Abstract
Cellular topoisomerases and helicases are thought to play an essential role in herpesvirus replication and gene expression and are considered to be potential targets for antiviral therapies. Topoisomerase I (Topo I) and Topo II inhibitors can selectively inhibit Epstein-Barr virus (EBV) lytic cycle DNA replication. We found that the Topo I inhibitor camptothecin and, to a lesser extent, the Topo II inhibitor etoposide are potent inhibitors of the transcription and replication function of the EBV-encoded immediate-early protein Zta (also referred to as ZEBRA, EB1, and BZLF1). Camptothecin inhibited the Zta transcription activation of endogenous and reporter-linked viral promoters. Small interfering RNA depletion of Topo I also inhibited the Zta-dependent activation of lytic cycle DNA replication. Topo I could be coimmunoprecipitated with Zta, but this interaction was restricted to EBV-positive cells, suggesting that other viral proteins stabilize the interaction between Zta and Topo I. We also found that the RecQL1 helicase, which is known to associate with Kaposi's sarcoma-associated herpesvirus (KSHV) OriLyt, interacts with EBV OriLyt. Treatment with camptothecin reduced both Zta and RecQL1 binding to OriLyt in vivo, suggesting that Topo I promotes replication protein assembly at OriLyt.
Epstein-Barr virus (EBV) is a human gammaherpesvirus that has been linked to various lymphoid and epithelial cell malignancies (reviewed in references 14 and 21). Although EBV exists predominantly as a multicopy episome in latently infected B lymphocytes, the productive infection of EBV is necessary for the propagation of infectious virus particles and the reinfection of new cells and hosts. Spontaneous lytic reactivation occurs during B-cell terminal differentiation into plasma cells but may also be triggered by other stress-related signaling pathways (reviewed in reference 1). Nearly 100 viral genes are expressed during the lytic cycle, and some of the lytic gene products may contribute to pathogenesis and early growth-transforming events. Lytic infection is directly linked to oral hairy leukoplakia in immunosuppressed individuals (9, 16) and to an increased risk of EBV-associated nasopharyngeal carcinoma (6). Moreover, viruses lacking the lytic activator Zta were compromised for tumor formation in mouse models (11, 12). For these reasons, inhibitors of lytic infection may be of therapeutic value for the prevention and treatment of EBV-associated disease.
The lytic cycle of EBV can be initiated by the expression of the immediate-early protein Zta (also referred to as BZLF1, ZEBRA, and EB1) (4, 5). Zta is a sequence-specific DNA binding protein with sequence similarity to the cellular b-zip proteins C/EBP, c-jun, and c-fos (15). Zta can interact with a variety of host cell factors including C/EBP, p53, and mitochondrial single-stranded DNA binding protein (26-28, 30). Zta is required for the transcription activation of numerous viral and cellular genes. Zta also binds to the origin of lytic replication (OriLyt) (2, 18) and nucleates a viral replisome (8, 17). Although Zta shares no obvious homology to the origin binding proteins of alpha- and betaherpesviruses, the components of the viral replisome that Zta recruits to OriLyt are largely conserved among the various herpesvirus families (7). This raises the question of how Zta functions as a viral origin binding protein and whether it recruits host cell factors that are necessary for the initiation of EBV lytic cycle replication.
Topoisomerases and helicases play essential roles in cellular and viral chromosome metabolism including DNA replication, recombination, and transcription (3, 22, 24). Previous studies have found that the topoisomerase I (Topo I) inhibitor camptothecin and the Topo II inhibitor ellipticine can inhibit EBV replication at concentrations that were not toxic to the host cell (13). Additionally, Topo I has been shown to play a direct role in the recombination-dependent DNA replication of herpes simplex virus (HSV), and the Topo II inhibitor ICRF-193 can inhibit HSV replication (10, 19). A more recent study has implicated Topo I and Topo II, as well as the helicase RecQL1, in OriLyt function for Kaposi's sarcoma-associated herpesvirus (KSHV) (25). The precise role of Topo I or Topo II in EBV lytic replication is not known but may be important for the future development of lytic cycle inhibitors. Topo I changes the DNA topology and linking number by introducing a transient single-strand break, while Topo II functions in the decatenation of tangled DNA strands by transient double-strand breaks (3, 24). Topo I and Topo II inhibitors have been used in cancer chemotherapy regimens but have not been fully explored as antiviral compounds (20). In this work, we investigate the role of Topo I and Topo II and their potential function in facilitating the assembly of other replication factors, like the RecQL1 helicase, during EBV lytic replication and reactivation from latency.
MATERIALS AND METHODS
Cells and plasmids.
ZKO-293 cells (a gift from H. J. Delecluse) are 293 cells transformed with a hygromycin-resistant EBV bacmid with a deletion of the BZLF1 gene and were grown in RPMI medium with 10% fetal bovine serum (FBS) and 100 μg/ml hygromycin. D98/HR1 cells (a gift from R. Glaser, Ohio State University) are an EBV-positive adherent cell line generated by the fusion of the P3HR1 Burkitt lymphoma line and HeLa cells and were grown in Dulbecco's modified Eagle medium with 10% FBS. 293 cells were grown in Dulbecco's modified Eagle medium with 10% FBS. p3xFLAG-Zta and p3xFLAG-Rta were generated by inserting Zta or Rta cDNA, respectively, as a HindIII-BamHI fragment into a p3xFLAG-myc-CMV24 vector (Sigma) for mammalian cell expression. Mp-Luc consists of the EBV BMRF1 promoter at positions −460 to +60, Hp-Luc consists of the EBV BHLF1 promoter at positions −400 to +30, and Zp-Luc consists of the EBV BZLF1 promoter at positions −220 to +12, cloned as NheI-HindIII fragments into pGL3-Basic. The topoisomerase inhibitors etoposide and camptothecin were purchased from Sigma. Topotecan and irinotecan were purchased from LKT Laboratories (St. Paul, MN). The pancaspase inhibitor Z-Val-Ala-Asp (OMe)-fluoromethylketone was purchased from Calbiochem.
Antibodies.
Anti-RecQL1 polyclonal antibody (provided by Perry Blackshear, NIEHS), anti-Topo I monoclonal antibody A (Santa Cruz Biotechnology), anti-Topo I monoclonal antibody B (provided by Daniel Simmons, University of Delaware), anti-Topo IIβ polyclonal antibody (provided by Gary Gorbsky, Oklahoma Medical Research Foundation), anti-Muts homologue 2 (MSH2) monoclonal antibody (BD Biosciences), anti-Zta polyclonal antibody, anti-EA-D monoclonal antibody (Advanced Biotechnologies), and anti-Rta monoclonal antibody (Argene) were used.
Western blotting.
ZKO-293cells were transfected with plasmid p3xFLAG-Zta or control vector p3xFLAG using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocol. The cells were treated with various concentrations (as indicated in each figure legend) of camptothecin or etoposide (Sigma) at 6 h posttransfection. Cells were harvested 48 h posttransfection and lysed in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) loading buffer by boiling for 10 min. Equal amounts of proteins in sample buffer were loaded onto SDS-PAGE gels and separated by gel electrophoresis. The gels were then transferred onto a nitrocellulose membrane and probed with anti-Zta, anti-EA-D, anti-Rta, and anti-Topo I antibodies. Signals were visualized by enhanced chemiluminescence (Amersham).
Transfections and reporter assays.
293 cells were transfected with Hp-luciferase plasmid or Mp-luciferase plasmid plus plasmid p3xFLAG-Zta or control vector p3xFLAG. The cells were treated with camptothecin or etoposide 6 h posttransfection. Transcription activation assays were performed by using the luciferase assay system (Promega). The results of luciferase assays were based on experiments performed in triplicate transfections. Expression levels of Zta were monitored by Western blot analysis.
siRNA and shRNA depletion.
A Topo I small interfering RNA (siRNA) mixture and control siRNA were purchased from Santa Cruz Biotechnology. siRNAs were transfected using Dharmacon transfection reagent as recommended by the manufacturer. The Mission short hairpin RNA (shRNA) gene set against RecQL (RecQ protein-like or DNA helicase Q1-like) was purchased from Sigma-Aldrich (GenBank accession number NM_002907.2).
A control vector (a nontargeting shRNA that activates the RNA interference pathway without targeting any known human gene, SHC002) was also purchased (Sigma-Aldrich). The best two RecQL1-targeting shRNA vectors and control vector were converted to lentivirus using packaging vectors (pHR′8.2ΔR and pCMV-VSV-G). MutuI cells were then transduced with shRNA encoding lentivirus in the presence of polybrene (4 mg/ml). Transduced cells were selected with puromycin (2 μg/ml) for a week. The efficacies of these shRNAs in the knockdown of the respective proteins were assayed by Western blotting with specific antibodies. MutuI cells stably expressing RecQL1/control shRNAs were treated with tetradecanoyl phorbol acetate (TPA) (40 ng/ml) and sodium butyrate (NaB) (3 mM) to induce EBV lytic replication. Two days postinduction, total DNA was assayed by real-time PCR to measure lytic replication.
Coimmunoprecipitations.
D98 cells were transfected with Zta plasmid or treated with TPA and NaB (Sigma). Approximately 48 h posttransfection, cells were washed with phosphate-buffered saline, lysed with NET buffer (20 mM Tris [pH 8.0], 100 mM NaCl, 0.1% NP-40, 1 mM EDTA) with cocktail protease inhibitors, placed on ice for 30 min, and sonicated for 5 s. After centrifugation at 10,000 × g for 10 min, the cell extract was preblocked with protein G beads (Dynal Biotech, Oslo, Norway) for 1 h at 4°C. Immunoprecipitation was performed by the addition of 2 μl of rabbit anti-Zta antibody or normal rabbit immunoglobulin G (IgG) to the cell lysates with agitation at 4°C overnight. Protein G beads were then added, and the mixtures were incubated at 4°C for 3 h. The beads were washed five times with immunoprecipitation buffer. Bound proteins were released by heating samples at 100°C for 5 min in SDS-PAGE sample buffer.
ChIP assay and quantitative real-time PCR.
Cells were cross-linked with 1% formaldehyde and lysed in a buffer containing 1% SDS, 10 mM EDTA, and 50 mM Tris-Cl (pH 8.0). Chromatin was sonicated to ∼600 bp and diluted with immunoprecipitation dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 20 mM Tris [pH 8.0], 167 mM NaCl) to a concentration of 1 × 106 cells/ml. Antibodies were added to 1 × 106 cell equivalents (1 ml of lysate). Input (total) DNA was obtained from samples not incubated with antibody. Chromatin immunoprecipitation (ChIP) DNA was analyzed by real-time PCR using an AB 7000 apparatus (Applied Biosystems). All values were normalized to the respective input controls and IgG corresponding to each sample. Real-time primers were designed using Primer Express (Applied Biosystems) to amplify OriLyt (forward primer TCGCCTTCTTTTATCCTCTTTTTG and reverse primer CCCAACGGGCTAAAATGACA) and actin (forward primer AACCCAGCCACACCACAAAG and reverse primer CACTGACTTGAGACCAGTTGAATAAAA).
RESULTS
Camptothecin inhibits Zta-dependent replication and lytic cycle gene activation.
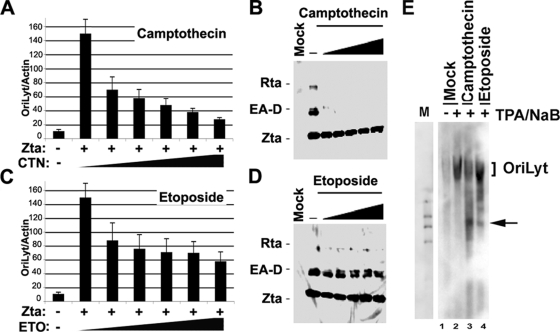
To explore the role of topoisomerase in EBV lytic replication, we first assayed the effect of topoisomerase inhibitors on Zta-dependent lytic replication and the reactivation of viral gene expression using stable cell lines containing EBV bacmids lacking the Zta gene (ZKO-293 cells) (Fig. 1). The transfection of Zta into ZKO-293 cells produced a ∼14-fold increase in viral genome copy numbers within 24 h posttransfection (Fig. 1A and C). We compared different doses of camptothecin, an inhibitor of Topo I (Fig. 1A and B), or etoposide, an inhibitor of Topo II (Fig. 1C and D), on Zta-dependent DNA amplification (Fig. 1A and C) or gene activation (Fig. 1B and D). We found that 2 μM camptothecin caused approximately a sevenfold reduction in viral DNA amplification (Fig. 1A), while a similar concentration of etoposide caused only approximately a twofold reduction (Fig. 1C). Camptothecin and etoposide had no effect on Zta levels expressed from the exogenous cytomegalovirus (CMV) promoter-driven plasmid (Fig. 1B and D). Camptothecin also inhibited Zta transcriptional activation of EA-D and Rta at concentrations as low as 0.1 μM (Fig. 1B), while etoposide showed no significant reduction of Zta-mediated viral gene activation (Fig. 1D). The effects of camptothecin on viral lytic replication were also examined more directly by Southern blot analysis of EBV DNA (Fig. 1E). EBV-positive D98/HR1 cells were treated with phorbol ester TPA (40 ng/ml) and NaB (3 mM) to induce lytic cycle replication. Cells were then treated (3 h postinduction) with 1 μM camptothecin or 10 μM etoposide or mock treated and assayed for viral DNA 24 h postinduction. We found that both camptothecin and etoposide reduced the amplification of OriLyt DNA, consistent with the findings from the real-time PCR analysis (Fig. 1A). Interestingly, we also found that both etoposide and especially camptothecin caused the formation of smaller, aberrant DNA products that were likely the result of abortive or incomplete viral DNA replication.
FIG. 1.
Camptothecin inhibits replication and reactivation of gene expression by Zta. (A) EBV DNA copy numbers were assayed by real-time PCR analysis of OriLyt relative to cellular actin. ZKO-293 cells were transfected with Zta (+) or with control vector (−) and then treated with either 0, 0.1, 0.25, 0.5, 1.0, or 2.0 μM camptothecin (CTN). (B) The same ZKO-293 cells treated as described above (A) were assayed by Western blotting with antibodies for Zta, EA-D, and Rta. (C) ZKO-293 cells were transfected as described above (A) and then treated with either 0, 0.5, 12.5, 25, 50, or 100 μg/ml of etoposide (ETO), as indicated. (D) ZKO-293 cells treated in C were assayed by Western blotting for Zta, EA-D, and Rta expression. (E) Southern blot analysis of EBV DNA in D98/HR1 cells induced with TPA and NaB and then treated with either mock treatment, 1 μM camptothecin, or 50 μg/ml etoposide at 3 h postinduction. DNA was isolated at 24 h postinduction. DNA was restricted with BamHI and visualized by hybridization with a probe to OriLyt. Arrows indicate incomplete products of incomplete viral DNA synthesis.
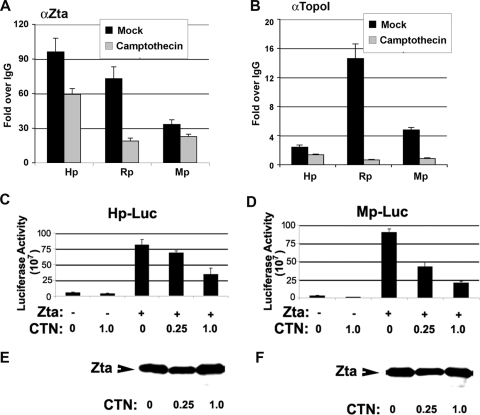
The effects of camptothecin on viral gene expression were further investigated by ChIP assays of endogenous viral promoters (Fig. 2A and B). Zta is known to bind directly to the BHRF1 (Hp), BMRF1 (Mp), and BRLF1 (Rp) promoters. We therefore tested whether the addition of camptothecin during the viral reactivation process inhibited Zta binding to these promoters (Fig. 2A). We found that 1 μM camptothecin partially inhibited Zta binding at Rp (∼28% of mock) but had a more modest effect at Hp (∼64% of mock) or Mp (∼72% of mock). We also tested whether Topo I bound directly to these viral promoters and whether camptothecin affected this binding (Fig. 2B). ChIP assays with Topo I antibody revealed that Topo I bound to the Zta-responsive promoters. Topo I was most enriched at Rp relative to Hp and Mp. The addition of 1 mM camptothecin caused a complete loss of Topo I binding to these promoters. This suggests that camptothecin inhibits the localization of Topo I with Zta at viral promoters. We further examined the effects of camptothecin on Zta-mediated transcription using luciferase reporter assays (Fig. 2C and D). We found that camptothecin partially inhibited the Zta transcriptional activation of Hp (threefold) and Mp (about fivefold) at 1 μM. A similar inhibitory activity was observed for the BZLF1 promoter (Zp) and the BRLF1 promoter (Rp) (data not shown). Zta protein expression levels were not affected by this concentration of camptothecin, indicating that camptothecin is not inhibiting the Zta expression vector (Fig. 2E and F). These results suggest that camptothecin inhibits Zta transcription activation function but that some promoters may be more sensitive to the Topo I inhibitor and also that transfected plasmids may not be as sensitive as the endogenous gene promoters (compare Fig. 1B and 2).
FIG. 2.
Camptothecin inhibits Zta-dependent transcription activation. (A) ZKO-293 cells were transfected with Zta and then treated with 1 μM camptothecin or mock treated. Zta binding to the viral promoters Hp, Rp, and Mp was assayed by ChIP with antibody to Zta (αZta) or with control IgG. ChIP DNA was quantified by real-time PCR and presented as change over IgG. (B) Same as in A except that ChIP assays were performed with antibody to Topo I. (C) 293 cells were transfected with Hp-Luc with either Zta expression vector (+) or control vector (−). Cells were then treated with 0, 0.25, or 1.0 μM camptothecin (CTN), as indicated. Luciferase assays were performed at 48 h posttransfection. (D) Same as in C except for the Mp-Luc reporter. (E and F) Western blot control for Zta expression levels for luciferase assays shown in C and D, respectively.
Topo I is required for lytic cycle replication.
Although camptothecin is a highly specific Topo I inhibitor, we tested whether the siRNA depletion of Topo I had an effect on lytic replication and transcription similar to that observed with pharmacological inhibition. Topo I was depleted with an siRNA mixture (Santa Cruz Biotechnology) and compared to a control siRNA with no predicted cellular targets (Fig. 3). Western blotting confirmed that Topo I protein levels were reduced by Topo I-specific siRNA and not by control siRNA (Fig. 3A). We assayed for Zta-dependent viral DNA replication using ZKO-293 cells (Fig. 3B). Zta stimulated EBV DNA amplification by ∼50-fold in these experiments. In cells that were cotransfected with siRNA targeting Topo I, viral DNA amplification was correspondingly reduced two- to fivefold. In contrast, no inhibition of viral DNA replication was observed in cells cotransfected with siRNA targeting the control. siRNA depletion of Topo I also inhibited the transcription activation function (Fig. 3C). The depletion of Topo I caused a ∼50% reduction in transcription activation function, while control siRNA had a ∼9% reduction. These findings indicate that the Topo I protein contributes to the Zta-dependent activation of EBV lytic replication and transcription.
FIG. 3.
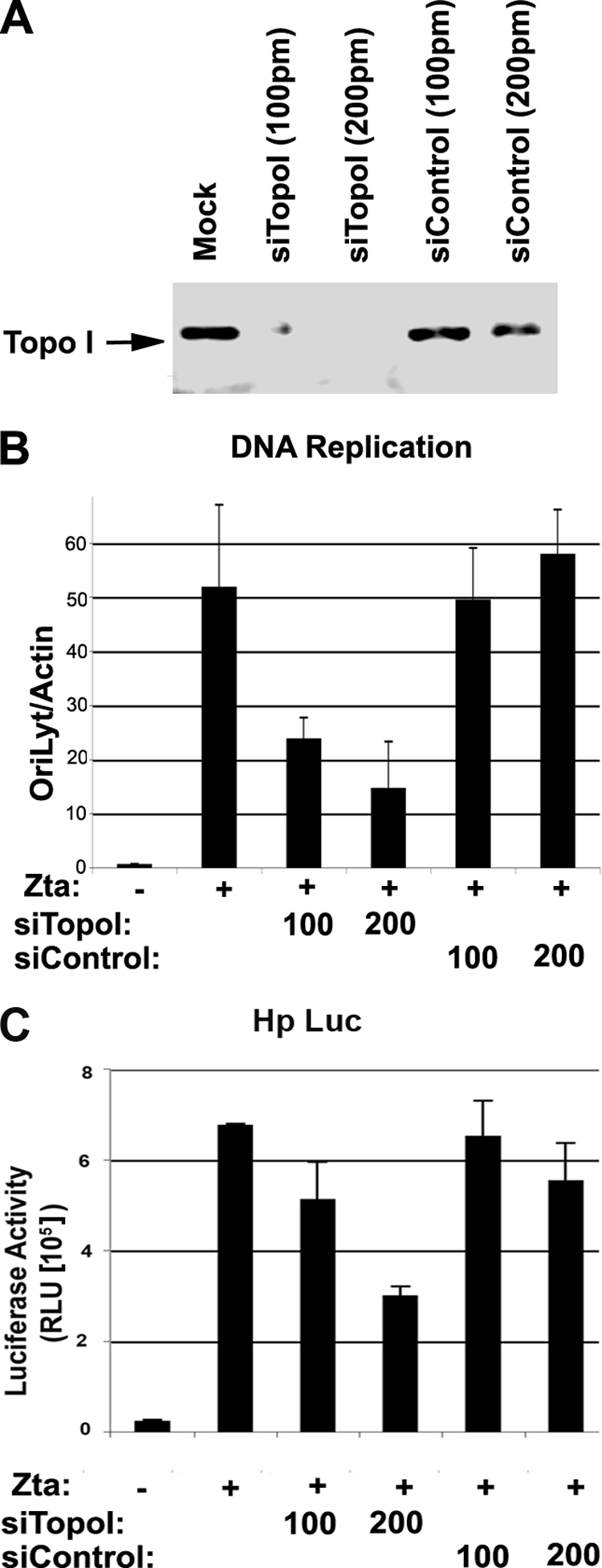
Depletion of Topo I inhibits Zta-mediated lytic replication. (A) ZKO-293 cells were transfected with siRNA targeting Topo I (siTopo I) or control siRNA at 100 or 200 pmol per transfection. Topo I expression levels were assayed by Western blotting. (B) ZKO-293 cells transfected as described above (A) were assayed for EBV genome copy numbers using real-time PCR analysis of EBV OriLyt DNA relative to cellular actin DNA. (C) 293 cells were transfected with Hp-Luc and either Zta expression vector (+) or control vector (−). Cells were then transfected with siRNA targeting Topo I (siTopo I) or control siRNA (siControl) as indicated. Luciferase assays were quantified as the mean values for three independent transfections and errors for standard deviations from the means. RLU, relative light units.
Topo I associates with Zta and OriLyt DNA.
Zta is known to interact with numerous cellular factors including several transcription coactivator complexes that might include Topo I. To test whether Topo I associates with Zta, we assayed Zta immunoprecipitates from either latently infected cells that were induced to express endogenous Zta using phorbol ester TPA combined with NaB or the same cells transfected with FLAG-Zta (Fig. 4). In untreated D98/HR1 cells, Topo I was not detected in the immunoprecipitates with Zta antibody or control antibody (IgG). In contrast, Topo I was detected in the immunoprecipitates from Zta antibody (Fig. 4A, lane 6) but not control IgG in TPA- and NaB-treated cells (Fig. 4A, lane 5). Similarly, Topo I was detected in FLAG immunoprecipitates (Fig. 4A, lane 9) but not in control IgG immunoprecipitates (Fig. 4A, lane 8) in cells that were transfected with FLAG-Zta. These experiments indicate that endogenous Zta, as well as ectopically expressed Zta, can form a stable complex with Topo I.
FIG. 4.
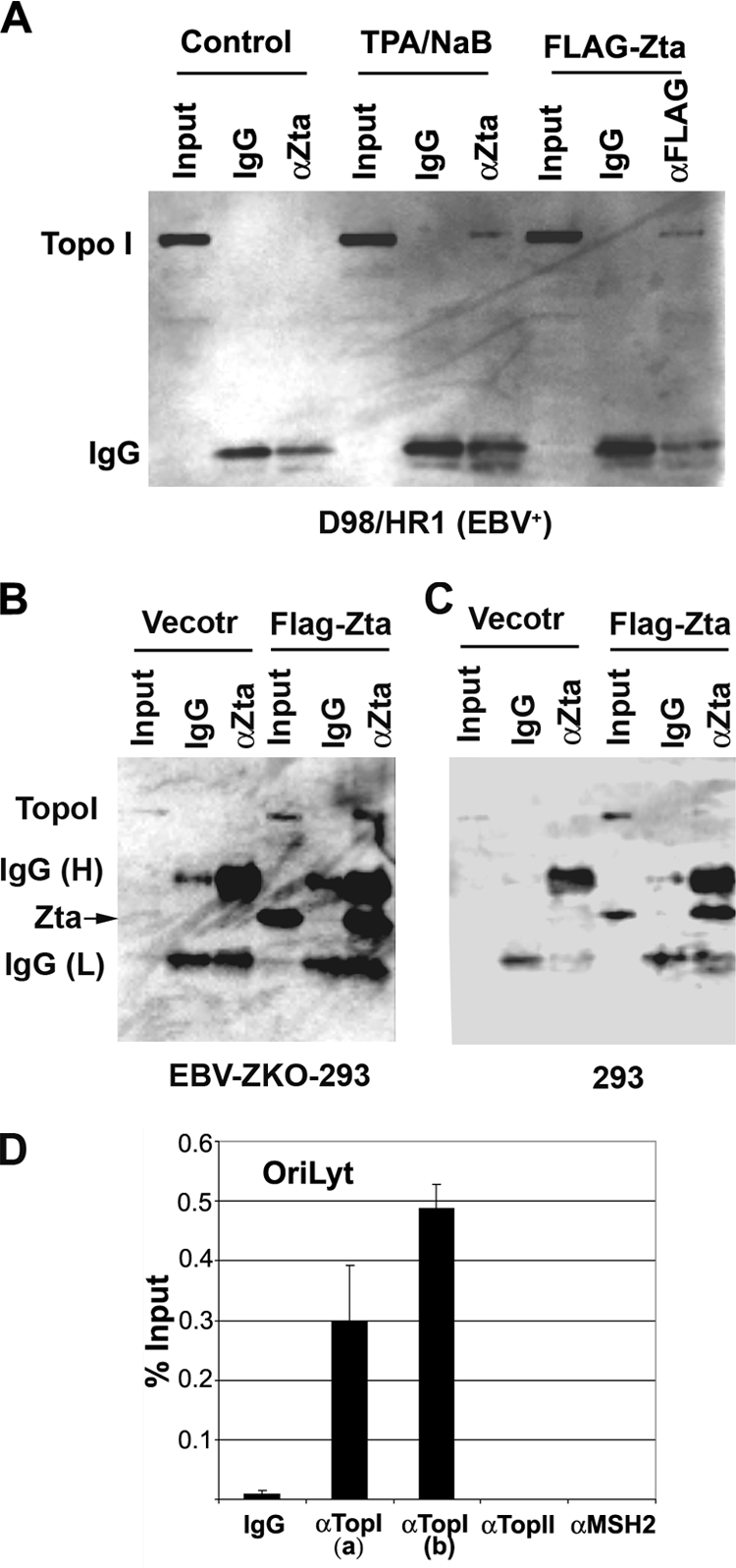
Topo I coimmunoprecipitates with Zta and colocalizes with OriLyt. (A) EBV-positive D98/HR1 cells were untreated (lanes 1 to 3), treated with TPA (40 ng/ml) and NaB (3 mM) to induce Zta and lytic replication (lanes 4 to 6), or transfected with FLAG-Zta expression vector (lanes 7 to 9). Cells were then assayed at 48 h posttreatment by immunoprecipitation with antibodies specific for Zta (αZta) (lanes 3 and 6), FLAG (lane 9), or control antibody (IgG) (lanes 2, 4, and 8). Input lanes represent 10% of the starting lysate for each immunoprecipitation. Immunoprecipitates were assayed by Western blotting with Topo I-specific antisera. (B) Western blotting of immunoprecipitates from EBV-positive ZKO-293 cells after transfection with CMV-FLAG vector or CMV-FLAG-Zta expression plasmids. Input (5%), IgG, and anti-Zta immunoprecipitates are indicated for each set of transfections. Western blots were probed with anti-Topo I-specific antibody and visualized by chemiluminescence.(C) Same as B except that EBV-negative 293 cells were used for transfection. (D) D98/HR1 cells treated with TPA and NaB as described above (A) were assayed by ChIP with two different antibodies (a or b) to Topo I, antibodies to Topo II or MSH2, or control IgG, as indicated. ChIP DNA was quantified for EBV OriLyt DNA as a percentage of the total input DNA.
To determine whether other viral proteins were required for the interaction of Topo I with Zta, we compared the abilities of Zta to coimmunoprecipitate with Topo I in cells with and without EBV genomes (Fig. 4B and C, respectively). EBV-positive ZKO-293 cells (Fig. 4B) or EBV-negative 293 cells (Fig. 4C) were transfected with vector or FLAG-Zta and then assayed by anti-Zta immunoprecipitation and anti-Topo I immunoblotting. We found that Topo I was readily detected in Zta immunoprecipitates from Zta-transfected ZKO-293 cells (Fig. 4B). However, Topo I was not readily detected in Zta immunoprecipitates from EBV-negative 293 cells (Fig. 4C). This suggests that the Zta interaction with Topo I may depend on other viral proteins induced during lytic replication.
Zta is known to recruit multiple viral and cellular factors to OriLyt during lytic cycle reactivation and replication. To test whether Topo I associates with OriLyt DNA during reactivation, we used a ChIP assay of D98/HR1 cells that were induced for lytic replication with TPA and NaB treatment (Fig. 4D). We found that Topo I was highly enriched (20- to 50-fold relative to control IgG) at OriLyt DNA using two different antibodies [TopI(a) and TopI(b) antibodies]. Antibodies to Topo II or the mismatch repair protein MSH2 showed no significant enrichment relative to control IgG at OriLyt. Previous studies and data not shown demonstrated that Zta is highly enriched at this region of OriLyt (∼200- to 500-fold relative to the IgG control). These findings indicate that Topo I can physically associate with Zta and OriLyt DNA during the lytic activation of EBV.
Multiple Topo I inhibitors block Zta transcription activation and DNA replication function.
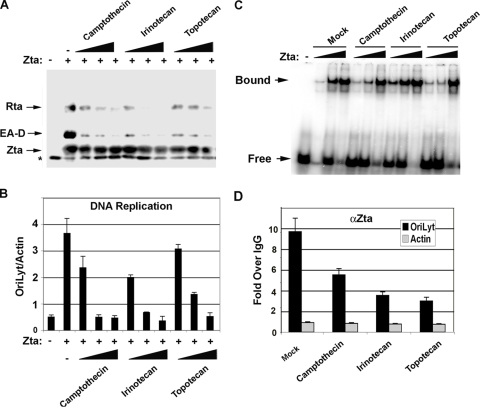
To further validate the role of Topo I in mediating Zta function during lytic reactivation and DNA replication, we compared the abilities of two other Topo II inhibitors, irinotecan and topotecan, to function similarly to camptothecin (Fig. 5). While irinotecan and topotecan are derivatives of camptothecin, they are thought to have altered target specificities and may interfere with different steps of the topoisomerase cleavage-ligation reaction (20). We found that irinotecan and topotecan, like camptothecin, inhibited the Zta transcriptional activation of EA-D and Rta after transient transfection of ZKO-293 cells (Fig. 5A). Similarly, irinotecan and topotecan inhibited EBV lytic cycle DNA replication comparably to camptothecin (Fig. 5B). To test whether any of these Topo I inhibitors may be blocking the inherent DNA binding ability of Zta, we isolated Zta from transfected cells using FLAG affinity purification of FLAG-tagged Zta. None of these Topo I inhibitors interfered with the DNA binding activity of Zta proteins by electrophoretic mobility shift assay (Fig. 5C). However, all of the Topo I inhibitors reduced Zta binding to OriLyt in ChIP assays (Fig. 5D). Camptothecin inhibited Zta binding to OriLyt to ∼60%, while irinotecan and topotecan reduced Zta binding to ∼30% of untreated samples. These results indicate that Topo I inhibition alters Zta binding to OriLyt in vivo but has no effect on the inherent Zta DNA binding properties after purification from cell extracts.
FIG. 5.
Multiple Topo I inhibitors block EBV replication. (A) Western blot analysis of Zta, EA-D, and Rta in ZKO-293 cells transfected without (−) or with (+) Zta expression vector and then treated with 0.1, 0.3, or 0.9 μM camptothecin, irinotecan, or topotecan (as indicated). (B) EBV DNA replication was assayed by real-time PCR with primers specific for OriLyt relative to cellular actin DNA primer amplification. Cells were treated with mock treatment (−), camptothecin, irinotecan, or topotecan as described above (A). (C) FLAG-Zta was affinity purified from ZKO-293 cells used in A and B and assayed for DNA binding activity by electrophoretic mobility shift assay. FLAG-Zta was assayed at 50, 150, and 450 μg/ml as indicated by the black triangles above each lane. Free and bound DNAs are indicated. (D) ChIP assay with anti-Zta (αZta) relative to control IgG measured at OriLyt (black bars) and control regions in cellular actin (gray bars). ChIP assays were performed with ZKO-293 cells transfected with Zta and then treated as described above (A) with 1 μM camptothecin, irinotecan, or topotecan, as indicated.
Camptothecin inhibits replication complex assembly at OriLyt.
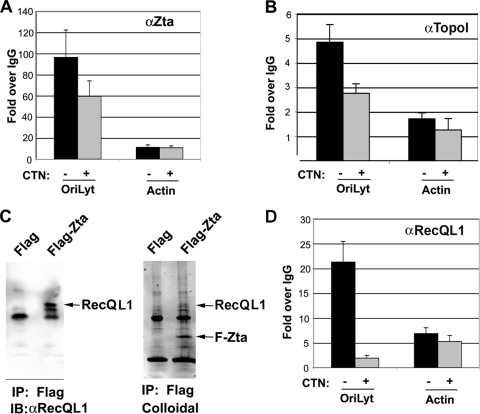
Camptothecin was next tested for its ability to interfere with steps subsequent to Zta binding to OriLyt DNA. Consistent with data described above (Fig. 5D), 1 μM camptothecin inhibited DNA replication by ∼85% but only modestly reduced Zta-OriLyt binding to ∼31% of that of mock-treated cells (Fig. 5B and 6A). Camptothecin had a similar modest effect of a ∼44% reduction in Topo I binding to OriLyt (Fig. 6B). To determine if other replication factors might be affected by camptothecin treatment, we utilized information recently obtained for proteins that assemble at the related KSHV lytic origin (25). In that study, the cellular helicase RecQL1 was found to associate physically and functionally with KSHV OriLyt. To determine if RecQL1 was also involved in Zta-dependent lytic replication, we first tested whether RecQL1 associated with Zta (Fig. 6B). We found that RecQL1 was highly enriched in immunoaffinity-purified FLAG-Zta but not in the control immunopurifications of transfected ZKO-293 cells (Fig. 6C). We next use ChIP assays to confirm that RecQL1 associated in vivo with EBV OriLyt during viral reactivation using the ChIP assay (Fig. 6D). RecQL1 was enriched ∼20-fold relative to control IgG. This indicates that RecQL1 associates with EBV OriLyt similarly to its association with KSHV OriLyt. To determine if camptothecin interfered with RecQL1 binding, we assayed RecQL1 binding by ChIP assays using cells treated with 1 μM camptothecin. We found that camptothecin reduced RecQL1 binding to EBV OriLyt by ∼86% of that of mock-treated cells (Fig. 6D). RecQL1 did not bind significantly to control DNA regions at the cellular actin locus.
FIG. 6.
Camptothecin inhibits Zta assembly of replisome at OriLyt. (A) ZKO-293 cells were transfected with Zta expression vector, followed by treatment with 1 μM camptothecin (CTN) (+) or vehicle control (−). Cells were then assayed by ChIP for Zta binding to viral OriLyt or to the cellular actin locus. (B) ZKO-293 cells transfected with Zta and then treated with (+) or without (−) camptothecin were assayed by ChIP with antibodies to Topo I (αTopoI) or control IgG. ChIP DNA was assayed at EBV OriLyt (left bars) or the control region in cellular actin (right bars). (C) ZKO-293 cells were transfected with FLAG-Zta or control FLAG expression vector and then subjected to FLAG immunopurification (IP). FLAG affinity-purified proteins were assayed by Western blotting (immunoblotting [IB]) for the cellular RecQL1 protein (left) or by colloidal blue staining of the affinity-purified proteins (right). FLAG-Zta is indicated by the arrow. (D) A ChIP assay was used to monitor RecQL1 binding to OriLyt in vivo in ZKO-293 cells transfected with FLAG-Zta followed by the addition of camptothecin (+) or vehicle control (−). ChIP DNA was assayed at the EBV OriLyt or cellular actin locus and quantified as change over control IgG.
To determine if the association of RecQL1 with Zta and OriLyt was functionally relevant for lytic replication, we generated two different shRNAs specific for RecQL1 (Fig. 7). The shRNAs were expressed from lentivirus containing a puromycin resistance gene. Control and RecQL1-specific shRNA lentiviruses were used to infect EBV-positive MutuI cells and then selected for puromycin resistance for 6 days. Puromycin-resistant pools were then treated with TPA and NaB to induce lytic reactivation and assayed by Western blotting with antibodies to RecQL1, Rta, EA-D, Zta, and the cellular loading control PCNA (Fig. 7A). The RecQL1 expression level was significantly reduced by the two shRNAs relative to those of viral lytic proteins and cellular PCNA. The depletion of RecQL1 did not cause a significant reduction in the level of expression of the Rta or Zta protein. However, EA-D levels were notably reduced by RecQL1 depletion. Similarly, lytic replication was notably reduced by RecQL1 depletion (Fig. 7B). RecQL1 shRNA reduced lytic replication by ∼49% and ∼67% as measured by real-time PCR analysis of EBV DNA relative to cellular DNA. Taken together, these findings suggest that RecQL1 associates with Zta at OriLyt and contributes to lytic replication. Moreover, Topo I activity is required for the efficient recruitment of RecQL1 to OriLyt.
FIG. 7.
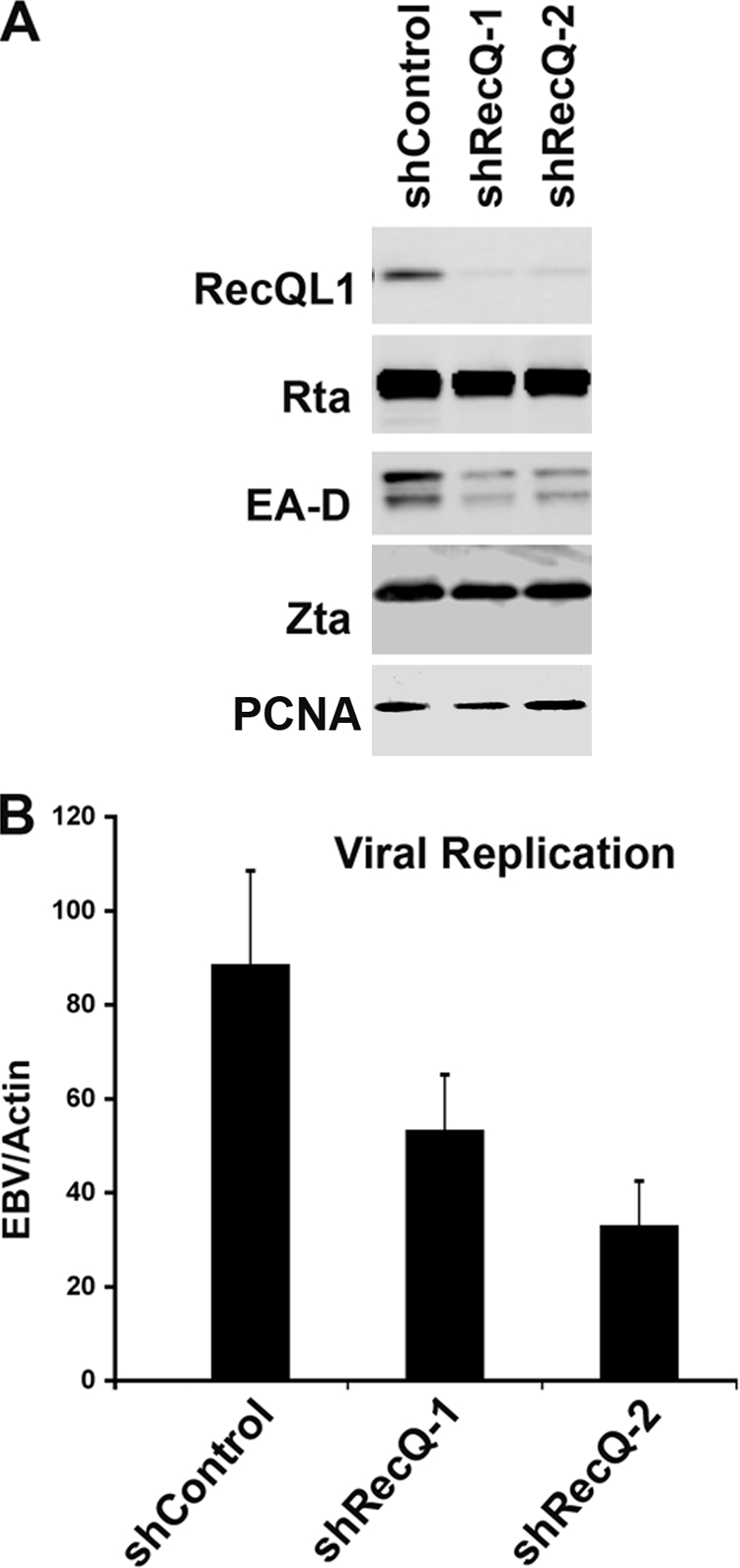
RecQL1 depletion compromises EBV lytic replication. (A) Western blotting of EBV-positive MutuI cells infected with lentivirus containing shRNA for control (shControl) or RecQL1-targeting sequences, as indicated. (B) MutuI cells infected with shRNA-containing lentivirus as shown above (A) were induced by TPA and NaB for lytic cycle replication. Viral DNA replication was measured by real-time PCR analysis of EBV DNA relative to cellular actin.
DISCUSSION
In this study, we investigated the mechanism through which topoisomerase inhibitors block EBV lytic cycle replication. We found that inhibitors for Topo I (e.g., camptothecin, topotecan, and irinotecan) and Topo II (e.g., etoposide) reduce viral lytic replication. Topo I inhibitors were more effective at reducing replication and lytic gene activation. Camptothecin efficiently blocked the Zta-dependent reactivation of viral transcription and DNA replication (Fig. 1). Furthermore, Topo I bound directly to Zta-responsive promoters and to OriLyt, as measured by ChIP assays (Fig. 2B, 4D, and 6B). Topo I siRNA depletion had an effect on viral replication and gene expression similar to that of pharmacological inhibitors (Fig. 3). The inhibition of Topo I reduced Zta binding to OriLyt and nearly eliminated the assembly of the cellular RecQL1 protein at OriLyt (Fig. 6D). Based on these findings, we conclude that Topo I plays a direct role in the transcription activation and DNA replication functions of Zta.
The inhibition of Topo I by camptothecin is likely to have broad effects on multiple viral and cellular processes. Therefore, it is likely that camptothecin may also block other essential steps in the viral reactivation pathway besides Zta-dependent transcription and replication activation. It is well known that viral reactivation also depends on the function of the other viral transcription factor, Rta. We also found that Topo I inhibitors are potent blockers of Rta transcription in transient reporter assays (data not shown). Thus, it remains possible that the inhibition of Rta transactivation is an important component of the inhibition of viral reactivation by Topo I inhibitors.
While Topo I is likely to affect multiple steps in the reactivation process, our data suggest that Topo I plays a direct role at OriLyt. We found that Topo I bound with Zta at OriLyt in ChIP assays (Fig. 4D) and formed a stable complex in lytic reactivated cells by coimmunoprecipitation (Fig. 4A and B). Interestingly, Zta did not form a stable complex with Topo I in EBV-negative 293 cells (Fig. 4C), suggesting that other factors cooperate to form a stable complex during lytic reactivation. We did not test whether Rta can form a stable complex with Topo I in EBV-negative cells, but it will be important to determine if Rta or other viral replication factors mediate the recruitment of Topo I to OriLyt through more direct binding to Topo I. The functional requirement for Topo I at OriLyt was demonstrated by several criteria. Multiple pharmacological inhibitors of Topo I blocked lytic replication (Fig. 5). Additionally, siRNA depletion of Topo I also blocked lytic replication (Fig. 3). Topo I inhibition also blocked the recruitment of the cellular helicase RecQL1 to OriLyt (Fig. 6). RecQL1 had been implicated at KSHV OriLyt, and we now show that this factor also binds EBV OriLyt and is required for optimal replication activity, as measured by the effects of the shRNA depletion of RecQL1 (Fig. 7). Based on these observations, we propose that Topo I is required for the recruitment of essential factors, like RecQL1, to OriLyt during lytic reactivation.
Topoisomerases have been implicated in herpesvirus lytic and latent cycle replication. Topo I was required for the biochemical reconstitution of HSV replication intermediates using highly purified viral replication proteins, where it is required for the relaxation of topologically constrained templates (19). Topo I is required for relieving torsional strain during transcription and replication (3, 20). The release of torsional strain may also be necessary to facilitate strand unwinding during the early stages of transcription and replication factor assembly at herpesvirus lytic origins. Topo I has been implicated in the replication function of several other viruses, including papillomaviruses (19) and vaccinia virus, which encodes its own type I topoisomerase (23). More recently, Topo I and Topo II were implicated both physically and functionally at KSHV OriLyt (25). Camptothecin and etoposide were also shown to inhibit KSHV lytic replication. That KSHV study also implicated a role for poly(ADP-ribose) polymerase 1 (PARP1) in OriLyt function, and others previously showed that PARP1 can form a complex with Topo I (29). These findings raise the possibility that Topo I-PARP1-associated DNA nicking and repair may play an essential role during the initiation of gammaherpesvirus lytic replication.
The DNA helicase RecQL1 has been implicated in homologous recombination and in regulating chromosomal replication (22). RecQL1 was found previously through a proteomic analysis of KSHV OriLyt DNA binding proteins and was shown to interact with KSHV Rta (25). We found that RecQL1 associates with the EBV Zta protein in EBV-positive cells, suggesting that other EBV proteins may be important for this interaction (Fig. 6C). The findings presented here indicate that Topo I activity is required for RecQL1 recruitment to OriLyt as well as for the transcription activation function of Zta and Rta. The common requirement for Topo I activity by multiple herpesviruses suggests that this factor plays a critical role in the lytic reactivation process. The availability of different classes of Topo I inhibitors may help to dissect the molecular mechanisms controlling lytic cycle transcription and replication as well as provide additional antiviral strategies to block herpesvirus infection.
Acknowledgments
We thank H.-J. Delecluse for ZKO-293 cells.
This work was supported by grants from the NIH to P.M.L. (grant CA86678) and to Y.Y. (grant AI052789) and from the Wistar Cancer Center (NCI) and PA Settlement for Tobacco Research. A.J.R. was supported by a predoctoral fellowship from a UPENN tumor virology training grant (grant 1T32 CA115299).
Footnotes
Published ahead of print on 3 June 2009.
REFERENCES
- 1.Amon, W., and P. J. Farrell. 2005. Reactivation of Epstein-Barr virus from latency. Rev. Med. Virol. 15149-156. [DOI] [PubMed] [Google Scholar]
- 2.Baumann, M., R. Feederle, E. Kremmer, and W. Hammerschmidt. 1999. Cellular transcription factors recruit viral replication proteins to activate the Epstein-Barr virus origin of lytic DNA replication, oriLyt. EMBO J. 186095-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champoux, J. J. 2001. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 70369-413. [DOI] [PubMed] [Google Scholar]
- 4.Chevallier, G. A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 53243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 824085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dardari, R., M. Khyatti, A. Benider, H. Jouhadi, A. Kahlain, C. Cochet, A. Mansouri, B. El Gueddari, A. Benslimane, and I. Joab. 2000. Antibodies to the Epstein-Barr virus transactivator protein (ZEBRA) as a valuable biomarker in young patients with nasopharyngeal carcinoma. Int. J. Cancer 8671-75. [DOI] [PubMed] [Google Scholar]
- 7.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1995. Replication of Epstein-Barr virus oriLyt: lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J. Virol. 692998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao, Z., A. Krithivas, J. E. Finan, O. J. Semmes, S. Zhou, Y. Wang, and S. D. Hayward. 1998. The Epstein-Barr virus lytic transactivator Zta interacts with the helicase-primase replication proteins. J. Virol. 728559-8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenspan, J. S., D. Greenspan, E. T. Lennette, D. I. Abrams, M. A. Conant, V. Petersen, and U. K. Freese. 1985. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N. Engl. J. Med. 3131564-1571. [DOI] [PubMed] [Google Scholar]
- 10.Hammarsten, O., X. Yao, and P. Elias. 1996. Inhibition of topoisomerase II by ICRF-193 prevents efficient replication of herpes simplex virus type 1. J. Virol. 704523-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong, G. K., M. L. Gulley, W. H. Feng, H. J. Delecluse, E. Holley-Guthrie, and S. C. Kenney. 2005. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J. Virol. 7913993-14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong, G. K., P. Kumar, L. Wang, B. Damania, M. L. Gulley, H. J. Delecluse, P. J. Polverini, and S. C. Kenney. 2005. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J. Virol. 7913984-13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawanishi, M. 1993. Topoisomerase I and II activities are required for Epstein-Barr virus replication. J. Gen. Virol. 74(10)2263-2268. [DOI] [PubMed] [Google Scholar]
- 14.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2343-2396. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 15.Kouzarides, T., G. Packham, A. Cook, and P. J. Farrell. 1991. The BZLF1 protein of EBV has a coiled coil dimerisation domain without a heptad leucine repeat but with homology to the C/EBP leucine zipper. Oncogene 6195-204. [PubMed] [Google Scholar]
- 16.Lau, R., J. Middeldorp, and P. J. Farrell. 1993. Epstein-Barr virus gene expression in oral hairy leukoplakia. Virology 195463-474. [DOI] [PubMed] [Google Scholar]
- 17.Liao, G., F. Y. Wu, and S. D. Hayward. 2001. Interaction with the Epstein-Barr virus helicase targets Zta to DNA replication compartments. J. Virol. 758792-8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieberman, P. M., J. M. Hardwick, J. Sample, G. S. Hayward, and S. D. Hayward. 1990. The Zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds to both AP-1 and ZRE sites in target promoter and enhancer regions. J. Virol. 641143-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimonkar, A. V., and P. E. Boehmer. 2004. Role of protein-protein interactions during herpes simplex virus type 1 recombination-dependent replication. J. Biol. Chem. 27921957-21965. [DOI] [PubMed] [Google Scholar]
- 20.Pommier, Y. 2006. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer 6789-802. [DOI] [PubMed] [Google Scholar]
- 21.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 22.Sharma, S., D. J. Stumpo, A. S. Balajee, C. B. Bock, P. M. Lansdorp, R. M. Brosh, Jr., and P. J. Blackshear. 2007. RECQL, a member of the RecQ family of DNA helicases, suppresses chromosomal instability. Mol. Cell. Biol. 271784-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shuman, S. 1998. Vaccinia virus DNA topoisomerase: a model eukaryotic type IB enzyme. Biochim. Biophys. Acta 1400321-337. [DOI] [PubMed] [Google Scholar]
- 24.Wang, J. C. 2002. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 3430-440. [DOI] [PubMed] [Google Scholar]
- 25.Wang, Y., H. Li, Q. Tang, G. G. Maul, and Y. Yuan. 2008. Kaposi's sarcoma-associated herpesvirus ori-Lyt-dependent DNA replication: involvement of host cellular factors. J. Virol. 822867-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiedmer, A., P. Wang, J. Zhou, A. J. Rennekamp, V. Tiranti, M. Zeviani, and P. M. Lieberman. 2008. Epstein-Barr virus immediate-early protein Zta coopts mitochondrial single-stranded DNA binding protein to promote viral and inhibit mitochondrial DNA replication. J. Virol. 824647-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu, F. Y., H. Chen, S. E. Wang, C. M. apRhys, G. Liao, M. Fujimuro, C. J. Farrell, J. Huang, S. D. Hayward, and G. S. Hayward. 2003. CCAAT/enhancer binding protein α interacts with ZTA and mediates ZTA-induced p21CIP-1 accumulation and G1 cell cycle arrest during the Epstein-Barr virus lytic cycle. J. Virol. 771481-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu, F. Y., S. E. Wang, H. Chen, L. Wang, S. D. Hayward, and G. S. Hayward. 2004. CCAAT/enhancer binding protein α binds to the Epstein-Barr virus (EBV) ZTA protein through oligomeric interactions and contributes to cooperative transcriptional activation of the ZTA promoter through direct binding to the ZII and ZIIIB motifs during induction of the EBV lytic cycle. J. Virol. 784847-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yung, T. M., S. Sato, and M. S. Satoh. 2004. Poly(ADP-ribosyl)ation as a DNA damage-induced post-translational modification regulating poly(ADP-ribose) polymerase-1-topoisomerase I interaction. J. Biol. Chem. 27939686-39696. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Q., D. Gutsch, and S. Kenney. 1994. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol. Cell. Biol. 141929-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]