Cardif−/− mice at 12 h (IFN-α, P = 0.0002; IFN-β, P = 0.0005), 18 h (IFN-α, P < 0.0001; IFN-β, P = 0.0011), and 24 h (IFN-α, P = 0.0051) were statistically significant using the unpaired t test. Baseline values in naive WT and Cardif−/− mice were 44.8 pg/ml and 41.9 pg/ml, respectively. (B) At the indicated times postinfection, WT and Cardif−/− mice infected with 1 × 1011 GE of S221 were sacrificed, and total RNA was extracted from spleens. Levels of IFN-α and IFN-β mRNA were determined by qRT-PCR using SYBR green (Applied Biosystems) and normalized to ribosomal gene L32 mRNA in parallel reactions. Differences in IFN mRNA between WT and Cardif−/− mice at 3 h (IFN-α, P = 0.0013; IFN-β, P = 0.0009), 6 h (IFN-α, P < 0.0001; IFN-β, P < 0.0001), 12 h (IFN-α, P < 0.0001; IFN-β, P < 0.0001), 18 h (IFN-α, P = 0.0012), and 36 h (IFN-α, P = 0.0022; IFN-β, P = 0.0032) were statistically significant using the unpaired t test. Each time point represents three to six mice per group, with means ± standard errors of the means. (C) WT and Cardif−/− mice infected with 1 × 1011 GE of S221 were sacrificed, and total RNA was extracted from PLN, MLN, and bone marrow. IFN-α levels were determined by qRT-PCR and normalized to L32 mRNA, as previously described. Differences in IFN mRNA between WT and Cardif−/− mice in the PLN (12 h, P = 0.0039), MLN (12 h, P = 0.0011; 18 h, P = 0.0013), and bone marrow (12 h, P = 0.0162; 18 h, P = 0.0196) were statistically significant using the unpaired t test. Each time point represents three to five mice per group, with means ± standard errors of the means, and the limit of detection for each (PLN, 0.68; MLN, 0.11; bone marrow, 1.18) is denoted by a dotted line.
Acknowledgments
This work was supported by an award from the Southeast Regional Center of Excellence for Emerging Infections and Biodefense (U54 AI057157; NIAID) and a Blasker Science and Technology grant from the San Diego Foundation (C-2008-00268) to S.S.
Footnotes
Published ahead of print on 3 June 2009.
REFERENCES
- 1.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 747814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamond, M. S., T. G. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 744957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiscott, J., R. Lin, P. Nakhaei, and S. Paz. 2006. MasterCARD: a priceless link to innate immunity. Trends Mol. Med. 1253-56. [DOI] [PubMed] [Google Scholar]
- 5.Imaizumi, T., S. Aratani, T. Nakajima, M. Carlson, T. Matsumiya, K. Tanji, K. Ookawa, H. Yoshida, S. Tsuchida, T. M. McIntyre, S. M. Prescott, G. A. Zimmerman, and K. Satoh. 2002. Retinoic acid-inducible gene-I is induced in endothelial cells by LPS and regulates expression of COX-2. Biochem. Biophys. Res. Commun. 292274-279. [DOI] [PubMed] [Google Scholar]
- 6.Jessie, K., M. Y. Fong, S. Devi, S. K. Lam, and K. T. Wong. 2004. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 1891411-1418. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, A. J., and J. T. Roehrig. 1999. New mouse model for dengue virus vaccine testing. J. Virol. 73783-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Krug, A., A. R. French, W. Barchet, J. A. Fischer, A. Dzionek, J. T. Pingel, M. M. Orihuela, S. Akira, W. M. Yokoyama, and M. Colonna. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21107-119. [DOI] [PubMed] [Google Scholar]
- 10.Kurane, I., and F. A. Ennis. 1988. Production of interferon alpha by dengue virus-infected human monocytes. J. Gen. Virol. 69445-449. [DOI] [PubMed] [Google Scholar]
- 11.Kurane, I., B. L. Innis, S. Nimmannitya, A. Nisalak, A. Meager, and F. A. Ennis. 1993. High levels of interferon alpha in the sera of children with dengue virus infection. Am. J. Trop. Med. Hyg. 48222-229. [DOI] [PubMed] [Google Scholar]
- 12.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, PA.
- 13.Loo, Y. M., J. Fornek, N. Crochet, G. Bajwa, O. Perwitasari, L. Martinez-Sobrido, S. Akira, M. A. Gill, A. Garcia-Sastre, M. G. Katze, and M. Gale, Jr. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 4371167-1172. [DOI] [PubMed] [Google Scholar]
- 15.Munoz-Jordan, J. L., M. Laurent-Rolle, J. Ashour, L. Martinez-Sobrido, M. Ashok, W. I. Lipkin, and A. Garcia-Sastre. 2005. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 798004-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz-Jordan, J. L., G. G. Sanchez-Burgos, M. Laurent-Rolle, and A. Garcia-Sastre. 2003. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 10014333-14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pichyangkul, S., T. P. Endy, S. Kalayanarooj, A. Nisalak, K. Yongvanitchit, S. Green, A. L. Rothman, F. A. Ennis, and D. H. Libraty. 2003. A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J. Immunol. 1715571-5578. [DOI] [PubMed] [Google Scholar]
- 18.Prestwood, T. R., D. M. Prigozhin, K. L. Sharar, R. M. Zellweger, and S. Shresta. 2008. A mouse-passaged dengue virus strain with reduced affinity for heparan sulfate causes severe disease in mice by establishing increased systemic viral loads. J. Virol. 828411-8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen, L., M. T. Drouet, and V. Deubel. 1999. Detection of dengue virus RNA by reverse transcription-polymerase chain reaction in the liver and lymphoid organs but not in the brain in fatal human infection. Am. J. Trop. Med. Hyg. 61720-724. [DOI] [PubMed] [Google Scholar]
- 20.Reference deleted.
- 21.Schneider, K., A. Loewendorf, C. De Trez, J. Fulton, A. Rhode, H. Shumway, S. Ha, G. Patterson, K. Pfeffer, S. A. Nedospasov, C. F. Ware, and C. A. Benedict. 2008. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe 367-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shresta, S., J. L. Kyle, H. M. Snider, M. Basavapatna, P. R. Beatty, and E. Harris. 2004. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J. Virol. 782701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shresta, S., K. L. Sharar, D. M. Prigozhin, P. R. Beatty, and E. Harris. 2006. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 8010208-10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shresta, S., K. L. Sharar, D. M. Prigozhin, H. M. Snider, P. R. Beatty, and E. Harris. 2005. Critical roles for both STAT1-dependent and STAT1-independent pathways in the control of primary dengue virus infection in mice. J. Immunol. 1753946-3954. [DOI] [PubMed] [Google Scholar]
- 25.Sun, P., S. Fernandez, M. A. Marovich, D. R. Palmer, C. M. Celluzzi, K. Boonnak, Z. Liang, H. Subramanian, K. R. Porter, W. Sun, and T. H. Burgess. 2009. Functional characterization of ex vivo blood myeloid and plasmacytoid dendritic cells after infection with dengue virus. Virology 383207-215. [DOI] [PubMed] [Google Scholar]
- 26.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 1013516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, J. P., P. Liu, E. Latz, D. T. Golenbock, R. W. Finberg, and D. H. Libraty. 2006. Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J. Immunol. 1777114-7121. [DOI] [PubMed] [Google Scholar]
- 28.Yoneyama, M., and T. Fujita. 2007. Function of RIG-I-like receptors in antiviral innate immunity. J. Biol. Chem. 28215315-15318. [DOI] [PubMed] [Google Scholar]