Abstract
TRIM5α mediates a potent retroviral restriction phenotype in diverse mammalian species. Here, we identify a TRIM5 transcript in cat cells with a truncated B30.2 capsid binding domain and ablated restrictive function which, remarkably, is conserved across the Feliformia. Cat TRIM5 displayed no restriction activity, but ectopic expression conferred a dominant negative effect against human TRIM5α. Our findings explain the absence of retroviral restriction in cat cells and suggest that disruption of the TRIM5 locus has arisen independently at least twice in the Carnivora, with implications concerning the evolution of the host and pathogen in this taxon.
One of the major determinants of host restriction of retroviral replication is the longest (alpha) isoform of the host protein TRIM5, a member of the tripartite motif protein family (34, 42). Human TRIM5α (huTRIM5α) inhibits preintegration stages of murine leukemia virus N-strain (MLV-N) infection, while rhesus macaque TRIM5α inhibits human immunodeficiency virus type 1 (HIV-1) infection (13, 16, 55). Recent studies have demonstrated retroviral restriction by TRIM5α species-specific variants from cows (Bos taurus) (41, 56) and rabbits (Oryctolagus cuniculus) (38), suggesting a common ancestor for mammalian TRIM5α with antiretroviral properties. Tripartite motif proteins typically comprise a RING domain with E3-ubiquitin ligase activity capable of autoubiquitination (54), a B-box 2 domain, and a coiled-coil domain, collectively know as the RBCC (34). Additionally, some TRIM proteins, including TRIM5α, possess a C-terminal B30.2 (PRY-SPRY) domain. TRIM5α blocks reverse transcription (RT) in most nonpermissive cells (16, 42), and evidence suggests that TRIM5α homodimers engage the incoming retroviral capsid in the cytoplasm via the B30.2 domain (18, 24, 40, 44). The resulting complex is then degraded rapidly by the proteasome (9, 47) (proteasomal inhibitors restore RT but not viral replication [3, 53]). Human splice variants TRIM5δ and TRIM5γ lack a B30.2 domain, which disrupts their ability to restrict (30, 42). Moreover, these short TRIM5 isoforms have a dominant negative effect, impairing the activity of full-length TRIM5α, presumably by the formation of heterodimers (24, 32).
Retroviruses have invaded the Felidae on several occasions. Feline leukemia virus (FeLV) and the endogenous retrovirus RD-114 are found in several species within the genus Felis, and recently cross-species transmission of FeLV into Iberian lynxes (Lynx pardinus) and Florida panthers (Puma concolor) has been described. In contrast, multiple species of Felidae and one species of Hyaenidae have tested seropositive for feline immunodeficiency virus (FIV) and many species harbor monophyletic strains of the virus (8, 50). FIV is unique among nonprimate lentiviruses in that it infects and depletes CD4+ T cells, leading to a syndrome analogous to human AIDS (1, 31, 46). Although FIV and HIV-1 are highly divergent at the nucleotide and protein levels, both viral capsids interact with host factor cyclophilin A (CypA) (20), indicative of shared postentry interactions between viral and cellular proteins. FIV is susceptible to both primate and nonprimate restriction factors (10, 14, 35, 38, 39, 51), and rhesus macaque TRIM5α determinants for HIV-1 and FIV restriction overlap, both involving the v1 region of the B30.2 domain (10). These data suggest shared capsid conformations between the feline and primate lentiviruses. The nature of cat TRIM5 (feTRIM5) is unknown; however, feline cells are highly permissive to vesicular stomatitis virus G pseudotyped retroviral vectors, suggesting a TRIM5-null phenotype (6, 13, 48). Accordingly, feline cells have been used widely as a negative control permissive cell line in which TRIM5 genes are ectopically expressed and assayed.
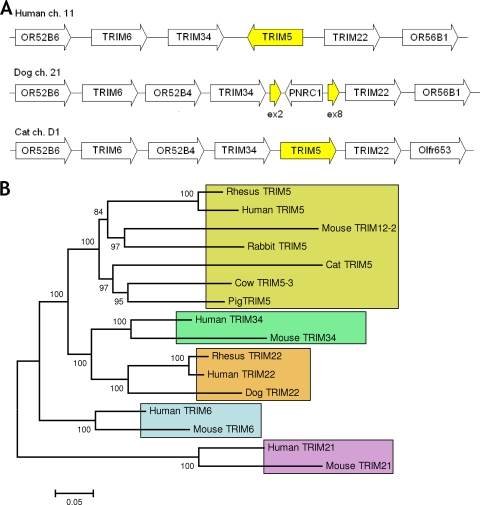
In order to explore the permissivity of feline cells to retroviral infection, primers corresponding to conserved regions of huTRIM5α were used to amplify TRIM5 from cDNA derived from the Mya-1 primary T-cell line (primers Ts3 [directed to exon 2, 5′-CATGTGGCCAACATAGTGGAG-3′] and Ts16 [directed to exon 8, 5′-CATAGTCTAGGAAAACTCCAACACG-3′]). The resulting product was sequenced and used to identify homologous contigs from the 1.9-fold cat genome (33) by using BLAST (2). Subsequently, primers wam4e from contig AANG01555594 (5′-CAGGGAATTCCTGCTATGGCTTCTGAACTCCTG-3′ [start codon in bold]) and wam13c against contig AANG01581224 (5′-TCATATTTTCGAATCAGTGTGGAATCACGTGAGC-3′) were used to amplify and clone feTRIM5 into expression vector CXCR. The transcript identified encodes a protein highly related to the primate TRIM5 RBCC (GenBank accession no. GQ183880; http://www.gla.ac.uk/media/media_120375_en.pdf). However, the cat transcript bears a stop codon at proximal exon 8 5′ to the v1 region and is thus truncated. Although regions of DNA bearing homology to human and rhesus B30.2 are found 3′ to the feTRIM5 stop codon, the sequence bears multiple stop codons in all of the reading frames. Predicted gene identities on the cat genome browser (GARFIELD; National Cancer Institute, Frederick, MD) reveal that the TRIM5 gene lies in a region of conserved synteny with TRIM5 paralogues TRIM6, TRIM22, and TRIM34 (Fig. 1A). A neighbor-joining tree was constructed by using codon-optimized DNA sequences of TRIM5 orthologues and other related TRIM genes (Fig. 1B), revealing domestic feTRIM5 to be monophyletic with TRIM5 (or TRIM12-2 in mice, one of an expanded cluster of murine TRIM5 genes [45]) from other mammalian species and was supported by high bootstrap values. Taken with the conserved synteny, these data provide strong evidence that the cat transcript identified is a true TRIM5 orthologue.
FIG. 1.
Conserved synteny and phylogenetic clustering indicate that feTRIM5 is a true TRIM5 orthologue. (A) Paralogues TRIM6, TRIM34, TRIM5, and TRIM22 are found in a cluster in the human and dog genomes and in the 1.9-fold cat genome project. In all of these cases, the cluster is surrounded by olfactory genes. Exons 2 (ex2) and 8 (ex8) of the pseudogenized dog TRIM5 sequence are shown. Other genes in cat as well as dog TRIM6 are predicted genes or regions of homology only and may not be expressed or functional. ch, chromosome. (B) Neighbor-joining tree of TRIM5 orthologues from several mammalian species. feTRIM5 clusters with other TRIM5 orthologues in preference to closely related paralogues TRIM34, TRIM6, and TRIM22 and the more distantly related paralogue TRIM21. Moreover, the TRIM5 phylogeny reflects established mammalian evolutionary relationships. Numbers indicate bootstrap values after 1,000 iterations, and branch length reflects the number of base substitutions per site. Nucleic acid sequences were codon optimized and aligned by using ClustalW with manual adjustment. All of the positions containing gaps were eliminated from the data set.
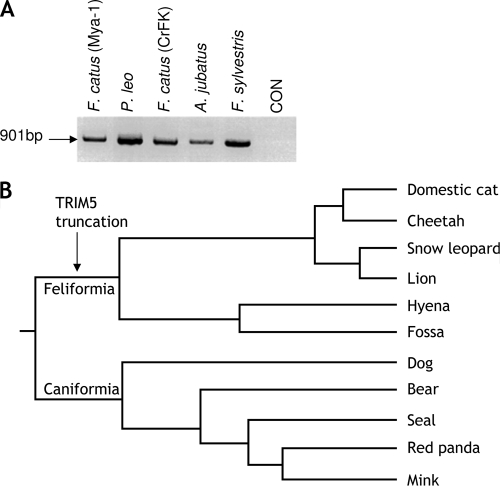
Next we compared TRIM5 expression and identity between members of the order Carnivora. RT-PCR with primers spanning all of the coding exons shows that expression of the transcript is maintained in all of the felid species tested (Fig. 2A). To discern the evolutionary history of TRIM5 in the felids, we used degenerate primers to amplify part of TRIM5 exon 8 from genomic DNAs of several carnivoran species to discern the point in evolution at which the truncating mutation occurred. Remarkably, all of the species tested from the feliform lineage bore a stop mutation in the same location as feTRIM5 (Fig. 2B), suggesting that the mutation occurred after the Caniformia-Feliformia divergence but before the Felidae-Hyaenidae split estimated at not before 47 million years ago (12) but transcription of the gene has been maintained. The homologous sequence in dog and mink cells was found to lack the stop codon but has been reported to be disrupted by the insertion of an unrelated gene, PNRC1 in the boxer breed genome (21, 36), and evidence of this insertion exists in the poodle genome (17) (insertion present in contig AACN010301967). Thus, two independent TRIM5 disruption events have taken place during carnivoran evolution. However, although the truncation of feTRIM5 is compatible with observations that cat cells lack restriction activity, other possibilities such as a readthrough transcript with downstream TRIM22, the use of an alternative downstream exon 8 splice acceptor or the splicing of another gene to the 3′ end of TRIM5 cannot be excluded. To address this issue, 3′ rapid amplification of cDNA ends (Roche, Burgess Hill, United Kingdom) was employed with exon 2-specific primer wam4e and an oligo(dT)-anchored primer from cDNA derived from cat cell lines Mya-1 and CrFK. The only transcript identified bore an open reading frame identical to that already cloned without 3′ modification. Furthermore, given recent findings that TRIM5-CypA fusions have arisen twice during primate evolution, reverse primers directed to feline CypA were used in conjunction with a range of TRIM5 forward primers in RT-PCRs, but no evidence of fusion products was found (data not shown).
FIG. 2.
TRIM5 expression is maintained in the felids, and the truncation is conserved across the Feliformia. (A) TRIM5 transcripts are present in cells from diverse members of the Feliformia. In addition to the Felis catus cell lines CrFK and Mya-1, exon 2 to exon 8 TRIM5 transcripts were amplified from cDNAs derived from cheetah (Acinonyx jubatus), lion (Panthera leo), and European wildcat (F. sylvestris) T cells. CON, control. (B) The 5′ end of TRIM5 exon 8 was sequenced from the genomic DNAs of several species to discern at which point in carnivoran evolution the truncation occurred. The stop codon TGA was found in all of the feliforms examined but absent from both the dog and mink sequences. When the findings are imposed on an established phylogenetic tree of the carnivorans (12, 15), the truncation of TRIM5 is shown to have taken place before the split of the Felidae and Hyaenidae lineages. An independent TRIM5 disruption is proposed to have taken place in the Caniformia lineage after the Feliformia-Caniformia split 53.8 million years ago and is present in modern dogs.
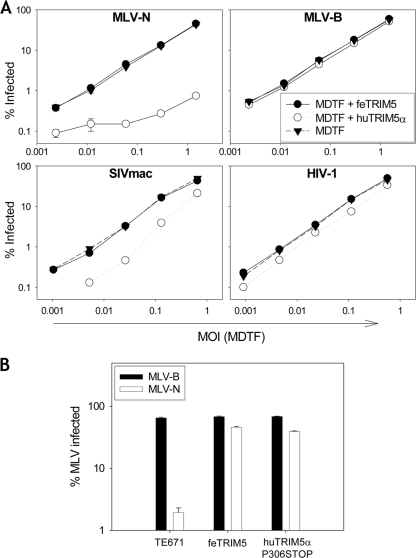
To assess the ability of feTRIM5 to restrict retroviruses in vitro, human and feTRIM5 orthologues were overexpressed in Fv1-null Mus dunni tail fibroblasts (5) (Fig. 3A). Expression of huTRIM5α conferred restriction of MLV-N but not MLV-B (48). However, feTRIM5 restricted neither MLV-N nor MLV-B, suggesting that, as predicted, the truncating mutation ablates antiretroviral activity. Similarly, no restriction of vesicular stomatitis virus G pseudotyped lentiviral vectors derived from HIV-1 (4) and simian immunodeficiency virus (27) from rhesus macaques was observed. Next, we investigated whether truncated feTRIM5 could act as a dominant negative agent for huTRIM5α, as has been reported for huTRIM5γ (19, 24, 32). A stop codon was introduced into huTRIM5α at the same position (P306) as in feTRIM5 as a positive control for dominant negative activity. feTRIM5 and huTRIM5 P306STOP were expressed in human TE671 cells, which express endogenous TRIM5α and restrict MLV-N (16) potently. Expression of either feTRIM5 or huTRIM5 P306STOP rescued the MLV-N titer (Fig. 3B), suggesting that, like huTRIM5γ and huTRIM5δ, feTRIM5 localizes to the cytoplasm and is able to heteromultimerize with functional TRIM5α to prevent optimal restriction.
FIG. 3.
Biological function of feTRIM5. (A) Mouse fibroblasts which lack restriction activity were transduced with domestic feTRIM5 or huTRIM5α. While huTRIM5α specifically restricts N-tropic but not B-tropic MLV, feTRIM5, which lacks a B30.2 domain, restricts neither MLV-N nor MLV-B. Nor is feTRIM5 able to restrict lentiviral vectors derived from HIV-1 and simian immunodeficiency virus from rhesus macaques. MDTF, M. dunni tail fibroblasts. (B) feTRIM5 acts as a dominant negative agent against huTRIM5α-mediated restriction. The TE671 cell line, which expresses endogenous TRIM5α, was stably transduced with feTRIM5 or with huTRIM5 P306STOP, which bears a stop codon at the residue corresponding to that in feTRIM5. Expression of feTRIM5 or huTRIM5 P306STOP rescued MLV-N infectivity. Error bars represent mean ± standard error (n = 3).
There is evidence of strong positive selection in primate TRIM5α B30.2 sequences and in their bovine and leporine orthologues (38, 41, 56), presumably driven by a genetic conflict between restriction factors and viruses over at least the past 33 million years (22, 37). The resulting orthologues are highly divergent in the variable regions of their B30.2 domains and have specific repertoires of restricted virus (26, 29, 43). Thus, it is likely that ancestral mammalian TRIM5α, as well as the ancestral carnivoran TRIM5α protein, possessed this ability and positive selection of TRIM5α may be a common feature among nonprimate mammals. Concomitantly, escaping TRIM5α restriction may be a widespread requirement for zoonosis and population invasion and the loss of antiretroviral TRIM5 in carnivorans has implications for the evolution and cross-species transmission of retroviruses. Although dogs are currently thought to be free of exogenous retroviruses, high titers of replicating FIV can be obtained in canine cells expressing the FIV receptor CD134 (52), suggesting a lack of intracellular defense (and potential vulnerability) to lentiviruses. Molecular phylogenies of FIV from divergent feliforms broadly reflect phylogenies of the host, suggesting that cross-species transmission is relatively rare (49). However, this may owe more to the lack of interspecies contact than to robust defenses, given that cross-species transmission of FIV has been observed frequently in captive animals (7, 50) and transmission of both FIV and FeLV has been observed in isolated examples of free-ranging animals (11, 23, 28).
The selective pressure to maintain nonantiretroviral TRIM5 alleles in the carnivorans is unclear. One possibility is the acquisition of a novel function of a truncated TRIM5 protein in cats. In this study, transcripts of the gene were detected in cDNAs derived from domestic cats, wildcats, lions, and cheetahs, indicating maintenance of TRIM5 gene expression. Thus, felid TRIM5 may have an alternative role that does not involve the B30.2 domain (analogous to huTRIM5γ and -δ). It has been reported that carnivorans have experienced relatively little endogenous retrovirus activity compared to other lineages: there are 13,000 long terminal repeat/endogenous retrovirus lineage-specific sequences in the dog genome, compared to 133,000 in the human genome and 470,000 in the mouse genome (21). Thus, the pressure to maintain antiretroviral TRIM5α in the Carnivora may have been reduced. Nonetheless, it is possible that other antiretroviral factors, such as the APOBEC3 family, tetherin, or unidentified restriction factors, may have compensated for the lack of TRIM5α. Indeed, the APOBEC3C genes have recently been shown to possess antiretroviral function and are under adaptive selection in the Felidae (25). Evolving in the absence of antiretroviral TRIM5α presumably has effects on retroviral evolution. FIV is particularly sensitive to TRIM5α-mediated restriction (35, 38, 51), and the absence of endogenous TRIM5α-like activity may have permitted the evolution of FIV toward structural optima that would otherwise be strongly restricted.
The lack of postentry retroviral restriction in the carnivorans contrasts strongly to the primates, where TRIM5α can reduce retroviral infectivity by several orders of magnitude in nonpermissive cells. Since primates and carnivorans are currently affected by closely related lentiviruses that infect and deplete similar cell populations, direct comparisons between these taxa may produce insights into the comparative roles of TRIM5 and other restriction factors in the evolution and cross-species transmission of lentiviruses.
Acknowledgments
This work was supported by Public Health Service grant AI049765 to B.J.W. and M.J.H. and by the Wellcome Trust (W.A.M. and G.J.T.).
We thank Melody Roelke for provision of hyena samples and John Lewis for provision of fossa samples.
Footnotes
Published ahead of print on 3 June 2009.
REFERENCES
- 1.Ackley, C. D., J. K. Yamamoto, N. Levy, N. C. Pedersen, and M. D. Cooper. 1990. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J. Virol. 645652-5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, J. L., E. M. Campbell, X. Wu, N. Vandegraaff, A. Engelman, and T. J. Hope. 2006. Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. J. Virol. 809754-9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bainbridge, J. W., C. Stephens, K. Parsley, C. Demaison, A. Halfyard, A. J. Thrasher, and R. R. Ali. 2001. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 81665-1668. [DOI] [PubMed] [Google Scholar]
- 5.Best, S., P. LeTissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382826-829. [DOI] [PubMed] [Google Scholar]
- 6.Bock, M., K. N. Bishop, G. Towers, and J. P. Stoye. 2000. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J. Virol. 747422-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter, M. A., E. W. Brown, M. Culver, W. E. Johnson, J. Pecon-Slattery, D. Brousset, and S. J. O'Brien. 1996. Genetic and phylogenetic divergence of feline immunodeficiency virus in the puma (Puma concolor). J. Virol. 706682-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter, M. A., and S. J. O'Brien. 1995. Coadaptation and immunodeficiency virus: lessons from the Felidae. Curr. Opin. Genet. Dev. 5739-745. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Griffero, F., X. Li, H. Javanbakht, B. Song, S. Welikala, M. Stremlau, and J. Sodroski. 2006. Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology 349300-315. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Griffero, F., A. Kar, M. Lee, M. Stremlau, E. Poeschla, and J. Sodroski. 2007. Comparative requirements for the restriction of retrovirus infection by TRIM5α and TRIMCyp. Virology 369400-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin, S. P., J. L. Troyer, J. A. Terwee, L. M. Lyren, W. M. Boyce, S. P. Riley, M. E. Roelke, K. R. Crooks, and S. VandeWoude. 2007. Frequent transmission of immunodeficiency viruses among bobcats and pumas. J. Virol. 8110961-10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaubert, P., and P. Cordeiro-Estrela. 2006. Phylogenetic systematics and tempo of evolution of the Viverrinae (Mammalia, Carnivora, Viverridae) within feliformians: implications for faunal exchanges between Asia and Africa. Mol. Phylogenet. Evol. 41266-278. [DOI] [PubMed] [Google Scholar]
- 13.Hatziioannou, T., D. Perez-Caballero, A. Yang, S. Cowan, and P. D. Bieniasz. 2004. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5α. Proc. Natl. Acad. Sci. USA 10110774-10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javanbakht, H. N., F. Diaz-Griffero, W. Yuan, D. F. Yeung, X. Li, B. Song, and J. Sodroski. 2007. The ability of multimerized cyclophilin A to restrict retrovirus infection. Virology 36719-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, W. E., E. Eizirik, J. Pecon-Slattery, W. J. Murphy, A. Antunes, E. Teeling, and S. J. O'Brien. 2006. The late Miocene radiation of modern Felidae: a genetic assessment. Science 31173-77. [DOI] [PubMed] [Google Scholar]
- 16.Keckesova, Z., L. M. J. Ylinen, and G. J. Towers. 2004. The human and African green monkey TRIM5α genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc. Natl. Acad. Sci. USA 10110780-10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkness, E. F., V. Bafna, A. L. Halpern, S. Levy, K. Remington, D. B. Rusch, A. L. Delcher, M. Pop, W. Wang, C. M. Fraser, and J. C. Venter. 2003. The dog genome: survey sequencing and comparative analysis. Science 3011898-1903. [DOI] [PubMed] [Google Scholar]
- 18.Langelier, C. R., V. Sandrin, D. M. Eckert, D. E. Christensen, V. Chandrasekaran, S. L. Alam, C. Aiken, J. C. Olsen, A. K. Kar, J. G. Sodroski, and W. I. Sundquist. 2008. Biochemical characterization of a recombinant TRIM5α protein that restricts human immunodeficiency virus type 1 replication. J. Virol. 8211682-11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, X., Y. Li, M. Stremlau, W. Yuan, B. Song, M. Perron, and J. Sodroski. 2006. Functional replacement of the RING, B-box 2, and coiled-coil domains of tripartite motif 5α (TRIM5α) by heterologous TRIM domains. J. Virol. 806198-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, T. Y., and M. Emerman. 2006. Cyclophilin A interacts with diverse lentiviral capsids. Retrovirology 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindblad-Toh, K., C. M. Wade, T. S. Mikkelsen, et al. 2005. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438803-819. [DOI] [PubMed] [Google Scholar]
- 22.Liu, H. L., Y. Q. Wang, C. H. Liao, Y. Q. Kuang, Y. T. Zheng, and B. Su. 2005. Adaptive evolution of primate TRIM5α, a gene restricting HIV-1 infection. Gene 362109-116. [DOI] [PubMed] [Google Scholar]
- 23.Meli, M. L., V. Cattori, F. Martinez, G. Lopez, A. Vargas, M. A. Simon, I. Zorrilla, A. Munoz, F. Palomares, J. V. Lopez-Bao, J. Pastor, R. Tandon, B. Willi, R. Hofmann-Lehmann, and H. Lutz. 2009. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus). PLoS ONE 4e4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mische, C. C., H. Javanbakht, B. Song, F. Diaz-Griffero, M. Stremlau, B. Strack, Z. Si, and J. Sodroski. 2005. Retroviral restriction factor TRIM5α is a trimer. J. Virol. 7914446-14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Münk, C., T. Beck, J. Zielonka, A. Hotz-Wagenblatt, S. Chareza, M. Battenberg, J. Thielebein, K. Cichutek, I. G. Bravo, S. J. O'Brien, M. Lochelt, and N. Yuhki. 2008. Functions, structure, and read-through alternative splicing of feline APOBEC3 genes. Genome Biol. 9R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama, E. E., H. Miyoshi, Y. Nagai, and T. Shioda. 2005. A specific region of 37 amino acid residues in the SPRY (B30.2) domain of African green monkey TRIM5α determines species-specific restriction of simian immunodeficiency virus SIVmac infection. J. Virol. 798870-8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nègre, D., P. E. Mangeot, G. Duisit, S. Blanchard, P. O. Vidalain, P. Leissner, A. J. Winter, C. Rabourdin-Combe, M. Mehtali, P. Moullier, J. L. Darlix, and F. L. Cosset. 2000. Characterization of novel safe lentiviral vectors derived from simian immunodeficiency virus (SIVmac251) that efficiently transduce mature human dendritic cells. Gene Ther. 71613-1623. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura, Y., Y. Goto, K. Yoneda, Y. Endo, T. Mizuno, M. Hamachi, H. Maruyama, H. Kinoshita, S. Koga, M. Komori, S. Fushuku, K. Ushinohama, M. Akuzawa, T. Watari, A. Hasegawa, and H. Tsujimoto. 1999. Interspecies transmission of feline immunodeficiency virus from the domestic cat to the Tsushima cat (Felis bengalensis euptilura) in the wild. J. Virol. 737916-7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohkura, S., M. W. Yap, T. Sheldon, and J. P. Stoye. 2006. All three variable regions of the TRIM5α B30.2 domain can contribute to the specificity of retrovirus restriction. J. Virol. 808554-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passerini, L. D., Z. Keckesova, and G. J. Towers. 2006. Retroviral restriction factors Fv1 and TRIM5α act independently and can compete for incoming virus before reverse transcription. J. Virol. 802100-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen, N. C., E. W. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 235790-793. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Caballero, D., T. Hatziioannou, A. Yang, S. Cowan, and P. D. Bieniasz. 2005. Human tripartite motif 5α domains responsible for retrovirus restriction activity and specificity. J. Virol. 798969-8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pontius, J. U., and S. J. O'Brien. 2007. Genome Annotation Resource Fields—GARFIELD: a genome browser for Felis catus. J. Hered. 98386-389. [DOI] [PubMed] [Google Scholar]
- 34.Reymond, A., G. Meroni, A. Fantozzi, G. Merla, S. Cairo, L. Luzi, D. Riganelli, E. Zanaria, S. Messali, S. Cainarca, A. Guffanti, S. Minucci, P. G. Pelicci, and A. Ballabio. 2001. The tripartite motif family identifies cell compartments. EMBO J. 202140-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saenz, D. T., W. Teo, J. C. Olsen, and E. M. Poeschla. 2005. Restriction of feline immunodeficiency virus by Ref1, Lv1, and primate TRIM5α proteins. J. Virol. 7915175-15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawyer, S. L., M. Emerman, and H. S. Malik. 2007. Discordant evolution of the adjacent antiretroviral genes TRIM22 and TRIM5 in mammals. PLoS Pathog. 3e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawyer, S. L., L. I. Wu, M. Emerman, and H. S. Malik. 2005. Positive selection of primate TRIM5α identifies a critical species-specific retroviral restriction domain. Proc. Natl. Acad. Sci. USA 1022832-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaller, T., S. Hue, and G. J. Towers. 2007. An active TRIM5 protein in rabbits indicates a common antiviral ancestor for mammalian TRIM5 proteins. J. Virol. 8111713-11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaller, T., L. M. J. Ylinen, B. L. J. Webb, S. Singh, and G. J. Towers. 2007. Fusion of cyclophilin A to Fv1 enables cyclosporine-sensitive restriction of human and feline immunodeficiency viruses. J. Virol. 8110055-10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sebastian, S., and J. Luban. 2005. TRIM5α selectively binds a restriction-sensitive retroviral capsid. Retrovirology 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Si, Z., N. Vandegraaff, C. O'Huigin, B. Song, W. Yuan, C. Xu, M. Perron, X. Li, W. A. Marasco, A. Engelman, M. Dean, and J. Sodroski. 2006. Evolution of a cytoplasmic tripartite motif (TRIM) protein in cows that restricts retroviral infection. Proc. Natl. Acad. Sci. USA 1037454-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427848-853. [DOI] [PubMed] [Google Scholar]
- 43.Stremlau, M., M. Perron, S. Welikala, and J. Sodroski. 2005. Species-specific variation in the B30.2(SPRY) domain of TRIM5α determines the potency of human immunodeficiency virus restriction. J. Virol. 793139-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stremlau, M., M. Perron, M. Lee, Y. Li, B. Song, H. Javanbakht, F. Diaz-Griffero, D. J. Anderson, W. I. Sundquist, and J. Sodroski. 2006. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5α restriction factor. Proc. Natl. Acad. Sci. USA 1035514-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tareen, S. U., S. L. Sawyer, H. S. Malik, and M. Emerman. 2009. An expanded clade of rodent Trim5 genes. Virology 385473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torten, M., M. Franchini, J. E. Barlough, J. W. George, E. Mozes, H. Lutz, and N. C. Pedersen. 1991. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J. Virol. 652225-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Towers, G. J. 2007. The control of viral infection by tripartite motif proteins and cyclophilin A. Retrovirology 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Towers, G., M. Bock, S. Martin, Y. Takeuchi, J. P. Stoye, and O. Danos. 2000. A conserved mechanism of retrovirus restriction in mammals. Proc. Natl. Acad. Sci. USA 9712295-12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Troyer, J. L., S. VandeWoude, J. Pecon-Slattery, C. McIntosh, S. Franklin, A. Antunes, W. Johnson, and S. J. O'Brien. 2008. FIV cross-species transmission: an evolutionary prospective. Vet. Immunol. Immunopathol. 123159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Troyer, J. L., J. Pecon-Slattery, M. E. Roelke, W. Johnson, S. VandeWoude, N. Vazquez-Salat, M. Brown, L. Frank, R. Woodroffe, C. Winterbach, H. Winterbach, G. Hemson, M. Bush, K. A. Alexander, E. Revilla, and S. J. O'Brien. 2005. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J. Virol. 798282-8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virgen, C. A., Z. Kratovac, P. D. Bieniasz, and T. Hatziioannou. 2008. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc. Natl. Acad. Sci. USA 1053563-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willett, B. J., E. L. McMonagle, S. Ridha, and M. J. Hosie. 2006. Differential utilization of CD134 as a functional receptor by diverse strains of feline immunodeficiency virus. J. Virol. 803386-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu, X., J. L. Anderson, E. M. Campbell, A. M. Joseph, and T. J. Hope. 2006. Proteasome inhibitors uncouple rhesus TRIM5α restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. USA 1037465-7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu, L. X., L. H. Yang, P. K. Moitra, K. Hashimoto, P. Rallabhandi, S. Kaul, G. Meroni, J. P. Jensen, A. M. Weissman, and P. D'Arpa. 2003. BTBD1 and BTBD2 colocalize to cytoplasmic bodies with the RBCC/tripartite motif protein, TRIM5δ. Exp. Cell Res. 28884-93. [DOI] [PubMed] [Google Scholar]
- 55.Yap, M. W., S. Nisole, C. Lynch, and J. P. Stoye. 2004. Trim5α protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. USA 10110786-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ylinen, L. M. J., Z. Keckesova, B. L. J. Webb, R. J. M. Gifford, T. P. L. Smith, and G. J. Towers. 2006. Isolation of an active Lv1 gene from cattle indicates that tripartite motif protein-mediated innate immunity to retroviral infection is widespread among mammals. J. Virol. 807332-7338. [DOI] [PMC free article] [PubMed] [Google Scholar]