Abstract
Estrogen-related receptors (ERRs) play critical roles in regulation of cellular energy metabolism in response to inducible coactivators such as peroxisome proliferator-activated receptor gamma (PPARγ) coactivator 1α (PGC-1α). A yeast two-hybrid screen led to the identification of the cytokine-stimulated transcriptional regulator, Bcl3, as an ERRα coactivator. Bcl3 was shown to synergize with PGC-1α to coactivate ERRα. Chromatin immunoprecipitation studies demonstrated that ERRα, PGC-1α, and Bcl3 form a complex on an ERRα-responsive element within the pyruvate dehydrogenase kinase 4 gene promoter in cardiac myocytes. Mapping studies demonstrated that Bc13 interacts with PGC-1α and ERRα, allowing for interaction with both proteins. Transcriptional profiling demonstrated that Bcl3 activates genes involved in diverse pathways including a subset involved in cellular energy metabolism known to be regulated by PGC-1α, ERRα, and a second nuclear receptor, PPARα. Consistent with the gene expression profiling results, Bcl3 was shown to synergistically coactivate PPARα with PGC-1α in a manner similar to ERRα. We propose that the cooperativity between Bcl3 and PGC-1α may serve as a point of convergence on nuclear receptor targets to direct programs orchestrating inflammatory and energy metabolism responses in heart and other tissues.
The nuclear receptor superfamily of transcription factors regulate the expression of genes involved in multiple cellular processes including metabolism, growth, differentiation, and inflammation (6, 14, 42). Canonical nuclear receptors are activated by small-molecule ligands which alter the structural conformation of the receptors, leading to recruitment of coactivator complexes that confer chromatin remodeling and promote assembly of the RNA polymerase II machinery on target gene promoters (36). The natural ligands for many nuclear receptors have been identified, providing a basis for subclassification of the superfamily (7). Nuclear receptor ligands can be classic hormones, such as for the thyroid and glucocorticoid receptors, or intermediary metabolites (e.g., liver X receptor and peroxisome proliferator-activated receptor [PPAR]).
The activating ligands for a subset of nuclear receptors, termed orphan receptors, have not been defined (7). Evidence is emerging that some members of this subgroup of nuclear receptors may not require ligand activation but, rather, are subject to control by specific corepressors and coactivators, depending on cell type and physiological context. The estrogen-related receptor α, or ERRα, one of the first orphan receptors to be cloned, is a member of this latter category (18). ERRα can activate or repress genes depending on cell type and promoter context. Recent studies have begun to define relevant ERRα coactivators and corepressors (18). The PPARγ coactivator 1α (PGC-1α) has been shown to serve an essential role as an ERRα coactivator (27, 30, 47). PGC-1α is expressed in a tissue-specific manner, with highest expression in tissues with high energy demands such as heart, and its expression is induced by various physiological stimuli (22, 27, 45). PGC-1α/ERRα targets include a wide array of genes involved in cellular energy metabolism including most mitochondrial energy transduction and ATP production pathways (13, 29). Whereas the role of PGC-1 coactivators in the control of ERRα activity is well established, it is likely that other regulatory factors participate in the physiological regulation of ERRα signaling.
Nuclear receptors direct gene-regulatory events involved in diverse biological processes including development, metabolism, and physiological responses. Accordingly, it is possible that this group of transcription factors, and their respective coregulators, serve as nodal points for cross talk with other cellular signaling pathways. Indeed, recent studies have provided evidence for this notion, showing that the nuclear receptor PPARγ is capable of exerting repressive effects on cytokine-triggered gene-regulatory events in macrophages by interacting with corepressors in a sumoylation-dependent manner (43). Other investigators have proposed mechanisms whereby cytokines converge on metabolism, energetics, and host immune response through ERRα, PGC-1α, and PGC-1β (44, 49, 53). These findings strongly suggest that in addition to directly regulating target genes, a subset of nuclear receptors exerts regulatory cross talk upon other cellular signaling pathways.
In this study, we sought to identify novel nuclear receptor coregulatory proteins. We were especially interested in the PGC-1/ERRα cascade, given its importance in controlling cellular energy metabolism pathways that are likely to respond to other biological signaling cascades. To this end, yeast two-hybrid screening studies were conducted to identify new ERRα coregulators. This screen identified the IκB family of proteins as potential interacting partners of ERRα in the cardiac myocyte. We found that one of the IκB family members, Bcl3, functions synergistically with PGC-1α to coactivate the nuclear receptors ERRα and PPARα in cardiac myocytes. The Bcl3-interacting domains within PGC-1α and ERRα are distinct, allowing for the formation of a multiprotein complex on target promoters. These results identify a nodal point for the convergence of inflammatory stress response signals and nuclear receptor-mediated regulation of genes involved in cellular energy metabolism.
MATERIALS AND METHODS
Yeast two-hybrid screen.
The yeast two-hybrid screen was performed using a Matchmaker Two-Hybrid System (Clontech) as per the manufacturer's instruction. Briefly, human ERRα cDNA corresponding to the ligand-binding domain (amino acids 197 to 422) was cloned into pAS2-1 vector and cotransformed with a human heart cDNA library (Clontech) into Saccharomyces cerevisiae strain CG-1945. A total of 1.3 million transformants were screened, and after the false positives were selected out using pAS2-1-lamin C as a negative control, 46 positive clones remained. Four of 46 were identified as IκBα.
Plasmid constructs. (i) Mammalian expression vectors.
pcDNA3.1-myc/his.PGC-1αWt (where PGC-1αWt is the full-length protein) has been previously described (54) and was used as a template to PCR-amplify cDNA of PGC-1α consisting of residues 1 to 701 (PGC-1α701) using the following primers: 5′-ATGGCTTGGGACATGTGCAG-3′ and 5′-TCATTCACCAAAAACTTCAAAGC-3′. The amplicon was subsequently cloned into pcDNA3.1-myc/his vector and checked by sequencing. The human Bcl3 cDNA (a kind gift from U. Sibenlist) was cloned into pcDNA3.1+ vector (Invitrogen). Construction of expression vectors consisting of pcDNA3.1 fused to full-length ERRα (ERRαWt,) and to COOH-terminal deletion mutants (ERRα403, ERRα359, ERRα144, consisting of residues 1 to 403, 1 to 359, and 1 to 144, respectively), pEFBOS-PPARα, pEFBOS-RXRα, pCMX-Gal4, and pCMX-Gal4-ERRα as well as the reporter constructs Vit2P36.Luc (11), mPDK4.Luc.2281 (where mPKD4 is the mouse pyruvate dehydrogenase kinase 4), NRptmutmPDK4.Luc.2281, (PPRE)X3TK.Luc, and (UAS)X3TK.Luc (where PPRE is peroxisome proliferator response element, TK is thymidine kinase, and UAS is upstream activation sequence) have been previously described (27, 46, 54, 56).
(ii) Bacterial expression vectors.
pGEX4T-3-ERRαWt has been described previously (27). pGEX4T-1-PPARα and pGEX4T-1-Bcl3 were made by cloning human cDNA for PPARα and Bcl3 into pGEX4T-1 (GE Healthcare), respectively.
(iii) Adenoviral expression vectors.
AdBcl3 was generated by cloning human Bcl3 cDNA into the AdTrack-CMV vector and recombined with pAd-Easy as previously described (24).
Mammalian cell culture, transient transfection, and luciferase reporter studies.
CV-1, COS7, and HEK 293 cells were cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Transient transfections were performed using FuGENE6 (Roche) as per the manufacturer's protocol. Briefly, 100 ng of reporter was cotransfected with 10 ng of expression vectors for nuclear receptors, 50 ng of expression vectors for nuclear receptor coactivators, and 10 ng of simian virus 40 promoter-driven Renilla luciferase to control for transfection efficiency. All cell culture wells were balanced for equal amounts of expression vector backbones. For luciferase reporter assays, cells were collected 36 to 48 h after cotransfection and analyzed using Dual-Glo (Promega) as per the manufacturer's protocol. Ventricular cardiac myocytes were prepared from 1-day-old Harlan-Sprague-Dawley rats as previously described (12). After 24 h in culture, cells were infected with adenovirus expressing green fluorescent protein (AdGFP) or GFP and Bcl3 (AdBcl3). An infection rate of 95% was achieved at 72 h postinfection. Subsequently, cells were collected for RNA isolation.
GST pulldown assays.
The glutathione S-transferase (GST) protein-protein interaction assay has been previously described (54). Briefly, all 35S-labeled proteins were synthesized in a TNT Quick Coupled transcription/translation system (Promega) as per the manufacturer's protocol. All GST fusion proteins were grown in BL21 competent bacterial cells (Stratagene) and purified on GSH-Sepharose (GE Healthcare). In the pulldown reaction, 3 μg of the fusion protein was incubated with 10 μl of 35S-labeled protein in 500 μl of binding buffer (20 mM Tris, pH 7.5, 100 mM KCl, 0.1m MEDTA, 0.05% Nonidet P-40, 10% glycerol, 1 mg/ml bovine serum albumin, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], and 1× Complete [Roche] with or without 5,8,11,14-eicosate traynoic acid [ETYA; Calbiochem]). The reaction mixture was incubated at 4°C for 2 h and then washed six to eight times with cold binding buffer. The fusion proteins and their potential interacting partners were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Coimmunoprecipitation and immunoblotting studies.
For coimmunoprecipitation studies, COS7 cells were transfected with mammalian expression vectors using FuGENE6 (Roche) per the manufacturer's protocol. After 48 h, cells were collected in lysis buffer (1× phosphate-buffered saline, 1 mM EDTA, 0.5% TritonX-100, 1× Complete, 1 mM PMSF). Cells were lysed with a sonicator, and 1 μg of M2 anti-FLAG (Sigma) or 9E10 anti-Myc (Santa Cruz) antibody was added to the extract with protein G-Sepharose (Zymed) and incubated for 24 h at 4°C. The Sepharose was washed three times with the lysis buffer, and the immunoprecipitated proteins were analyzed by immunoblotting.
Immunoblotting was performed as previously described (28). Briefly, protein extracts were resolved on SDS-PAGE gels and transferred onto nitrocellulose membranes. Detection of ERRα and PGC-1α was performed using antibodies as previously described (27, 32, 56). Detection of Bcl3 was performed using commercially available antibodies (Santa Cruz).
ChIP assays.
Chromatin-immunoprecipitation (ChIP) assays were performed as previously described (56). Briefly, rat ventricular cardiac myocytes were cultured for 72 h. Cells were cross-linked with 1% formaldehyde (10 min), followed by the addition of 0.125 M glycine to halt the cross-linking. Cells were collected and lysed in nuclear lysis buffer (50 mM Tris, pH 8.0, 10 mM EDTA, 1% SDS, 10 mM sodium butyrate, 300 μM PMSF, and 1× Complete). Chromatin was collected, sonicated, and incubated with immunoglobulin G (IgG) or with polyclonal antibodies against PGC-1α (32), ERRα (56), or Bcl3 (Santa Cruz) at 4°C for 2 h before protein A-Sepharose (Zymed) was added for an overnight incubation. The Sepharose was washed and eluted, and the eluted chromatin was analyzed by PCR using the following primers: ratPDK4P(f) (5′-TGATTGGCTACTGTAAAAGTCCCG-3′) and ratPDK4p(r) (5′-GTCCCAGGTCGCCCGGGGCTTCAGG-3′), which correspond to −462 to −297 of the rat PDK4 promoter; ratPDK4IC(f) (5′-ACACTGTCTCCCTCTCCTC-3′) and ratPDK4IC(r) (5′-TCCATGCTTGTGAGATTCTG-3′), which correspond to −1924 to −2144 of the rat PDK4 promoter; and 36B4 primers which have been previously described (56). Qualitative PCR was performed by amplifying the immunoprecipitated chromatin with the primers described above under the following cycling conditions: 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s for 26 cycles; the reaction was analyed using 1% agarose gel electrophoresis. This experiment was quantified using SYBR green real-time quantitative PCR (Applied Biosystem, Foster City, CA). The PCR conditions used were the same as above except the cycle number was increased to 40. For ChIP-reimmunoprecipitation studies, the primary immunoprecipitation was performed using ERRα antibody as described above. The complexes were eluted in 10 mM dithiothreitol at 37°C for 30 min and diluted 1:10 in immunoprecipitation buffer (48). All subsequent steps were as described above.
siRNA studies.
Adenovirus expressing small interfering RNA (siRNA) against rat ERRα (siERRα) and its backbone control (Super) were generous gifts from A. Kralli (25). Primary rat cardiac myocytes were infected with these viruses for 24 to 48 h at a multiplicity of infection of 50 prior to the harvesting of cells. siRNAs against rat Bcl3 and the control were purchased from Ambion (control-AM4611, Bcl3#1-s183606, Bcl3#2-s189734, and Bcl3#3-s183605). One microgram of mPDK4.Luc.2281 with 5 picomoles of siRNA was transfected into primary rat cardiac myocytes using the calcium phosphate coprecipitation method (20).
Gene expression array analyses.
Total RNA was isolated from rat neonatal cardiac myocytes with RNAzol (Tel-Test Inc.) after adenoviral infection as described above. cRNA was synthesized from total RNA as previously described (29). The Alvin Siteman Cancer Center's Multiplexed Gene Analysis Core at Washington University School of Medicine performed the hybridization to the Affymetrix rat U34A chip. Spotfire for Functional Genomics software was used for the initial analysis and background normalization. Subsequent data analyses were performed on Excel. Pathway analyses were performed on GenMAPP2 and MAPPFinder2 software. Inclusion criteria for regulated genes are provided in the footnote to Table 1.
TABLE 1.
Gene expression array results for Bcl3-regulated genes in cardiac myocytesa
| GO pathway | No. of genes changed |
|---|---|
| Cell cycle process | 109 |
| Immune system process | 108 |
| Peptidase activity | 100 |
| Cell adhesion | 97 |
| Oxidoreductase activity | 93 |
| Ubiquitin cycle | 69 |
| Vasculature development | 55 |
| Generation of precursor metabolites and energy | 50 |
| Growth factor activity | 38 |
| DNA replication | 32 |
| Electron transport | 31 |
| GTPase activity | 25 |
| Cofactor metabolic process | 19 |
| Sphingolipid metabolic process | 14 |
Gene expression array was performed with RNA isolated from neonatal rat cardiac myocytes after infection with either adenoviral AdGFP or AdBcl3. Data were normalized to find the relative change in expression for each gene (AdBcl3/AdGFP). Upregulated genes were identified with a relative change of ≥2-fold. Downregulated genes were identified with a relative change of ≤0.5-fold. (P ≤ 0.05; n = 4). Spotfire and MAPPFinder 2 were used for analyses of gene expression array. Of 22,280 genes, Spotfire identified 496 upregulated genes and 17 downregulated genes. MAPPFinder 2 identified changes in 266 of 6,780 gene pathways. Boldface indicates Gene Ontology (GO) pathways involved in energy metabolism.
qRT-PCR.
Total RNA was isolated from rat neonatal cardiac myocytes after adenoviral infection as described above, and real-time quantitative reverse transcription-PCR was performed as previously described (29, 56). Primers for rat fatty acid binding protein 3 (rFABP3) and rat very-low-density lipoprotein receptor (rVLDLR) were as follows: rFABP3(f) (5′-AAGCCCGGCTCACATTGA-3′) and rFABP3(r) (5′-CCACTGAACTTTTCCATTGGT-3′); rVLDLR(f) (5′-TCATCATCTGTGCTTACA-3′) and rVLDLR(r) (5′-ACTTACAGTGAGACAAAAG-3′).
Statistical analyses.
Transient transfection results were analyzed using one-way analysis of variance with subsequent post hoc Tukey's pairwise analyses. All real-time quantitative PCR results were analyzed using a Student's t test. The statistical analyses for the gene chip studies were performed using Spotfire and MAPPFinder2 software, which consist of Student's t test and nonparametric bootstrapping/Westfall-Young adjustment for multiple testing, respectively. Data are presented as means ± standard error of the mean (SEM), with statistically significant differences as P value less than or equal to 0.05.
RESULTS
The IκB family member Bcl3 interacts with and coactivates the nuclear receptor ERRα.
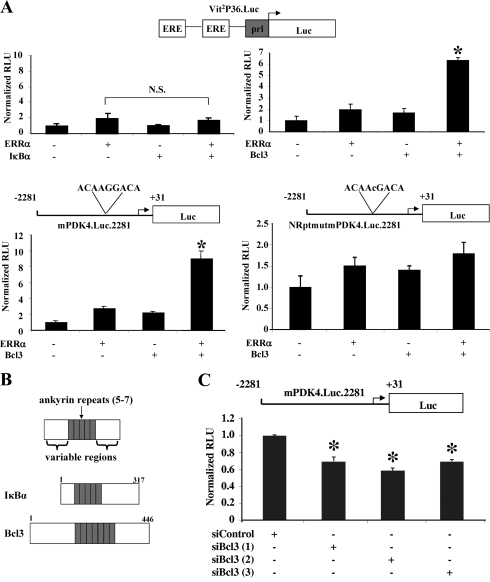
A yeast two-hybrid screen was conducted to identify proteins that interact with the ligand-binding domain of ERRα. Approximately 1.3 million independent clones of a human heart cDNA library were screened, resulting in isolation of 46 positive clones, 17 of which were redundant clones of known ERRα-interacting proteins (ERRα and SRC1) and 4 of which encoded the IκB family member, IκBα (see Table S1 in the supplemental material). The functional significance of the potential interaction of IκBα with ERRα was assessed in cotransfection studies in which an ERRα-responsive luciferase reporter (Vit2P36.Luc) was transfected into HEK 293 cells in the presence and absence of expression vectors for IκBα. IκBα did not have any effect on the ERRα-mediated activation of Vit2P36.Luc; moreover, it did not influence PGC-1α-mediated coactivation of ERRα (Fig. 1A and data not shown). These results are consistent with previous observations that IκBα is predominantly a cytoplasmic protein, whereas ERRα is a transcription factor whose intracellular localization is likely to be predominantly nuclear although this has not been fully substantiated (21, 33). However, another member of the IκB family, Bcl3 (Fig. 1B), which shares homology with IκBα, is known to enter the nucleus and can function as a transcriptional coactivator for other transcription factors including NF-κB (1, 15) and the nuclear receptor RXRα (38). Accordingly, cotransfection experiments were repeated to determine whether Bcl3 could interact with ERRα in a functionally relevant manner. Whereas neither Bcl3 nor ERRα alone had a significant activating effect on the reporter, together they activated the reporter sixfold (Fig. 1A). Similarly, Bcl3 and ERRα synergistically activated a second ERRα-responsive reporter, mPDK4.Luc.2281 (56), which contains the promoter of the ERRα-responsive mouse PDK4 gene. This synergism was completely abolished when a single point mutation was placed in the ERRα-responsive element within mPDK4.Luc.2281 (Fig. 1A). To explore the relevance of the Bcl3-mediated transcriptional regulation of the ERRα target PDK4, Bcl3 knockdown studies were conducted in primary neonatal rat cardiac myocytes, which express Bcl3 (data not shown). Three independent siRNAs against Bcl3 significantly reduced basal mPDK4.Luc.2281 promoter activity by 30 to 40% (Fig. 1C).
FIG. 1.
(A) Bc13, but not IκBα, coactivates ERRα. (Top) A schematic representation of Vit2P36.Luc heterologous promoter-luciferase reporter construct with two copies of the estrogen receptor response element (ERE) derived from the vitellogenin promoter linked to prolactin promoter (prl) and luciferase (Luc) reporter. This reporter was cotransfected into HEK 293 cells with combinations of ERRα, IκBα, and Bcl3 as indicated below the graph. (Bottom) Schematic representations of mPDK4.Luc.2281 and NRptmutmPDK4.Luc.2281 homologous promoter-reporter constructs containing the ERRα response element or its nuclear receptor point mutant equivalent within the −2281 to +31 region of the mouse PDK4 promoter linked to luciferase. These reporters were cotransfected into CV-1 cells with combinations of ERRα and Bcl3 as indicated. The bars represent mean (± SEM) relative light units (RLU) corrected for Renilla luciferase activity, a measure of transfection efficiency, and normalized to basal Vit2P36.Luc, mPDK4.Luc.2281, or NRptmut mPDK4.Luc.2281 activity (set at 1.0). All values represent the results of a minimum of three independent transfections conducted in triplicate. *, significant difference compared to reporter activity with ERRα alone (P < 0.05); N.S., not significant. (B) Schematic representation of IκBα and Bcl3 proteins. The numbers represent amino acids, and gray boxes denote individual ankyrin repeat domains. (C) Endogenous Bcl3 activates the mPDK4.Luc.2281 reporter in primary rat cardiac myocytes. The mPDK4.Luc.2281 reporter was transfected into primary rat cardiac myocytes with either control siRNA or three independent siRNAs directed against Bcl3. The bars represent mean (± SEM) relative light units (RLU) corrected for Renilla luciferase activity and normalized to basal mPDK4.Luc.2281 reporter and siRNA control (set at 1.0). All values represent the results of two independent transfections conducted in triplicate. *, significant difference compared to reporter plus control siRNA (P < 0.05).
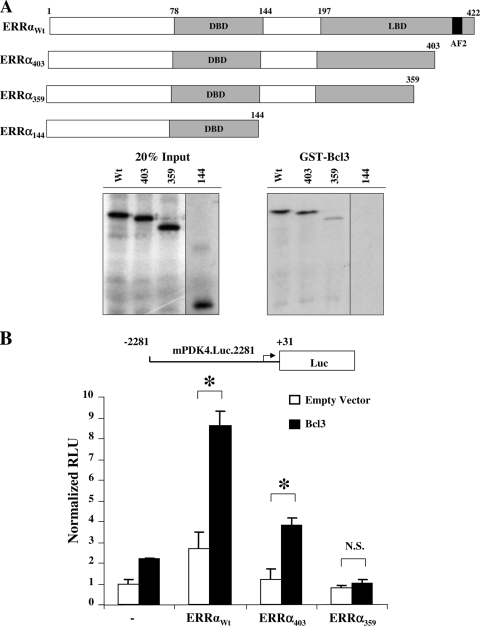
To determine whether Bcl3 interacts directly with ERRα, GST pulldown assays were performed. Full-length ERRα (ERRαWt) bound a GST-Bcl3 fusion protein (Fig. 2A). ERRα COOH-terminal deletion mutants (ERRα403, ERRα359, and ERRα144) were next assessed to map the Bcl3-interacting region. Bcl3 bound similarly to ERRαWt and ERRα403; however, only minimal residual binding was observed with ERRα359 (Fig. 2A). Bcl3 binding was completely abolished with ERRα144. Taken together, these results identify at least two Bcl3 interacting regions within amino acids 359 to 403 and 144 to 359 of the ERRα protein (Fig. 2A). These findings were surprising, given that all known coactivator interacting sites in the ERRα molecule involve the COOH-terminal AF-2 domain (18). Consistent with the results of the binding studies, Bcl3 coactivated both ERRαWt and ERRα403 but not ERRα359 in cell cotransfection studies (Fig. 2B).
FIG. 2.
Bcl3 interacts with ERRα. (A) Schematic representations of ERRα and its truncation mutants used in GST pulldowns to localize the ERRα interaction with Bcl3. The gray regions represent the major domains: the DNA binding domain (DBD) and the ligand binding domain (LBD) with the numbers corresponding to amino acid positions. Bacterially expressed GST or GST-Bcl3 fusion proteins were used with 35S-labeled full-length or truncated mutants of ERRα in GST pulldown assays. Twenty percent of the radiolabeled proteins used in the binding reaction (20% Input) and GST fusion proteins with their potential binding partners were analyzed by SDS-PAGE (proteins are indicated by their subscripts).The result shown is representative of three independent experiments. (B) Transient transfection experiments using mPDK4.Luc.2281 reporter was performed in CV-1 cells with either no nuclear receptor, ERRα, or its truncation mutants (ERRα403 and ERRα359) and cotransfected with empty vector (white bars) or Bcl3 (black bars). The bars represent mean (± SEM) relative light units (RLU) corrected for Renilla luciferase activity and normalized to basal mPDK4.Luc.2281 activity (set at 1.0). All values represent the results of three independent transfections conducted in triplicate. *, significant difference (P < 0.05) between bracketed values; N.S., not significant.
Bcl3 synergizes with PGC-1α to coactivate ERRα.
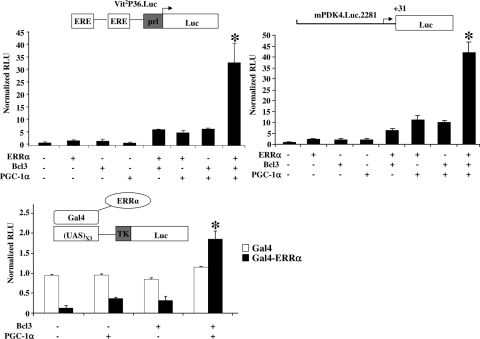
To explore the possibility that Bcl3 influences the coregulation of ERRα by PGC-1α, ERRα reporter cotransfection experiments were repeated in the absence and presence of PGC-1α. Individually, PGC-1α and Bcl3 modestly coactivated (three to sevenfold) ERRα on either Vit2P36.Luc or mPDK4.Luc.2281 (Fig. 3). However, when added together, PGC-1α and Bcl3 mediated a marked (30-fold) synergistic coactivation of ERRα (Fig. 3). PGC-1α also modestly boosted the Bcl3-mediated activation of mPDK4.Luc.2281 (Fig. 3), an effect likely mediated through an endogenous transcription factor other than ERRα, given that ERRα is not immunodetectable in this cell line (data not shown). The synergism between PGC-1α and Bcl3 was also seen in studies using the Gal4 hybrid system in which a Gal4 DNA binding domain was fused to ERRα (Gal4-ERRα). The (UAS)X3TK.Luc reporter, which contains three copies of Gal4 binding elements, was repressed by Gal4-ERRα by approximately 80%. The addition of either PGC-1α or Bcl3 modestly activated Gal4-ERRα, but together the proteins conferred a ninefold activation (Fig. 3). These results strongly suggest that Bcl3 and PGC-1α form a functionally cooperative complex with ERRα.
FIG. 3.
Bcl3 interacts cooperatively with PGC-1α to coactivate ERRα. Results of transient transfection experiments using Vit2P36.Luc in HEK 293, mPDK4.Luc.2281, or (UAS)X3TK.Luc reporters in CV-1 cells. The reporters were cotransfected with combinations of Gal4, Gal4-ERRα, ERRα, Bcl3, and PGC-1α as indicated at the bottom. The bars represent mean (± SEM) relative light units (RLU) corrected for Renilla luciferase activity and normalized to basal Vit2P36.Luc, mPDK4.Luc.2281, or (UAS)X3TK.Luc activity (set at 1.0). All values represent the results of a minimum of three independent transfections conducted in triplicate. *, significant difference compared to reporter activity with ERRα plus Bcl3 and ERRα plus PGC-1α or Gal4-ERRα plus Bcl3 and Gal4-ERRα plus PGC-1α (P < 0.05).
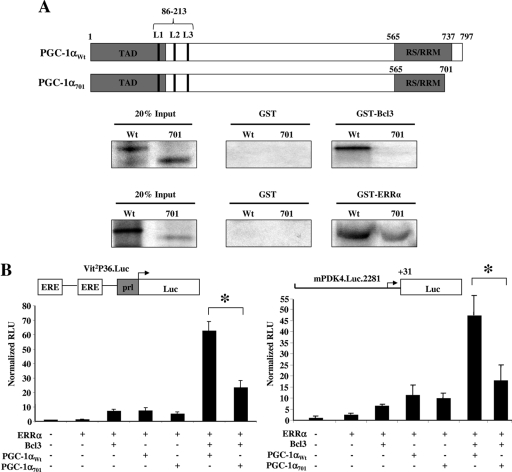
GST pull-down assays were performed to determine whether, as suggested by the observed functional cooperativity, PGC-1α and Bcl3 interact directly. Full-length PGC-1α protein (PGC-1Wt) bound to Bcl3 (Fig. 4A). Surprisingly, a C-terminal deletion mutant (PGC-1α701) was unable to bind to Bcl3 (Fig. 4A). This latter result was surprising given that previously identified protein-protein interaction domains within the PGC-1α molecule do not involve this region. PGC-1α701 still retained its ability to bind to ERRα, given that the ERR-interacting domains are still present in this truncated mutant (Fig. 4A) (27). The functional significance of the PGC-1α-Bcl3 interaction was tested in cotransfection experiments using Vit2P36.Luc and mPDK4.Luc.2281. PGC-1αWt and Bcl3 coactivated ERRα on the two reporters 60-fold and 45-fold, respectively. In contrast, activation by PGC-1α701 and Bcl3 was only 20-fold and 15-fold, respectively (additive rather than synergistic) (Fig. 4B). These results indicate that PGC-1α and Bcl3 interact directly through a novel protein interaction domain within the COOH-terminal 96 amino acids of the PGC-1α protein.
FIG. 4.
Bcl3 interacts with PGC-1α. (A) Schematic representations of PGC-1α (PGC-1αWt) and its truncation mutant (consisting of residues 1 to 701; PGC-1α701) used in GST pulldowns to localize the Bcl3 interaction with PGC-1α. The gray regions represent the major domains: the transactivating domain (TAD) and the serine/arginine-rich domain/RNA recognition motif (RS/RRM). Vertical lines L1, L2, and L3 represent leucine-rich motifs LXXLL or LLXXL necessary for ERRα binding. The numbers correspond to amino acid positions. Bacterially expressed GST, GST-ERRα, or GST-Bcl3 fusion proteins were used with 35S-labeled full-length (Wt) or truncated (701) PGC-1α in GST pull-down assays. Twenty percent of the radiolabeled proteins (20% Input) used in the binding reactions and the GST fusion proteins with their potential binding partners were analyzed. This experiment was repeated three times, and a representative result is shown. (B) Transient transfection experiments with Vit2P36.Luc and mPDK4.Luc.2281 were performed in HEK 293 (left) and CV-1 cells (right), respectively. These reporters were cotransfected with combinations of ERRα, Bcl3, PGC-1αWt, and PGC-1α701 as indicated. The bars represent mean (± SEM) relative light units (RLU) corrected for Renilla luciferase activity and normalized to basal Vit2P36.Luc activity (set at 1.0). All values represent the results of three independent transfections conducted in triplicate. *, significant difference between bracketed bars (P < 0.05).
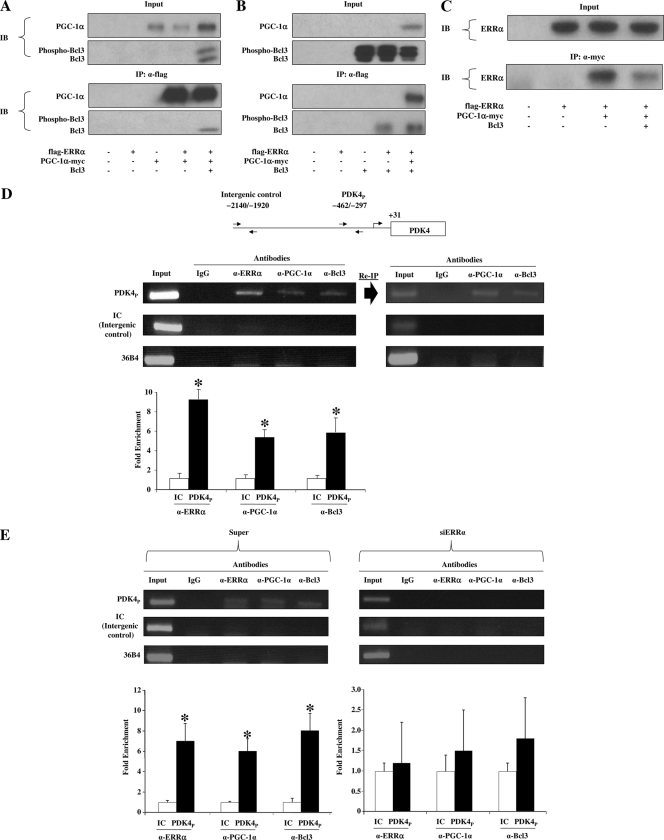
We next sought to determine whether ERRα, PGC-1α, and Bcl3 form a complex in cells. To address this, coimmunoprecipitation experiments were conducted in COS7 cells overexpressing Flag epitope-tagged ERRα (Flag-ERRα), myc epitope-tagged PGC-1α (PGC-1α-myc), and Bcl3 to assess a combination of two-way interactions. As expected, PGC-1α or Bcl3 was individually immunoprecipitated with ERRα (Fig. 5A and B). Importantly, when all three proteins were overexpressed, anti-Flag antibody coimmunoprecipitated both PGC-1α and Bcl3 along with ERRα (Fig. 5A and B). Interestingly, only the dephosphorylated form of Bcl3 (the nuclear-localized form) (3, 4, 40) coimmunoprecipitated with ERRα (Fig. 5A and B). We also noted that the level of coimmunoprecipitation between Flag-ERRα and one coactivator was not perturbed by the presence of the other coactivator (Fig. 5A and B). Immunoprecipitation of PGC-1α-myc also pulled out ERRα. However, in this latter experiment, the amount of ERRα coimmunoprecipitated with PGC-1α was reduced by the presence of Bcl3 (Fig. 5C), suggesting that Bcl3 interfered with the PGC-1α-myc/α-myc interaction.
FIG. 5.
ERRα/Bcl3/PGC-1α form a trimeric complex on an ERRα-responsive element in cardiac myocytes. Coimmunoprecipitation experiments were performed by cotransfecting combinations of Flag-ERRα, PGC-1α-myc, and Bcl3 in COS7 cells as indicated. Antibodies against the Flag epitope (A and B) and myc-epitope (C) were used for coimmunoprecipitation. The extracts (Input) from COS7 cells and the proteins from the immunoprecipitations (IP) were analyzed by immunoblotting (IB) as noted. Each coimmunoprecipitation was repeated three times, and a representative result is shown. (D) A schematic representation of the rat PDK4 promoter region is shown at the top of the panel with arrows representing primers used for ChIP studies. PDK4P represents the region containing the ERRα responsive element, and the intergenic control (IC) represents an upstream region that does not contain any putative ERRα-responsive consensus element (negative control). The numbers indicate the corresponding nucleotides. The middle panel shows a ChIP assay performed on chromatin extracted from rat neonatal cardiac myocytes using IgG or antibodies against ERRα, PGC-1α, and Bcl3 as labeled. Reimmunoprecipitation (Re-IP) was performed on chromatin immunoprecipitated by antibody against ERRα and subsequently reimmunoprecipitated using IgG or antibodies against PGC-1α and Bcl3. Crude chromatin (Input) and immunoprecipitated chromatin were analyzed by PCR with primers (arrows) for IC, PDK4P, and rat 36B4 genome. Quantification using SYBR green real-time PCR is shown in the bottom panel. The relative increase in enrichment represents fluorescent signal generated by PDK4P (calculated as a percentage of input) divided by fluorescent signal generated by IC (calculated as a percentage of input and set to 1.0) for each antibody. Bars represent the mean relative increase in enrichment (± SEM) from three independent experiments. *, significant differences between IC and PDK4P values for each antibody. (E) ChIP was performed on chromatin extracted from rat neonatal cardiac myocytes that had been infected with adenovirus expressing siERRα or its backbone control (Super) and immunoprecipitated with IgG or antibodies against ERRα, PGC-1α, and Bcl3 as labeled. All analyses were as described above. α, anti.
To determine whether endogenous ERRα serves as a platform for the assembly of Bcl3 and PGC-1α on target gene promoters, ChIP experiments were performed using the PDK4 gene promoter. Primary rat cardiac myocytes were chosen for these experiments because PDK4 levels are relatively high in this cell type (2). Immunoprecipitation with antibodies to ERRα, PGC-1α, or Bcl3 resulted in significant enrichment of the relevant ERRα response region within the PDK4 promoter region. To establish that both coactivators occupied this region with ERRα, chromatin immunoprecipitated with antibody against ERRα was reimmunoprecipitated with antibodies against PGC-1α or Bcl3, resulting in reenrichment of the ERRα response region (Fig. 5D). Moreover, when this ChIP experiment was conducted in the presence of an adenovirus expressing siERRα, enrichment of the ERRα response region by antibodies against either PGC-1α or Bcl3 was abolished (Fig. 5E). Taking the cotransfection and immunoprecipitation results together, we conclude that PGC-1α and Bcl3 interact cooperatively in a complex with ERRα to activate the transcription of at least a subset of ERRα target genes.
Bcl3 activates cardiac myocyte genes involved in cellular metabolism and additional biological pathways.
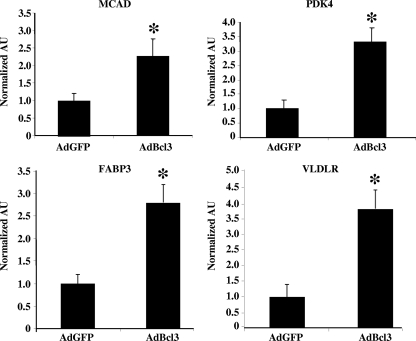
To identify potential target genes of Bcl3 in cardiac myocytes, a recombinant adenovirus expressing Bcl3 was used to infect rat neonatal cardiac myocytes with consequential gene expression profiling. A total of 496 upregulated genes and 17 downregulated genes met inclusion criteria in our initial analysis (Table 1; see also Tables S2 and S3 in the supplemental material). The gene expression changes clustered around a subset of biological processes defined by 266 pathways identified using an unbiased pathway analytic tool (Table 1) (see Materials and Methods). A predominance of pathways involved in cellular fuel and mitochondrial energy metabolism were upregulated by Bcl3, including fatty acid oxidation, oxidation phosphorylation, and glucose metabolism (Table 1; see also Tables S2 and S3 in the supplemental material). Notably, ERRα and PGC-1α have been established as important regulators of many genes in these pathways, and several of the genes identified in this analysis are known ERRα target genes (13, 27, 29). Further validation of these results was provided by qRT-PCR studies demonstrating that mitochondrial fatty acid oxidation (MCAD), PDK4 (negative regulator of glucose oxidation), FABP3, and VLDLR mRNA levels were significantly upregulated by Bcl3 in cardiac myoctyes (Fig. 6; see also Tables S2 and S3 in the supplemental material). These results strongly suggest that Bcl3 serves as a bona fide coregulator of metabolic pathways downstream of PGC-1α/ERRα. In addition to energy metabolism, target genes involved in several other biological processes, including cell cycle control, the immune response, peptidase activity, cell adhesion, ubiquitination, vascular development, and growth programs, were regulated by Bcl3 overexpression (Table 1).
FIG. 6.
Bcl3 induces expression of genes involved in glucose and fatty acid metabolism in rat neonatal cardiac myocytes. Graph represents mean (± SEM) MCAD, PDK4, FABP3, and VLDLR mRNA levels in arbitrary units (AU) after adenoviral infection of either GFP alone (AdGFP) or Bcl3 (AdBcl3) as determined by qRT-PCR from three independent experiments. Values shown are corrected to 36B4 and normalized (1.0) to AdGFP values. *, significant difference compared to AdGFP (P ≤ 0.05).
Bcl3 cooperates with PGC-1α to coactivate PPARα.
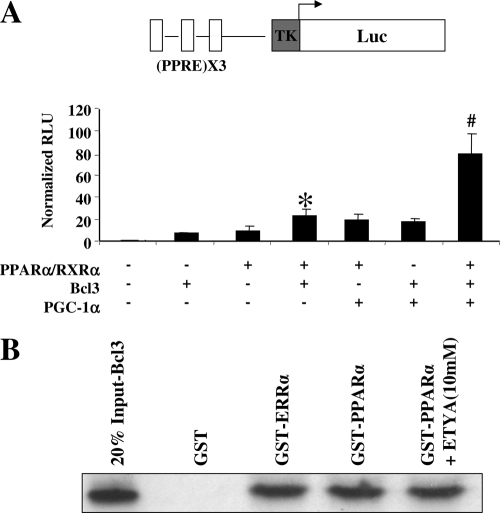
The gene expression array data indicated that Bcl3 regulated a significant subset of genes involved in cellular fatty acid oxidation, many of which are known to be PPARα targets including MCAD (Acadm) (Fig. 6), carnitine palmitoyltransferase-1a and -1b (Cpt1a and -b), and long-chain acyl-coenzyme A synthetase 1 (Acsl1) (see Tables S2 and S3 in the supplemental material). In addition, expression of the PDK4 gene, another known PPARα target (51) involved in the regulation of glucose oxidation, was activated by Bcl3 (Fig. 6; see also Tables S2 and S3 in the supplemental material). Given that PPARα is known to be coactivated by PGC-1α (54) and that PPARα gene expression is regulated by ERRα (29), we sought to determine whether Bcl3 cooperated with PGC-1α to coactivate PPARα. The PPARα-responsive reporter (PPRE)X3TK.Luc was cotransfected into CV-1 cells along with various combinations of expression vectors for Bcl3, PPARα and its heterodimeric partner, RXRα, and PGC-1α. Bcl3 or PGC-1α independently boosted the PPAR/RXR-mediated regulation of the reporter by a modest degree (approximately 2.0- to 2.5-fold), resulting in 22-fold activation over baseline transcriptional activity (Fig. 7A). In contrast, addition of PGC-1α together with Bcl3 resulted in a marked coactivation (to 80-fold) (Fig. 7A). GST pull-down assays confirmed that Bcl3 bound directly to PPARα and that this interaction is not influenced by addition of ETYA, a synthetic PPARα ligand (Fig. 7B). Taken together, these results indicate that Bcl3 and PGC-1α cooperate to coactivate PPARα in a manner that produces effects similar to those on ERRα. This finding is notable, given the known overlap of genes regulated by ERRα and PPARα (29).
FIG. 7.
Bcl3 cooperates with PGC-1α to coactivate PPARα. (A) A schematic representation of (PPRE)X3TK.Luc heterologous promoter-reporter construct with three copies of a PPARα-responsive element (PPRE) derived from the peroxisomal acyl-coenzyme A oxidase gene linked to a minimal TK promoter and luciferase (Luc) is shown at the top. This reporter construct was cotransfected into CV-1 cells with combinations of PPARα, RXRα, Bcl3, and PGC-1α as indicated. The bars represent mean (± SEM) relative light units (RLU) corrected for Renilla luciferase activity and normalized to basal (PPRE)X3TK.Luc activity (set at 1.0). All values represent the results of five independent transfections conducted in triplicate. *, significant differences compared to reporter activity with PPARα/RXRα alone (P < 0.05); #, significant differences compared to reporter activities with PPARα/RXRα plus PGC-1α and PPARα/RXRα plus Bcl3 (P < 0.05). (B) Bacterially expressed GST, GST-ERRα, and GST-PPARα fusion proteins were used with 35S-labeled Bcl3 in GST pull-down assays with and without ETYA, a synthetic PPARα ligand, as indicated. Twenty percent of the radiolabeled proteins used in the binding reaction (20% Input) and GST fusion proteins with their potential binding partners were analyzed by SDS-PAGE. This experiment was repeated three times, and a representative result is shown.
DISCUSSION
Emerging evidence indicates that an important function of nuclear receptor coregulators is to mediate the interface between extracellular signals and gene-regulatory responses. The PGC-1 family (PGC-1α, PGC-1β, and PGC-1-related coactivator or PRC) fulfill this role as transcriptional coactivators that are dynamically induced by developmental, tissue-specific, and physiological cues to reprogram the cell to meet energy metabolism demands imposed by diverse physiological conditions such as cold exposure, exercise, and fasting (22). The activity of the orphan nuclear receptor ERRα is dependent on coactivators such as PGC-1α to exert gene-regulatory control of cellular energy metabolism pathways. In this study we sought to identify additional coregulators of ERRα and found that Bcl3, a component of the cellular cytokine-induced inflammatory signaling cascade, is a potent ERRα coactivator. Our results indicate that Bcl3 interacts cooperatively with PGC-1α to synergistically boost the activity of ERRα and PPARα, resulting in an increase in the expression of target genes involved in cellular energy metabolism.
IκB proteins regulate the Rel family of transcription factors (p65, p50, p52, c-Rel, and RelB) (19, 21). The IκB family, which mediates key components of the cellular response to inflammation, consists of seven members: IκBα, IκBβ, IκBɛ, IκBζ, p100, p105, and Bcl3 (19, 21, 23, 31, 59, 60). The best-characterized family member, IκBα, sequesters the cytokine-stimulated transcription factor, NF-κB (p65/p50 heterodimer), in the cytoplasm. Upon activation by tumor necrosis factor alpha or other cytokines, IκBα is phosphorylated by IκBα kinase, which leads to ubiquitination and proteasomal degradation of the IκBα complex. Once IκBα is degraded, NF-κB translocates to the nucleus and initiates changes in gene expression (8, 21). Interestingly, some of the gene targets of the NF-κB pathway are members of the IκB family, including Bcl3 (3, 16). Bcl3, in turn, preferentially binds to p50 and p52 homodimers, functioning as a transcriptional coactivator (1, 15). Bcl3 has also been shown to coactivate AP-1 and the nuclear receptor RXRα (38, 39). Our results demonstrate that ERRα and PPARα are also targets of Bcl3-mediated coactivation.
The classical model of nuclear receptor activation involves the exchange of coactivators for corepressors initiated by ligand binding to a pocket formed largely by COOH-terminal residues, resulting in a conformational change of the COOH-terminal AF-2 domain and realignment of helix 12, events that are believed to be critical for nuclear receptor-coactivator stabilization (42). Surprisingly, in contrast to most nuclear receptor-coactivator interactions, our results indicate that binding of Bcl3 to ERRα does not require the AF-2 domain. Instead, the Bcl3-ERRα interaction requires a region adjacent to the AF-2 on the NH2-terminal side. This unusual lack of dependence on the AF-2 region is also true of the previously defined Bcl3-RXRα interaction (38) with several notable differences. The Bcl3-RXRα interaction is influenced by the presence of 9-cis-retinoic acid (38), whereas ligand does not appear to be necessary for the Bcl3-ERRα or the Bcl3-PPARα interactions. In addition, the Bcl3-RXRα interaction involves the NH2-terminal ABC domain of RXRα (38), whereas the Bcl3-ERRα interaction requires domains within the hinge region (between the DNA binding domain and the ligand binding domain) and the first two-thirds of the ligand binding domain (Fig. 2A). We propose that the AF-2 domain-independent interaction of Bcl3 with ERRα may allow for simultaneous interaction of Bcl3 with PGC-1α and perhaps other coregulators (27, 47, 58).
We found that Bcl3 and PGC-1α interact in a cooperative manner. Mapping of this interaction revealed the surprising finding that the Bcl3 interaction requires the COOH-terminal 96 amino acids of PGC-1α, a domain that, to our knowledge, has not previously been assigned a specific function. Again, this interaction site may allow for PGC-1α to interact simultaneously with Bcl3 and nuclear receptor partners (the latter via leucine-rich domains that exist in the amino terminal part of the molecule). Indeed, immunoprecipitation results demonstrate that ERRα, PGC-1α, and Bc13 form a complex. It is also of interest that the TRAP/Mediator complex interacts with PGC-1α through a domain near the Bcl3-interacting region within the arginine/serine (RS) domain (55). Recently, the interaction of PGC-1α with the transcriptional factor Yin-Yang 1 has also been reported to occur in the COOH-terminal half of PGC-1α, but the interaction domain(s) has not been delineated (9). Both Bcl3 and PGC-1α are coactivators without known enzymatic activity (10, 50), i.e., class II coactivators. Class II coactivators are thought to work by recruiting coactivators with histone acetyltransferase, histone methyltransferase, or histone kinase activity, i.e., class I coactivators. It is interesting that despite the lack of known enzymatic activity, Bcl3 and PGC-1α act synergistically to coactivate the transcriptional regulatory functions of ERRα and PPARα. It is tempting to speculate that the PGC-1α/Bcl3 complex recruits additional coregulators with such activity (e.g., SRC-1) with greater efficiency and avidity than either factor alone. Interestingly, Bcl3 has previously been reported to function with SRC-1, which possesses histone acetyltransferase activity (38). It is also possible that this cooperative activity is due to efficient recruitment of TRAP/Mediator components that, in turn, increase recruitment of the RNA polymerase II machinery. Lastly, our data also cannot exclude the possibility that Bcl3 interacts with the RNA recognition motif domain of PGC-1α because it was also disrupted by the COOH-terminal deletion.
Bcl3 was originally discovered as a putative oncogene involved in B-cell lymphocytic leukemia (41). Later, it was discovered to be a regulator of the NF-κB signaling cascade, placing it within the cellular inflammatory response (57). However, in contrast to most other members of the ΙκΒ family, Bcl3 can localize to the nucleus, where it serves as a coactivator of transcription factors involved in NF-κB signaling. Our results suggest that in addition to coactivating transcription factors relevant to the NF-κB pathway, Bcl3 coactivates PGC-lα, ERRα, and PPARα, key regulators of cellular fuel utilization pathways. PGC-1α is a logical Bcl3-interacting partner, given that it is a master regulator of genes involved in energy metabolism and, therefore, serves as a key nodal control point. It is possible that this response allows the cell to adapt energetically to the cellular stress of inflammation, providing bursts of ATP production. Accordingly, this regulatory cross talk could serve as an adaptive response to physiological stressors imposed on the heart. Moreover, this response is likely to be relevant to pathological states such as cardiac hypertrophy and ischemic insult, both of which trigger cytokine signals and activation of the NF-κB pathway (34, 35, 52).
Our gene expression array results provide additional information about the potential biological roles of Bcl3 in cardiac myocytes. The subset of genes we found to be activated by Bcl3 in this unbiased screen are consistent with its role as a coregulator of ERRα and PPARα, given that many of the activated genes are known targets for these nuclear receptors. Specifically, pathways involved in fatty acid oxidation, electron transport chain/oxidative phosphorylation, and oxidative stress responses were upregulated. However, the expression of other genes previously reported as direct ERRα targets, identified by a combination of ChIP and genomic DNA array, or ChIP-on-chip technique (13), were not regulated by Bcl3. Indeed, relatively few genes in our data set are known ERRα gene targets, yet many of the pathways are shared. This result could reflect differences in sensitivity and specificity between the gene expression array and ChIP-on-chip approaches, but it also raises the intriguing possibility that Bcl3 serves as a coactivator of a distinct subset of the PGC-1α/ERRα regulatory pathway, as a unique component of the inflammatory response. The gene expression array results also suggest that Bcl3 regulates cardiac gene-regulatory programs involved in the immune response, cell cycle control, and growth. Additional Bc13-regulated pathways of interest are those involved in peptidase activity and ubiquitin action, given that NF-κB signaling and Bcl3 have both been implicated in muscle atrophy programs (5, 26, 37). It will be of interest to delineate the possible role of nuclear receptors involved in these novel pathways.
In summary, we have shown that Bcl3 is a novel nuclear receptor coregulator that synergizes with the inducible coactivator PGC-1α to drive expression of ERRα and PPARα target genes in cardiac myocytes. Given the established role of Bcl3 in cytokine-triggered signaling, we propose that the Bcl3-PGC-1α interaction serves as a key interface for regulatory cross talk between inflammation and cellular energy metabolism, an adaptive cardiac stress response.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health to D.P.K. (R01 DK045416, R01 HL058493, and P50 HL077113) and J.Y. (KO8 HL076452). The Digestive Diseases Research Core Center is funded by NIH grant P30 DK52574; the Clinical Nutrition Research Unit Core Center is funded by NIH grant P30 DK56341.
We thank Mary Wingate and Shonna Hyde for assistance with manuscript preparation and Teresa Leone for critical review of the manuscript.
Footnotes
Published ahead of print on 18 May 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bours, V., G. Franzoso, V. Azarenko, S. Park, T. Kanno, K. Brown, and U. Siebenlist. 1993. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell 72729-739. [DOI] [PubMed] [Google Scholar]
- 2.Bowker-Kinley, M. M., W. I. Davis, P. Wu, R. A. Harris, and K. M. Popov. 1998. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem. J. 329191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasier, A. R., M. Lu, T. Hai, Y. Lu, and I. Boldogh. 2001. NF-κB-inducible BCL-3 expression is an autoregulatory loop controlling nuclear p50/NF-κB1 residence. J. Biol. Chem. 27632080-32093. [DOI] [PubMed] [Google Scholar]
- 4.Bundy, D. L., and T. W. McKeithan. 1997. Diverse effects of BCL3 phosphorylation on its modulation of NF-κB p52 homodimer binding to DNA. J. Biol. Chem. 27233132-33139. [DOI] [PubMed] [Google Scholar]
- 5.Cai, D., J. D. Frantz, N. E. Tawa, Jr., P. A. Melendez, B. C. Oh, H. G. Lidov, P. O. Hasselgren, W. R. Frontera, J. Lee, D. J. Glass, and S. E. Shoelson. 2004. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119285-298. [DOI] [PubMed] [Google Scholar]
- 6.Chambon, P. 2005. The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol. Endocrinol. 191418-1428. [DOI] [PubMed] [Google Scholar]
- 7.Chawla, A., J. J. Repa, R. M. Evans, and D. J. Mangelsdorf. 2001. Nuclear receptors and lipid physiology: opening the X-files. Science 2941866-1870. [DOI] [PubMed] [Google Scholar]
- 8.Chen, G., and D. V. Goeddel. 2002. TNF-0R1 signaling: a beautiful pathway. Science 2961634-1635. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham, J. T., J. T. Rodgers, D. H. Arlow, F. Vazquez, V. K. Mootha, and P. Puigserver. 2007. mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450736-740. [DOI] [PubMed] [Google Scholar]
- 10.Dechend, R., F. Hirano, K. Lehmann, V. Heissmeyer, S. Ansieau, F. G. Wulczyn, C. Scheidereit, and A. Leutz. 1999. The Bcl-3 oncoprotein acts as a bridging factor between NF-κB/Rel and nuclear coregulators. Oncogene 183316-3323. [DOI] [PubMed] [Google Scholar]
- 11.Dibbs, K. I., Y. Sadovsky, X. J. Li, S. S. Koide, S. Adler, and A. R. Fuchs. 1995. Estrogenic activity of RU 486 (mifepristone) in rat uterus and cultured uterine myocytes. Am. J. Obstet. Gynecol. 173134-140. [DOI] [PubMed] [Google Scholar]
- 12.Disch, D. L., T. A. Rader, S. Cresci, T. C. Leone, P. M. Barger, R. Vega, P. A. Wood, and D. P. Kelly. 1996. Transcriptional control of a nuclear gene encoding a mitochondrial fatty acid oxidation enzyme in transgenic mice: role for nuclear receptors in cardiac and brown adipose expression. Mol. Cell. Biol. 164043-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dufour, C. R., B. J. Wilson, J. M. Huss, D. P. Kelly, W. A. Alaynick, M. Downes, R. M. Evans, M. Blanchette, and V. Giguere. 2007. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 5345-356. [DOI] [PubMed] [Google Scholar]
- 14.Evans, R. M. 2005. The nuclear receptor superfamily: a Rosetta stone for physiology. Mol. Endocrinol. 191429-1438. [DOI] [PubMed] [Google Scholar]
- 15.Fujita, T., G. P. Nolan, H. C. Liou, M. L. Scott, and D. Baltimore. 1993. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-κB p50 homodimers. Genes Dev. 71354-1363. [DOI] [PubMed] [Google Scholar]
- 16.Ge, B., O. Li, P. Wilder, A. Rizzino, and T. W. McKeithan. 2003. NF-κB regulates BCL3 transcription in T lymphocytes through an intronic enhancer. J. Immunol. 1714210-4218. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16225-260. [DOI] [PubMed] [Google Scholar]
- 18.Giguere, V. 2008. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr. Rev. 29677-696. [DOI] [PubMed] [Google Scholar]
- 19.Gilmore, T. D., and P. J. Morin. 1993. The I kappa B proteins: members of a multifunctional family. Trends Genet. 9427-433. [DOI] [PubMed] [Google Scholar]
- 20.Gorman, C. 1985. High efficiency gene transfer into mammalian cells, p. 143-190. In D. M. Glover (ed.), DNA cloning, a practical approach, vol. II. IRL Press, Oxford, United Kingdom. [Google Scholar]
- 21.Hacker, H., and M. Karin. 2006. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006:re13. [DOI] [PubMed] [Google Scholar]
- 22.Handschin, C., and B. M. Spiegelman. 2006. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 27728-735. [DOI] [PubMed] [Google Scholar]
- 23.Haruta, H., A. Kato, and K. Todokoro. 2001. Isolation of a novel interleukin-1-inducible nuclear protein bearing ankyrin-repeat motifs. J. Biol. Chem. 27612485-12488. [DOI] [PubMed] [Google Scholar]
- 24.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 952509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzog, B., J. Cardenas, R. K. Hall, J. A. Villena, P. J. Budge, V. Giguere, D. K. Granner, and A. Kralli. 2006. Estrogen-related receptor alpha is a repressor of phosphoenolpyruvate carboxykinase gene transcription. J. Biol. Chem. 28199-106. [DOI] [PubMed] [Google Scholar]
- 26.Hunter, R. B., and S. C. Kandarian. 2004. Disruption of either the Nfkb1 or the Bcl-3 gene inhibits skeletal muscle atrophy. J. Clin. Investig. 1141504-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huss, J. M., R. P. Kopp, and D. P. Kelly. 2002. Peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ. Identification of novel leucine-rich interaction motif within PGC-1α. J. Biol. Chem. 27740265-40274. [DOI] [PubMed] [Google Scholar]
- 28.Huss, J. M., F. H. Levy, and D. P. Kelly. 2001. Hypoxia inhibits the peroxisome proliferator-activated receptor alpha/retinoid X receptor gene regulatory pathway in cardiac myocytes: a mechanism for O2-dependent modulation of mitochondrial fatty acid oxidation. J. Biol. Chem. 27627605-27612. [DOI] [PubMed] [Google Scholar]
- 29.Huss, J. M., I. P. Torra, B. Staels, V. Giguere, and D. P. Kelly. 2004. Estrogen-related receptor α directs peroxisome proliferator-activated receptor α signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol. Cell. Biol. 249079-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamei, Y., H. Ohizumi, Y. Fujitani, T. Nemoto, T. Tanaka, N. Takahashi, T. Kawada, M. Miyoshi, O. Ezaki, and A. Kakizuka. 2003. PPARγ coactivator 1β/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc. Natl. Acad. Sci. USA 10012378-12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitamura, H., K. Kanchira, K. Okita, M. Morimatsu, and M. Saito. 2000. MAIL, a novel nuclear IκB protein that potentiates LPS-induced IL-6 production. FEBS Lett. 48553-56. [DOI] [PubMed] [Google Scholar]
- 32.Lehman, J. J., P. M. Barger, A. Kovacs, J. E. Saffitz, D. M. Medeiros, and D. P. Kelly. 2000. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Investig. 106847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, and R. M. Evans. 1995. The nuclear receptor superfamily: the second decade. Cell 83835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann, D. L. 2003. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu. Rev. Physiol. 6581-101. [DOI] [PubMed] [Google Scholar]
- 35.Mann, D. L., and M. R. Bristow. 2005. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 1112837-2849. [DOI] [PubMed] [Google Scholar]
- 36.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108465-474. [DOI] [PubMed] [Google Scholar]
- 37.Mourkioti, F., P. Kratsios, T. Luedde, Y. H. Song, P. Delafontaine, R. Adami, V. Parente, R. Bottinelli, M. Pasparakis, and N. Rosenthal. 2006. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J. Clin. Investig. 1162945-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Na, S. Y., H. S. Choi, J. W. Kim, D. S. Na, and J. W. Lee. 1998. Bcl3, an IκB protein, as a novel transcription coactivator of the retinoid X receptor. J. Biol. Chem. 27330933-30938. [DOI] [PubMed] [Google Scholar]
- 39.Na, S. Y., J. E. Choi, H. J. Kim, B. H. Jhun, Y. C. Lee, and J. W. Lee. 1999. Bcl3, an IκB protein, stimulates activating protein-1 transactivation and cellular proliferation. J. Biol. Chem. 27428491-28496. [DOI] [PubMed] [Google Scholar]
- 40.Nolan, G. P., T. Fujita, K. Bhatia, C. Huppi, H. C. Liou, M. L. Scott, and D. Baltimore. 1993. The bcl-3 proto-oncogene encodes a nuclear IκB-like molecule that preferentially interacts with NF-κB p50 and p52 in a phosphorylation-dependent manner. Mol. Cell. Biol. 133557-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohno, H., G. Takimoto, and T. W. McKeithan. 1990. The candidate proto-oncogene Bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell 60991-997. [DOI] [PubMed] [Google Scholar]
- 42.O'Malley, B. W. 2005. A life-long search for the molecular pathways of steroid hormone action. Mol. Endocrinol. 191402-1411. [DOI] [PubMed] [Google Scholar]
- 43.Pascual, G., A. L. Fong, S. Ogawa, A. Gamliel, A. C. Li, V. Perissi, D. W. Rose, T. M. Willson, M. G. Rosenfeld, and C. K. Glass. 2005. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature 437759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puigserver, P., J. Rhee, J. Lin, Z. Wu, J. C. Yoon, C. Y. Zhang, S. Krauss, V. K. Mootha, B. B. Lowell, and B. M. Spiegelman. 2001. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARγ coactivator-1. Mol. Cell 8971-982. [DOI] [PubMed] [Google Scholar]
- 45.Puigserver, P., Z. Wu, C. W. Park, R. Graves, M. Wright, and B. M. Spiegelman. 1998. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92829-839. [DOI] [PubMed] [Google Scholar]
- 46.Robinson, C. E., X. Wu, D. C. Morris, and J. M. Gimble. 1998. DNA bending is induced by binding of the peroxisome proliferator-activated receptor gamma 2 heterodimer to its response element in the murine lipoprotein lipase promoter. Biochem. Biophys. Res. Commun. 244671-677. [DOI] [PubMed] [Google Scholar]
- 47.Schreiber, S. N., D. Knutti, K. Brogli, T. Uhlmann, and A. Kralli. 2003. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRα). J. Biol. Chem. 2789013-9018. [DOI] [PubMed] [Google Scholar]
- 48.Shang, Y., X. Hu, J. DiRenzo, M. A. Lazar, and M. Brown. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103843-852. [DOI] [PubMed] [Google Scholar]
- 49.Sonoda, J., J. Laganiere, I. R. Mehl, G. D. Barish, L. W. Chong, X. Li, I. E. Scheffler, D. C. Mock, A. R. Bataille, F. Robert, C. H. Lee, V. Giguere, and R. M. Evans. 2007. Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors of IFN-gamma-induced host defense. Genes Dev. 211909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soyal, S., F. Krempler, H. Oberkofler, and W. Patsch. 2006. PGC-1α: a potent transcriptional cofactor involved in the pathogenesis of type 2 diabetes. Diabetologia 491477-1488. [DOI] [PubMed] [Google Scholar]
- 51.Sugden, M. C., and M. J. Holness. 2003. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Phys. Endocrinol. Metab. 284E855-E862. [DOI] [PubMed] [Google Scholar]
- 52.Valen, G., Z. Yan, and G. K. Hansson. 2001. Nuclear factor kappa-B and the heart. J. Am. Coll. Cardiol. 38307-314. [DOI] [PubMed] [Google Scholar]
- 53.Vats, D., L. Mukundan, J. I. Odegaard, L. Zhang, K. L. Smith, C. R. Morel, R. A. Wagner, D. R. Greaves, P. J. Murray, and A. Chawla. 2006. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 413-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vega, R. B., J. M. Huss, and D. P. Kelly. 2000. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 201868-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallberg, A. E., S. Yamamura, S. Malik, B. M. Spiegelman, and R. G. Roeder. 2003. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1α. Mol. Cell 121137-1149. [DOI] [PubMed] [Google Scholar]
- 56.Wende, A. R., J. M. Huss, P. J. Schaeffer, V. Giguere, and D. P. Kelly. 2005. PGC-1α coactivates PDK4 gene expression via the orphan nuclear receptor ERRα: a mechanism for transcriptional control of muscle glucose metabolism. Mol. Cell. Biol. 2510684-10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wulczyn, F. G., M. Naumann, and C. Scheidereit. 1992. Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-κB. Nature 358597-599. [DOI] [PubMed] [Google Scholar]
- 58.Xie, W., H. Hong, M. M. Yang, R. J. Lin, C. M. Simon, M. R. Stallcup, and R. M. Evans. 1999. Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2. Mol. Endocrinol. 132151-2162. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto, M., S. Yamazaki, S. Uematsu, S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Kuwata, O. Takeuchi, K. Takeshige, T. Saitoh, S. Yamaoka, N. Yamamoto, S. Yamamoto, T. Muta, K. Takeda, and S. Akira. 2004. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature 430218-222. [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki, S., T. Muta, and K. Takeshige. 2001. A novel IκB protein, IκB-ζ, induced by proinflammatory stimuli, negatively regulates nuclear factor-κB in the nuclei. J. Biol. Chem. 27627657-27662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.