Abstract
Hepadnaviruses replicate via reverse transcription of an RNA template, the pregenomic RNA (pgRNA). Although hepadnaviral polymerase (Pol) and retroviral reverse transcriptase are distantly related, some of their features are distinct. In particular, Pol contains two additional N-terminal subdomains, the terminal protein and spacer subdomains. Since much of the spacer subdomain can be deleted without detrimental effects to hepatitis B virus (HBV) replication, this subdomain was previously thought to serve only as a spacer that links the terminal protein and reverse transcriptase subdomains. Unexpectedly, we found that the C terminus of the spacer subdomain is indispensable for the encapsidation of pgRNA. Alanine-scanning mutagenesis revealed that four conserved cysteine residues, three at the C terminus of the spacer subdomain and one at the N terminus of the reverse transcriptase subdomain, are critical for encapsidation. The inability of the mutant Pol proteins to incorporate into nucleocapsid particles, together with other evidence, argued that the four conserved cysteine residues are critical for RNA binding. One implication is that these four cysteine residues might form a putative zinc finger motif. Based on these findings, we speculate that the RNA binding activity of HBV Pol may be mediated by this newly identified putative zinc finger motif.
Hepatitis B virus (HBV) infection is a major global public health problem, with more than 300 million chronically infected patients worldwide (17). A significant subset of these HBV carriers progresses to severe liver disease, such as hepatocellular carcinoma, which is estimated to cause up to 1 million deaths per year. Current treatment protocols for chronic HBV infections have shown only limited success, which emphasizes the need for new therapeutic strategies (22). HBV is the prototype member of the hepadnavirus family, which includes woodchuck hepatitis virus (WHV) and duck hepatitis B virus (17). Although hepadnaviruses contain a DNA genome, the replication strategy utilizes the reverse transcription of an RNA template, the pregenomic RNA (pgRNA). Reverse transcription is catalyzed by HBV polymerase (Pol), which is distantly related to retroviral reverse transcriptases (RT) (14).
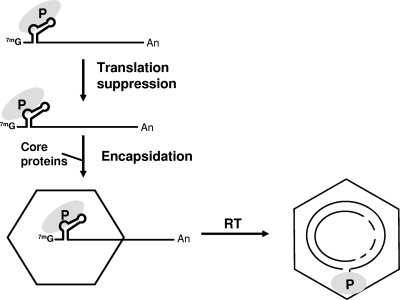
A key event for viral genome replication is the binding of Pol to a stem-loop structure (ɛ), which is an encapsidation signal located near the 5′ end of pgRNA (Fig. 1). The interaction between Pol and the 5′ ɛ directs the nucleocapsid assembly and the specific incorporation of both pgRNA and Pol into nascent particles (Fig. 1). Viral replication occurs entirely within nucleocapsids by the reverse transcription of pgRNA to produce a single-stranded DNA (ssDNA) copy, which in turn serves as the template for second-strand DNA synthesis. Complete HBV replication results in a circular double-stranded DNA genome, termed relaxed circular DNA (RC DNA) (Fig. 1). Another important consequence of the Pol-5′ ɛ interaction is that it suppresses the translation of pgRNA (16) (Fig. 1), a process that is proposed to regulate the switch from translation to encapsidation.
FIG. 1.
Schematic illustrating the steps of hepadnaviral genome replication. The pgRNA with the 5′ stem-loop structures (ɛ) is shown at the top. The recognition of the 5′ ɛ structure by Pol (P) triggers multiple events, including translation suppression, nucleocapsid assembly, and Pol-primed initiation of reverse transcription (2). First, the Pol-5′ ɛ interaction is necessary and sufficient for the suppression of the translation of the pgRNA (16). The resulting Pol-5′ ɛ ribonucleoprotein complex then recruits core proteins to assemble nascent nucleocapsids that incorporate the Pol-5′ ɛ ribonucleoprotein complex. Viral reverse transcription (RT) takes place entirely within nucleocapsids. As a result of the protein priming mechanism, the Pol remains linked to the 5′ end of the RC DNA minus-strand via the Pol TP subdomain. 7mG, cap; An, poly(A) tail.
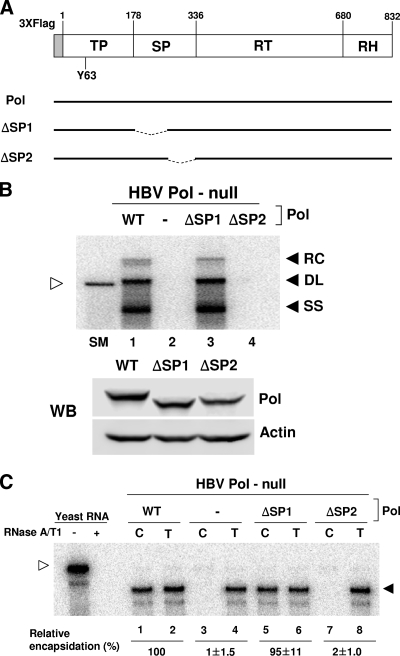
Based upon its similarity to retroviral RTs, HBV Pol can be divided into four subdomains, which are (from N terminus to C terminus) the terminal protein (TP), spacer (SP), RT, and RNase H subdomains (15) (Fig. 2A). Notably, the two N-terminal subdomains have no counterparts in the retroviral RTs. The TP subdomain utilizes an invariant tyrosine residue (i.e., Y63) to function as a protein primer to initiate reverse transcription (20) (Fig. 2A). In contrast, no specific function has been attributed to the SP subdomain, because much of it can be deleted without affecting Pol functions (6, 15). Further, the SP subdomain is a highly divergent region of the viral genome among hepadnavirus members. Therefore, it was believed that the SP subdomain simply serves as a tether that links the TP and RT subdomains (14).
FIG. 2.
The SP deletion mutants of HBV Pol were defective in pgRNA encapsidation. (A) Diagram illustrating the HBV Pol expression plasmids used for this study. Four subdomains of HBV Pol are shown, which are (from the N terminus) the TP, SP, RT, and RNase H (RH) domains. The first amino acid number of each subdomain is denoted above, according to the standard numbering of genotype D (ayw subtype) (19). HBV Pol contains three copies of the FLAG epitope (3XFlag) at its N terminus as a tag for detection, as described previously (16). An invariant tyrosine residue (Y63) of the TP domain, which is critical for protein priming, is denoted. Two deletion mutants of the SP subdomain, ΔSP1 and ΔSP2, are depicted below WT Pol. (B) Southern blot (top panel) and Western blot (WB; bottom panel) analysis. Cells were cotransfected with the HBV Pol-null construct along with WT Pol or Pol mutant expression constructs, as indicated above each lane. Southern blot analysis was performed to detect the HBV replication intermediates produced by Pol. Three replication DNA intermediates, RC DNA (RC), duplex-linear DNA (DL), and ssDNA (SS), are denoted. A restriction fragment representing one HBV genomic unit, 3.2 kb in size, serves as a size marker (SM) (unfilled arrowhead). Western blot analysis of HBV Pol was performed using an anti-FLAG antibody to demonstrate equal Pol expression levels. Actin served as a loading control. (C) RPA. Cells are fractionated into the capsid-associated fraction (C) and total cytoplasmic fraction (T), as described in Materials and Methods. RPA was performed to determine the level of pgRNA encapsidation by Pol. The pgRNA is denoted by a filled arrowhead, whereas the riboprobe is denoted by an unfilled arrowhead. Yeast RNA, which served as a negative control, was analyzed with (+) and without (−) RNase treatment.
While the crystal structure of retroviral RT has been solved, no structural information is yet available for hepadnaviral Pol. The main challenge has been obtaining high-level expression of a biochemically active recombinant Pol (14). Instead, structural insights on HBV Pol could be obtained primarily through genetic analysis. In the process of attempting to define the HBV Pol subdomain(s) that contributes to pgRNA encapsidation, unexpectedly, we found that the SP subdomain is not entirely dispensable. Alanine-scanning mutational analysis identified four highly conserved cysteine residues that are critical for encapsidation. Three of the cysteines are in the SP subdomain and the fourth one lies in the neighboring RT domain, and the organization of the four cysteine residues in the primary sequence is consistent with a zinc finger motif. The mutation of the cysteine residues abolished the ability of Pol to bind RNA, suggesting that these residues mediate the RNA binding activity of Pol. Overall, these findings indicate that a putative zinc finger motif in Pol is critical for the ability of Pol to recognize and encapsidate pgRNA during hepadnaviral assembly.
MATERIALS AND METHODS
Cell culture and transfection.
Huh7 cells and HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (GIBCO-BRL) and 10 μg/ml of gentamicin at 37°C in 5% CO2 and were split every third day. Calcium phosphate precipitate-mediated transfection was used for the transfection of Huh7 cells, as described previously (8). HEK293 cells were transfected using polyethylenimine (25 kDa; Aldrich) as previously described (16).
Plasmid construction.
The HBV Pol-null replicon construct was described previously (16). It was made by introducing two mutations in the HBV replicon: (i) a frameshift mutation by deletion of the T nucleotide of the second ATG of the P open reading frame (ORF) and (ii) a point mutation that changed the first ATG of the P ORF into ACG without altering the amino acid encoded by the overlapping C ORF (16). pCMV-Pol, an HBV polymerase expression construct, was described previously (16), from which HBV Pol with three copies of the FLAG epitope at the N terminus is expressed. pCMV-WHV Pol was made and expresses WHV Pol harboring three copies of FLAG tag at its N terminus. The HBV Pol-null-ΔL42 construct was previously reported and encodes the capsid assembly-defective core protein (16). All substitution and deletion mutants were generated by overlap extension PCR protocols as previously described (13). The details of the molecular cloning of any plasmid construct will be provided upon request. All mutants were sequenced to confirm the base change.
Southern blot analysis.
Southern blot analysis was performed as previously described (18). Briefly, the extracted viral DNA was separated by electrophoresis through a 1.3% agarose gel in a 0.5× Tris-acetate-EDTA buffer and then transferred onto a nylon membrane. The nylon membrane was prehybridized and hybridized with a 32P-labeled full-length HBV DNA probe in a hybridization solution for 16 h at 65°C. Images were obtained using a phosphorimager (BAS-2500; Fujifilm).
Western blot analysis.
Western blot analysis was performed essentially as described previously (16). The protein samples were resolved through 10% sodium dodecyl sulfate-polyacrylamide gels and then transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore). After blocking, the membrane was incubated with a rabbit anti-core antibody (1:2,000; Dako) or a mouse anti-FLAG M2 antibody (1:5,000; Sigma), followed by anti-rabbit or anti-mouse immunoglobulin G horseradish peroxidase-linked antibody (1:5,000; Amersham), respectively. β-Actin was detected by using a rabbit anti-actin antibody (1:5,000; Sigma). The proteins were visualized using Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer). The images were quantified using an LAS-3000 imaging system (Fujifilm).
RNA extraction and RNase protection analysis.
RNA from whole cells and cytoplasmic capsids was extracted 3 days after transfection as described previously (8). Approximately three times more cell equivalents of the capsid fraction than the total cytoplasmic fraction were loaded. RNA was analyzed by RNase protection analysis, according to the manufacturer's procedure (Ambion). The riboprobe was derived from the core region (nucleotides 1903 to 2140), as described previously (16). Briefly, each sample of RNA was hybridized with 105 cpm of [α-32P]UTP (3,000 Ci/mmol; Amersham)-labeled probe for 16 h at 42°C. RNase digestion was carried out with a mixture of RNase A and RNase T1 for 30 min at 37°C. The digested products were separated on 5% acrylamide-8 M urea gels. Images were obtained using a phosphorimager (BAS-2500; Fujifilm).
Density gradient analysis.
Density gradient analysis was performed by using an OptiPrep velocity gradient (60% [wt/vol] iodixanol; Axis-Shield), essentially as described previously (9). The OptiPrep density gradients were prepared in lysis buffer as five steps in 10% increments and ranged from 10 to 50%. The cell lysates were then treated with proteinase K (Sigma) at a concentration of 400 μg/ml for 20 min at 37°C. An aliquot of 200 μl cell lysates was carefully loaded onto the top of the gradient and centrifuged for 45 min at 55 krpm in a TLS 55 swing-out rotor (Beckman Instruments) at 20°C.
RESULTS
The C terminus SP subdomain is important for pgRNA encapsidation.
To determine whether the SP subdomain is entirely dispensable for viral genome replication, we tested the ability of a Pol mutant that lacked the SP subdomain to support pgRNA encapsidation and subsequent viral genome replication. We generated two deletion mutants of the SP subdomain, ΔSP1 and ΔSP2, that lacked either the N-terminal or C-terminal half of the SP subdomain, respectively (Fig. 2A). The functionalities of the SP deletion mutants were evaluated by their abilities to rescue an HBV Pol-null mutant construct, which was shown to lead to viral genome replication if a functional Pol is provided in trans (16). The Huh7 cell line, a human hepatocellular carcinoma cell line, was transfected with the HBV Pol-null construct and an expression plasmid for either wild-type (WT) Pol or one of the SP deletion mutants (Fig. 2B). Three days following the transfection, the viral DNA replication intermediates were extracted from capsids and analyzed by Southern blot analysis. As anticipated, WT Pol complemented the HBV Pol-null construct, as evidenced by the detection of all three of the viral DNA replication intermediates: RC DNA, double-stranded linear DNA, and ssDNA (Fig. 2B, lane 1). The ΔSP1 mutant lacking the N-terminal half of the SP subdomain supported viral genome replication similarly to WT Pol (Fig. 2B, lane 3). In contrast, the ΔSP2 mutant, which lacked the C-terminal half of the SP subdomain, failed to support viral genome replication (Fig. 2B, lane 4), suggesting that the C-terminal half is indispensable for viral genome replication. Western blot analysis of the cell lysates from a parallel experiment confirmed that the expression of the ΔSP2 Pol protein was comparable to the expression of WT Pol (Fig. 2B).
Next, we examined whether the ΔSP2 mutant is defective in pgRNA encapsidation, which is the step prior to genome replication. Huh7 cells were transfected with the HBV Pol-null construct and an expression plasmid for either the WT or one of the SP deletion mutants, as indicated (Fig. 2C). Three days after transfection, both capsid-associated RNA and total RNA were extracted and analyzed by RNase protection analysis (RPA) using a riboprobe specific for the detection of pgRNA. The RPA assay showed that WT Pol successfully complemented the HBV Pol-null construct, as evidenced by the detection of pgRNA in the capsid fraction (Fig. 2C, lane 1). As anticipated, the ΔSP1 mutant was able to support the encapsidation of pgRNA to a level comparable to that of WT Pol (Fig. 2C, lane 5). In contrast, the ΔSP2 mutant failed to support encapsidation (Fig. 2C, lane 7). Thus, we concluded that the C-terminal half of the SP subdomain is essential for pgRNA encapsidation.
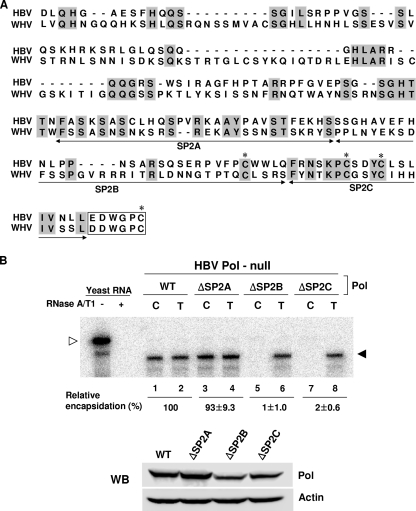
To gain further insight into the function(s) of the SP region, the amino acid sequence of HBV Pol was compared with that of WHV Pol SP subdomains. The alignment analysis indicated that the C-terminal half of the SP subdomain is relatively well conserved between HBV and WHV Pol, but the N-terminal half of the SP subdomain has a higher degree of divergence and several gaps (Fig. 3A). However, no significant alignment was obtained between the HBV and duck hepatitis B virus Pol SP subdomains, reflecting divergence between mammalian and avian hepadnaviruses (data not shown). To further define the critical sequence within the C-terminal half of the SP subdomain, it was further divided into three parts, the SP2A, SP2B, and SP2C regions (Fig. 3A). Three deletion mutants, lacking one of three C-terminal SP subdomain regions, were constructed and designated ΔSP2A (Δ80 to 108), ΔSP2B (Δ109 to 139), and ΔSP2C (Δ140 to 158). These three deletion mutants were evaluated by the RPA assay, as described above (Fig. 3B). The RPA assays showed that while the ΔSP2A mutant supported encapsidation (Fig. 3B, lane 3), the ΔSP2B and ΔSP2C mutants were unable to encapsidate pgRNA (Fig. 3B, lane 5 and 7). Western blot analysis performed in parallel showed that the expression levels of the ΔSP2B and ΔSP2C Pol proteins were comparable to that of WT Pol (Fig. 3B). Thus, we concluded that both the SP2B and SP2C regions of the SP subdomain are important for encapsidation.
FIG. 3.
The C terminus of the SP region of HBV Pol is critical for encapsidation. (A) Diagram showing conserved amino acid residues of the SP subdomain of mammalian hepadnaviral Pol. Conserved amino acids between HBV and WHV Pol are denoted by gray boxes. The C-terminal half was subdivided into three regions, SP2A, SP2B, and SP2C, as denoted by double-headed arrows. The first six amino acid residues of the neighboring RT subdomain are denoted by an open box. The four conserved cysteine residues are denoted by asterisks above the letters. (B) RPA and Western blot (WB) analysis were performed as described in the legend to Fig. 2.
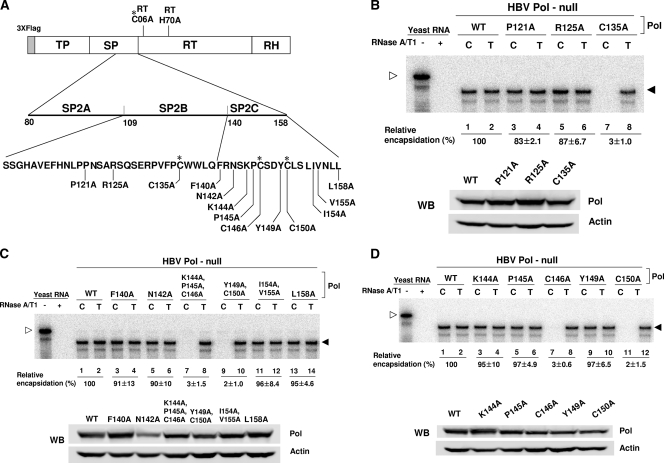
Three cysteine residues of the SP subdomain are critical for encapsidation.
To define specific amino acid residues critical for the encapsidation process, 13 amino acid residues that are conserved between the HBV and WHV Pol proteins were individually substituted by alanine (Fig. 4A). These alanine substitution mutants were each examined for their ability to support pgRNA encapsidation. Of the three mutants within the SP2B region (Fig. 4B), the RPA data indicated that only the C135A mutant failed to support encapsidation (Fig. 4B, lane 7). Western blot analysis confirmed that the Pol protein levels derived from three mutants were comparable (Fig. 4B), suggesting that the C135 residue is the only residue in the SP2B region critical for encapsidation. Next, we tested six alanine substitution mutants of the SP2C region, which included some double or triple substitution mutants (Fig. 4C). RPA analysis of this group of mutants showed that only the Pol mutants that harbor a cysteine residue mutation (i.e., C146A and C150A) failed to support encapsidation (Fig. 4C, lanes 7 and 9). Western blot analysis confirmed that the expression levels of the mutant Pol proteins were comparable (Fig. 4C). To determine the specific residues within the double and triple mutants responsible for the inability to encapsidate pgRNA, five individual residues were substituted with alanine (Fig. 4D). As before, RPA analysis was performed to determine the ability of these mutants to encapsidate pgRNA. The results indicate that the two cysteine-substituted mutants were defective in RNA packaging (Fig. 4D, lanes 7 and 11). Again, Western blot analysis confirmed that the expression levels of the Pol mutant proteins were comparable (Fig. 4D). Overall, these results indicated that the three cysteine residues in the C-terminal half of the SP subdomain are critically important for encapsidation, but the mutation of the other residues in the SP2B or SP2C regions had no effect on encapsidation. Further, the mutation of any one of the cysteine residues results in defective pgRNA encapsidation.
FIG. 4.
Three conserved cysteine residues are critical for encapsidation. (A) Diagram showing the residues of the C-terminal half of the SP subdomain that were mutated. Alanine-scanning mutagenesis was performed to change individually 13 conserved residues to alanine. The amino acid is represented by a single-letter abbreviation along with its number starting from its N terminus of the SP domain. The four conserved cysteine residues are denoted by asterisks. (B) Alanine-scanning mutagenesis of the SP2B region. RPA and Western blot (WB) analysis of the SP2B region mutants were performed as shown in Fig. 2. (C) Alanine-scanning mutagenesis of the SP2C region. Note that three mutants harbor either double or triple substitutions. (D) Alanine-scanning mutagenesis of the SP2C region, which was performed similarly to the assays described above.
The cysteine mutants failed to suppress the translation of pgRNA.
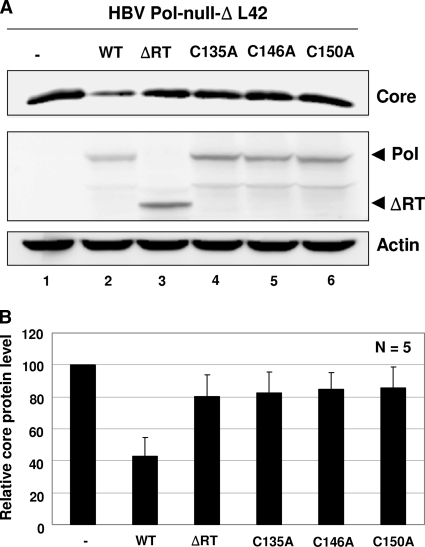
The inability of the cysteine mutants of HBV Pol to support the encapsidation of pgRNA suggested that these Pol mutants may have lost RNA binding activity. To assess the interaction between Pol and the 5′ ɛ stem-loop, we sought to exploit our recent observation, in which we demonstrated that HBV Pol suppresses the translation of pgRNA by binding to the 5′ ɛ stem-loop structure (16). In this published work, the suppression of the translation of pgRNA was measured by the Pol-mediated reduction of the C (core) protein levels. To prevent capsid assembly, the capsid assembly-defective ΔL42 allele of the core protein, which is a deletion of the 42nd leucine residue in the core protein, was employed in the HBV Pol-null background, as described previously (16). Cells were transfected with expression constructs encoding either the WT or one of the Pol mutant constructs, along with the HBV Pol-null-ΔL42 construct, as indicated (Fig. 5A). The core protein level was measured by Western blot analysis. In cells transfected with WT Pol, as expected, the amount of the core protein was substantially reduced, indicating that WT Pol suppressed the translation of pgRNA (Fig. 5A, lane 1 versus 2). ΔRT, a deletion mutant of the RT subdomain that served as a negative control, failed to suppress the translation of the core protein (Fig. 5A, lane 3), which is consistent with its lack of RNA binding ability (6). Three cysteine mutants also failed to suppress the translation of pgRNA (Fig. 5A, lanes 4 to 6, and Fig. 5B), suggesting that these mutants were unable to bind pgRNA. Comparable expression of the Pol mutants further corroborated this finding. Thus, these data indicated that three conserved cysteine residues within the SP subdomain are critical for the RNA binding activity of Pol.
FIG. 5.
The conserved cysteine residues are essential for the ability of HBV Pol to suppress the translation of core protein. (A) Western blot analysis of core protein levels. Cells were cotransfected with the HBV Pol-null-ΔL42 construct along with the Pol expression plasmids as indicated (note that three times the cysteine mutant DNA was transfected relative to that of the WT and ΔRT [1.5 μg or 0.5 μg per 35-mm plate, respectively]). The Pol and ΔRT proteins were detected with anti-Flag antibodies and are denoted by arrows. The reduction in core protein levels indicated translation suppression by HBV Pol (16). Actin served as a loading control. (B) Quantitation. Relative core protein levels were obtained from five independent transfections. The value for the HBV Pol-null construct was set at 100. Error bars represent the standard deviations of the results from five independent experiments.
The cysteine mutants did not impede the ability of WT Pol to support viral genome replication.
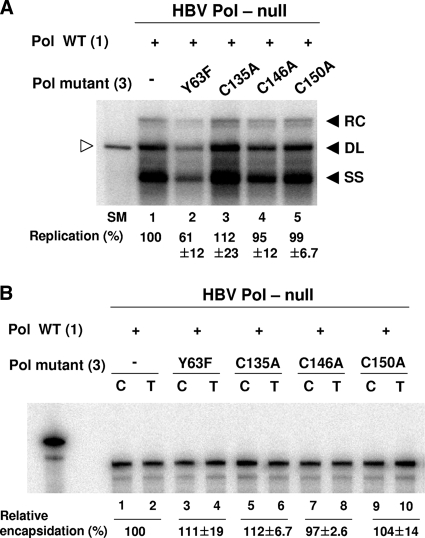
One expectation of the above result is that the Pol mutants, when ectopically cotransfected, would not inhibit WT Pol from supporting viral genome replication, owing to the lack of the RNA binding ability. To substantiate this expectation, we examined the viral DNA synthesis following the transfection of cells with 1:3 ratios of WT Pol and Pol mutant expression plasmids, along with the HBV Pol-null construct, as indicated in Fig. 6A. In addition, the Y63F mutant, in which an invariant tyrosine residue of the TP domain critical for protein priming has been changed to a phenylalanine (Fig. 2A), was included for comparison. Unlike the cysteine mutants, the Y63F mutant, which is defective in RT activity but retains RNA binding ability, would inhibit WT Pol from supporting viral genome replication (15). Consistent with the expectation, Southern blot analysis showed that the cotransfection of the Y63F mutant, in addition to WT Pol, led to significantly reduced viral DNA synthesis, suggesting that the Y63F mutant affected the ability of WT Pol to support viral DNA synthesis in a dominant-negative manner (Fig. 6A, lane 1 versus 2). By contrast, the cotransfection of any of three cysteine mutants did not affect viral DNA synthesis (Fig. 6A, lanes 3 to 5). Importantly, the observation that the cysteine mutants did not manifest as a dominant-negative inhibitor of WT Pol in this competition experiment led us to interpret these data indicating that the cysteine mutants failed to bind to the RNA. RPA performed in parallel corroborated that WT Pol expressed in the transfected cells was not limiting, since encapsidation proceeded comparably, whether Pol mutants were cotransfected or not (Fig. 6B). Based on these results, together with the result depicted in Fig. 5, we concluded that the conserved cysteine residues are critically important for the RNA binding ability of HBV Pol.
FIG. 6.
HBV Pol harboring the cysteine mutation did not inhibit the ability of WT Pol to support viral genome replication. (A) Southern blot analysis. Cells were cotransfected with 1:3 ratios of WT Pol (1 μg) and Pol mutant expression plasmids (3 μg), along with the HBV Pol-null construct (16 μg) per 100-mm plate. Southern blot analysis was performed as described in the legend to Fig. 2B. Symbols are same as described in the legend to Fig. 2. (B) RPA. Transfection was performed as described for panel A. RPA was performed as described in the legend to Fig. 2C. Symbols are the same as described in the legend to Fig. 2.
A cysteine residue in the RT subdomain is also critical for encapsidation.
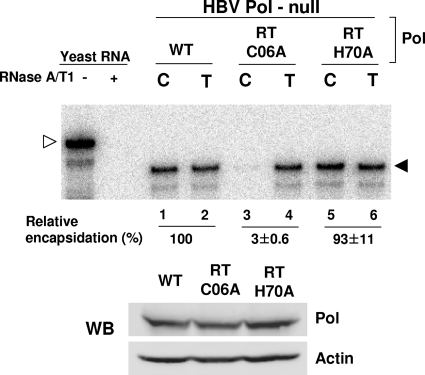
One possibility is that the three cysteine residues could be part of a zinc finger motif, a protein fold that is frequently found in RNA binding proteins as well as DNA binding proteins (11, 12). This hypothesis predicts that there should be another cysteine or histidine residue within HBV Pol to constitute the putative zinc finger motif. However, no additional conserved cysteine or histidine residue was found in the C-terminal half of the SP subdomain, so for this hypothesis to be addressed, the fourth residue must be in another subdomain of Pol. We sought to identify the putative fourth residue of the zinc finger within the N terminus of the neighboring RT subdomain (Fig. 4A). As before, we made an alanine substitution mutation of the cysteine residue at position 6 and the histidine residue at position 70 of the RT subdomain. To determine the effect of the mutations on pgRNA encapsidation, RPA analysis was performed (Fig. 7). The RPA data indicated that while the RT H70A mutant fully supported encapsidation, the RT C06A mutant failed to encapsidate pgRNA (Fig. 7, lanes 3 and 5). Again, Western blot analysis showed that the Pol proteins from two mutants were expressed to comparable levels (Fig. 7). The data indicated that the cysteine residue at position 6 of the RT subdomain is critical for pgRNA encapsidation, a finding that uncovered the fourth cysteine residue of the putative zinc finger motif.
FIG. 7.
A cysteine residue at the N terminus of the RT subdomain is critical for encapsidation. A schematic representation of 2 amino acid residues mutated in the RT subdomain (Fig. 4A). RT C06 represents cysteine 6, while RT H70 represents histidine 70 of the RT subdomain; the numbering is relative to the N terminus of the RT subdomain. RPA and Western blot (WB) analysis of the RT subdomain mutants were performed as described in the legend to Fig. 2.
The cysteine mutants failed to incorporate into nucleocapsids.
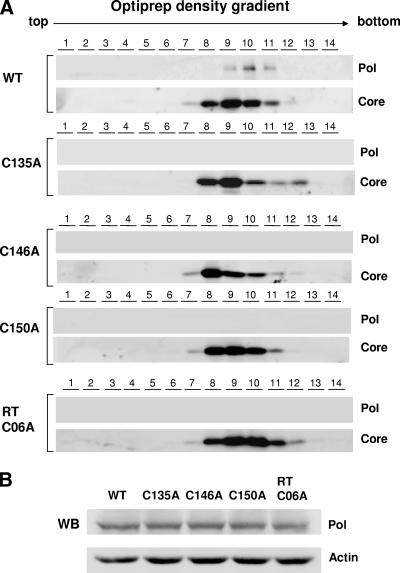
Another expectation is that, unlike WT Pol, the Pol mutants would not incorporate into capsid particles due to a lack of RNA binding ability. To substantiate this notion, we carried out density gradient analysis of nucleocapsids. Cells were transfected with the HBV Pol-null construct, along with either the WT Pol or Pol mutant expression plasmid, as before. To demonstrate the resistance of the encapsidated Pol to mild proteinase K treatment, cell lysates were treated with proteinase K prior to the OptiPrep density gradient, as detailed in Materials and Methods. HBV Pol and the core protein in each fraction were detected by immunoblotting with their respective antibodies (Fig. 8A). An immunoblot analysis indicated that HBV Pol in capsid fractions remained detectable (Fig. 8A, fractions 9 to 11). Thus, the resistance to proteinase K treatment, as evidenced by the detection of Pol in the capsid fractions following proteinase K treatment, is consistent with the interpretation that HBV Pol is indeed incorporated into capsid particles (Fig. 8A). Likewise, all four cysteine mutants were examined by density gradient analysis following proteinase K treatment. The result indicated that unlike WT Pol, all four Pol mutant proteins were not incorporated into capsid particles (Fig. 8A). The comparable expression of the Pol protein mutant validated this finding (Fig. 8B). Overall, the result of the density gradient analysis further strengthened the above conclusion that the conserved cysteine residues are critical for RNA binding.
FIG. 8.
No incorporation of HBV Pol harboring the cysteine mutation into nucleocapsid particles. (A) OptiPrep density gradient analysis was performed as detailed in Materials and Methods. HEK293 cells were cotransfected with the HBV Pol-null construct and either WT Pol or Pol mutant expression plasmid, as indicated. Three days following transfection, the cells were lysated with NP-40 lysis buffer, and the cell lysates were treated with proteinase K. Then, the lysates were subjected to a 10 to 50% OptiPrep density gradient. Fourteen 140-μl fractions from the top were collected, and HBV Pol and core proteins were detected by Western blot analysis with anti-Flag and anti-core antibodies, respectively. (B) Western blot (WB) analysis of the cell lysate was performed as described in the legend to Fig. 2B.
The four conserved cysteine residues in WHV Pol are also critical for encapsidation.
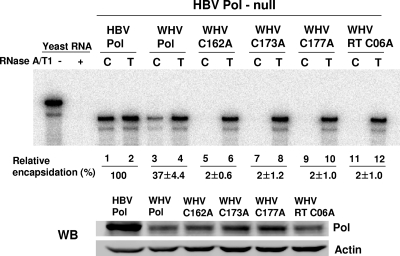
Because WHV and HBV share substantial homology, about 60% at the nucleotide level (10), the WHV model could provide an opportunity to substantiate the findings made for HBV. Thus, we sought to explore the transcomplementation that has been established by an earlier study, which demonstrated that WHV proteins (i.e., core and Pol) modestly support the encapsidation of HBV pgRNA and vice versa (21). To examine the importance of the four conserved cysteine residues of WHV Pol, we generated four WHV Pol mutants, in which each of the four conserved cysteine residues was mutated to alanine (Fig. 3A). As before, Huh7 cells were transfected, and the RNA was extracted and analyzed by RPA (Fig. 9). The RPA data showed that WT WHV Pol supported the encapsidation of HBV pgRNA, albeit the level of encapsidation was somewhat reduced compared to the level of pgRNA encapsidation mediated by HBV Pol (Fig. 9, lanes 3 to 4), which is consistent with the previous report (21). As was observed with the HBV mutants, all four WHV Pol mutants failed to support HBV pgRNA encapsidation (Fig. 9, lanes 5 to 12), indicating that the four cysteine residues of WHV Pol are also critical for encapsidation. Western blot analysis showed that WHV Pol was expressed at a low level in comparison to HBV Pol (Fig. 9), which could, in part, account for the reduced encapsidation level mediated by WHV Pol (Fig. 9, lane 3). However, the lack of encapsidation mediated by the four cysteine mutants was not attributable to the lower protein level, since all four mutant Pol proteins were expressed comparably to the level of WT WHV Pol. Overall, the identification of the putative zinc finger motif in HBV Pol was substantiated by parallel findings made with WHV Pol. Therefore, we concluded that the four conserved cysteine residues within the SP-RT subdomain junction of mammalian hepadnaviral Pol are critical for RNA binding and pgRNA encapsidation.
FIG. 9.
The four conserved cysteine residues in WHV Pol are also critical for encapsidation. The four alanine substitution mutants of WHV Pol (C162A, C173A, C177A, and C06A) were made in parallel, and the positions of the four conserved cysteine residues are indicated by asterisks in Fig. 3A. The four WHV Pol mutants were tested for their ability to support the encapsidation of HBV pgRNA by complementing the HBV Pol-null construct. RPA and Western blot (WB) analysis were performed as described in the legend to Fig. 2.
DISCUSSION
It was previously reported that the SP subdomain of HBV Pol can be largely deleted without detrimental effect. Unexpectedly, in contrast with the prevailing notion, we found that the conserved cysteine residue in the SP subdomain is critical for pgRNA encapsidation. Genetic analysis of HBV Pol revealed that the four conserved cysteine residues that span the junction of the SP and RT subdomains of HBV Pol are critical for pgRNA encapsidation. Based on the fact that the four cysteine residues are clustered within 30 amino acids (i.e., C-X10-C-X3-C-X13-C), which is consistent with a feature of a zinc finger motif (11), we postulated that the four conserved cysteines constituted a putative zinc finger motif that is essential for the recognition of the 5′ ɛ stem-loop structure by HBV Pol.
A notion that the conserved cysteine residues are critical not only for encapsidation but also for RNA binding was supported by three independent experiments. First, we exploited the recently described Pol-mediated suppression of pgRNA translation, which was shown to depend on the interaction between Pol and 5′ ɛ (16). The result indicated that the four cysteine residues are essential for the suppression of pgRNA translation by HBV Pol (Fig. 5 and data not shown). Second, the cysteine mutants, when ectopically cotransfected, did not adversely affect the ability of WT Pol to support viral genome replication (Fig. 6), a finding that is also consistent with the lack of RNA binding. Third, via density gradient analysis, we demonstrated that the Pol cysteine mutants were not encapsidated into capsid particles, unlike WT Pol (Fig. 8). Importantly, this density gradient analysis can be considered a direct measurement of RNA binding, since the incorporation of HBV Pol relies on its ability to recognize the 5′ ɛ stem-loop structure (1). Overall, the results of the above three experiments are consistent with the interpretation that the conserved cysteine residues are critical for RNA binding. On the other hand, our RNA binding analyses presented here were confined to in vivo measurement. A parallel in vitro examination of the RNA binding of Pol mutants merits further investigation by employing the in vitro reconstitution of E. coli-derived Pol (6).
Nonetheless, it is important to note that the possibility that the cysteine residues could be involved in disulfide bond formation has not been excluded. In addition, it is formally possible that the mislocalization of the Pol mutant in the cell could be responsible for the lack of pgRNA encapsidation. An immunofluorescence analysis showed that WT Pol was predominantly localized in the cytoplasm, possibly enriched near the nuclear periphery (data not shown), which is in good agreement with a previous report (3). Importantly, the subcellular localization of the Pol mutant was indistinguishable from that of WT Pol, excluding this formal possibility (data not shown).
An earlier genetic analysis suggested that the SP subdomain of Pol is largely dispensable for proper Pol function. For instance, a deletion analysis has shown that about 90 internal amino acids of the SP subdomain could be deleted without detrimental effect (15), leading to the conclusion that the SP subdomain is not essential for Pol functions. Nevertheless, this study has demonstrated that the C-terminal 45 residues of the SP subdomain are critically involved in RNA binding and pgRNA encapsidation, because the deletion of this region led to a lack of Pol activity of nucleocapsid particles (15). On the other hand, it was reported that the SP subdomain was not essential for the ability of Pol to bind the 5′ ɛ stem-loop structure by using E. coli-expressed Pol (5, 7). However, in fact, the minimal Pol domain capable of ɛ RNA binding retains the residual C-terminal part of the SP region (i.e., 45 amino acids), including all three cysteine residues we examined here (5). Overall, the data presented here are consistent with these in vitro studies (5, 15).
The HBV Pol protein plays multiple critical functions in viral assembly and replication and has been the focus of intense efforts to develop anti-HBV drugs (4). Due to challenges in obtaining appropriate amounts of soluble protein necessary to obtain a crystal structure, hepadnaviral Pol remains refractory to structural analysis (14). In the absence of structural information, genetic analysis is an alternative mean to obtain information about the structure of HBV Pol. The data presented here revealed a potentially novel structural role of the SP subdomain, which was previously thought to be an unstructured region that simply tethers the TP and RT domains together. The challenge for future research lies in demonstrating that these cysteine residues in HBV Pol indeed coordinate a zinc ion. If this holds, it would represent a novel feature of hepadnaviral Pol that is distinct from retroviral RT. Further, this putative zinc finger motif presents a novel antiviral target for therapeutic intervention against HBV-induced disease.
Acknowledgments
We thank Byung-Yoon Ahn (Korea University) for reading and critically evaluating the manuscript.
This work was supported by a Korea Research Foundation grant (KRF-2009-C00271) and, in part, by grant A050748 from the Korea Health 21 R & D Project, Ministry of Health & Welfare.
Footnotes
Published ahead of print on 10 June 2009.
REFERENCES
- 1.Bartenschlager, R., and H. Schaller. 1992. Hepadnaviral assembly is initiated by polymerase binding to the encapsidation signal in the viral RNA genome. EMBO J. 113413-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck, J., and M. Nassal. 2007. Hepatitis B virus replication. World J. Gastroenterol. 1348-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao, F., and J. E. Tavis. 2004. Detection and characterization of cytoplasmic hepatitis B virus reverse transcriptase. J. Gen. Virol. 853353-3360. [DOI] [PubMed] [Google Scholar]
- 4.Feld, J., J. Y. Lee, and S. Locarnini. 2003. New targets and possible new therapeutic approaches in the chemotherapy of chronic hepatitis B. Hepatology 38545-553. [DOI] [PubMed] [Google Scholar]
- 5.Hu, J., and M. Boyer. 2006. Hepatitis B virus reverse transcriptase and epsilon RNA sequences required for specific interaction in vitro. J. Virol. 802141-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu, J., D. Flores, D. Toft, X. Wang, and D. Nguyen. 2004. Requirement of heat shock protein 90 for human hepatitis B virus reverse transcriptase function. J. Virol. 7813122-13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu, J., D. Toft, D. Anselmo, and X. Wang. 2002. In vitro reconstitution of functional hepadnavirus reverse transcriptase with cellular chaperone proteins. J. Virol. 76269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong, J. K., G. S. Yoon, and W. S. Ryu. 2000. Evidence that the 5′-end cap structure is essential for encapsidation of hepatitis B virus pregenomic RNA. J. Virol. 745502-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kock, J., M. Kann, G. Putz, H. E. Blum, and F. Von Weizsacker. 2003. Central role of a serine phosphorylation site within duck hepatitis B virus core protein for capsid trafficking and genome release. J. Biol. Chem. 27828123-28129. [DOI] [PubMed] [Google Scholar]
- 10.Kodama, K., N. Ogasawara, H. Yoshikawa, and S. Murakami. 1985. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: evolutional relationship between hepadnaviruses. J. Virol. 56978-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishna, S. S., I. Majumdar, and N. V. Grishin. 2003. Structural classification of zinc fingers: survey and summary. Nucleic Acids Res. 31532-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laity, J. H., B. M. Lee, and P. E. Wright. 2001. Zinc finger proteins: new insights into structural and functional diversity. Curr. Opin. Struct. Biol. 1139-46. [DOI] [PubMed] [Google Scholar]
- 13.Lee, J., H.-J. Lee, M.-K. Shin, and W.-S. Ryu. 2004. Versatile PCR-mediated insertion or deletion mutagenesis. BioTechniques 36398-400. [DOI] [PubMed] [Google Scholar]
- 14.Nassal, M. 2008. Hepatitis B viruses: reverse transcription a different way. Virus Res. 134235-249. [DOI] [PubMed] [Google Scholar]
- 15.Radziwill, G., W. Tucker, and H. Schaller. 1990. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J. Virol. 64613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu, D. K., S. Kim, and W. S. Ryu. 2008. Hepatitis B virus polymerase suppresses translation of pregenomic RNA via a mechanism involving its interaction with 5′ stem-loop structure. Virology 373112-123. [DOI] [PubMed] [Google Scholar]
- 17.Seeger, C., F. Zoulim, and W. Mason. 2007. Hepadnaviruses, p. 2977-3029. In D. M. Knipe and P. M. Howley (ed.), Field's virology, 5th ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 18.Shin, M.-K., J. Lee, and W.-S. Ryu. 2004. A novel cis-acting element facilitates minus-strand DNA synthesis during reverse transcription of the hepatitis B virus genome. J. Virol. 786252-6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuyver, L. J., S. A. Locarnini, A. Lok, D. D. Richman, W. F. Carman, J. L. Dienstag, and R. F. Schinazi. 2001. Nomenclature for antiviral-resistant human hepatitis B virus mutations in the polymerase region. Hepatology 33751-757. [DOI] [PubMed] [Google Scholar]
- 20.Wang, G. H., and C. Seeger. 1992. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell 71663-670. [DOI] [PubMed] [Google Scholar]
- 21.Ziermann, R., and D. Ganem. 1996. Homologous and heterologous complementation of HBV and WHV capsid and polymerase functions in RNA encapsidation. Virology 219350-356. [DOI] [PubMed] [Google Scholar]
- 22.Zoulim, F. 2006. Antiviral therapy of chronic hepatitis B. Antivir. Res. 71206-215. [DOI] [PubMed] [Google Scholar]