Abstract
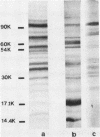
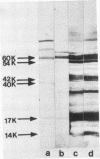
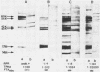
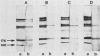
The utility of sodium dodecyl sulfate-polyacrylamide gel electrophoresis immunoblotting as a serological tool in the diagnosis of human syphilitic infections was examined. In model experiments, rabbits were immunized with Treponema pallidum or T phagedenis, and the antisera were tested for cross-reactivities with both sets of antigens. A major T. pallidum antigen with a molecular weight of ca. 17,000 appeared to be the most reliable specific antigenic marker as assessed by the immunoblotting technique with peroxidase-labeled second antibodies. Antibodies to this antigen were never detected in hyperimmune rabbit anti-T. phagedenis sera or in the sera of nonsyphilitic humans. In contrast, reactive antibodies were found in all syphilitic human sera and also in liquor samples that were positive in the passive hemagglutination test. Differentiation between immunoglobulin M and immunoglobulin G antibodies was directly possible by applying the respective specific second antibodies. Immunoblotting tests were performed with sera exhibiting low passive hemagglutination test titers and equivocal fluorescent treponemal antibody and rapid plasma reagin card reactions. In more than 60% of these cases, immunoblot positivity with respect to the 17,000-molecular-weight antigen was found. The same results were obtained with partially purified 17,000-molecular-weight antigen. The immunoblot technique should be useful as an additional diagnostic tool for differentiating between true and false-positive serological reactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker-Zander S. A., Lukehart S. A. Molecular basis of immunological cross-reactivity between Treponema pallidum and Treponema pertenue. Infect Immun. 1983 Nov;42(2):634–638. doi: 10.1128/iai.42.2.634-638.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Adams C. B., Musher D. M. Circulating immune complexes in experimental syphilis: identification of treponemal antigens and specific antibodies to treponemal antigens in isolated complexes. Infect Immun. 1983 Nov;42(2):585–593. doi: 10.1128/iai.42.2.585-593.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Klump O. The terminal membrane C5b-9 complex of human complement. Evidence for the existence of multiple protease-resistant polypeptides that form the trans-membrane complement channel. J Immunol. 1980 May;124(5):2451–2457. [PubMed] [Google Scholar]
- Hanff P. A., Bishop N. H., Miller J. N., Lovett M. A. Humoral immune response in experimental syphilis to polypeptides of Treponema pallidum. J Immunol. 1983 Oct;131(4):1973–1977. [PubMed] [Google Scholar]
- Hanff P. A., Fehniger T. E., Miller J. N., Lovett M. A. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J Immunol. 1982 Sep;129(3):1287–1291. [PubMed] [Google Scholar]
- Hanff P. A., Miller J. N., Lovett M. A. Molecular characterization of common treponemal antigens. Infect Immun. 1983 May;40(2):825–828. doi: 10.1128/iai.40.2.825-828.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Gubish E. R., Jr Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J Immunol. 1982 Aug;129(2):833–838. [PubMed] [Google Scholar]
- Moskophidis M., Müller F. Molecular analysis of immunoglobulins M and G immune response to protein antigens of Treponema pallidum in human syphilis. Infect Immun. 1984 Jan;43(1):127–132. doi: 10.1128/iai.43.1.127-132.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. S., Axelsen N. H., Jørgensen B. B., Petersen C. S. Antibodies in secondary syphilis against five of forty Reiter treponeme antigens. Scand J Immunol. 1980;11(6):629–633. doi: 10.1111/j.1365-3083.1980.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Pedersen N. S., Axelsen N. H., Petersen C. S. Antigenic analysis of Treponema pallidum: cross-reactions between individual antigens of T. pallidum and T. Reiter. Scand J Immunol. 1981;13(2):143–150. doi: 10.1111/j.1365-3083.1981.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Petersen C. S., Pedersen N. S., Axelsen N. H. Purification of a Reiter treponemal protein antigen that is immunologically related to an antigen in Treponema pallidum. Infect Immun. 1982 Mar;35(3):974–978. doi: 10.1128/iai.35.3.974-978.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]