Abstract
The Epstein-Barr virus (EBV) SM protein is essential for lytic EBV DNA replication and virion production. When EBV replication is induced in cells infected with an SM-deleted recombinant EBV, approximately 50% of EBV genes are expressed inefficiently. When EBV replication is rescued by transfection of SM, SM enhances expression of these genes by direct and indirect mechanisms. While expression of most EBV genes is either unaffected or enhanced by SM, expression of several genes is decreased in the presence of SM. Expression of BHRF1, a homolog of cellular bcl-2, is particularly decreased in the presence of SM. Investigation of the mechanism of BHRF1 downregulation revealed that SM downregulates expression of the immediate-early EBV transactivator R. In EBV-infected cells, R-responsive promoters, including the BHRF1 and SM promoters, were less active in the presence of SM, consistent with SM inhibition of R expression. SM decreased spliced R mRNA levels, supporting a posttranscriptional mechanism of R inhibition. R and BHRF1 expression were also found to decrease during later stages of EBV lytic replication in EBV-infected lymphoma cells. These data indicate that feedback regulation of immediate-early and early genes occurs during the lytic cycle of EBV regulation.
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus that infects epithelial and lymphoid cells and is associated with several human malignancies (for a review, see reference 59). EBV undergoes lytic replication in epithelial cells during primary infection and during reactivation from latent infection in B lymphocytes. The switch from latency to lytic replication is regulated by two EBV transcriptional activators: Z (also known as BZLF1, Zta, or ZEBRA) and R (also known as BRLF1 or Rta) (for a review, see reference 68). SM (also known as Mta, EB2, or BMLF1) is an RNA-binding EBV protein that is expressed following Z and R and enhances EBV gene expression by multiple posttranscriptional mechanisms (4, 11, 28, 29, 35, 61, 65). SM is essential for the lytic phase of EBV replication and virion production (21). We previously used an epithelial cell line carrying an EBV recombinant deleted for SM (293 BMLF1-KO) to compare gene expression in the presence and absence of SM (25). When cells carrying recombinant SM-deleted EBV were transfected with the EBV transactivator BZLF1 gene, approximately 50% of EBV genes were expressed poorly but were rescued by cotransfection with SM, resulting in EBV DNA replication and virion production (25). The block in EBV DNA replication is at least partly due to inefficient expression of EBV DNA polymerase and primase in the absence of the SM protein. Transfection of EBV DNA polymerase and primase genes with BZLF1 allows EBV DNA replication but does not rescue virion production, indicating that additional essential EBV mRNAs directly require SM for efficient expression (25).
When EBV lytic replication was rescued by SM transfection, expression of most EBV genes either was upregulated or was not significantly changed, but BHRF1 mRNA became significantly less abundant (25). The BHRF1 gene encodes a bcl-2 homolog expressed early in the lytic phase of EBV replication (50, 51). BHRF1 blocks apoptosis induced by a variety of stimuli, including growth factor withdrawal (13, 27), granzyme B (9), γ irradiation (43), chemotherapeutic drugs (36), deregulated c-Myc (10), and p53 (69, 70). Although BHRF1 is dispensable for replication of EBV and for transformation of B lymphocytes in vitro, the BHRF1 homolog expressed by the murine gammaherpesvirus MHV68 is required for efficient virus reactivation from latency ex vivo and persistent replication in vivo, suggesting that BHRF1 could play an important role during EBV lytic replication in vivo (15, 38, 42). The finding that the SM protein may negatively regulate BHRF1 expression during lytic replication led us to investigate the effect of SM on BHRF1 expression further.
Autoregulation of immediate-early (IE) and delayed-early genes occurs during herpes simplex virus (HSV) replication and occurs by several mechanisms (16, 31, 39, 48). Feedback regulation of gene expression during lytic replication in lymphocryptoviruses has not been extensively studied. Synchronous and efficient lytic EBV replication is difficult to induce in latently EBV-infected cells, contributing to the relatively limited knowledge of mechanisms by which IE and early gene expression may be curtailed during later phases of the lytic cycle. The experiments described below reveal mechanisms by which the SM protein may contribute to the orderly progression of the lytic cascade during lytic EBV reactivation by downregulating IE and early gene expression.
MATERIALS AND METHODS
Cell lines.
293 is a cell line derived from human embryonic kidney cells (19). 293 BMLF1-KO cells are 293 cells carrying SM-deleted recombinant EBV expressing green fluorescent protein, a kind gift of H. Gruffat and W. Hammerschmidt (21). B95-8 is a marmoset B-lymphocyte cell line derived by immortalization with human EBV (45). The P3HR-1 ZHT cell line was derived from P3HR-1 (53) by transfection with a plasmid expressing a tamoxifen-inducible EBV Zta activator of lytic gene expression (pCDNA3-ZHT), followed by selection in neomycin. All cell lines were cultured in Dulbecco's modified Eagle's medium or RPMI supplemented with Glutamax (Invitrogen) and 10% fetal bovine serum. 293 cell transfections were performed with TransIT293 (Mirus) reagent according to the manufacturer's protocols. 4-Hydroxy-tamoxifen (Sigma) was added to the culture medium at a final concentration of 100 nM to induce lytic replication in P3HR-1 ZHT cells.
Plasmids.
To generate the BHRF1 expression plasmid, 1.639 kb of EBV DNA containing the entire BHRF1 gene including 5′ and 3′ untranslated regions (accession number NC_007605; nucleotides [nt] 41492 to 43130) was amplified by high-fidelity PCR. The amplified fragment was cloned into the mammalian expression vector pCDNA3 (Invitrogen) at the EcoRV site. pCDNA3-ZHT was made by fusing Zta to a 4-hydroxytamoxifen-responsive mutated estrogen receptor hormone-binding domain (ZHT) to make a 4-hydroxytamoxifen-dependent inducer of EBV replication as described previously (33). The SM expression plasmid was constructed by PCR amplification from B95-8 EBV DNA as described previously (61). Plasmid pCMV-Z containing Zta cloned in pCDNA3 was a kind gift of P. Lieberman (73). To generate an Rta expression vector, 1.818 kb of B95-8 EBV R cDNA (nt 91078 to 92895) was amplified by high-fidelity PCR and cloned into pCDNA3 at the EcoRV site. An R and Z bicistronic expression vector containing BRLF1 and BZLF1 was made by PCR amplification of 2.974 kb of B95-8 EBV genomic DNA (nt 89922 to 92895; accession no. NC_007605.1) and cloned into pCDNA3 at the EcoRV site. This R-Z construct contains the coding R exon and all exons and introns of Z but does not contain the upstream noncoding exon and intron of R. The plasmid BHRF1p-luc was constructed by PCR amplification of 320 bp containing the EBV BHRF1 promoter (nt 41200 to 41520) and cloned into the SmaI sites of pGL3Basic (Promega) upstream of the luciferase gene. The BHRF1 promoter with the oriLyt p-luc plasmid was made by PCR amplification of 1.218 kb EBV DNA including the BHRF1 promoter and additional oriLyt sequence (nt 40303 to 41520) and cloned into the SmaI site of pGL3Basic. SMp-luc was generated by cloning 500 bp of PCR-amplified EBV SM promoter sequence (nt 72500 to 72001) in the SmaI site of pGL3Basic. Plasmid Rp-luc was kindly provided to us by Shannon C. Kenney and has been described previously (1). All plasmid DNA was purified with Qiagen columns as described by the manufacturer's protocols (Qiagen). The structures of all plasmids generated in our laboratory were verified by DNA sequencing.
Oligonucleotide primers.
The following primers were used to generate the plasmids and probes used in this study. For the BHRF1 genomic construct, BHRF1 forward primer 5′-AGTTGCCTGTTTCATCAC-3′ and BHRF1 reverse primer 5′-CTGGGCTGCAGTATAGGCTC-3′ were used; for the BHRF1 promoter, forward primer 5′GACCGATGCTCGCCACTTCCTG-3′ and reverse primer 5′-GCCCGGGGTTAGTGATGAAAC-3′ were used; for the BHRF1 promoter with oriLyt, forward primer 5′-CCCAGCGCGCCCCGTTCA-3′ and reverse primer 5′-GCCCGGGGTTAGTGATGAAAC-3′ were used; for the SM promoter, forward primer 5′-CGCGGATGATGGGCGTGACTATCTAA-3′ and reverse primer 5′-CTTGTCTGTCTCTACGA-3′ were used; for RTA, forward primer 5′-ATGAGGCCTAAAAAGGATGGCTTGG-3′ and reverse primer 5′-CTAAAATAAGCTGGTGTCAAAAATAG-3′ were used; for the bicistronic R-Z construct, forward primer 5′-ATGAGGCCTAAAAAGGATGGCTTGG-3′ and reverse primer 5′-TTAGAAATTTAAGAGATCCTCGTGTAAAACAT-3′ were used; for reverse transcription-PCR (RT-PCR) analysis of R transcripts, primer P3 (5′-GCCCGTCTTCTTACCCTGTTGTTTCG), primer P1 (5′-CTCACTACACAAACAGACGC-3′), and primer P2 (5′-ATTCAGGGATCTATAGCCACCATC-3′) were used.
RNA extraction, RT-PCR, and Northern blot analysis.
Transfected cells were harvested at various times after transfection by trypsinization and centrifugation. Cells were washed with phosphate-buffered saline and lysed in RNA-bee (Tel-Tech, Inc.), and RNA was isolated using RNeasy mini columns as per the manufacturers protocol (Qiagen). RNA was quantitated by spectrophotometry, and RT-PCR was performed using AccessQuik RT-PCR reagents (Promega) or by reverse transcription with strand-specific primers, followed by DNA PCR. For Northern blotting, 5 μg of unfractionated RNA samples were subjected to electrophoresis through denaturing formaldehyde-agarose gels. RNA was transferred overnight to charged nylon membrane through capillary action and hybridized with gene-specific probes labeled with [32P]dCTP as described previously (61). Gene-specific probes were generated by PCR amplification and gel purification of fragments or isolation from previously cloned plasmids. Quantitative PCR was performed with a StepOne real-time PCR thermocycler and SYBR green PCR master mix (Applied Biosystems).
Western blot analysis.
Cells were washed with phosphate-buffered saline and lysed in sodium dodecyl sulfate sample buffer. The extracts were boiled at 95°C for 5 min, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, proteins were transferred to nitrocellulose membranes (Bio-Rad) and probed with specific antibodies. The following antibodies were used for immunoblotting: for BRLF1, 1:100 dilution of mouse monoclonal antibody (11-008; Argene), for BZLF1, 1:20 dilution of mouse monoclonal antibody (M 7005; Dako), for β actin, 1:5,000 dilution of mouse monoclonal antibody (A5441; Sigma), for BHRF1, 1:100 dilution of mouse monoclonal antibody (5B11Δ). Blots were incubated with secondary antibodies conjugated to horseradish peroxidase (Amersham) diluted to 1:1,000 for BRLF1 and BZLF1, 1:5,000 for actin, and 1:7,500 for BHRF1 antibodies. Antigen and antibody complexes were then detected with enhanced chemiluminescence detection reagents (Pierce).
Reporter assays.
293 cells were transfected with plasmids DNA using the TransIT293 reagent. Cells were harvested and washed with phosphate-buffered saline 48 h after transfection. Cell pellets were lysed at room temperature for 15 min in 1× reporter lysis buffer (Promega). The lysed cell suspension was centrifuged, and cleared supernatant was transferred to new tubes. Luciferase assays were performed using luciferase assay reagent and a Turner luminometer as per the manufacturer's protocol (Promega). Protein concentrations were measured by Bradford assay and 5 μg of protein was used for each luciferase reaction. Each transfection was performed at least twice, and all assays were performed in triplicate.
RESULTS
SM decreases BHRF1 mRNA expression from the EBV genome but not from a heterologous promoter.
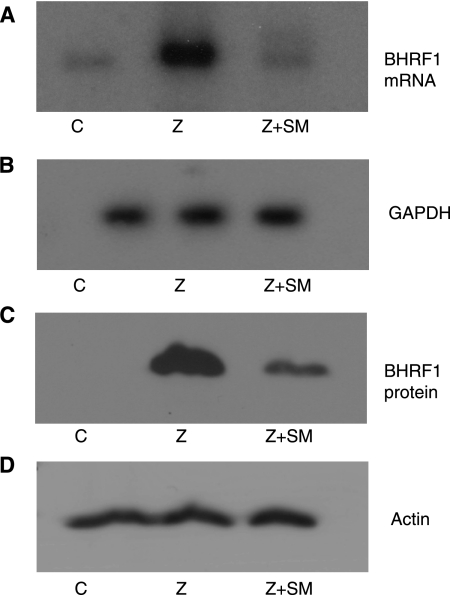
When EBV deleted for SM is induced to replicate lytically by transfection of Z, only a subset of EBV lytic genes is expressed, efficient DNA replication does not occur, and replication is abortive (21, 25). When SM is cotransfected with Z, the full lytic gene repertoire is expressed and virus production is rescued. Interestingly however, when measured by an EBV DNA microarray, expression of a few genes, and BHRF1 in particular, is lower when SM is expressed (25). In order to confirm previous microarray results indicating that SM resulted in lower levels of BHRF1 and to investigate the nature of BHRF1 mRNAs produced in the presence of SM, we analyzed BHRF1 mRNA in epithelial cells infected with SM-deleted recombinant EBV (293-BMLF1KO) by Northern blotting. As shown in Fig. 1A, transfection of Z strongly induced BHRF1, whereas coexpression of SM inhibited BHRF1 expression to levels comparable to those in cells that had not been induced to permit lytic EBV replication. In order to measure SM effects on BHRF1 protein expression, protein lysates were made from the same transfected cells and immunoblotted with anti-BHRF1 antibody. BHRF1 protein expression was also significantly lower when SM was expressed, consistent with our previous results (Fig. 1C) (25).
FIG. 1.
Effect of SM on BHRF1 expression. (A) BHRF1 mRNA expression in 293 BMLF1-KO cells. 293 BMLF1-KO cells were transfected with either empty vector plasmid (C), Z expression plasmid (Z), or Z and SM expression plasmid DNA (Z+SM). RNA was isolated, and Northern blotting was performed with a BHRF1-specific probe. (B) The blot in panel A was probed with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe as a loading control. (C) BHRF1 protein expression in BMLF1-KO cells. BMLF1-KO cells were transfected as for panel A, and protein lysates were analyzed by immunoblotting with anti-BHRF1 antibody. Lane 1, BMLF1KO cells transfected with vector plasmid DNA (C); lane 2, BMLF1KO cells transfected with Z expression plasmid DNA (Z); lane 3, BMLF1KO cells transfected with Z and SM expression plasmid DNA (Z+SM). (D) The blot in panel C was stripped and reprobed with actin antibody.
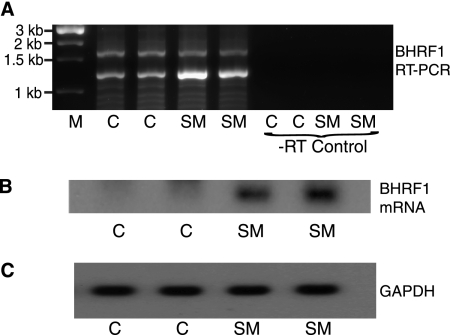
SM is known to inhibit expression of some spliced genes by multiple mechanisms, including decreasing the stability of the intron-containing transcript and enhancing export of unspliced RNA (11, 61). The BHRF1 gene contains an intron 5′ to the single open reading frame, suggesting the possibility that SM inhibited BHRF1 expression posttranscriptionally (51). In addition, SM has not been shown to inhibit transcription when its effects on transcription have been directly examined by nuclear run-on assays (47, 61). We therefore asked whether SM would inhibit BHRF1 expression when the intron-containing gene was transcribed from a heterologous promoter. A 1.6-kb region of EBV DNA containing the entire BHRF1 gene was cloned downstream of the cytomegalovirus (CMV) IE promoter in the expression vector pCDNA3. This minigene construct was transfected into 293 cells with or without SM. RNA was isolated 48 h after transfection and analyzed by RT-PCR using BHRF1 primers. In contrast to its effect on BHRF1 expressed from the EBV genome, SM increased accumulation of spliced BHRF1 mRNA transcribed from the CMV promoter (Fig. 2A). It should be noted that the larger and the smaller amplification products were cloned and sequenced. The larger band is derived from pre-mRNA, and the smaller band corresponds to spliced BHRF1 mRNA (intron from nt 41607 to 42046, NCBI reference EBV sequence NC_007605.1). Interestingly, the splice donor site in this BHRF1 cDNA is different from that reported in the reference sequence, although the splice acceptor site is identical. The donor splice site in the cDNA reported here is 22 nt upstream of that reported in the reference sequence (nt 41629) and may represent differences in splicing in epithelial cells versus that in B lymphocytes.
FIG. 2.
Effect of SM on BHRF1 transcribed from a heterologous promoter. (A) EBV DNA containing the BHRF1 gene including the 5′UTR and intron was cloned into the mammalian expression vector pCDNA3 and transfected into 293 cells with either empty vector plasmid (C) or SM expression plasmid DNA (SM). RNA was isolated 48 h after transfection and analyzed by RT-PCR using BHRF1 primers. One-kbp DNA markers (M) are shown in lane 1. PCR without reverse transcription is shown at right (-RT Control). (B) RNA from samples in panel A above were analyzed by Northern blotting with a BHRF1 probe. Lanes 1 and 2, RNA from 293 cells transfected with BHRF1 and empty vector plasmids (C); Lanes 3 and 4, RNA from 293 cells transfected with BHRF1 and SM expression plasmids (SM). (C) RNA from samples in panel A were probed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
The effect of SM on BHRF1 mRNA accumulation was confirmed by Northern blotting with the BHRF1 probe, which showed that BHRF1 mRNA expression from the CMV promoter was increased by SM (Fig. 2B). Since CMV promoter activity is not affected by SM (47, 61), these results indicate that SM could actually enhance accumulation of BHRF1 mRNA posttranscriptionally, similar to its effect on the majority of EBV transcripts (25). Nevertheless, since the net effect of SM on BHRF1 expression during EBV replication was inhibitory, it appeared likely that SM inhibits the activity of the BHRF1 promoter in the EBV genome during lytic replication.
SM inhibits BHRF1 promoter activity in EBV-positive but not EBV-negative cells.
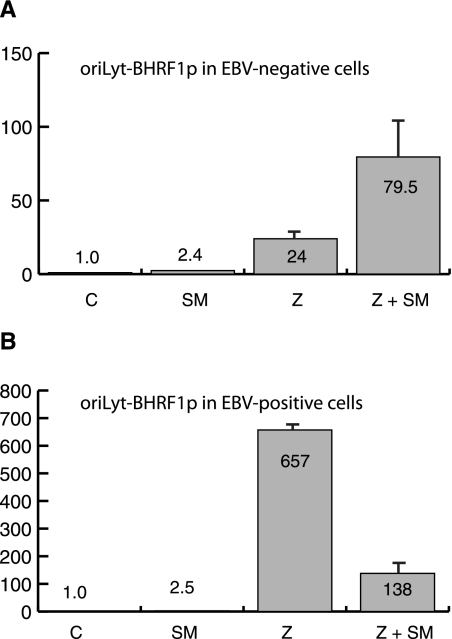
SM could inhibit the BHRF1 promoter (BHRF1p) by affecting the levels or function of transcription factors acting on BHRF1p. In addition, we considered the possibility that SM could indirectly affect BHRF1 promoter activity by enhancing DNA replication from the origin of lytic EBV DNA replication (oriLyt), which is immediately upstream of BHRF1p (24, 50). We have previously demonstrated that while the majority of early genes do not require SM for expression, DNA replication does not occur efficiently in the absence of SM (25). The dependence of EBV lytic DNA replication on SM is due primarily to inefficient expression of EBV DNA polymerase and primase in the absence of SM (25). Thus, lytic EBV DNA replication facilitated by SM might interfere with BHRF1 promoter function, thereby indirectly inhibiting BHRF1 expression. Therefore, in order to test the effect of SM on BHRF1p, we constructed a luciferase reporter plasmid with a fragment of the B95-8 genome which included oriLyt and the BHRF1 promoter upstream of luciferase (oriLyt-BHRF1p-luc). In order to study potential direct effects of SM on BHRF1 promoter activity, as well as effects mediated via enhancement of oriLyt replication, EBV-infected BMLF1KO 293 cells and EBV-uninfected 293 cells were transfected with oriLyt-BHRF1p-luc and either empty vector, Z (to activate the promoter), SM alone, or Z and SM plasmids in combination, and promoter activity was analyzed by luciferase assay.
As shown in Fig. 3A, SM did not inhibit BHRF1 promoter activity in EBV-negative 293 cells; in fact, it had a moderate enhancing effect on luciferase (∼2.5-fold). As we have previously shown using the CMV promoter (47, 61), these effects on luciferase in transfection assays are posttranscriptional and therefore promoter independent. Expression of Z increased oriLyt-BHRF1p activity by ∼25-fold, and in the presence of Z and SM, activity was increased by ∼80-fold (Fig. 3A) due to the combined effect of Z on the promoter and SM on the transcript.
FIG. 3.
Effect of SM on activity of BHRF1 promoter linked to oriLyt. (A) 293 cells were transfected with a reporter plasmid in which luciferase was transcribed from an oriLyt-BHRF1 promoter (oriLyt-BHRF1p) and either empty vector (C), Z, or Z+SM expression plasmid. Lysates were prepared 48 h after transfection and assayed for luciferase activity. n-fold activation is shown on the y axis. (B) BMLF1-KO-293 cells (EBV positive) were transfected and assayed for luciferase expression as in panel A above.
In contrast, when these transfection assays were performed with BMLF1KO 293 cells to determine whether SM would exert an inhibitory effect on the BHRF1 promoter in the context of EBV lytic replication, BHRF1p activity was significantly induced by Z (∼650-fold) and reduced to 138-fold in the added presence of SM (Fig. 3B). Thus, oriLyt-BHRF1p activity in BMLF1KO cells recapitulated the behavior of BHRF1 gene expression from the viral genome, in which EBV lytic replication was rescued by SM. These data therefore indicate that SM inhibited BHRF1 expression by inhibiting BHRF1 transcription but also that the mechanism required active EBV lytic replication or other EBV proteins.
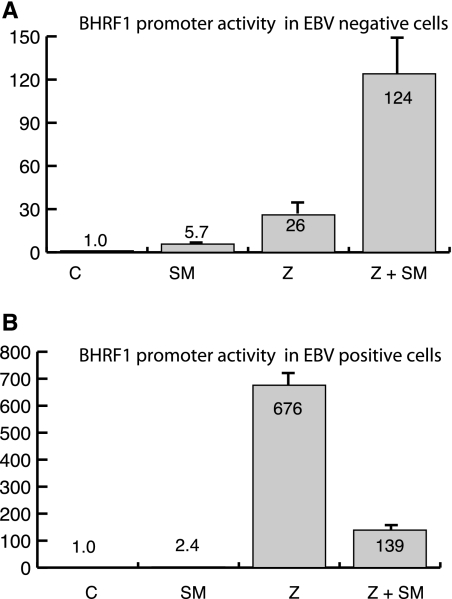
Effect of SM on BHRF1 promoter activity is independent of oriLyt.
In order to test whether the inhibitory effect of SM on BHRF1 promoter activity was dependent on linkage to oriLyt, we constructed a reporter plasmid in which oriLyt was not present and only BHRF1p was driving luciferase transcription (BHRF1p-luc). Both BMLF1KO 293 and EBV-negative 293 cells were transfected with BHRF1p-luc and induced to begin lytic DNA replication by cotransfection of a Z expression plasmid. In EBV-negative 293 cells, BHRF1p-luc activity was not inhibited by SM (Fig. 4A), as was the case with oriLyt-BHRF1p. In BMLF1 KO-293 (EBV-positive) cells, BHRF1 promoter activity was increased ∼670-fold in the presence of Z compared to results with the control plasmid (Fig. 4B), a level of induction almost identical to that seen with oriLyt-BHRF1p-luc (Fig. 3B). Again, similar to the results obtained with oriLyt-BHRF1p-luc in EBV-positive cells, cotransfection of SM with Z decreased BHRF1p-luc activity to ∼140-fold. These results indicate that inhibition of the BHRF1 promoter by SM is oriLyt independent and therefore is not due to SM's ability to activate EBV DNA replication. Thus, the inhibitory effect of SM on the BHRF1 promoter requires EBV-specific factors but does not require the presence of oriLyt in cis.
FIG. 4.
Effect of SM on activity of BHRF1 promoter unlinked to oriLyt. (A) 293 cells (EBV negative) were transfected with BHRF1p-luciferase reporter plasmid and either empty vector (C) or Z or Z+SM expression plasmid. Lysates were prepared 48 h after transfection and assayed for luciferase activity. n-fold activation is shown on the y axis. (B) BMLF1KO 293 (EBV-positive) cells were transfected and assayed for luciferase expression as in panel A above.
SM decreases expression of EBV R transactivator.
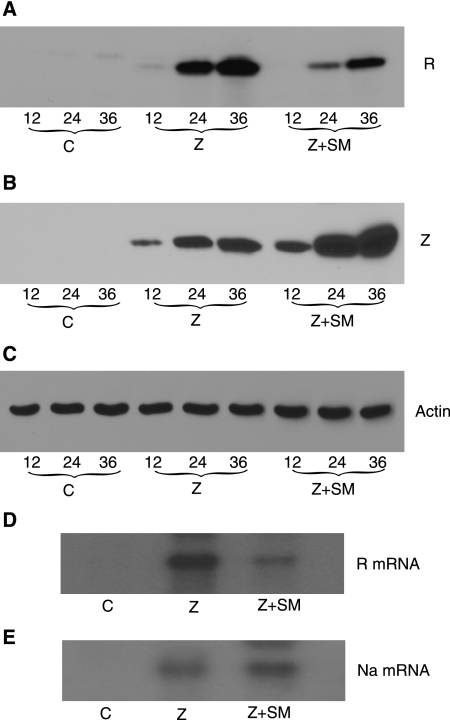
The reporter assays also revealed that BHRF1p activity was much greater in EBV-positive cells than in EBV-negative 293 cells, although both cell lines were transfected with exogenous Z expression plasmid (cf. Fig. 3A and B and 4A and B). In fact, the activity of BHRF1p was approximately 25-fold greater in EBV-positive cells than in EBV-negative cells. This suggested that other EBV transcriptional activators or cellular transcription factors induced by lytic EBV replication were responsible for the greater BHRF1p activity observed in EBV-positive BMLF1KO 293 cells. Reactivation and lytic replication in latently EBV-infected cells are primarily controlled by the two transactivator proteins Z and R (for a review, see reference 68). Z and R act cooperatively to activate the transcription of EBV early genes, including BHRF1, and the BHRF1 promoter contains both Z and R response elements to which Z and R bind directly (26, 34, 40, 54, 56). Since it appeared that SM's inhibitory effect on the BHRF1 promoter might be exerted through another EBV protein, we examined the effect of SM on both Z and R expression. We performed immunoblot analyses for R expression with protein cell lysates from BMLF1KO cells transfected with either empty vector, Z, or Z plus SM. Cells transfected with Z and SM expressed less R protein than cells transfected with Z alone (Fig. 5A). In contrast, SM did not inhibit Z expression and in fact increased total Z protein expression (Fig. 5B) in the same cell lysate. These data therefore indicate that SM may limit BHRF1 expression by inhibiting R expression at later times during EBV replication.
FIG. 5.
Effect of SM on Rta expression in EBV-positive 293 cells. (A) Effect of SM on RTA protein expression in EBV-positive 293 cells. BMLF1KO 293 cells were transfected with either control plasmid (C), Z expression plasmid (Z), or Z and SM expression plasmid DNA (Z+SM). Protein lysates were made from cells harvested at 12, 24, and 36 h after transfection and analyzed by immunoblotting with anti-R antibody. The time posttransfection is shown below each lane. (B) Effect of SM on Z expression in EBV-positive 293 cells. The lysates used in panel A to measure R expression were similarly immunoblotted with anti-Z antibody. (C) Actin expression in transfected cells. The immunoblot used to measure R expression in panel A was stripped and reprobed with antiactin antibody as a loading control. (D) Effect of SM on R mRNA levels in EBV-positive 293 cells. BMLF1KO cells were transfected with either empty vector (C), Z expression plasmid (Z), or Z and SM expression plasmids (Z+SM). RNA was isolated and analyzed by Northern blotting with R-specific probe. (E) Effect of SM on BRRF1 (Na) mRNA expression in BMLF1KO 293 cells. BMLF1KO cells were transfected with empty vector (C), Z expression plasmid (Z), or Z and SM expression plasmids (Z+SM). RNA was isolated and analyzed by Northern blotting with BRRF1-specific probe.
Based on these results, it appeared that SM inhibited R gene expression from the endogenous EBV genome. In order to determine whether SM inhibited R expression at the RNA level, mRNA isolated from BMLF1KO cells transfected with either empty vector alone, Z plasmid, or Z and SM plasmids was analyzed by Northern blotting. When SM was coexpressed with Z, R mRNA levels were significantly decreased (Fig. 5D, lane 3) compared to those when Z alone was transfected (Fig. 5D, lane 2). These results therefore indicate that SM downregulates R mRNA and protein levels during EBV replication.
The existence of a third transcriptional activator important for induction of lytic EBV replication has recently been described (32, 64). The product of the BRRF1 gene, also known as Na, is encoded in the opposite direction in the intron of R (BRLF1). Na cooperates with R to activate Z and enhances the efficiency of lytic EBV gene expression (32). Therefore, it was possible that the negative autoregulatory effects of SM might also be mediated via negative effects on Na expression. In order to examine the effect of SM on Na expression during lytic replication, we compared mRNA levels for BRRF1 in BMLF1KO cells transfected with Z or with Z and SM. As shown in Fig. 5E, SM did not inhibit Na expression. Rather, SM led to a slightly increased level of Na expression, indicating that the effects of SM on R expression are selective.
SM inhibits R mRNA processing activity in EBV-infected cells.
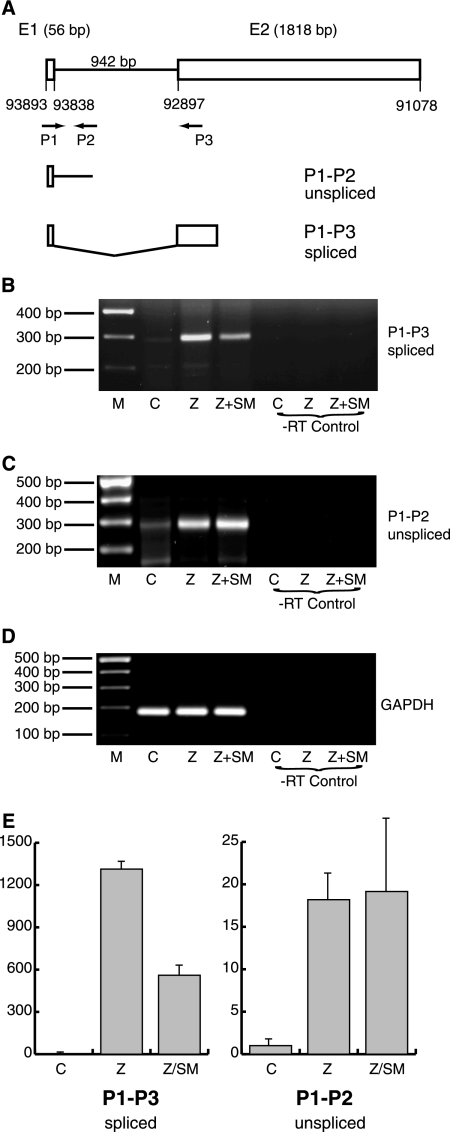
The R gene is transcribed as a bicistronic R-Z mRNA in which R is encoded as a single open reading frame and Z is spliced from three exons (41). The majority of Z expression from the viral genome occurs from a second promoter, Zp, located proximal to the Z start codon (12, 41). The R promoter, Rp, is located approximately 1,000 nt upstream of the R open reading frame. A short noncoding exon located near Rp is spliced to the second exon, which contains the single R open reading frame (Fig. 6A). Because SM is known to exert pleiotropic effects on expression of spliced genes, including decreasing transcript accumulation, as well as facilitating alternative splicing (11, 60, 61, 71), we wished to investigate whether the ability of SM to inhibit R mRNA accumulation was posttranscriptional. In order to examine potential SM effects on processing of the R transcript, we analyzed RNA from BMLF1-KO 293 cells transfected with empty vector, Z, or Z plus SM by RT-PCR. Exon and intron primers were used that distinguish between spliced R mRNA and pre-mRNA (Fig. 6A). Consistent with decreased levels of R mRNA detected by Northern blotting in the presence of SM, spliced R mRNA detected by RT-PCR was also decreased compared to the level seen in the presence of Z alone (Fig. 6B). The level of unspliced product derived from pre-mRNA was unchanged or slightly increased in the presence of SM, indicating that SM was acting to inhibit the production of spliced mRNA (Fig. 6C). These results were confirmed by quantitative RT-PCR (Fig. 6E).
FIG. 6.
Effects of SM on R gene splicing. (A) Diagram of R splicing patterns. The first noncoding exon, E1, of the R mRNA is separated by a 942-nt intron from the second exon, E2, which contains the entire R coding region. Primers used to amplify E1-E2 spliced products (P1-P3) and pre-mRNA (P1-P2) are also shown. (B) RT-PCR was performed with primers to detect spliced E1-E2 product using RNA from BMLF1KO 293 cells transfected with either empty vector (C), Z, or Z plus SM and analyzed by gel electrophoresis. Controls without reverse transcriptase (-RT) are shown at right. (C) RT-PCR was performed and analyzed as described above using primers to detect unspliced pre-mRNA using E1 primer P1 and intron primer P2. (D) RT-PCR was performed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers as an internal control and analyzed as above. (E) Quantitative RT-PCR was performed on RNA samples analyzed in panels B and C above.
It has recently been reported that in Akata cells, R transcripts may also be initiated from an additional promoter upstream of Rp (20). These transcripts appear to include an upstream exon, E0, and may be alternatively spliced to include or exclude E1 before splicing to the coding E2 exon. Primers in E0 and downstream primers in either intron or in E2 were employed in an attempt to detect potential spliced or unspliced transcripts that included E0. Despite several attempts using RNA from which E1-E2 spliced forms were detectable, no E0-containing spliced products were detected (data not shown).
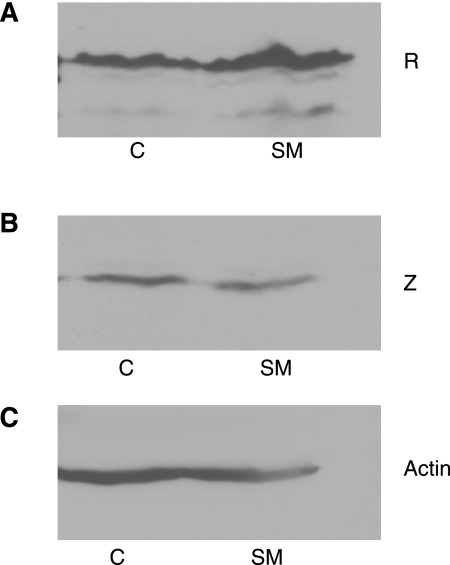
In order to determine whether SM affected accumulation of R mRNA due to effects dependent on splicing of the Z exons distal to R, an R-Z minigene was constructed. This minigene contains the entire E2 R coding exon and the Z exons and introns but does not include the R promoter, the large intron, or E1. This minigene was cloned downstream of a CMV promoter, thereby eliminating any potential effects of SM on the R promoter. The CMV-R-Z expression vector was then transfected into 293 cells with or without SM, and expression of both R and Z was measured by immunoblotting (Fig. 7). SM did not inhibit expression of R from the R-Z gene when transcribed from the CMV promoter in 293 cells, indicating that SM effects on R mRNA are not related to splicing of Z in the bicistronic gene but that SM only targets the splicing of the first and second exons of the genome-encoded R-Z bicistronic mRNA.
FIG. 7.
Effect of SM on R and Z expression from a bicistronic R-Z gene transcribed from the CMV IE promoter. CMV-RZ, which contains a BRLF1 and BZLF1 bicistronic gene driven by the CMV IE promoter, was transfected into 293 cells either with empty vector (C/SM) or with SM expression plasmid (lanes 2 and 4). Protein lysates were made 48 h after transfection and analyzed by immunoblotting using anti-R antibody (A) anti-Z antibody (B), or antiactin antibody as a loading control (C).
SM indirectly autoregulates its own promoter.
R activates lytic EBV promoters through both direct DNA binding mechanisms and indirect mechanisms (6, 8, 22, 23, 34, 52). Several classes of lytic genes have been identified which are differentially dependent on R (5, 56). Some promoters are activated by R by mechanisms that do not involve direct R-DNA interactions, whereas other R-responsive promoters have been identified that contain direct R-binding response elements characterized by a GC-rich motif (23). Such an R-binding response element is present in the promoter of BHRF1. The BHRF1 promoter also belongs to a group of EBV lytic promoters that is synergistically responsive to the actions of Z and R (56) and particularly responsive to Rta (26). The particular sensitivity of BHRF1 to SM suggested that promoters with direct R-binding response elements might be more R dependent and therefore susceptible to feedback regulation by SM during lytic replication. A direct R-binding response element is present in the SM promoter as well (22, 34). Since SM downregulated R activity, one would expect that SM might inhibit its own promoter in EBV-positive cells. The effect of SM on its own promoter (SMp) was therefore tested by cloning SMp in a luciferase reporter plasmid and comparing the activity of the promoter in both EBV-infected and uninfected 293 cells. Transfection of Z activated the SM promoter as expected in both EBV-positive and EBV-negative cells and to a greater degree in EBV-positive cells (Fig. 8). The more-pronounced effect of Z on SMp is also expected because of the production of endogenous R in EBV-positive cells. Whereas SM did not inhibit SM promoter activity in EBV-negative cells (Fig. 8A), it had a significant inhibitory effect in EBV-positive cells (Fig. 8B), consistent with R responsiveness of the SM promoter and inhibition of R protein expression by SM.
FIG. 8.
Effect of SM on the SM promoter. EBV-negative 293 cells (A) or BMLF1KO 293 (EBV-positive) cells (B) were transfected with SMp-luc reporter plasmid and either empty vector, Z, or Z plus SM expression plasmids. Cells were harvested 48 h posttransfection, and luciferase assays were performed. n-fold activation is shown on the y axis.
R and BHRF1 protein levels decline during late lytic replication in P3HR1 cells.
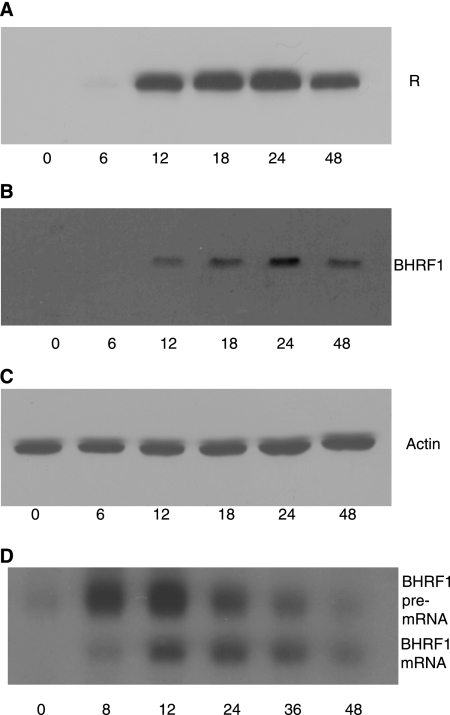
SM expression and rescue of SM-deleted recombinant EBV allowed isolation and analysis of effects of SM on other lytic genes during replication as described above. In order to examine whether the levels of R and BHRF1 protein decline during the course of lytic replication in EBV-infected lymphoma cells, as would be predicted if there were a negative autoregulatory loop in effect, we measured R and BHRF1 expression by immunoblotting at several time points after induction of lytic replication in Burkitt lymphoma cells. In order to achieve robust lytic replication, we employed a derivative of the P3HR-1 cell line in which a tamoxifen-dependent Z-gene fusion is stably expressed. As shown in Fig. 9A, R levels began to be detectable at 6 h after addition of tamoxifen and continued to increase but decreased after 24 h. Immunoblotting with anti-BHRF1 antibody demonstrated that BHRF1 levels also declined at later times during lytic replication. BHRF1 mRNA accumulation in P3HR1-ZHT cells was also examined in a similar experiment. RNA harvested at serial times after induction of lytic replication was analyzed by Northern blotting. As shown in Fig. 9D, BHRF1 pre-mRNA levels increased initially and subsequently declined, followed by a parallel increase and decline in BHRF1 mRNA. These data indicate that negative autoregulation of IE and early genes may occur during the course of EBV lytic replication in B lymphocytes.
FIG. 9.
Rta and BHRF1 expression in P3HR1-ZHT cells during lytic replication. Protein cell lysates were made at 0, 6, 12, 18, 24, and 48 h after tamoxifen treatment to induce lytic EBV replication. Western blot analyses were performed with anti-R (A), anti-BHRF1 (B), or antiactin (C) antibody. (D) RNA was made from P3HR1-ZHT cells at indicated times after induction of lytic EBV replication and probed with BHRF1 probe.
DISCUSSION
The essential role of SM in EBV replication, like that of its homologs in other herpesviruses, is multifactorial. The multiple roles played by each of these proteins are uniquely evolved to facilitate the replication of the parent virus. The distinct regulatory niche of each protein is evinced by the fact that although they perform similarly in many functional assays that measure target gene activation or mRNA export, they nevertheless fail to complement each other in the ability to rescue virus replication (3, 7, 21, 46, 74). In this study, we show that in addition to enhancing expression of the majority of EBV lytic genes, SM is involved in downregulation of IE and early gene expression. The particular effect on BHRF1 appears to be at least partly mediated by downregulation of R at the posttranscriptional level. However, as R autoactivates the R promoter (55), any posttranscriptional inhibitory effects of SM on R are also likely to result in decreased R transcription. Control of R transcription is complex, and Z is an important and central activator of Rp, which acts preferentially on methylated Rp (2, 40, 66). Cellular transcriptional activators important in Rp regulation include YY1, Sp1, and EGR-1 (40, 55, 75). In the experiments reported here, it is unlikely that SM exerts effects on R levels or activity by downregulating Z or Na, since both Z and Na expression actually increased during lytic replication in the presence of SM. These experiments do not address the possibility of SM acting to modulate Z expression during the course of spontaneous lytic replication in vivo as Z was transfected to induce replication.
BHRF1 downregulation as lytic replication progresses may serve several functions. While BHRF1 is not required for reactivation from latency in vitro, it is likely that it prevents apoptosis of infected cells in vivo. There is a significant induction of BHRF1 with the onset of EBV lytic replication, which may maintain cell viability during early lytic replication. It is possible that reducing BHRF1 synthesis during later stages of lytic replication facilitates viral production by allowing cell death to occur. Interestingly, BHRF1 has been shown to be a potent epitope for CD4+ cytotoxic T lymphocytes directed against EBV-infected cells (37), and downregulation of BHRF1 may thus be important in helping EBV evade destruction by the immune system during the later stages of virus production. It is also possible that BHRF1 transcription may facilitate DNA replication from oriLyt, similar to the enhancement of Kaposi's sarcoma-associated herpesvirus lytic origin usage by transcription in this region (72). Downregulation of BHRF1 promoter activity in this region could then act to curtail DNA replication during the late phase of lytic replication.
Feedback regulation of IE and early lytic gene expression during progression of the lytic cascade in HSV is well described (30, 31). ICP4 may participate in autoregulation by inhibiting transcription from its own promoter (39). ICP8, the single-stranded DNA binding protein, inhibits ICP4 transcription and that of other HSV early genes (17, 18). The mRNA degradation activity of vhs operates on HSV mRNAs and cellular mRNA, and this activity acts to modulate levels of lytic HSV mRNAs of all classes, as shown by the temporal profiles of lytic RNA expression in vhs mutant-infected cells (48, 49, 57). Perhaps most relevant to the role of SM in modulating EBV lytic gene expression is the repressive function of HSV ICP27 on IE gene expression during HSV replication (44, 58, 62, 67). Many of ICP27's repressive effects may be indirect, mediated through activation of other HSV genes, such as ICP8, for example. As in the case of HSV, it is likely that negative regulatory effects of SM include indirect effects. Since mRNA levels of approximately 40% of EBV lytic genes are severely reduced and EBV DNA replication is limited in the absence of SM (25), there are likely many cell and viral genes affected by SM which could act to inhibit IE and early gene expression.
The interactions between cellular transcriptional activators, Z, R, and Na generate a complex system of lytic gene regulation with multiple points of control. The finding that R levels decrease during lytic replication and that BHRF1 and SM promoters appear to be particularly susceptible to SM-mediated inhibition suggests that promoters that bind R directly may be differentially regulated. Indeed, the particular activity of Rta on the BHRF1 promoter was observed in early experiments characterizing Rta function (8, 26). It should be noted that unlike HSV lytic replication occurring during primary infection, reactivation of EBV from latency requires exogenous chemical induction or transfection of Z. Thus, Z levels may also be subject to negative autoregulation, which would not be detected in experiments in which exogenous Z is expressed by transfection. RAZ, a protein encoded by an alternatively spliced product of the R-Z bicistronic mRNA, may also exert a dominant-negative effect on Z function (14, 63). Such feedback control of IE and early genes is likely to be an integral component of an efficient and ordered lytic cascade of gene expression in herpesviruses. The data reported here suggest that feedback control of IE and early gene expression occurs during EBV replication and that SM may play a negative as well as positive role in lytic gene expression.
Acknowledgments
This work was supported by grants RO1 CA81133 (SS) from the NCI, National Institutes of Health, and a Howard Hughes Medical Institute Early Career Award (to E.J.).
We thank Dorit Mueller for expert technical assistance and Zhao Han and Melusine Gaillard for advice and assistance with experimental design.
Footnotes
Published ahead of print on 10 June 2009.
REFERENCES
- 1.Bhende, P. M., S. J. Dickerson, X. Sun, W. H. Feng, and S. C. Kenney. 2007. X-box-binding protein 1 activates lytic Epstein-Barr virus gene expression in combination with protein kinase D. J. Virol. 817363-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhende, P. M., W. T. Seaman, H. J. Delecluse, and S. C. Kenney. 2004. The EBV lytic switch protein, Z, preferentially binds to and activates the methylated viral genome. Nat. Genet. 361099-1104. [DOI] [PubMed] [Google Scholar]
- 3.Boyer, J. L., S. Swaminathan, and S. J. Silverstein. 2002. The Epstein-Barr virus SM protein is functionally similar to ICP27 from herpes simplex virus in viral infections. J. Virol. 769420-9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle, S. M., V. Ruvolo, A. K. Gupta, and S. Swaminathan. 1999. Association with the cellular export receptor CRM 1 mediates function and intracellular localization of Epstein-Barr virus SM protein, a regulator of gene expression. J. Virol. 736872-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, L. W., P. J. Chang, H. J. Delecluse, and G. Miller. 2005. Marked variation in response of consensus binding elements for the Rta protein of Epstein-Barr virus. J. Virol. 799635-9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevallier-Greco, A., H. Gruffat, E. Manet, A. Calender, and A. Sergeant. 1989. The Epstein-Barr virus (EBV) DR enhancer contains two functionally different domains: domain A is constitutive and cell specific, domain B is transactivated by the EBV early protein R. J. Virol. 63615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, J. I., T. Krogmann, J. P. Ross, L. Pesnicak, and E. A. Prikhod'ko. 2005. Varicella-zoster virus ORF4 latency-associated protein is important for establishment of latency. J. Virol. 796969-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox, M. A., J. Leahy, and J. M. Hardwick. 1990. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J. Virol. 64313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, J. E., V. R. Sutton, M. J. Smyth, and J. A. Trapani. 2000. Dependence of granzyme B-mediated cell death on a pathway regulated by Bcl-2 or its viral homolog, BHRF1. Cell Death Differ. 7973-983. [DOI] [PubMed] [Google Scholar]
- 10.Fanidi, A., D. C. Hancock, and T. D. Littlewood. 1998. Suppression of c-Myc-induced apoptosis by the Epstein-Barr virus gene product BHRF1. J. Virol. 728392-8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farjot, G., M. Buisson, M. Duc Dodon, L. Gazzolo, A. Sergeant, and I. Mikaelian. 2000. Epstein-Barr virus EB2 protein exports unspliced RNA via a Crm-1-independent pathway. J. Virol. 746068-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flemington, E. K., A. E. Goldfeld, and S. H. Speck. 1991. Efficient transcription of the Epstein-Barr virus immediate-early BZLF1 and BRLF1 genes requires protein synthesis. J. Virol. 657073-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foghsgaard, L., and M. Jaattela. 1997. The ability of BHRF1 to inhibit apoptosis is dependent on stimulus and cell type. J. Virol. 717509-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furnari, F. B., V. Zacny, E. B. Quinlivan, S. Kenney, and J. S. Pagano. 1994. RAZ, an Epstein-Barr virus transdominant repressor that modulates the viral reactivation mechanism. J. Virol. 681827-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangappa, S., L. F. van Dyk, T. J. Jewett, S. H. Speck, and H. W. Virgin IV. 2002. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 195931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelman, I. H., and S. Silverstein. 1986. Coordinate regulation of herpes simplex virus gene expression is mediated by the functional interaction of two immediate early gene products. J. Mol. Biol. 191395-409. [DOI] [PubMed] [Google Scholar]
- 17.Godowski, P. J., and D. M. Knipe. 1983. Mutations in the major DNA-binding protein gene of herpes simplex virus type 1 result in increased levels of viral gene expression. J. Virol. 47478-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godowski, P. J., and D. M. Knipe. 1986. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc. Natl. Acad. Sci. USA 83256-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 3659-74. [DOI] [PubMed] [Google Scholar]
- 20.Gray, K. S., R. D. Allen III, M. L. Farrell, J. C. Forrest, and S. H. Speck. 2009. Alternatively initiated gene 50/RTA transcripts expressed during murine and human gammaherpesvirus reactivation from latency. J. Virol. 83314-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruffat, H., J. Batisse, D. Pich, B. Neuhierl, E. Manet, W. Hammerschmidt, and A. Sergeant. 2002. Epstein-Barr virus mRNA export factor EB2 is essential for production of infectious virus. J. Virol. 769635-9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruffat, H., N. Duran, M. Buisson, F. Wild, R. Buckland, and A. Sergeant. 1992. Characterization of an R-binding site mediating the R-induced activation of the Epstein-Barr virus BMLF1 promoter. J. Virol. 6646-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruffat, H., E. Manet, A. Rigolet, and A. Sergeant. 1990. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence specific DNA binding protein. Nucleic Acids Res. 186835-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammerschmidt, W., and B. Sugden. 1988. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell 55427-433. [DOI] [PubMed] [Google Scholar]
- 25.Han, Z., E. Marendy, Y. D. Wang, J. Yuan, J. T. Sample, and S. Swaminathan. 2007. Multiple roles of Epstein-Barr virus SM protein in lytic replication. J. Virol. 814058-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardwick, J. M., P. M. Lieberman, and S. D. Hayward. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 622274-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson, S., D. Huen, M. Rowe, C. Dawson, G. Johnson, and A. Rickinson. 1993. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc. Natl. Acad. Sci. USA 908479-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiriart, E., L. Bardouillet, E. Manet, H. Gruffat, F. Penin, R. Montserret, G. Farjot, and A. Sergeant. 2003. A region of the Epstein-Barr virus (EBV) mRNA export factor EB2 containing an arginine-rich motif mediates direct binding to RNA. J. Biol. Chem. 27837790-37798. [DOI] [PubMed] [Google Scholar]
- 29.Hiriart, E., G. Farjot, H. Gruffat, M. V. C. Nguyen, A. Sergeant, and E. Manet. 2003. A novel nuclear export signal and a REF interaction domain both promote mRNA export by the Epstein-Barr virus EB2 protein. J. Biol. Chem. 278335-342. [DOI] [PubMed] [Google Scholar]
- 30.Honess, R. W., and B. Roizman. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. USA 721276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 148-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong, G. K., H.-J. Delecluse, H. Gruffat, T. E. Morrison, W. Feng, and S. C. Kenney. 2004. The BRRF1 early gene of Epstein-Barr virus encodes a transcription factor that enhances induction of lytic infection by BRLF1. J. Virol. 784983-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johannsen, E., M. Luftig, M. R. Chase, S. Weicksel, E. Cahir-McFarland, D. Illanes, D. Sarracino, and E. Kieff. 2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 10116286-16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenney, S., E. Holley-Guthrie, E. C. Mar, and M. Smith. 1989. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J. Virol. 633878-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Key, S. C., T. Yoshizaki, and J. S. Pagano. 1998. The Epstein-Barr virus (EBV) SM protein enhances pre-mRNA processing of the EBV DNA polymerase transcript. J. Virol. 728485-8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khanim, F., C. Dawson, C. A. Meseda, J. Dawson, M. Mackett, and L. S. Young. 1997. BHRF1, a viral homologue of the Bcl-2 oncogene, is conserved at both the sequence and functional level in different Epstein-Barr virus isolates. J. Gen. Virol. 782987-2999. [DOI] [PubMed] [Google Scholar]
- 37.Landais, E., X. Saulquin, E. Scotet, L. Trautmann, M. A. Peyrat, J. L. Yates, W. W. Kwok, M. Bonneville, and E. Houssaint. 2004. Direct killing of Epstein-Barr virus (EBV)-infected B cells by CD4 T cells directed against the EBV lytic protein BHRF1. Blood 1031408-1416. [DOI] [PubMed] [Google Scholar]
- 38.Lee, M. A., and J. L. Yates. 1992. BHRF1 of Epstein-Barr virus, which is homologous to human proto-oncogene bcl2, is not essential for transformation of B cells or for virus replication in vitro. J. Virol. 661899-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leopardi, R., N. Michael, and B. Roizman. 1995. Repression of the herpes simplex virus 1 alpha 4 gene by its gene product (ICP4) within the context of the viral genome is conditioned by the distance and stereoaxial alignment of the ICP4 DNA binding site relative to the TATA box. J. Virol. 693042-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, P., and S. H. Speck. 2003. Synergistic autoactivation of the Epstein-Barr virus immediate-early BRLF1 promoter by Rta and Zta. Virology 310199-206. [DOI] [PubMed] [Google Scholar]
- 41.Manet, E., H. Gruffat, M. C. Trescol-Biemont, N. Moreno, P. Chambard, J. F. Giot, and A. Sergeant. 1989. Epstein-Barr virus bicistronic mRNAs generated by facultative splicing code for two transcriptional trans-activators. EMBO J. 81819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchini, A., B. Tomkinson, J. I. Cohen, and E. Kieff. 1991. BHRF1, the Epstein-Barr virus gene with homology to Bc12, is dispensable for B-lymphocyte transformation and virus replication. J. Virol. 655991-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy, N. J., S. A. Hazlewood, D. S. Huen, A. B. Rickinson, and G. T. Williams. 1996. The Epstein-Barr virus gene BHRF1, a homologue of the cellular oncogene Bcl-2, inhibits apoptosis induced by gamma radiation and chemotherapeutic drugs. Adv. Exp. Med. Biol. 40683-97. [DOI] [PubMed] [Google Scholar]
- 44.McMahan, L., and P. A. Schaffer. 1990. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J. Virol. 643471-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller, G., and M. Lipman. 1973. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc. Natl. Acad. Sci. USA 70190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moriuchi, H., M. Moriuchi, H. A. Smith, and J. I. Cohen. 1994. Varicella-zoster virus open reading frame 4 protein is functionally distinct from and does not complement its herpes simplex virus type 1 homolog, ICP27. J. Virol. 681987-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicewonger, J., G. Suck, D. Bloch, and S. Swaminathan. 2004. Epstein-Barr virus (EBV) SM protein induces and recruits cellular Sp110b to stabilize mRNAs and enhance EBV lytic gene expression. J. Virol. 789412-9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oroskar, A. A., and G. S. Read. 1987. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J. Virol. 61604-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oroskar, A. A., and G. S. Read. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 631897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearson, G. R., J. Luka, L. Petti, J. Sample, M. Birkenbach, D. Braun, and E. Kieff. 1987. Identification of an Epstein-Barr virus early gene encoding a second component of the restricted early antigen complex. Virology 160151-161. [DOI] [PubMed] [Google Scholar]
- 51.Pfitzner, A. J., E. C. Tsai, J. L. Strominger, and S. H. Speck. 1987. Isolation and characterization of cDNA clones corresponding to transcripts from the BamHI H and F regions of the Epstein-Barr virus genome. J. Virol. 612902-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinlivan, E. B., E. A. Holley-Guthrie, M. Norris, D. Gutsch, S. L. Bachenheimer, and S. C. Kenney. 1993. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 211999-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rabson, M., L. Gradoville, L. Heston, and G. Miller. 1982. Non-immortalizing P3J-HR1 Epstein-Barr virus: a deletion mutant of its transforming parent, Jijoye. J. Virol. 48834-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 727978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ragoczy, T., and G. Miller. 2001. Autostimulation of the Epstein-Barr virus BRLF1 promoter is mediated through consensus Sp1 and Sp3 binding sites. J. Virol. 755240-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ragoczy, T., and G. Miller. 1999. Role of the Epstein-Barr virus RTA protein in activation of distinct classes of viral lytic cycle genes. J. Virol. 739858-9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 46498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rice, S. A., L. S. Su, and D. M. Knipe. 1989. Herpes simplex virus alpha protein ICP27 possesses separable positive and negative regulatory activities. J. Virol. 633399-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rickinson, A. B., and E. Kieff. 2007. Epstein-Barr virus, p. 2655-2700. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 2. Wolters Kluwer/Lippincott Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 60.Ruvolo, V., L. Sun, K. Howard, S. Sung, H. J. Delecluse, W. Hammerschmidt, and S. Swaminathan. 2004. Functional analysis of Epstein-Barr virus SM protein: identification of amino acids essential for structure, transactivation, splicing inhibition, and virion production. J. Virol. 78340-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruvolo, V., E. Wang, S. Boyle, and S. Swaminathan. 1998. The Epstein-Barr virus nuclear protein SM is both a post-transcriptional inhibitor and activator of gene expression. Proc. Natl. Acad. Sci. USA 958852-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sacks, W. R., C. C. Greene, D. P. Aschman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Segouffin, C., H. Gruffat, and A. Sergeant. 1996. Repression by RAZ of Epstein-Barr virus bZIP transcription factor EB1 is dimerization independent. J. Gen. Virol. 771529-1536. [DOI] [PubMed] [Google Scholar]
- 64.Segouffin-Cariou, C., G. Farjot, A. Sergeant, and H. Gruffat. 2000. Characterization of the Epstein-Barr virus BRRF1 gene, located between early genes BZLF1 and BRLF1. J. Gen. Virol. 811791-1799. [DOI] [PubMed] [Google Scholar]
- 65.Semmes, O. J., L. Chen, R. T. Sarisky, Z. Gao, L. Zhong, and S. D. Hayward. 1998. Mta has properties of an RNA export protein and increases cytoplasmic accumulation of Epstein-Barr virus replication gene mRNA. J. Virol. 729526-9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sinclair, A. J., M. Brimmell, F. Shanahan, and P. J. Farrell. 1991. Pathways of activation of the Epstein-Barr virus productive cycle. J. Virol. 652237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 18674-86. [DOI] [PubMed] [Google Scholar]
- 68.Swaminathan, S., and S. Kenney. 2009. The Epstein-Barr virus lytic lifecycle. In B. Damania and J. Pipas (ed.), DNA tumor viruses. Springer, Berlin, Germany.
- 69.Tarodi, B., T. Subramanian, and G. Chinnadurai. 1994. Epstein-Barr virus BHRF1 protein protects against cell death induced by DNA-damaging agents and heterologous viral infection. Virology 201404-407. [DOI] [PubMed] [Google Scholar]
- 70.Theodorakis, P., C. D'Sa-Eipper, T. Subramanian, and G. Chinnadurai. 1996. Unmasking of a proliferation-restraining activity of the anti-apoptosis protein EBV BHRF1. Oncogene 121707-1713. [PubMed] [Google Scholar]
- 71.Verma, D., and S. Swaminathan. 2008. Epstein-Barr virus SM protein functions as an alternative splicing factor. J. Virol. 827180-7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang, Y., Q. Tang, G. G. Maul, and Y. Yuan. 2006. Kaposi's sarcoma-associated herpesvirus ori-Lyt-dependent DNA replication: dual role of replication and transcription activator. J. Virol. 8012171-12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiedmer, A., P. Wang, J. Zhou, A. J. Rennekamp, V. Tiranti, M. Zeviani, and P. M. Lieberman. 2008. Epstein-Barr virus immediate-early protein Zta co-opts mitochondrial single-stranded DNA binding protein to promote viral and inhibit mitochondrial DNA replication. J. Virol. 824647-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winkler, M., S. A. Rice, and T. Stamminger. 1994. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J. Virol. 683943-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zalani, S., A. Coppage, E. Holley-Guthrie, and S. Kenney. 1997. The cellular YY1 transcription factor binds a cis-acting, negatively regulating element in the Epstein-Barr virus BRLF1 promoter. J. Virol. 713268-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]