Abstract
Integrin signaling promotes, through p21-activated kinase, phosphorylation and inactivation of the tumor suppressor merlin, thus removing a block to mitogenesis in normal cells. However, the biochemical function of merlin and the effector pathways critical for the pathogenesis of malignant mesothelioma and other NF2-related malignancies are not known. We report that integrin-specific signaling promotes activation of mTORC1 and cap-dependent mRNA translation. Depletion of merlin rescues mTORC1 signaling in cells deprived of anchorage to a permissive extracellular matrix, suggesting that integrin signaling controls mTORC1 through inactivation of merlin. This signaling pathway controls translation of the cyclin D1 mRNA and, thereby, cell cycle progression. In addition, it promotes cell survival. Analysis of a panel of malignant mesothelioma cell lines reveals a strong correlation between loss of merlin and activation of mTORC1. Merlin-negative lines are sensitive to the growth-inhibitory effect of rapamycin, and the expression of recombinant merlin renders them partially resistant to rapamycin. Conversely, depletion of merlin restores rapamycin sensitivity in merlin-positive lines. These results indicate that integrin-mediated adhesion promotes mTORC1 signaling through the inactivation of merlin. Furthermore, they reveal that merlin-negative mesotheliomas display unregulated mTORC1 signaling and are sensitive to rapamycin, thus providing a preclinical rationale for prospective, biomarker-driven clinical studies of mTORC1 inhibitors in these tumors.
The NF2 gene, which encodes the FERM domain protein merlin, was identified in 1993 as the tumor suppressor inactivated in the familial cancer predisposition syndrome neurofibromatosis type 2 (50, 60). Since then, biallelic NF2 mutations have been found not only in sporadic schwannomas and meningiomas, but also in a large fraction of malignant mesotheliomas and, less frequently, in other tumor types. Furthermore, conditional inactivation experiments have indicated that merlin has a broad tumor-suppressive function in mice (29, 39).
Merlin is a member of the ezrin/radixin/moesin (ERM) family of proteins, which link membrane receptors to the cortical actin cytoskeleton (4). ERM proteins consist of an N-terminal FERM domain followed by a coiled-coil domain and a C-terminal domain containing an actin-binding motif. They are believed to switch between a closed conformation, which is inactive, and an open conformation, which mediates the linkage of certain cell adhesion proteins to the actin cytoskeleton. In contrast to classical ERM proteins, merlin does not contain a standard actin-binding motif. In addition, although merlin also switches between an open and a closed conformation, it is the closed form that suppresses tumorigenesis and is thereby considered active (25, 37, 57). The serine/threonine kinase p21-activated kinase (PAK) inactivates merlin by phosphorylating its C-terminal tail at Ser 518 and thereby disrupting the intramolecular interaction between the FERM domain and the C-terminal tail, which maintains the protein in the closed conformation (21, 56, 67). Conversely, the protein phosphatase MYPT1-PP1δ is thought to promote merlin-mediated growth inhibition by reversing the phosphorylation of Ser 518 (19).
Distinct adhesion-dependent stimuli converge on merlin to regulate cell proliferation. Cadherin-dependent cell-to-cell adhesion and CD44 engagement by hyaluronic acid activate MYPT1-PP1δ and inhibit PAK, causing an accumulation of dephosphorylated, closed-conformation merlin (19, 33, 38, 55). In contrast, integrin-dependent adhesion to the extracellular matrix activates PAK, causing inactivation of merlin and thereby presumably removing a block to cell cycle progression (38). These results suggest that merlin functions as a phosphorylation-dependent switch downstream of cell adhesion receptors. Although significant progress has been made toward elucidating the mechanisms that act upon merlin to regulate growth suppression, the biochemical function of merlin and the critical effector pathways through which it suppresses tumorigenesis have remained elusive.
Several studies have provided evidence that the closed form of merlin inhibits Rac signaling (22, 34, 38, 56). In this model, PAK-mediated inactivation of merlin would enhance Rac signaling, either by removing a block to the recruitment of Rac to the plasma membrane (38) or by alleviating direct inhibition of PAK (22). Indeed, NF2-deficient schwannoma cells display large lamellipodia and membrane ruffles, which are distinctive of activated Rac signaling (44). Other studies have documented effects of merlin on the Ras-extracellular signal-regulated kinase (ERK) pathway (19, 34), the phosphatidylinositol 3-kinase (PI-3K)-AKT pathway (49), and focal adhesion kinase signaling (46). Recently, it has been shown that merlin binds to the cytoplasmic tails of receptor tyrosine kinases (RTKs) in contact-inhibited cells and that, although merlin decreases the rate at which the RTKs are internalized, it hinders their abilities to initiate signaling (7). In apparent contrast, studies of the Drosophila fruit fly have indicated that merlin does not decrease, but rather increases, the rate of internalization of RTKs and other cell surface receptors, thus reducing their signaling activities (27). Finally, the results of genetic epistasis experiments with the same model indicate that merlin cooperates with the related ERM protein Expanded to activate the Hippo tumor suppressor pathway, which impinges on the transcriptional activator Yorkie (5, 16, 43). Although it is possible and, indeed, likely that loss of merlin activates several mitogenic pathways, it remains unclear whether any of the identified pathways are critical for NF2-dependent tumorigenesis.
Integrins bind to extracellular matrix components and cooperate with RTKs and other cytokine receptors to regulate cell survival and cell cycle progression (8, 13, 63). Most of the evidence to date suggests that the integrins control cell fate by regulating transcription. However, increasing evidence suggests that integrin-mediated adhesion also influences mRNA translation. Maeshima and colleagues have shown that tumstatin, an antiangiogenic fragment of collagen type IV, binds to the αvβ3 integrin and suppresses mTORC1 signaling and cap-dependent translation in endothelial cells (26). Furthermore, it has been shown that the engagement of αIIbβ3 by fibrinogen induces translation of the mRNA encoding Bcl-3 in activated platelets through a rapamycin-sensitive pathway (42) and that α6β4 enhances translation of the mRNA encoding vascular endothelial growth factor in breast carcinoma cells through phosphorylation of 4EBP1 (6). However, it has also been reported that adhesion to fibronectin affects cap-dependent translation independently of mTORC1 (14). These results suggest that integrin signaling controls cap-dependent translation. However, the mechanisms underlying this effect and its generality and scope currently remain unclear.
The initiation of mRNA translation is controlled by the evolutionarily conserved TCS1-TCS2-Rheb-mTORC1 signaling axis (15, 48). In this pathway, the TCS1-TCS2 complex exerts GTPase-activating protein activity toward the GTPase Rheb, which in turn activates the mTORC1 kinase complex. Whereas hypoxia and energy deprivation activate TCS1-TCS2 through REDD1/REDD2 and AMP-activated protein kinase, respectively, RTK signaling inhibits TCS1-TCS2 predominantly through AKT-mediated phosphorylation. Upon release from TCS1-TCS2 inhibition, mTORC1 phosphorylates the eIF4E inhibitory protein 4EBP1 and the kinase S6K1, thereby promoting cap-dependent translation (48). Several oncoproteins, including Ras, PI-3K, and AKT, and tumor suppressor proteins, such as LKB1, NF1, PTEN, and TCS1-TCS2, function upstream of or along this signaling axis, leading to frequent dysregulation of mTORC1 signaling and enhanced cap-dependent mRNA translation in human cancer. Both genetic and pharmacologic evidence suggests that this signaling axis plays an essential role in the initiation and maintenance of the transformed phenotype, providing a rationale for the use of mTOR inhibitors in certain cancer types (15, 54).
In this study, we provide evidence that integrin-specific signaling promotes activation of mTORC1 and cap-dependent translation through inactivation of merlin. We show that this signaling mechanism contributes to the regulation of cell survival and cell proliferation. Through examination of multiple mesothelioma cell lines, we provide evidence that the loss of merlin causes activation of mTORC1 signaling in malignant mesothelioma cells. Finally, we provide evidence that merlin mutant malignant mesothelioma cells are selectively sensitive to the growth-inhibitory effect of rapamycin.
MATERIALS AND METHODS
Cell lines, transfections, transductions, and constructs.
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics and cultured in serum-free medium (SFM; Gibco BRL) supplemented with serum and growth factors. LP9 cells were obtained from Coriell Cell Repositories (Camden, NJ). Meso-9 and Meso-33 cells were a gift from Suresh Jhanwar (Memorial Sloan-Kettering Cancer Center [MSKCC], New York, NY). VAMT and H2452 cells were a gift from Yuman Fong (MSKCC, New York, NY). 211H, H28, H2052, JMN, and Meso-10 cells were a gift from M. Ladanyi (MSKCC, New York, NY). Primary mouse embryonic fibroblasts (MEFs) harboring the conditional-mutant allele NF2flox/flox were isolated from embryos at embryonic day 12.5. Mice carrying this mutation were a kind gift from T. Jacks (Massachusetts Institute of Technology, Boston, MA).
For transfection of HUVECs, 5 × 106 cells were resuspended in 300 μl of SFM with the vectors indicated below and subjected to electroporation at 300 V and 450 μF. The small interfering RNA (siRNA) oligonucleotides si-Mer-1 (TGGCCAACGAAGCACTGAT) and si-Mer-2 (GUCUCAAGCCCAAAGAGCATT) were designed to target human merlin and were synthesized by the RNA Interference Facility of MSKCC. The siRNA oligonucleotide for TSC2 (TSC2 siRNA I) was purchased from Cell Signaling Technology. Cells were cultured in six-well plates (Transwell; Costar) until 40% confluent and transfected with 1 ml of Opti-MEM (Gibco BRL) containing 4 μl of Oligofectamine (Invitrogen) and 5 μl of 20 μM siRNA for 4 h. Cells were then incubated in SFM supplemented with 20% fetal bovine serum and growth factors for 24 h and deprived of growth factors for 24 h before the assays. pLKO.1 plasmids (clones TRCN0000018338 and TRCN0000039977) encoding short hairpin RNAs (shRNAs) targeting merlin were from Open Biosystems. Viral supernatants were generated by transfecting 293-FT cells with the shRNA constructs in combination with the packaging vectors pVSVG and pDR2. 211H cells stably expressing the shRNAs were selected by growth in puromycin for 1 week. VAMT cells were infected with amphotropic retroviruses pBABE-puro and pBABE-puro-NF2 and selected for 7 days in medium containing puromycin.
The reporter plasmid pRL-HCV-FL was provided by Martin Krüger (Medizinische Hochschule Hannover, Hannover, Germany) and has been described previously (24). The vector pCMV-TAG3b-pHAS-I, encoding 4EBP1, was provided by John Lawrence (University of Virginia Health System, Charlottesville, VA). pCMV-TAG3b-4EBP1-4A was generated by site-directed mutagenesis using the QuikChange kit according to the instructions of the manufacturer (Stratagene). The vectors pXJ40-HA-PakCAAX, which encodes a constitutively active form of PAK, and pXJ40-HA-PakCAAX-K298R, which encodes a dominant negative form of PAK, have been described previously (38). The vector pXJ40-HA-MerlinI and vectors encoding S518A and S518D merlin mutants have been described previously (38).
Immunoblotting analysis and kinase assays.
Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose (Schleicher and Schuell), and probed with the antibodies indicated below. Anti-cyclin D1 (A12), antimerlin (A19), anti-p27, anti-AKT, and anti-PAK (N20) were purchased from Santa Cruz Biotechnology. Anti-eIF4E was purchased from BD Biosciences. Anti-4EBP1, anti-ERK, anti-p65-4EBP1, anti-phosphorylated AKT, anti-phosphorylated ERK, anti-phosphorylated p70S6K, anti-p70S6K, anti-mTOR, and anti-TSC2 were from Cell Signaling Technology. PAK was immunoprecipitated from cell lysates with anti-Pak1 antibodies (N20; Santa Cruz Biotechnology, Inc.) and subjected to a kinase assay with myelin basic protein as a substrate, as described previously (38).
DNA microarray analysis.
Double-stranded cDNA was synthesized from total or polysomal RNA (1 to 10 μg). Linear amplification with T7 RNA polymerase (Ambion) and labeling with biotin (Enzo) were performed by in vitro transcription according to standard procedures. The resulting biotin-labeled cRNA was fragmented and hybridized to the Affymetrix human genome U133A oligonucleotide microarray chip for 16 h at 45°C. Following hybridization, the probe array was washed and stained on a fluidics station and immediately scanned on a Hewlett-Packard GeneArray scanner. The data generated from the scan were then analyzed using the MicroArray suite software (M.A.S. 5.0).
Bicistronic luciferase assay.
HUVECs were transfected with the pRL-HCV-FL reporter and the siRNAs indicated below. After 24 h, the cells were detached and plated onto fibronectin or laminin 1 in the presence or absence of growth factors. Luciferase activity was measured using a dual-luciferase reporter assay system (Promega) and a luminometer (Berthold) according to the instructions of the manufacturers.
Polyribosome analysis.
For preparation of cytoplasmic extracts, cells (25 to 30% confluent) from three 15-cm-diameter tissue culture plates were treated with cycloheximide (100 μg/ml; Sigma) for 5 min at 37°C, washed with phosphate-buffered saline containing cycloheximide, and harvested by trypsinization. Polyribosomes were prepared as described previously (51) with slight modifications. The cells were pelleted by centrifugation, swollen for 2 min in 375 μl of low-salt buffer (20 mM Tris [pH 7.5], 10 mM NaCl, and 3 mM MgCl2) containing 1 mM dithiothreitol and 50 U of recombinant RNasin (Promega), and lysed by the addition of 125 μl of lysis buffer (1× low-salt buffer-0.2 M sucrose-1.2% Triton N-101) followed by 10 strokes with a Dounce homogenizer. The nuclei were pelleted by centrifugation in a microcentrifuge at top speed for 30 s. The supernatants (cytoplasmic extracts; 500 μl each) were poured into new tubes containing a mixture of 50 μl of heparin (10 mg/ml; Sigma), 15 μl of 5 M NaCl, and 1 mM dithiothreitol. The cytoplasmic extracts were layered over continuous 15 to 40% sucrose gradients and centrifuged at 38,000 rpm for 2 h in an SW41 rotor. Gradient samples were divided into 10 fractions, and the RNA from each fraction was extracted and analyzed by Northern blotting.
Cap-binding assay.
Cells were lysed in TGN buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Tween 20, 0.3% NP-40, 1 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitors). Cell lysates were incubated with 7-methyl-GTP (m7GTP)-Sepharose beads for 2 h at room temperature or overnight at 4°C. The m7GTP beads were washed once with modified TGN buffer containing 50 mM NaCl, 0.25% Tween 20, and 0.05% NP-40 and twice with cold phosphate-buffered saline. Proteins were eluted from the beads by addition of SDS-PAGE loading buffer and subjected to SDS-PAGE.
RESULTS
Integrin-specific signaling controls activation of mTORC1 and cap-dependent translation during the G1 phase.
The recruitment of the 43S ribosomal subunit and initiation of cap-dependent translation are mediated by the eIF4F complex, which consists of the scaffold protein eIF4G, the RNA helicase eIF4A, and the cap-binding protein eIF4E (48). mTORC1 regulates the ordered assembly of the different components of this complex through two major mechanisms. It phosphorylates a family of regulatory proteins named 4EBPs, which compete with eIF4G for the binding to eIF4E, thus inhibiting the formation of an active eIF4F complex. In addition, it phosphorylates and activates S6K1, which in turn phosphorylates PDCD4 and targets it for degradation, alleviating its inhibition of eIF4A (15).
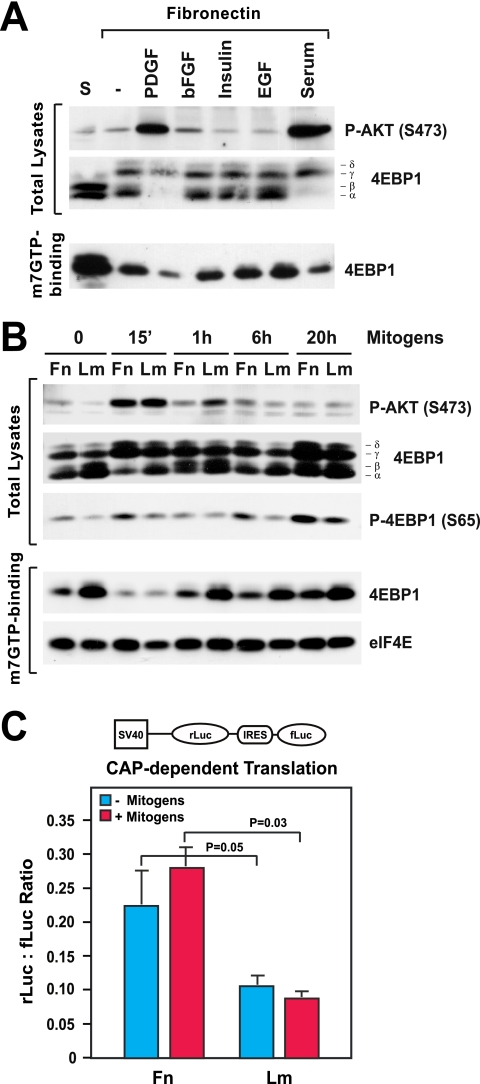
To examine if adhesion to the extracellular matrix is sufficient for activation of mTORC1 and cap-dependent translation, primary MEFs were detached and either kept in suspension or replated onto fibronectin for 6 h in the absence or the presence of various mitogens. The results of mobility shift and cap-binding assays revealed that adhesion to fibronectin is sufficient to promote partial phosphorylation of 4EBP1 and dissociation of 4EBP1 from the cap in the absence of growth factors (Fig. 1A). Notably, adhesion to fibronectin did not cause any apparent increase in the activation of AKT under these circumstances. Mitogenic concentrations of basic fibroblast growth factor (bFGF), insulin, or epidermal growth factor (EGF) did not activate AKT or increase the phosphorylation of 4EBP1 or the dissociation of 4EBP1 from the cap in cells adhering to fibronectin (Fig. 1A). In contrast, platelet-derived growth factor or serum caused robust activation of AKT and significant increases in the phosphorylation of 4EBP1 and dissociation of 4EBP1 from the cap in cells attaching to fibronectin. These results suggest that matrix adhesion is sufficient to activate mTORC1 signaling but that RTK-mediated activation of AKT is necessary to promote complete phosphorylation of 4EBP1 and dissociation of 4EBP1 from the cap in cells stimulated with growth factors.
FIG. 1.
Integrin-specific adhesion promotes mTORC1 signaling and cap-dependent mRNA translation. (A) Matrix adhesion promotes phosphorylation of 4EBP1 without causing significant activation of AKT. MEFs were detached and kept in suspension (S) or plated onto fibronectin in the presence of platelet-derived growth factor (PDGF; 10 ng/ml), bFGF (20 ng/ml) along with 1 μg/ml heparin, insulin (10 ng/ml), EGF (10 ng/ml), or fetal calf serum (10%) for 6 h. Equal amounts of total proteins or proteins binding to m7GTP-Sepharose were subjected to immunoblotting with antibodies to the indicated antigens. −, control with no serum or growth factor; P-AKT (S473), AKT phosphorylated at S473. (B) Integrin-specific signaling sustains the activation of mTORC1 during the G1 phase. HUVECs were plated onto fibronectin (Fn) or laminin 1 (Lm) in the presence mitogens (20 ng/ml bFGF, 1 μg/ml heparin, 10 ng/ml insulin, 10 μg/ml transferrin, and 10 ng/ml EGF) for the indicated times. Equal amounts of total proteins or proteins binding to m7GTP-Sepharose were subjected to immunoblotting with antibodies to the indicated antigens. 15′, 15 min; P-4EBP1 (S65), 4EBP1 phosphorylated at S65. (C) HUVECs were transfected with the bicistronic reporter construct depicted above the graph, deprived of serum, and plated onto either fibronectin or laminin 1 in the absence (−) or presence (+) of mitogens for 24 h. The graph shows the mean ratios ± standard deviations (SD) between Renilla luciferase (rLuc) and firefly luciferase (fLuc) bioluminescence levels in the indicated samples. SV40, simian virus 40; IRES, internal ribosome entry site.
To examine the role of integrin-specific signaling in the activation of mTORC1 and phosphorylation of 4EBP1, primary HUVECs were synchronized in G0 and plated onto either fibronectin or laminin 1 in the presence of mitogenic concentrations of growth factors for various amounts of time. In HUVECs, integrin α5β1-mediated adhesion to fibronectin promotes progression through the G1 phase in response to growth factor stimulation whereas α2β1-mediated adhesion to laminin 1 does not, and this effect has been linked to the ability of the α5β1 integrin to promote translation of the mRNA encoding cyclin D1 (31, 64, 65). As shown in Fig. 1B, adhesion to fibronectin was sufficient to promote phosphorylation of 4EBP1, as detected by both a mobility shift assay and immunoblotting with anti-phosphorylated 4EBP1 (S65), and dissociation of 4EBP1 from cap-bound eIF4E, as measured by a cap-binding assay. In contrast, adhesion to laminin 1 did not exert this effect. Growth factor stimulation caused a similarly large, transient increase in activation of AKT in cells plated onto fibronectin or laminin 1, and at 15 min, when AKT activation was at its peak, 4EBP1 was highly phosphorylated and almost completely dissociated from the cap in cells on both fibronectin and laminin 1 (Fig. 1B). However, after 1 h of mitogenic stimulation, AKT activation in cells on both substrates had declined substantially. During the remaining portion of the G1 phase, adhesion to fibronectin enabled a higher level of phosphorylation of 4EBP1 and a more profound dissociation of 4EBP1 from the cap than adhesion to laminin 1 (Fig. 1B). No significant differences in the total levels of eIF4E and eIF4G between cells plated onto fibronectin and those on laminin 1 were observed (data not shown). Taken together, these observations suggest that α5β1-mediated adhesion sustains the activation of mTORC1 and phosphorylation of 4EBP1 independently of AKT during the G1 phase and that α2β1-mediated adhesion is unable to exert this effect.
To estimate the effect of integrin signaling on cap-dependent translation, HUVECs were transfected with a previously described dual-luciferase reporter system (24). In this system, the Renilla luciferase gene is translated in a cap-dependent fashion whereas the firefly luciferase gene is translated via a viral internal ribosome entry site independently of initiation factors. As shown in Fig. 1C, HUVECs plated onto fibronectin in the absence of mitogens displayed an approximately twofold-higher level of cap-dependent translation activity than those plated onto laminin 1 under the same conditions. Concurrent growth factor stimulation increased cap-dependent translation to a modest extent in cells on fibronectin but not in those on laminin 1 (Fig. 1C). These results suggest that integrin-mediated signaling is necessary and sufficient to sustain cap-dependent translation during the G1 phase of the cell cycle.
Integrin-specific signaling controls the translation of several mRNAs and protein synthesis.
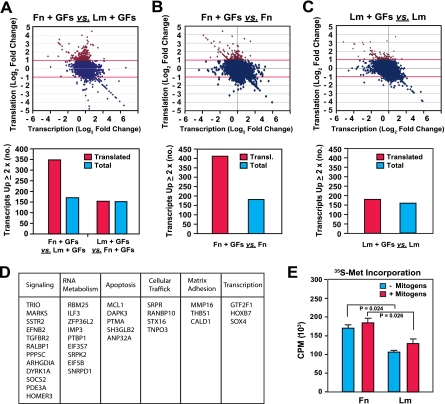
To estimate the impact of integrin signaling on mRNA translation, we examined the translational profile of HUVECs plated onto fibronectin or laminin 1 in the presence or absence of mitogens. Total poly(A)+ mRNAs and polysome-bound mRNAs were isolated from cells under these conditions and subjected to DNA microarray analysis in parallel to determine their transcriptomic and translational profiles, respectively (see Fig. S1A in the supplemental material). Adhesion to fibronectin in the presence of growth factors resulted in a significant elevation of mRNA translation compared to adhesion to laminin 1 under the same conditions (Fig. 2A). In fact, approximately twice as many genes were upregulated by twofold or more at the translational level in cells plated onto fibronectin in the presence of mitogens as in those plated onto laminin 1 under the same conditions. In contrast, about the same numbers of genes were upregulated by twofold or more at the transcriptional level in cells plated onto fibronectin in the presence of mitogens and in those plated onto laminin 1 under the same conditions (Fig. 2A). Furthermore, growth factor stimulation enhanced mRNA translation in cells plated onto fibronectin by approximately twofold compared to that in cells plated onto laminin 1 (Fig. 2B and C). In contrast, exposure to mitogens induced the transcription of about the same numbers of genes in cells plated onto fibronectin and in those plated onto laminin 1 (Fig. 2B and C). The effect of adhesion to fibronectin on mRNA translation was highly significant, as assessed by statistical analysis (see Fig. S1B to D in the supplemental material). These results indicate that α5β1-mediated adhesion to fibronectin and ensuing mTORC1 signaling promote the translation of a large set of mRNAs during the G1 phase.
FIG. 2.
Integrin-specific signaling promotes the translation of a subset of cellular mRNAs. (A to C) Integrin-specific adhesion controls mRNA translation in cells exposed to growth factors. Total and polysome-bound mRNAs from HUVECs plated onto fibronectin (Fn) or laminin 1 (Lm) for 16 h in the absence or presence of growth factors (GFs) as described in the legend to Fig. 1B were hybridized to Affymetrix human genome U133A oligonucleotide microarray chips. Panel A shows the comparison between cells plated onto fibronectin and those plated onto laminin 1 in the presence of growth factors, panel B shows the comparison between cells plated onto fibronectin in the presence and absence of growth factors, and panel C shows the comparison between cells plated onto laminin 1 in the presence and absence of growth factors. (Top panels) The dot matrix graphs plot log2 changes in translation levels against the corresponding log2 changes in transcription levels for all genes represented on the array. Data for genes upregulated at the translational level by twofold or more are denoted in red. (Bottom panels) The bar graphs illustrate the total numbers of genes regulated at the translational (transl.) and transcriptional levels in the indicated comparisons. (D) The products of genes that were upregulated more than fourfold at the translational level in cells on fibronectin in the presence of growth factors compared to those in cells on laminin 1 under the same conditions were assigned to functional categories. (E) Integrin-specific signaling promotes protein biosynthesis. HUVECs were synchronized in G0, detached, and plated onto fibronectin or laminin for 6 h in SFM supplemented (+) or not (−) with growth factors and for 1 h in methionine-cysteine-free medium supplemented or not with growth factors (Gibco BRL). They were then labeled for 1 h with 200 μCi/ml [35S]methionine-cysteine (Tran35S-Label; ICN). The graph shows the mean levels ± SD of radioactivity present in identical aliquots of total lysates from the indicated samples.
To obtain insight into the physiological significance of integrin-specific activation of mTORC1 signaling and cap-dependent translation, the genes upregulated by fourfold or more at the translational level in cells plated onto fibronectin in the presence of mitogens compared to those in cells plated onto laminin 1 under the same conditions were assigned to functional categories. As shown in Fig. 2D, adhesion to fibronectin promoted translation of mRNAs encoding proteins involved in cell signaling, RNA metabolism, and apoptosis and, to a lower degree, translation of mRNAs encoding proteins involved in cellular trafficking, matrix adhesion and remodeling, and transcription. Of note, multiple mRNA-binding proteins and components of initiation factors were found to be under translational control, suggesting a potential mechanism for the reinforcement of this process.
To assess the impact of integrin signaling on total protein biosynthesis, we measured the incorporation of [35S]methionine into total proteins in HUVECs plated onto fibronectin or laminin 1 in the presence or absence of mitogens. As shown in Fig. 2E, adhesion to fibronectin in the absence of mitogens enabled a significantly higher level of protein biosynthesis than adhesion to laminin 1. Concurrent growth factor stimulation increased protein biosynthesis to a modest extent in both cells on fibronectin and cells on laminin 1 (Fig. 2E). These observations suggest that integrin-mediated adhesion and signaling are major determinants of cap-dependent translation and, thereby, protein biosynthesis.
Integrin-specific adhesion controls mTORC1 signaling through inactivation of merlin.
Mitogenic stimuli control mTORC1 activation through AKT and possibly ERK (15). However, growth factor stimulation caused similar levels of activation of AKT and ERK in HUVECs plated onto fibronectin and those plated onto laminin 1 (Fig. 1B) (31), suggesting that integrin-specific signaling controls the activation of mTORC1 through a novel mechanism. Since we had shown previously that α5β1-dependent adhesion to fibronectin is necessary for activation of Rac and translation of cyclin D1 mRNA in HUVECs (31), we examined the potential involvement of Rac signaling in the activation of mTORC1.
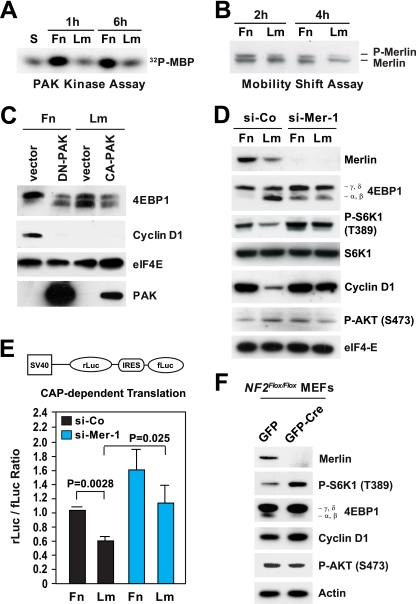
We specifically focused on the Rac target-effector PAK, because it can promote cell cycle progression through phosphorylation and, thereby, inactivation of merlin (21, 38, 67) and/or activation of Jun N-terminal protein kinase (JNK) (41). PAK assays indicated that adhesion to fibronectin enables significant activation of PAK in HUVECs plated onto fibronectin in the presence of growth factors but not in those plated onto laminin 1 under the same conditions (Fig. 3A). Since RTK activation does not contribute to a significant extent to the activation of Rac and PAK in HUVECs (31, 38), we infer that α5β1-mediated adhesion promotes the activation of PAK and that α2β1-mediated adhesion does not. In agreement with this hypothesis, the results of electrophoresis mobility shift assays indicated that merlin becomes phosphorylated to a greater extent in cells plated onto fibronectin in the presence of mitogens than in those plated onto laminin 1 under the same conditions (Fig. 3B). Furthermore, glutathione S-transferase-Jun kinase assays indicated that JNK is activated transiently but to significant levels in HUVECs plated onto fibronectin in the presence of growth factors but not in those plated onto laminin 1 under the same conditions (see Fig. S2A in the supplemental material). In agreement with the recent finding that JNK is activated also during late G2 and promotes entry into mitosis (40), JNK was reactivated at 20 h after release from G0 in cells plated onto fibronectin. This reactivation was not observed in HUVECs plated onto laminin 1, consistent with the observation that these cells do not progress through the cell cycle and enter into mitosis on laminin 1 (31).
FIG. 3.
Inactivation of merlin mediates integrin-dependent mTORC1 signaling. (A) Integrin-specific adhesion controls activation of PAK. HUVECs synchronized in G0 were detached and kept in suspension (S) or plated onto fibronectin (Fn) or laminin 1 (Lm) in the presence of mitogens for the indicated times. Equal amounts of total proteins were subjected to immunoprecipitation with antibodies to PAK, followed by an in vitro kinase assay using 32P-labeled myelin basic protein (32P-MBP) as a substrate. (B) Integrin-specific adhesion promotes phosphorylation of merlin. HUVECs were transfected with a plasmid encoding wild-type merlin. After synchronization in G0, the cells were plated onto fibronectin or laminin 1 in the presence of mitogens for the indicated times. Equal amounts of proteins were subjected to high-resolution SDS-PAGE, followed by immunoblotting with antimerlin. P-merlin, phosphorylated merlin. (C) PAK activity is necessary for phosphorylation of 4EBP1 and expression of cyclin D1 on fibronectin. HUVECs were transfected with an empty vector or vectors encoding dominant negative (DN) or constitutively active (CA) PAK. After synchronization in G0, the cells were plated onto fibronectin or laminin 1 in the presence of mitogens for 24 h and lysed. Equal amounts of total proteins were subjected to immunoblotting with antibodies to the indicated antigens. (D) Depletion of merlin rescues phosphorylation of 4EBP1 and expression of cyclin D1 on laminin 1. HUVECs were transfected with an siRNA oligonucleotide targeting human merlin (si-Mer-1) or control siRNA (si-Co), synchronized in G0, detached, and plated onto fibronectin or laminin 1 in the presence of mitogens for 24 h. Total lysates were analyzed by immunoblotting using antibodies to the indicated antigens. P-S6K1 (T389), S6K1 phosphorylated at T389; P-AKT (S473), AKT phosphorylated at S473. (E) Depletion of merlin promotes cap-dependent translation. HUVECs were transfected with the bicistronic reporter construct depicted above the graph, in combination with an siRNA oligonucleotide targeting human merlin or control siRNA. Cells were synchronized in G0 and plated onto fibronectin or laminin 1 in the presence of growth factors for 24 h. The graph shows the mean ratios ± SD between the Renilla luciferase (rLuc) and firefly luciferase (fLuc) bioluminescence levels in the indicated samples. SV40, simian virus 40; IRES, internal ribosome entry site. (F) Genetic inactivation of NF2 promotes mTORC1 signaling without activating AKT in primary fibroblasts. NF2flox/flox MEFs were infected with adenoviruses encoding green fluorescent protein-Cre (GFP-Cre) or GFP alone. Two days later, cells were synchronized in G0, plated onto fibronectin for 20 h in the presence of 20 ng/ml bFGF-1 μg/ml heparin, and subjected to immunoblotting using antibodies to the indicated antigens.
Since there are multiple PAK proteins, we used a dominant negative approach to examine the role of PAK in mTORC1 signaling. As shown in Fig. 3C, overexpression of a kinase-dead form of PAK suppressed phosphorylation of 4EBP1 and expression of cyclin D1 in HUVECs plated onto fibronectin in the presence of growth factors. However, moderate expression of a constitutively active form of PAK did not restore phosphorylation of 4EBP1 and expression of cyclin D1 in cells plated onto laminin 1. These results suggest that PAK is required but not sufficient for integrin-mediated activation of mTORC1 signaling.
To examine the hypothesis that PAK controls mTORC1 signaling by phosphorylating and thereby inactivating merlin, we used the S518D phosphorylation site mimetic mutant form of merlin, which is stabilized in the open conformation and capable of exerting a dominant negative effect, and the unphosphorylatable S518A mutant form of merlin, which is stabilized in the closed conformation and thought to be constitutively active (4). As shown in Fig. S3 in the supplemental material, overexpression of S518D mutant merlin rescued activation of S6K1 and expression of cyclin D1 in HUVECs plated onto laminin 1 in the presence of mitogens. This effect occurred in the absence of detectable activation of AKT. Conversely, overexpression of S518A mutant merlin suppressed the activation of S6K1 in HUVECs plated onto fibronectin under the same conditions (see Fig. S3 in the supplemental material). These results implicate the inactivation of merlin in mTORC1 signaling. Although we have not directly examined this hypothesis, it is possible that constitutively active PAK does not rescue phosphorylation of 4EBP1 and expression of cyclin D1 in cells adhering to laminin 1 because expression of this construct does not cause full inactivation of merlin.
To directly test the hypothesis that integrin-specific adhesion controls mTORC1 signaling through inactivation of merlin, we silenced merlin in HUVECs plated onto fibronectin or laminin 1 in the presence of mitogens. As shown in Fig. 3D, depletion of merlin fully rescued phosphorylation of 4EBP1, activation of S6K1, and expression of cyclin D1 in cells plated onto laminin 1. This effect occurred without the apparent involvement of AKT (Fig. 3D), and it was specific, as it was observed with two distinct siRNAs targeting merlin (see Fig. S4 in the supplemental material). In addition, the results of dual-luciferase reporter assays showed that depletion of merlin with two distinct siRNAs rescues cap-dependent translation on laminin 1 (Fig. 3E; see also Fig. S5 in the supplemental material). Finally, silencing of merlin also led to the downregulation of the cyclin-dependent kinase (CDK) inhibitor p27 in HUVECs plated onto laminin 1 (see Fig. S4 in the supplemental material), suggesting that inactivation of merlin is sufficient to promote progression through G1 in cells plated onto an otherwise nonpermissive matrix substrate. To examine if JNK also affects mTORC1 signaling, we silenced JNK1, JNK2, or both by using siRNAs (see Fig. S2B in the supplemental material). However, depletion of JNK1, JNK2, or both did not inhibit the phosphorylation of 4EBP1 in HUVECs plated onto fibronectin in the presence of growth factors (see Fig. S2B in the supplemental material), suggesting that PAK does not control mTORC1 through JNK.
To examine if loss of merlin controls mTORC1 signaling in other cell types, we infected primary NF2flox/flox MEFs with an adenovirus encoding Cre or with a control virus. As shown in Fig. 3E, Cre-mediated deletion of NF2 and loss of expression of merlin enhanced phosphorylation of 4EBP1 and activation of S6K1 in MEFs without affecting the activation of AKT (Fig. 3F). Taken together, the results of siRNA-mediated depletion and genetic inhibition suggest that integrin-specific adhesion promotes mTORC1 signaling and cap-dependent translation through inactivation of merlin.
Integrin-specific activation of mTORC1 promotes cell cycle progression.
HUVECs synchronized in G0 and plated onto laminin 1 fail to translate the mRNA encoding cyclin D1 and hence to assemble active cyclin D1-CDK4-CDK6 complexes in response to growth factor stimulation (31). Since depletion of merlin activates mTORC1 signaling and at the same time induces expression of cyclin D1 and downregulation of p27 in these cells (Fig. 3D; see also Fig. S4 in the supplemental material), we examined the hypothesis that integrin signaling controls cell cycle progression at least in part through inactivation of merlin and activation of mTORC1 signaling.
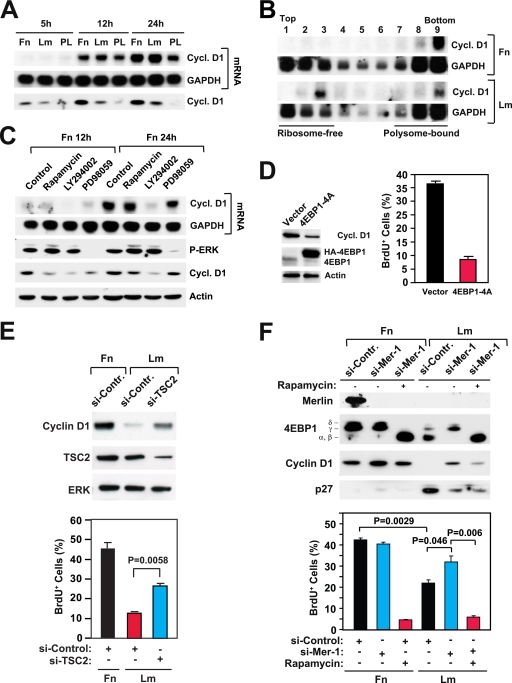
To examine the role of integrin-specific mTORC1 signaling in cell cycle progression, we examined if this signaling pathway controls the translation of cyclin D1 mRNA. In agreement with prior results (31), we found that growth factor stimulation induces similarly high levels of cyclin D1 mRNA in HUVECs plated onto fibronectin and in those plated onto laminin 1 (Fig. 4A). However, whereas cells plated onto fibronectin accumulate high levels of cyclin D1 protein, those plated onto laminin 1 express significantly lower levels of this protein (Fig. 4A). Cells plated onto the control substrate poly-l-lysine accumulated reduced levels of the cyclin D1 mRNA compared to cells plated onto laminin 1 or fibronectin and failed to express detectable levels of cyclin D1 protein (Fig. 4A). To determine if cells plated onto laminin 1 fail to accumulate cyclin D1 protein due to translational inhibition, we fractionated samples of total mRNA from cells plated onto fibronectin or laminin 1 by sucrose density gradient centrifugation and subjected the resulting fractions to Northern blotting. The results indicated that the cyclin D1 mRNA is almost entirely associated with polysomes in cells plated onto fibronectin but is equally distributed in the ribosome-free and the polysome fractions in cells plated onto laminin 1 (Fig. 4B). In contrast, the mRNA encoding GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was similarly associated with the ribosome-free and polysome fractions in cells plated onto each of the two substrates. These results confirm that integrin-specific signaling controls the translation of cyclin D1 mRNA in HUVECs.
FIG. 4.
Integrin-specific mTORC1 signaling controls the translation of cyclin D1 mRNA and promotes cell cycle progression. (A) Integrin-specific signaling is not required for the transcription of cyclin D1. HUVECs were plated onto fibronectin (Fn), laminin 1 (Lm), or the control substrate poly-l-lysine (PL) in the presence of mitogens. Northern blotting was used to examine the levels of mRNAs encoding cyclin D1 (cycl. D1) and GAPDH at the indicated time points. (B) Integrin-specific signaling promotes association of the mRNA encoding cyclin D1 with polysomes. Samples of total mRNA from HUVECs plated onto fibronectin or laminin 1 for 16 h in the presence of growth factors (20 ng/ml bFGF, 1 μg/ml heparin, 10 ng/ml insulin, 10 μg/ml transferrin, and 10 ng/ml EGF) were fractionated on sucrose density gradients. Northern blotting was used to examine the levels of mRNAs encoding cyclin D1 and GAPDH in each gradient fraction. (C) Rapamycin inhibits translation of the mRNA encoding cyclin D1. HUVECs synchronized in G0 were detached and plated onto fibronectin in the presence of growth factors without or with 5 nM rapamycin, 20 μM LY294002, or 40 μM PD98059. At the indicated time points, cells were lysed and subjected to either Northern analysis with the indicated probes (upper two panels) or immunoblotting with antibodies to the indicated antigens (lower two panels). P-ERK, phosphorylated ERK. (D) Disruption of cap-dependent translation inhibits induction of cyclin D1 and cell cycle progression. HUVECs were transfected with an empty vector or a vector encoding a hemagglutinin (HA)-tagged form of the phosphorylation-defective mutant 4EBP1-4A. After 48 h, cells were synchronized in G0, detached, and plated onto fibronectin in the presence of mitogens and BrdU for 24 h. (Left) Total cell lysates were subjected to immunoblotting with antibodies to the indicated antigens. (Right) The graph shows the percentages of cells entering S phase under the indicated conditions. The experiment was done three times. (E) Depletion of TSC2 partially rescues expression of cyclin D1 and progression through the cell cycle on laminin 1. HUVECs were transfected with the indicated siRNAs, synchronized in G0, and plated onto fibronectin or laminin 1 for 24 h. (Top) Total lysates were subjected to immunoblotting using antibodies to the indicated antigens. (Bottom) Cells were subjected to BrdU incorporation and anti-BrdU staining. The graph illustrates the mean percentages ± SD of BrdU-positive (BrdU+) cells. si-Contr., control siRNA; si-TSC2, siRNA targeting TSC2; +, present; −, absent. (F, top) Depletion of merlin partially rescues expression of cyclin D1 on laminin 1 through a rapamycin-sensitive pathway. HUVECs were transfected with the indicated siRNAs, synchronized in G0, and then plated onto fibronectin or laminin 1 in the presence of growth factors for 24 h without (−) or with (+) 5 nM rapamycin. Equal amounts of total proteins were subjected to immunoblotting with antibodies to the indicated antigens. (Bottom) Depletion of merlin partially rescues progression through G1 on laminin 1 through a rapamycin-sensitive pathway. HUVECs were transfected with a control siRNA (si-Contr., or si-Control) or an siRNA targeting merlin (si-Mer-1), synchronized in G0, and plated onto fibronectin or laminin 1 in the presence of growth factors and BrdU for 24 h without or with 5 nM rapamycin. The graph shows the mean percentages ± SD of BrdU+ cells under the indicated conditions.
To examine if integrin-mediated adhesion controls expression of cyclin D1 through activation of mTORC1, we tested if the mTOR inhibitor rapamycin inhibits the accumulation of cyclin D1 in HUVECs plated onto fibronectin. As shown in Fig. 4C, treatment with rapamycin inhibited the accumulation of cyclin D1 in cells plated onto fibronectin. This inhibition was particularly evident after 12 h of growth factor stimulation but still detectable after 24 h. Interestingly, cells plated onto fibronectin in the presence of rapamycin exhibited levels of cyclin D1 similar to those of cells plated onto laminin 1 at both 12 and 24 h of growth factor stimulation (compare Fig. 4A and C), suggesting that the inhibition of mTOR generates a phenocopy of adhesion to laminin 1. As anticipated, rapamycin did not affect the expression of the mRNA encoding cyclin D1 in cells plated onto fibronectin (Fig. 4C). However, treatment with the PI-3K inhibitor LY294002 completely suppressed the expression of cyclin D1 mRNA in these cells, suggesting that PI-3K signaling is necessary for transcription of cyclin D1 in HUVECs. The MEK inhibitor PD98059 also inhibited transcription of cyclin D1 but to a lower degree (Fig. 4C). These results suggest that mTORC1 controls expression of cyclin D1 by a posttranscriptional mechanism.
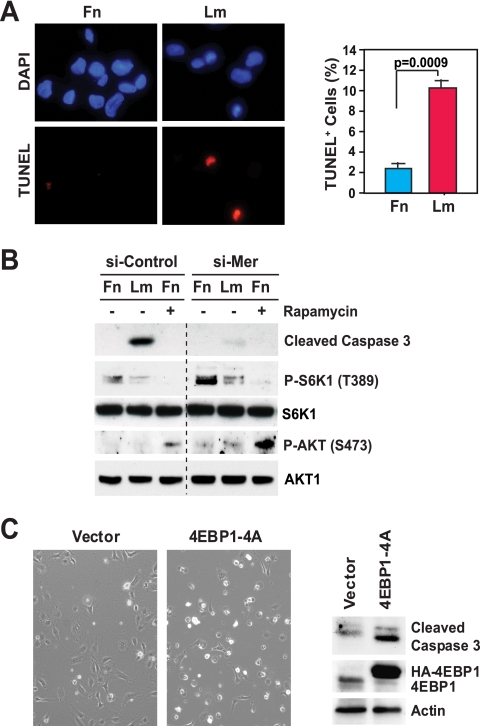
mTORC1-mediated phosphorylation of 4EBP1 causes its dissociation from eIF4E, enabling the assembly of the initiation complex. To further examine the role of mTORC1 in the control of translation of cyclin D1 mRNA, we transduced HUVECs with a mutant form of 4EBP1 carrying alanine substitutions at its major phosphorylation sites (4EBP1-4A). Prior studies have indicated that this mutant form of 4EBP1 binds constitutively to the cap and thereby impairs proper assembly of the eIF4F complex (11). As shown in Fig. 4D, ectopic expression of this 4EBP1 mutant inhibited expression of cyclin D1, progression through G1, and entry into S phase in HUVECs plated onto fibronectin. These results suggest that mTORC1 signaling controls the expression of cyclin D1 at least in part by inhibiting 4EBP1 and thereby promoting cap-dependent translation.
To further examine the hypothesis that HUVECs do not progress through the G1 phase and enter into S phase on laminin 1 because of inefficient activation of mTORC1, we silenced TSC2 in HUVECs and plated them onto laminin 1 in the presence of mitogens. As shown in Fig. 4E, depletion of TSC2 rescued expression of cyclin D1 and entry into the S phase in cells plated onto laminin 1 to a partial, but significant, extent. This result indicates that inefficient mTORC1 signaling contributes to the inhibition of cell cycle progression in HUVECs adhering to laminin 1 and implies that merlin functions upstream of TSC1-TSC2 to inhibit mTORC1 signaling.
We next examined if inactivation of merlin promotes expression of cyclin D1 and cell cycle progression through activation of mTORC1. Control HUVECs and HUVECs in which merlin was silenced were plated onto fibronectin or laminin 1 either in the absence or in the presence of rapamycin. As shown in Fig. 4F, knockdown of merlin rescued phosphorylation of 4EBP1 completely and expression of cyclin D1 to a partial but significant extent in cells on laminin 1. Interestingly, treatment with rapamycin suppressed both events. Knockdown of merlin also caused significant downregulation of the CDK inhibitor p27 in cells plated onto laminin 1, but inhibition of mTOR did not reverse this effect, suggesting that inactivation of merlin downregulates p27 independently of mTORC1. These results suggest that loss of merlin promotes expression of cyclin D1 through activation of mTORC1.
To examine the role of integrin-specific mTORC1 signaling in cell proliferation, control HUVECs and those in which merlin was silenced were synchronized in G0, plated onto fibronectin or laminin 1 with mitogens in the absence or presence of rapamycin, and subjected to a bromodeoxyuridine (BrdU) incorporation assay. Whereas rapamycin suppressed entry into S phase in cells plated onto fibronectin, knockdown of merlin partially restored progression through G1 and entry into S phase in cells plated onto laminin 1 (Fig. 4F). Interestingly, rapamycin blocked this restoration. Together, these results provide evidence that integrin-specific adhesion controls mTORC1 signaling, translation of cyclin D1 mRNA, and cell cycle progression through inactivation of merlin.
Integrin-specific activation of mTORC1 promotes cell survival.
To examine the potential role of integrin-specific mTORC1 signaling in suppression of apoptosis and cell survival, we measured the percentage of HUVECs undergoing apoptosis at 24 h after growth factor stimulation on fibronectin or laminin 1. The results of a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay indicated that virtually all cells plated onto fibronectin in the presence of growth factors remained viable at 24 h but that about 10% of those plated onto laminin 1 under the same conditions were apoptotic at this time point (Fig. 5A). This finding probably underestimates the extent of cell death occurring on laminin 1, as we consistently recovered fewer cells from this substrate at 24 h, in agreement with the hypothesis that many cells had died earlier and detached.
FIG. 5.
Integrin-specific mTORC1 signaling promotes cell survival. (A) Integrin-specific adhesion promotes cell survival. HUVECs were synchronized in G0, plated onto fibronectin or laminin 1 in the presence of growth factors for 48 h, and subjected to a TUNEL assay and counterstaining with DAPI (4′,6-diamidino-2-phenylindole). (Left) The pictures show representative fields of cells on fibronectin (Fn) or laminin 1 (Lm). (Right) The graph shows the mean percentages ± SD of TUNEL-positive cells under the indicated conditions. The experiment was done two times. (B) Knockdown of merlin protects cells plated onto laminin 1 from apoptosis. HUVECs were transfected with the indicated siRNAs, synchronized in G0, detached, and plated in the presence of mitogens for 48 h without (−) or with (+) 5 nM rapamycin. Equal amounts of total proteins were subjected to immunoblotting with antibodies to the indicated antigens. si-Control, control siRNA; si-Mer, siRNA targeting Mer; P-S6K1 (T389), S6K1 phosphorylated at T389; P-AKT (S473), AKT phosphorylated at S473. (C) Disruption of cap-dependent translation causes apoptosis in cells plated onto fibronectin. HUVECs were transfected with an empty vector or a vector encoding a hemagglutinin (HA)-tagged form of the phosphorylation-defective mutant 4EBP1-4A. After 48 h, cells were synchronized in G0, detached, and plated onto fibronectin in the presence of mitogens for 24 h. (Left) The pictures show phase-contrast images of representative fields of cells on fibronectin or laminin 1. (Right) Equal amounts of total proteins were subjected to immunoblotting with antibodies to the indicated antigens.
To investigate if integrin-specific signaling controls cell survival through inactivation of merlin, we silenced merlin in HUVECs plated onto either fibronectin or laminin 1. As anticipated, immunoblotting with antibodies to cleaved caspase 3 confirmed that cells plated onto fibronectin in the presence of growth factors do not undergo apoptosis and that a significant fraction of those plated onto laminin 1 under the same conditions undergo this process (Fig. 5B). Depletion of merlin rescued cells plated onto laminin 1 from apoptosis, indicating that inactivation of merlin promotes cell survival. However, although treatment with rapamycin suppressed mTORC1 signaling, as measured by immunoblotting with antibodies to phosphorylated S6K1, it did not induce death among HUVECs plated onto fibronectin (Fig. 5B). Since mTORC1 signaling is constrained by a negative feedback loop, whereby activated mTORC1 suppresses AKT (15), we reasoned that rapamycin might have induced the activation of AKT in cells plated onto fibronectin, thereby protecting these cells from apoptosis. Indeed, immunoblotting revealed that rapamycin induced activation of AKT in cells plated onto fibronectin (Fig. 5B). Interestingly, inhibition of mTORC1 caused stronger activation of AKT in cells in which merlin was silenced than in control cells plated onto fibronectin, perhaps because constitutive activation of mTORC1 signaling establishes a stronger negative feedback loop in cells in which merlin is silenced.
In order to avoid potential interpretation problems caused by the negative feedback loop, we decided to test if inhibition of cap-dependent translation induces apoptosis in HUVECs plated onto fibronectin. As shown in Fig. 5C, phase-contrast microscopy and immunoblotting with antibodies to cleaved caspase 3 indicated that overexpression of the phosphorylation-resistant version of 4EBP1 induces a significant degree of apoptosis among HUVECs plated onto fibronectin in the presence of growth factors. Taken together, these results are consistent with the hypothesis that the inactivation of merlin protects HUVECs from apoptosis at least in part through activation of mTORC1 and cap-dependent translation.
Loss of merlin function activates mTORC1 signaling in malignant mesothelioma.
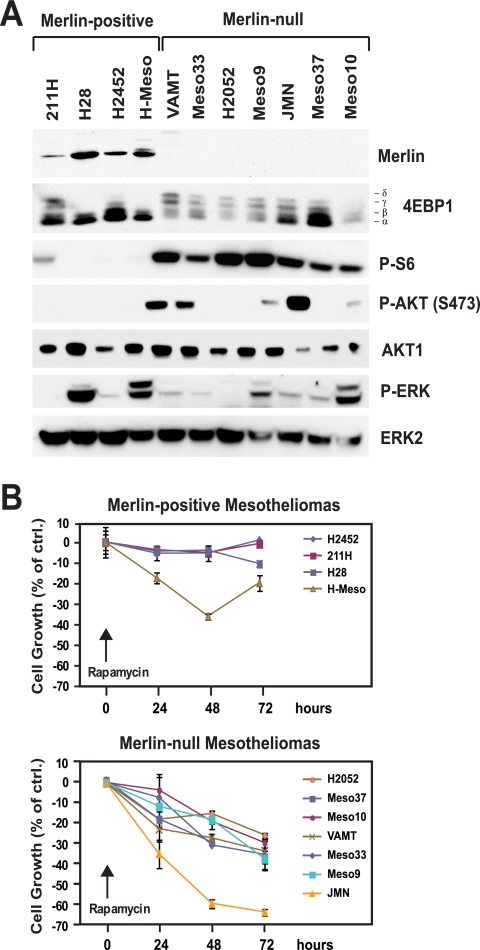
The observation that integrin-specific signaling controls cell cycle progression and cell survival through inactivation of merlin and, thereby, induction of mTORC1 signaling suggests that this signaling pathway may be constitutively active in tumor cells carrying loss-of-function mutations in NF2. To examine this hypothesis, we analyzed a panel of human malignant mesothelioma cell lines. The large majority of NF2 mutations that have been identified in malignant mesothelioma are predicted to lead to complete loss of expression of merlin (3, 52). We therefore used immunoblotting to classify the cell lines as merlin positive or merlin null. Of 11 cell lines examined, 4 were found to express an apparently wild-type form of merlin and 7 were found to lack merlin (Fig. 6A). This ratio is in general agreement with the reported mutational rate of NF2 in primary malignant mesothelioma (3, 52). To monitor the activation of mTORC1, ERK, and AKT, the samples were subjected to immunoblotting. Interestingly, all the merlin null cell lines were found to exhibit phosphorylated 4EBP1, as judged by mobility shift assays, and phosphorylated S6, as measured by immunoblotting with anti-phosphorylated S6, whereas all the merlin-positive cell lines did not. In contrast, although a subset of cell lines exhibited elevated levels of phosphorylated ERK or phosphorylated AKT, the activation of ERK or AKT did not correlate with the loss of merlin or the activation of mTORC1. In agreement with the hypothesis that inactivation of merlin promotes mTORC1 signaling independently of AKT or ERK in cells of mesothelial origin, depletion of merlin caused activation of S6K1 but not AKT or ERK in LP9 normal mesothelial cells (see Fig. S6 in the supplemental material; also data not shown). Together, these observations provide evidence that the loss of merlin causes activation of mTORC1 signaling in malignant mesothelioma.
FIG. 6.
Loss of merlin correlates with activation of mTORC1 signaling and sensitivity to rapamycin in malignant mesothelioma. (A) Lack of expression of merlin correlates with activation of mTORC1 but not AKT or ERK. The indicated mesothelioma cell lines were cultured in complete medium, and equal amounts of total proteins were subjected to immunoblotting using antibodies to the indicated antigens. P-S6, phosphorylated S6; P-AKT (S473), AKT phosphorylated at S473; P-ERK, phosphorylated ERK. (B) Lack of expression of merlin correlates with sensitivity to rapamycin. The indicated mesothelioma cell lines were plated at subconfluent densities into complete medium and treated with 1 nM rapamycin or vehicle alone for the indicated times. The number of viable cells at each time point was estimated by using the crystal violet assay. The graphs show the percentages of cells remaining viable in the presence of rapamycin relative to the number of control cells (ctrl.) cultured in the absence of the drug. The results at 72 h were subjected to Student's t test to assess the statistical significance of the differential sensitivities of merlin-negative mesothelioma cell lines to the growth-inhibitory effect of rapamycin (P = 0.0018). The average doubling times of merlin-positive and of merlin null mesothelioma lines were not dissimilar (merlin positive, 48.5 h; merlin null, 44.1 h).
Malignant mesothelioma cells lacking merlin are sensitive to rapamycin.
The observation that cancer cells are addicted to oncogene signaling suggests that drug sensitivity can be used to identify the signaling pathways that maintain the oncogenicity of specific tumor types (53). We reasoned that, if mTORC1 signaling is critical for NF2-dependent tumorigenesis, the merlin-deficient malignant mesothelioma cells should be selectively sensitive to the growth-inhibitory effect of rapamycin. To test this hypothesis, we monitored the abilities of merlin-positive and merlin null malignant mesothelioma cells to grow in the presence of rapamycin. As shown in Fig. 6B, whereas mesothelioma cell lines expressing merlin showed modest sensitivities to rapamycin (7.6% ± 9.6% growth inhibition at 72 h), those lacking merlin were more sensitive to the growth-inhibitory effect of the drug (36.4% ± 12.7% growth inhibition at 72 h; P = 0.0018). The selective sensitivity of merlin null lines to rapamycin was not a consequence of accelerated cell cycle progression because the average doubling time of these lines in the absence of the drug (44.1 h) was not significantly shorter than that of merlin-positive lines under the same conditions (48.5 h). These results indicate that malignant mesothelioma cells carrying NF2 mutations are selectively sensitive to drugs targeting mTORC1 and suggest that the mTORC1 signaling pathway is critical for malignant mesothelioma.
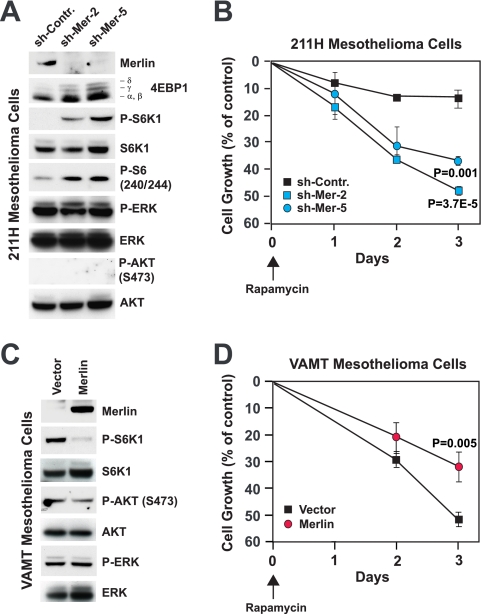
To obtain further evidence that loss of merlin function causes activation of mTORC1 signaling and underlies sensitivity to rapamycin, we infected merlin-positive 211H mesothelioma cells with lentiviral vectors encoding shRNAs targeting merlin. As shown in Fig. 7A, stable knockdown of merlin induced phosphorylation of 4EBP1, S6K1, and S6, indicating that loss of merlin causes the activation of mTORC1 in malignant mesothelioma cells. Notably, depletion of merlin did not increase activation of ERK or AKT in these cells, in agreement with the hypothesis that loss of merlin activates mTORC1 independently of AKT or ERK. To determine if loss of merlin renders mesothelioma cells sensitive to rapamycin, we examined the rapamycin sensitivities of 211H control cells and 211H cells in which merlin was silenced. As shown in Fig. 7B, rapamycin affected the growth of control cells only modestly but inhibited that of the cells in which merlin was silenced to a significant extent. As a complementary approach, we expressed merlin in merlin-negative VAMT malignant mesothelioma cells. As anticipated, expression of merlin inhibited activation of S6K1 without perturbing activation of AKT or ERK in these cells (Fig. 7C). In addition, expression of merlin rendered VAMT cells less sensitive to rapamycin (Fig. 7D). Collectively, these results indicate that loss of merlin sensitizes malignant mesothelioma cells to the growth-inhibitory effect of rapamycin.
FIG. 7.
Loss of merlin causes activation of mTORC1 and induces sensitivity to rapamycin in malignant mesothelioma. (A) Depletion of merlin causes the activation of mTORC1 in 211H cells. Cells were infected with lentiviruses encoding the indicated shRNAs, synchronized in G0, and plated into complete medium for 24 h. Equal amounts of total proteins were subjected to immunoblotting with antibodies to the indicated antigens. sh-Contr., control shRNA; sh-Mer-2 and sh-Mer-5, shRNAs targeting merlin; P-S6K1, phosphorylated S6K1; P-S6 (240/244), S6 phosphorylated at positions 240 and 244; P-ERK, phosphorylated ERK; P-AKT (S473), AKT phosphorylated at S473. (B) Depletion of merlin induces sensitivity to rapamycin in 211H cells. Cells were plated at subconfluent densities into complete medium and treated with 1 nM rapamycin or vehicle alone for the indicated times. The number of viable cells at each time point was estimated by using the crystal violet assay. The graph shows the percentages of cells remaining viable in the presence of rapamycin relative to the population of viable control cells cultured in the absence of the drug. (C) Expression of merlin suppresses mTORC1 in VAMT cells. Cells were infected with a retrovirus encoding merlin or with an empty vector, synchronized in G0, and plated into complete medium for 24 h. Equal amounts of total proteins were subjected to immunoblotting with antibodies to the indicated antigens. (D) Expression of merlin reduces sensitivity to rapamycin in VAMT cells. Cells were plated at subconfluent densities into complete medium and treated with 1 nM rapamycin or vehicle alone for the indicated times. The number of viable cells at each time point was estimated by using the crystal violet assay. The graph shows the percentages of cells remaining viable in the presence of rapamycin relative to the population of control cells cultured in the absence of the drug. Doubling times were as follows: H2452 cells, 72 h; 211H cells, 40 h; H28 cells, 55 h; H-Meso cells, 27 h; H2052 cells, 32 h; Meso-37 cells, 29 h; Meso-10 cells, 72 h; VAMT cells, 36 h; Meso-33 cells, 48 h; Meso-9 cells, 77 h; and JMN cells, 15 h.
DISCUSSION
Our study provides evidence that integrin-specific adhesion promotes mTORC1 signaling through inactivation of the tumor suppressor merlin. In addition, we link the loss of merlin to the activation of mTORC1 and sensitivity to rapamycin in malignant mesothelioma. These findings reveal that merlin is a negative regulator of mTORC1 signaling and suggest that loss-of-function mutations in NF2 contribute to tumor initiation and maintenance through activation of mTORC1.
Although it has long been known that matrix adhesion is necessary for mRNA translation (1, 10), it has remained unclear whether integrin signaling exerts specific control on mRNA translation and, if so, through which mechanism and with what consequences. Our results indicate that the α5β1 integrin promotes, through PAK, inactivation of merlin, thereby controlling mTORC1 signaling and cap-dependent mRNA translation, whereas the α2β1 integrin is unable to activate this signaling pathway. In addition, we have observed that integrin-specific signaling is required to sustain mRNA translation during the mid- to late G1 phase, when the effect of growth factor stimulation on the activation of AKT has subsided. If integrin-specific signaling is not activated during the mid- to late G1 phase, several mRNAs are not translated and total protein biosynthesis declines. Under these conditions, endothelial cells undergo cell cycle arrest and eventually apoptosis. Presumably, other cell types in which cyclin D is under translational control (17, 23, 35, 68) may undergo a similar demise if plated onto an inappropriate matrix. These results confirm and extend those of Maeshima and colleagues, who have found that the angiogenesis inhibitor tumstatin induces apoptosis in endothelial cells that are stably adherent to a complex matrix by interfering with the ability of the αvβ3 integrin to activate mTORC1 signaling (26). Together, the findings of these studies highlight the impact of integrin-specific signaling through mTORC1 on cell survival and proliferation.
It is known that RTK activation controls mTORC1 signaling through AKT- and possibly ERK-dependent phosphorylation of TSC1-TSC2 (15). In this report, we show that integrin-specific adhesion controls mTORC1 through PAK, which phosphorylates and inactivates merlin. In addition, depletion or ablation of merlin activates mTORC1 in several cell types, and this effect appears to occur independently of AKT or ERK. Since the biochemical function and direct target-effectors of merlin are not known, we have not investigated in this study the mechanism through which inactivation of merlin activates mTORC1. We have, instead, focused on the biological consequences of this new signaling connection.
Extracellular stimuli converge on cyclin D to control cell cycle progression (28). Our study suggests that, although joint integrin-RTK signaling controls the transcription of cyclin D (63), integrin-specific activation of mTORC1 is necessary for the translation of cyclin D mRNA and hence progression through the G1 phase of the cell cycle. This observation suggests the possibility that matrix adhesion coordinately regulates cell growth, i.e., increase in cell size, and cell cycle progression through the activation of mTORC1. Prior studies have suggested that cell cycle progression is dependent on cell growth, leading to the hypothesis of a cell growth checkpoint for cell cycle progression (20). Our findings suggest that cell growth and cell cycle progression are coupled via the translational control of cyclin D1. In this model, cyclin D1 would function as a sensor of cap-dependent translation and, consequently, of the growth rate and as a cell cycle regulator. The regulation of the budding yeast G1 cyclin CLN3 provides additional support for this model. In this system, a short upstream open reading frame in the CLN3 mRNA 5′ leader sequence attenuates the translation of the full-length CLN3 coding region under suboptimal growth conditions, when the ribosome content is limiting (45).
Our results indicate that integrin-specific signaling through mTORC1 also contributes to sustain cell survival. It is well established that lack of proper attachment to the matrix results in apoptosis, a phenomenon that has been named anoikis, and earlier studies have identified a variety of mechanisms underlying the induction of anoikis (12, 47). We have observed that endothelial cells adhering to laminin 1 undergo apoptosis even if they are exposed to otherwise mitogenic concentrations of growth factors. It is unlikely that defective activation of AKT or ERK contributes to cell death on laminin 1, as these signaling effectors are activated to comparable levels on fibronectin and laminin 1. Two lines of evidence suggest instead that endothelial cell survival is dependent on mTORC1 signaling. First, knockdown of merlin activates mTORC1 signaling and rescues endothelial cells plated onto laminin 1 from apoptosis and treatment with rapamycin reverses this effect. Second, overexpression of 4EBP1 induces apoptosis in cells plated onto fibronectin. These results are consistent with the findings of prior studies showing that a constitutively active form of mTOR prevents apoptosis in normal cells deprived of growth factors (9) and that inhibition of mTORC1 by rapamycin induces apoptosis in tumor cells (2, 32, 59).
mTORC1 is abnormally activated in several tumor types, owing to activation of Ras or PI-3K or inactivation of NF1, PTEN, TSC, or LKB1 (15, 54). Our results provide strong evidence that loss-of-function mutations in NF2 activate mTORC1 signaling in malignant mesothelioma. In the accompanying paper, James and colleagues show that loss of merlin activates mTORC1 signaling in meningiomas and that this occurs, as we have also shown here, independently of AKT or ERK (18). Together, the results of these studies indicate that the activation of mTORC1 signaling is a key feature of NF2 mutant tumors. Heterozygous NF2 mutations cause type 2 neurofibromatosis, which belongs to a group of cancer predisposition syndromes characterized by neurocutaneous manifestations, the phakomatoses (62). Interestingly, several other phakomatoses are caused by heterozygous mutations in tumor suppressor genes (NF1 for type 1 neurofibromatosis, PTEN for Cowden disease, LKB1 for Peutz-Jeghers syndrome, and TSC1 and TSC2 for tuberous scleroses 1 and 2) that negatively regulate mTORC1 signaling. These observations suggest the possibility that activated mTORC1 signaling contributes to the neurocutaneous manifestations of phakomatoses.
Experiments with mouse models and the remarkable efficacy of certain oncogene-targeted therapies have led to the belief that cancer cells are addicted to oncogene signaling (66). Accordingly, recent studies have employed drug sensitivity to infer oncogene pathway activation in tumor cells (30, 36, 58). Our observation that merlin-deficient malignant mesothelioma cells are selectively sensitive to the growth-inhibitory effect of rapamycin suggests that mTORC1 signaling contributes to the expansion of NF2 mutant tumors in vivo. Although future studies using mouse models will be required to validate this model, the observation that blockage of mTORC1 inhibits in vitro two distinct tumor types caused by NF2 mutations corroborates the hypothesis that a major function of merlin is to suppress mTORC1 signaling.
Malignant mesothelioma is an aggressive tumor type that responds poorly to standard chemotherapy or radiation therapy (61). In fact, most patients die within 4 to 12 months from the onset of the first symptoms. The observation that loss of merlin predicts sensitivity to rapamycin identifies mTORC1 as a therapeutic target in the large fraction of malignant mesotheliomas that carry NF2 mutations. We suggest that clinical trials employing mTORC1 inhibitors for malignant mesothelioma are warranted and that patients should be stratified according to NF2 status in preparation for such trials.
Supplementary Material
Acknowledgments
We thank Yuman Fong, Tyler Jacks, Suresh Jhanwar, Martin Krüger, and John Lawrence for reagents, Marc Ladanyi and Shigeki Shimizu for reagents and discussions, María Carmen Cerviño for help with 4EBP1 transfection experiments, Gaia Schiavón for help with the analysis of DNA microarray data, the Genomics Core Facility of MSKCC for assistance, and members of the Giancotti laboratory for discussions.
This work was supported by grants from the NIH (R01 78901 to F.G.G. and P30 CA08748 to Harold Varmus) and the Baker Street Foundation (to F.G.G.).
Footnotes
Published ahead of print on 18 May 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Benecke, B. J., A. Ben-Ze'ev, and S. Penman. 1978. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell 14931-939. [DOI] [PubMed] [Google Scholar]
- 2.Beuvink, I., A. Boulay, S. Fumagalli, F. Zilbermann, S. Ruetz, T. O'Reilly, F. Natt, J. Hall, H. A. Lane, and G. Thomas. 2005. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell 120747-759. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi, A. B., S. I. Mitsunaga, J. Q. Cheng, W. M. Klein, S. C. Jhanwar, B. Seizinger, N. Kley, A. J. Klein-Szanto, and J. R. Testa. 1995. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc. Natl. Acad. Sci. USA 9210854-10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bretscher, A., K. Edwards, and R. G. Fehon. 2002. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3586-599. [DOI] [PubMed] [Google Scholar]
- 5.Cho, E., Y. Feng, C. Rauskolb, S. Maitra, R. Fehon, and K. D. Irvine. 2006. Delineation of a Fat tumor suppressor pathway. Nat. Genet. 381142-1150. [DOI] [PubMed] [Google Scholar]
- 6.Chung, J., R. E. Bachelder, E. A. Lipscomb, L. M. Shaw, and A. M. Mercurio. 2002. Integrin α6β4 regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells. J. Cell Biol. 158165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curto, M., B. K. Cole, D. Lallemand, C. H. Liu, and A. I. McClatchey. 2007. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J. Cell Biol. 177893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danen, E. H., and K. M. Yamada. 2001. Fibronectin, integrins, and growth control. J. Cell. Physiol. 1891-13. [DOI] [PubMed] [Google Scholar]
- 9.Edinger, A. L., and C. B. Thompson. 2004. An activated mTOR mutant supports growth factor-independent, nutrient-dependent cell survival. Oncogene 235654-5663. [DOI] [PubMed] [Google Scholar]
- 10.Farmer, S. R., A. Ben-Ze'av, B. J. Benecke, and S. Penman. 1978. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell 15627-637. [DOI] [PubMed] [Google Scholar]
- 11.Fingar, D. C., S. Salama, C. Tsou, E. Harlow, and J. Blenis. 2002. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 161472-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisch, S. M., and R. A. Screaton. 2001. Anoikis mechanisms. Curr. Opin. Cell Biol. 13555-562. [DOI] [PubMed] [Google Scholar]
- 13.Giancotti, F. G., and G. Tarone. 2003. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 19173-206. [DOI] [PubMed] [Google Scholar]
- 14.Gorrini, C., F. Loreni, V. Gandin, L. A. Sala, N. Sonenberg, P. C. Marchisio, and S. Biffo. 2005. Fibronectin controls cap-dependent translation through β1 integrin and eukaryotic initiation factors 4 and 2 coordinated pathways. Proc. Natl. Acad. Sci. USA 1029200-9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guertin, D. A., and D. M. Sabatini. 2007. Defining the role of mTOR in cancer. Cancer Cell 129-22. [DOI] [PubMed] [Google Scholar]
- 16.Hamaratoglu, F., M. Willecke, M. Kango-Singh, R. Nolo, E. Hyun, C. Tao, H. Jafar-Nejad, and G. Halder. 2006. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 827-36. [DOI] [PubMed] [Google Scholar]
- 17.Hizli, A. A., A. R. Black, M. A. Pysz, and J. D. Black. 2006. Protein kinase C alpha signaling inhibits cyclin D1 translation in intestinal epithelial cells. J. Biol. Chem. 28114596-14603. [DOI] [PubMed] [Google Scholar]
- 18.James, M. F., S. Han, C. Polizzano, S. R. Plotkin, B. D. Manning, A. O. Stemmer-Rachamimov, J. F. Gusella, and V. Ramesh. 2009. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol. Cell. Biol. 294250-4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, H., T. Sperka, P. Herrlich, and H. Morrison. 2006. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature 442576-579. [DOI] [PubMed] [Google Scholar]
- 20.Johnston, G. C., J. R. Pringle, and L. H. Hartwell. 1977. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp. Cell Res. 10579-98. [DOI] [PubMed] [Google Scholar]
- 21.Kissil, J. L., K. C. Johnson, M. S. Eckman, and T. Jacks. 2002. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J. Biol. Chem. 27710394-10399. [DOI] [PubMed] [Google Scholar]
- 22.Kissil, J. L., E. W. Wilker, K. C. Johnson, M. S. Eckman, M. B. Yaffe, and T. Jacks. 2003. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol. Cell 12841-849. [DOI] [PubMed] [Google Scholar]
- 23.Koziczak, M., and N. E. Hynes. 2004. Cooperation between fibroblast growth factor receptor-4 and ErbB2 in regulation of cyclin D1 translation. J. Biol. Chem. 27950004-50011. [DOI] [PubMed] [Google Scholar]
- 24.Kruger, M., C. Beger, P. J. Welch, J. R. Barber, M. P. Manns, and F. Wong-Staal. 2001. Involvement of proteasome α-subunit PSMA7 in hepatitis C virus internal ribosome entry site-mediated translation. Mol. Cell. Biol. 218357-8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, Q., M. R. Nance, R. Kulikauskas, K. Nyberg, R. Fehon, P. A. Karplus, A. Bretscher, and J. J. Tesmer. 2007. Self-masking in an intact ERM-merlin protein: an active role for the central alpha-helical domain. J. Mol. Biol. 3651446-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeshima, Y., A. Sudhakar, J. C. Lively, K. Ueki, S. Kharbanda, C. R. Kahn, N. Sonenberg, R. O. Hynes, and R. Kalluri. 2002. Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science 295140-143. [DOI] [PubMed] [Google Scholar]
- 27.Maitra, S., R. M. Kulikauskas, H. Gavilan, and R. G. Fehon. 2006. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr. Biol. 16702-709. [DOI] [PubMed] [Google Scholar]
- 28.Massague, J. 2004. G1 cell-cycle control and cancer. Nature 432298-306. [DOI] [PubMed] [Google Scholar]
- 29.McClatchey, A. I., and M. Giovannini. 2005. Membrane organization and tumorigenesis—the NF2 tumor suppressor, Merlin. Genes Dev. 192265-2277. [DOI] [PubMed] [Google Scholar]
- 30.McDermott, U., S. V. Sharma, L. Dowell, P. Greninger, C. Montagut, J. Lamb, H. Archibald, R. Raudales, A. Tam, D. Lee, S. M. Rothenberg, J. G. Supko, R. Sordella, L. E. Ulkus, A. J. Iafrate, S. Maheswaran, C. N. Njauw, H. Tsao, L. Drew, J. H. Hanke, X. J. Ma, M. G. Erlander, N. S. Gray, D. A. Haber, and J. Settleman. 2007. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc. Natl. Acad. Sci. USA 10419936-19941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mettouchi, A., S. Klein, W. Guo, M. Lopez-Lago, E. Lemichez, J. K. Westwick, and F. G. Giancotti. 2001. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol. Cell 8115-127. [DOI] [PubMed] [Google Scholar]
- 32.Mills, J. H., L. F. Thompson, C. Mueller, A. T. Waickman, S. Jalkanen, J. Niemela, L. Airas, and M. S. Bynoe. 2008. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 1059325-9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison, H., L. S. Sherman, J. Legg, F. Banine, C. Isacke, C. A. Haipek, D. H. Gutmann, H. Ponta, and P. Herrlich. 2001. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 15968-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison, H., T. Sperka, J. Manent, M. Giovannini, H. Ponta, and P. Herrlich. 2007. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 67520-527. [DOI] [PubMed] [Google Scholar]
- 35.Muise-Helmericks, R. C., H. L. Grimes, A. Bellacosa, S. E. Malstrom, P. N. Tsichlis, and N. Rosen. 1998. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 27329864-29872. [DOI] [PubMed] [Google Scholar]
- 36.Neve, R. M., K. Chin, J. Fridlyand, J. Yeh, F. L. Baehner, T. Fevr, L. Clark, N. Bayani, J. P. Coppe, F. Tong, T. Speed, P. T. Spellman, S. DeVries, A. Lapuk, N. J. Wang, W. L. Kuo, J. L. Stilwell, D. Pinkel, D. G. Albertson, F. M. Waldman, F. McCormick, R. B. Dickson, M. D. Johnson, M. Lippman, S. Ethier, A. Gazdar, and J. W. Gray. 2006. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10515-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen, R., D. Reczek, and A. Bretscher. 2001. Hierarchy of merlin and ezrin N- and C-terminal domain interactions in homo- and heterotypic associations and their relationship to binding of scaffolding proteins EBP50 and E3KARP. J. Biol. Chem. 2767621-7629. [DOI] [PubMed] [Google Scholar]
- 38.Okada, T., M. Lopez-Lago, and F. G. Giancotti. 2005. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J. Cell Biol. 171361-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada, T., L. You, and F. G. Giancotti. 2007. Shedding light on Merlin's wizardry. Trends Cell Biol. 17222-229. [DOI] [PubMed] [Google Scholar]
- 40.Oktay, K., E. Buyuk, O. Oktem, M. Oktay, and F. G. Giancotti. 2008. The c-Jun N-terminal kinase JNK functions upstream of Aurora B to promote entry into mitosis. Cell Cycle 7533-541. [DOI] [PubMed] [Google Scholar]
- 41.Oktay, M., K. K. Wary, M. Dans, R. B. Birge, and F. G. Giancotti. 1999. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J. Cell Biol. 1451461-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pabla, R., A. S. Weyrich, D. A. Dixon, P. F. Bray, T. M. McIntyre, S. M. Prescott, and G. A. Zimmerman. 1999. Integrin-dependent control of translation: engagement of integrin αIIbβ3 regulates synthesis of proteins in activated human platelets. J. Cell Biol. 144175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellock, B. J., E. Buff, K. White, and I. K. Hariharan. 2007. The Drosophila tumor suppressors Expanded and Merlin differentially regulate cell cycle exit, apoptosis, and Wingless signaling. Dev. Biol. 304102-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelton, P. D., L. S. Sherman, T. A. Rizvi, M. A. Marchionni, P. Wood, R. A. Friedman, and N. Ratner. 1998. Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human Schwannoma cells. Oncogene 172195-2209. [DOI] [PubMed] [Google Scholar]
- 45.Polymenis, M., and E. V. Schmidt. 1997. Coupling of cell division to cell growth by translational control of the G1 cyclin CLN3 in yeast. Genes Dev. 112522-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poulikakos, P. I., G. H. Xiao, R. Gallagher, S. Jablonski, S. C. Jhanwar, and J. R. Testa. 2006. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene 255960-5968. [DOI] [PubMed] [Google Scholar]
- 47.Reddig, P. J., and R. L. Juliano. 2005. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 24425-439. [DOI] [PubMed] [Google Scholar]
- 48.Richter, J. D., and N. Sonenberg. 2005. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433477-480. [DOI] [PubMed] [Google Scholar]
- 49.Rong, R., X. Tang, D. H. Gutmann, and K. Ye. 2004. Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc. Natl. Acad. Sci. USA 10118200-18205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouleau, G. A., P. Merel, M. Lutchman, M. Sanson, J. Zucman, C. Marineau, K. Hoang-Xuan, S. Demczuk, C. Desmaze, B. Plougastel, S. M. Pulst, G. Lenoir, E. Bijlsma, L. Fashold, J. Dumanski, P. de Jong, D. Parry, R. Eldrige, A. Aurias, O. Delattre, and G. Thomas. 1993. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 363515-521. [DOI] [PubMed] [Google Scholar]
- 51.Rousseau, D., R. Kaspar, I. Rosenwald, L. Gehrke, and N. Sonenberg. 1996. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc. Natl. Acad. Sci. USA 931065-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekido, Y., H. I. Pass, S. Bader, D. J. Mew, M. F. Christman, A. F. Gazdar, and J. D. Minna. 1995. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res. 551227-1231. [PubMed] [Google Scholar]
- 53.Sharma, S. V., and J. Settleman. 2007. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 213214-3231. [DOI] [PubMed] [Google Scholar]
- 54.Shaw, R. J., and L. C. Cantley. 2006. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441424-430. [DOI] [PubMed] [Google Scholar]
- 55.Shaw, R. J., A. I. McClatchey, and T. Jacks. 1998. Regulation of the neurofibromatosis type 2 tumor suppressor protein, merlin, by adhesion and growth arrest stimuli. J. Biol. Chem. 2737757-7764. [DOI] [PubMed] [Google Scholar]
- 56.Shaw, R. J., J. G. Paez, M. Curto, A. Yaktine, W. M. Pruitt, I. Saotome, J. P. O'Bryan, V. Gupta, N. Ratner, C. J. Der, T. Jacks, and A. I. McClatchey. 2001. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev. Cell 163-72. [DOI] [PubMed] [Google Scholar]
- 57.Sherman, L., H. M. Xu, R. T. Geist, S. Saporito-Irwin, N. Howells, H. Ponta, P. Herrlich, and D. H. Gutmann. 1997. Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene 152505-2509. [DOI] [PubMed] [Google Scholar]
- 58.Solit, D. B., L. A. Garraway, C. A. Pratilas, A. Sawai, G. Getz, A. Basso, Q. Ye, J. M. Lobo, Y. She, I. Osman, T. R. Golub, J. Sebolt-Leopold, W. R. Sellers, and N. Rosen. 2006. BRAF mutation predicts sensitivity to MEK inhibition. Nature 439358-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teachey, D. T., D. A. Obzut, J. Cooperman, J. Fang, M. Carroll, J. K. Choi, P. J. Houghton, V. I. Brown, and S. A. Grupp. 2006. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood 1071149-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trofatter, J. A., M. M. MacCollin, J. L. Rutter, J. R. Murrell, M. P. Duyao, D. M. Parry, R. Eldridge, N. Kley, A. G. Menon, K. Pulaski, V. H. Haase, C. M. Ambrose, D. Munroe, C. Bove, J. L. Haines, R. L. Martuza, M. E. MacDonald, B. R. Seizinger, M. P. Short, A. J. Buckler, and J. F. Gusella. 1993. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 72791-800. [DOI] [PubMed] [Google Scholar]
- 61.Tsiouris, A., and R. K. Walesby. 2007. Malignant pleural mesothelioma: current concepts in treatment. Nat. Clin. Pract. Oncol. 4344-352. [DOI] [PubMed] [Google Scholar]
- 62.Tucker, M., A. Goldstein, M. Dean, and A. Knudson. 2000. National Cancer Institute Workshop Report: the phakomatoses revisited. J. Natl. Cancer Inst. 92530-533. [DOI] [PubMed] [Google Scholar]
- 63.Walker, J. L., and R. K. Assoian. 2005. Integrin-dependent signal transduction regulating cyclin D1 expression and G1 phase cell cycle progression. Cancer Metastasis Rev. 24383-393. [DOI] [PubMed] [Google Scholar]
- 64.Wary, K. K., F. Mainiero, S. J. Isakoff, E. E. Marcantonio, and F. G. Giancotti. 1996. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87733-743. [DOI] [PubMed] [Google Scholar]
- 65.Wary, K. K., A. Mariotti, C. Zurzolo, and F. G. Giancotti. 1998. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94625-634. [DOI] [PubMed] [Google Scholar]
- 66.Weinstein, I. B. 2002. Cancer addiction to oncogenes—the Achilles heal of cancer. Science 29763-64. [DOI] [PubMed] [Google Scholar]
- 67.Xiao, G. H., A. Beeser, J. Chernoff, and J. R. Testa. 2002. p21-activated kinase links Rac/Cdc42 signaling to merlin. J. Biol. Chem. 277883-886. [DOI] [PubMed] [Google Scholar]
- 68.Xu, Y., S. Y. Chen, K. N. Ross, and S. P. Balk. 2006. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 667783-7792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.