Abstract
The p38 mitogen-activated protein kinase (MAPK) pathway is required for differentiation of skeletal myoblasts, but how the pathway is activated during this process is not well understood. One mechanism involves the cell surface receptor Cdo (also known as Cdon), which binds to Bnip-2 and JLP, scaffold proteins for Cdc42 and p38, respectively; formation of these complexes results in Bnip-2/Cdc42-dependent activation of p38. It has been reported that the tyrosine kinase Abl promotes myogenic differentiation in a manner dependent on its cytoplasmic localization, but the cytoplasmic signaling proteins with which it interacts to achieve this effect are unidentified. We report that Abl associates with both Cdo and JLP during myoblast differentiation. Abl binds a proline-rich motif in Cdo via its SH3 domain, and these regions of Abl and Cdo are required for their promyogenic effects. Cdo is important for full Abl kinase activity, and Abl is necessary for full activation of p38 MAPK, during myogenic differentiation. As seen with myoblasts depleted of Cdo, the diminished differentiation displayed by Abl-depleted cells is rescued by the expression of an activated form of the immediate upstream p38-activating kinase MAPK kinase 6. Abl's promyogenic effect is therefore linked to a multiprotein cell surface complex that regulates differentiation-dependent p38 activation.
The process of cell differentiation involves the acquisition by a precursor cell of a specialized transcriptional program that results in tissue-specific structure and function. Differentiation of skeletal myoblasts, a widely studied model system, is orchestrated by the myogenic regulatory factors of the MyoD family (36, 45). Expression of MyoD in many nonmuscle cell types converts such cells to skeletal muscle cells, revealing its ability to act as a master regulator in driving tissue-specific transcription and cell differentiation (45). MyoD's ability to function in this manner occurs in conjunction with non-muscle-specific factors, such as E proteins, Mef2 family members, transcriptional coactivators and corepressors, and chromatin-remodeling factors (45). Furthermore, MyoD activity is dependent on signal transduction pathways that influence these interactions. The extracellular-signal-activated p38α/β mitogen-activated protein kinase (MAPK) pathway plays a particularly prominent role in this regard. There is a persistent rise in p38α/β (hereafter simply p38) activity during myogenesis, and inhibition of p38 expression or activity blocks induction of select muscle-specific genes and myogenic differentiation (3, 11, 29, 35, 50). p38 activity regulates myogenesis at several levels, including cell cycle control, MyoD dimerization with E proteins, Mef2 transcriptional activity, chromatin remodeling at muscle-specific genes, and stability of myogenic mRNAs (6, 12, 28, 38, 40, 41, 50). However, despite the documented role of the p38 MAPK pathway in myogenesis, the signaling mechanisms by which it becomes activated during this process are not well understood.
Cdo (also known as Cdon) is a multifunctional cell surface receptor that harbors Ig and FnIII repeats in its ectodomain and a 260-amino-acid intracellular region that lacks significant sequence resemblance to other proteins (22). Cdo is a vertebrate member of a subfamily of the immunoglobulin (Ig) superfamily that also includes Boc in vertebrates and Ihog and Boi in Drosophila (23, 51). Mice lacking Cdo display delayed skeletal muscle development, and myoblasts derived from such mice differentiate defectively in culture (9). Similarly, C2C12 myoblasts depleted of Cdo by RNA interference (RNAi) differentiate inefficiently, while overexpression of Cdo in such cells accelerates and enhances differentiation (24, 43). Cdo's promyogenic effects are exerted mainly through activation of the p38 MAPK pathway, via a distinctive mechanism. Furthermore, the activation of p38 MAPK that occurs in differentiating myoblasts is largely, but not completely, dependent on Cdo (43). During myoblast differentiation, the Cdo intracellular region binds to Bnip-2, a scaffold-like protein for the small GTPase Cdc42, and to JLP, a scaffold protein for the p38 MAPK pathway (20, 43). The Cdo-Bnip-2-Cdc42 interaction stimulates Cdc42 activity, which is, in turn, required for the differentiation-dependent increase in p38 activity. Bnip-2 and JLP associate indirectly in a Cdo-dependent manner, implying that Cdc42 bound to Cdo via Bnip-2 signals to activate p38 bound to Cdo via JLP (20). A similar pathway regulates neuronal differentiation in vitro (34), and we have proposed that formation of this signaling complex represents one mechanism for differentiation-specific activation of p38 MAPK. It is therefore of obvious interest to identify additional components and regulators of Cdo-containing signaling complexes involved in cell differentiation.
Abl is a ubiquitously expressed nonreceptor tyrosine kinase involved in many signaling processes and contains, in addition to its kinase domain, SH2, SH3, DNA-binding, and actin-binding domains (16). Abl shuttles between the nucleus and cytoplasm and has various biological roles that depend on its subcellular localization and the initiating stimulus (42, 47). Activation of nuclear Abl occurs following DNA damage or death receptor signaling and plays a role in checkpoint responses and apoptosis. In contrast, activation of cytoplasmic Abl in response to platelet-derived growth factor promotes cell proliferation. Additionally, Abl binds directly to the intracellular region of the Ig/FnIII repeat-containing axon guidance receptor Robo and regulates cytoskeleton dynamics and cell-cell adhesion of axonal growth cones (2, 31).
Proliferating myoblasts contain nuclear and cytoplasmic Abl, but when induced to differentiate, Abl rapidly exits the nucleus and accumulates in the cytoplasm (13, 37). Acetylation of Abl by histone acetyltransferases is involved in this process, and cytoplasmic, but not nuclear, Abl promotes myogenic differentiation (13). These results imply that Abl interacts with other cytoplasmic proteins that are also involved in promoting myogenesis, but such proteins are as yet unidentified. We report here that Abl binds to the cytoplasmic tail of Cdo and also to the p38 MAPK scaffold JLP. Abl binds a proline-rich motif in Cdo via its SH3 domain, and structure-function analyses reveal that these regions of Abl and Cdo are important for their promyogenic effects. Furthermore, Cdo is involved in the activation of Abl, and Abl is necessary for full activation of p38 MAPK, during myogenic differentiation. Myoblasts depleted of Cdo or Abl by RNAi have a similar phenotype, and as reported for Cdo (43), the diminished differentiation displayed by Abl-depleted cells is rescued by expression of an activated form of the immediate upstream p38 activating kinase MAPK kinase 6 (MKK6). Thus, Abl's promyogenic effect is linked to a multiprotein signaling complex that forms at the cytoplasmic face of a cell surface receptor to regulate differentiation-dependent p38 activation.
MATERIALS AND METHODS
Cell culture and expression vectors.
C2C12 cells and 293T cells were cultured as previously described (9, 20, 24, 25). C2C12 cells were induced to differentiate at near confluence in Dulbecco modified Eagle medium containing 2% horse serum (differentiation medium [DM]), and myotube formation in stable and transient assays was quantified as previously described (25). Statistical analysis of myotube formation was done with Student's t test. To generate C2C12 cell lines that stably overexpress Cdo, mutant forms of Cdo, Abl, mutant forms of Abl, or small interfering RNAs (siRNAs) against Cdo or Abl, cells were transfected with the indicated expression vectors and FuGene6, and cultures were selected in puromycin- or hygromycin-containing medium. Drug-resistant cells were pooled and analyzed for Western blotting or myosin heavy chain (MHC) staining. For experiments in which mutant forms of Cdo were assessed for the ability to rescue the effects of siRNA against Cdo, a transient coexpression approach was employed as described previously (20). Briefly, C2C12 cells were cotransfected with pSuper driving a previously validated Cdo siRNA sequence (no. 1) or an irrelevant siRNA sequence (20, 52), the full-length Cdo or mutant forms of Cdo expressed via pBabePuro (pBP) (22, 43), and pQ-lacZ, a vector driving the expression of nucleus-localized β-galactosidase (β-Gal). Forty-eight hours after transfection, the cells were transferred into DM and, 48 h later, fixed and stained for both MHC expression and β-Gal activity. Transfection efficiencies for these experiments were ∼10% to minimize the fusion of independent β-Gal+ transfectants. The expression constructs for Abl, kinase-deficient Abl (Abl-KD), and deletion mutant forms of Abl (48) were kindly provided by J. Y. J. Wang (University of California San Diego, La Jolla). Where noted, cultures were treated with either 1.4 mM EGTA (18) or 10 μM anisomycin (Sigma).
The Cdo intracellular region was amplified by PCR by using pBP-Cdo as a template and the following primers: sense, 5′-ATAGGATCCTGGAAGAGTCGCCAACAG-3′; antisense, 5′-ATGGTACCTCAGGTCTCTTGGGCTTG-3′. The PCR fragment was cut with BamHI and KpnI and cloned into the pEBG-3X vector. The CdoP/A point mutant protein was constructed by PCR mutagenesis by using pBP-Cdo as a template and the primer 5′-AAGACAGCGAGGTCTGCCGCTGGAGCTGCACTTGACGGGCTGTCG-3′. For Abl siRNA studies, the following sequence was inserted into the pSilencer 2.1-U6 hygro and pSuper-puro vectors: 5′-GCAACAAGCCCACTATCTA-3′.
Western blot analysis and immunoprecipitation.
Western blot analyses were performed as previously described (22). Briefly, cells were lysed in extraction buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM EGTA, 1% Triton, 10 mM NaF, 1 mM Na3VO4, Complete protease inhibitor cocktail [Roche]) and sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed. The primary antibodies used were anti-Cdo (Zymed), anti-pan-cadherin (Sigma), anti-β-tubulin (Zymed), anti-p38 (Sigma), anti-pp38 (the phosphorylated, active form of p38; Cell Signaling Technology), anti-MHC (MF-20; Developmental Studies Hybridoma Bank), anti-myogenin (Santa Cruz Biotechnology, Inc.), anti-Abl (Santa Cruz Biotechnology, Inc.), anti-phospho-Abl(pY245) (Zymed), anti-S-probe (Santa Cruz Biotechnology, Inc.), and anti-JLP (Abcam). For glutathione S-transferase (GST) pulldown experiments, 293T cells were transfected with the combination of full-length Abl or deletion mutant forms of Abl and the GST-Cdo intracellular domain construct, as indicated. Forty-eight hours later, whole-cell extracts were incubated overnight at 4°C with 20 μl of a 50% slurry of glutathione-Sepharose 4B beads. Beads were washed three times with, and suspended in, extraction buffer, and samples were analyzed by Western blotting. To study the formation of Abl-Cdo and Abl-JLP complexes, coimmunoprecipitation was performed as described previously (23).
Yeast two-hybrid assay.
cDNA fragments encoding either the SH2 or the SH3 domain of Abl were ligated to the Gal4 DNA-binding domain in the pGBT9 vector, and a cDNA encoding the Cdo intracellular domain was ligated to the Gal4 activation domain in the pGAD vector. Yeast strain AH109 (Clontech) was sequentially transformed with these vectors, and β-Gal activity was detected by colony lift filter assay.
Abl kinase assay.
Abl kinase assays were performed as described by Choi et al. (8). C2C12 cell extract (500 μg) was immunoprecipitated with anti-Abl antibody (2 μg/ml) at 4°C for 4 h. The immunoprecipitates were washed twice with kinase reaction buffer (50 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol, 2.5 mM EGTA, 1 mM NaF, 0.1 mM Na3VO4, 10 mM β-glycerophosphate) and resuspended in 20 μl of kinase reaction buffer. The assay was initiated by the addition of 20 μl of kinase reaction buffer containing 10 μg of myelin basic protein (MBP) substrate and 2 μCi of [γ-32P]ATP (ICN). The reactions were carried out at 30°C for 30 min and terminated by addition of SDS sample buffer, followed by SDS-polyacrylamide gel electrophoresis and autoradiography.
Immunocytochemistry and microscopy.
Immunostaining for MHC expression and β-Gal activity was performed as described previously (25), and images were captured and processed with a Nikon ECLIPSE TE-2000U and NIS-Elements F software. For the results shown in Fig. 6, C2C12 cells in 12-well plates were cotransfected with 100 ng of enhanced green fluorescent protein (GFP) expression vector and 900 ng of the indicated DNA constructs for 2 days, fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.5% Triton X-100 in phosphate-buffered saline, blocked, and stained with anti-pp38MAPK, anti-p38MAPK, or anti-MHC, followed by an Alexa Fluor 568-conjugated secondary antibody (Molecular Probes). Images were obtained on a Zeiss LSM-510 Meta confocal microscope. Quantification of the fluorescent signal for p38 and pp38 was performed with Image-Gauge software (Fuji Film, Tokyo, Japan).
FIG. 6.
Abl promotes p38 MAPK activation during myogenic differentiation. (A) C2C12 cells were transiently transfected with Abl siRNA or control (pSilencer) vectors plus a GFP vector to mark transfectants. Confluent cultures that had not been fed with fresh medium for 48 h were then fixed and stained with antibodies to activated p38 MAPK (pp38) (red) and visualized for GFP expression (green). Cell nuclei were visualized by staining with 4′,6-diamidino-2-phenylindole (DAPI; blue). White arrows indicate untransfected cells, and red arrows indicate transfected cells. (B) Quantification of cultures shown in panel A. GFP+ cells were scored as positive or negative for pp38 staining. Values represent means of triplicate determinations ± 1 standard deviation. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at P < 0.01. (C) C2C12 cells were transiently transfected with Abl siRNA, Cdo siRNA, or control vectors plus a GFP vector to mark transfectants. Cultures were incubated in DM for 48 h and then fixed and stained with antibodies to pp38 MAPK (top; red) or total p38 (bottom; red) and visualized for GFP expression (green). White arrows indicate transfected cells. (D) Quantification of cultures shown in panel C. The intensity of the immunofluorescent pp38 and p38 signals in GFP+ and GFP− cells was quantified, with the values obtained from control vector-transfected cultures set to 1.0. Values represent means of triplicate determinations ± 1 standard deviation. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at P < 0.01. (E) Lysates of C2C12 cells that stably express Abl siRNA or control (pSilencer) vectors were cultured in DM for 48 h and Western blotted with antibodies to Abl, β-tubulin (as a loading control), pp38, and total p38. (F) C2C12 cells that stably express Abl siRNA (+) or control (−) vectors in GM were treated with 10 μM anisomycin (Aniso) or the dimethyl sulfoxide vehicle as a control (Con) for 30 min. Lysates were Western blotted with antibodies to pp38 and total p38.
RESULTS
Cdo interacts with Abl.
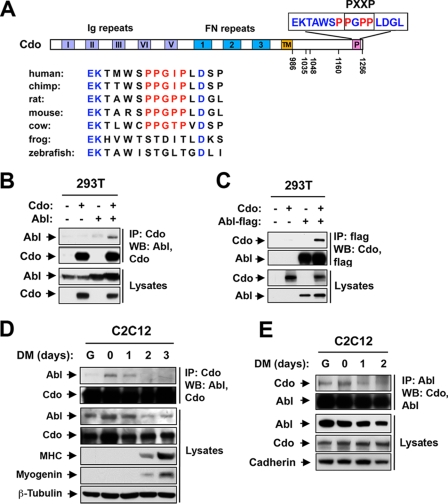
Sequence analysis of the Cdo intracellular region revealed a PXXP motif within a context predicted by Scansite (33) to function as a ligand for the Abl SH3 domain. This motif is present near the carboxyl terminus and is conserved in mammals but not other vertebrates (Fig. 1A). Because Cdo and Abl each promote myogenic differentiation, we asked whether they interact. 293T cells were transiently transfected with expression vectors for Cdo and Abl, and lysates were immunoprecipitated with antibodies to Cdo or Abl and then blotted for the reciprocal proteins; Abl coprecipitated with Cdo and Cdo coprecipitated with Abl (Fig. 1B and C). To assess whether these proteins interact endogenously during myogenesis, Cdo or Abl was immunoprecipitated from proliferating and differentiating C2C12 myoblasts and precipitates were analyzed for the presence of Abl or Cdo, respectively. While some association was observed in proliferating cultures, Cdo and Abl coprecipitated most efficiently when the cells were nearly confluent (day 0) and during the first 24 h in DM; the interaction diminished thereafter, as did the overall level of Abl protein (Fig. 1D and E).
FIG. 1.
Cdo interacts with Abl. (A) Schematic of the Cdo intracellular region, highlighting the PXXP motif and surrounding sequences from several species. Numbering is from rat Cdo, from which expression constructs are derived. Note that the frog (Xenopus laevis) and zebrafish (Danio rerio) Cdo sequences lack this motif, despite conserved amino acids that flank the motif. Chicken (Gallus gallus) Cdo lacks a region that clearly aligns with these sequences (not shown). TM, transmembrane. (B) Lysates of 293T cells transiently transfected with Cdo, Abl, or control (−) expression vectors, as indicated, were immunoprecipitated (IP) with an antibody to Cdo and then Western blotted (WB) with Cdo or Abl antibodies. Note that Cdo is not expressed endogenously in 293T cells (22). (C) Lysates of 293T cells transiently transfected with Cdo, Flag-tagged Abl, or control (−) expression vectors, as indicated, were immunoprecipitated with an antibody to the Flag epitope and then Western blotted with Cdo or flag antibodies. (D) Lysates of C2C12 cells that were proliferating in GM (lane G), at near confluence (0) or in DM for the indicated times were immunoprecipitated with antibodies to Cdo and Western blotted with Abl or Cdo antibodies. Straight lysates were also Western blotted with antibodies to Abl and Cdo, to myogenin and MHC to monitor differentiation, and to β-tubulin as a loading control. (E) Same as in panel D, except that lysates were immunoprecipitated with antibodies to Abl and Western blotted with Abl or Cdo antibodies, and straight lysates were blotted with antibodies to Abl and Cdo and to pan-cadherin as a loading control.
We next determined whether the Abl SH3 domain and the Cdo PXXP motif are involved in their association. A yeast two-hybrid assay was performed with the Cdo intracellular region plus the Abl SH2 or SH3 domain, and interaction was revealed by β-Gal production. Cdo interacted with the SH3, but not the SH2, domain (Fig. 2A). Consistent with this result, in transiently transfected 293T cells, a deletion mutant Abl protein that lacked the SH3 domain was pulled down only very weakly by a GST-Cdo intracellular region fusion protein, whereas a deletion mutant Abl protein that lacked the SH2 domain was pulled down as efficiently as full-length Abl (Fig. 2B; Abl was not pulled down when GST alone was expressed in transfectants [data not shown]). These results indicate that the Abl SH3 domain is sufficient and necessary for association with Cdo. Mutant forms of Cdo that contain deletions in the cytoplasmic tail were then assessed for the ability to coimmunoprecipitate with Abl in 293T cell transfectants. Mutant forms of Cdo that lacked amino acids in the juxtamembrane region (CdoΔ986-1048) or middle portion (CdoΔ1035-1160) of the cytoplasmic tail precipitated with Abl as efficiently as did full-length Cdo (Fig. 2C). In contrast, a mutant protein lacking the C-terminal 90 amino acids (CdoΔ1160-1256), which include the PXXP motif, precipitated with Abl inefficiently. To test whether the proline residues in the PXXP motif were directly involved in Abl binding, they were changed to alanine residues within the context of the GST-Cdo intracellular region fusion construct (the mutant protein was designated CdoP/A). In transiently transfected 293T cells, wild-type GST-Cdo pulled down Abl more efficiently than did GST-CdoP/A, though the latter still displayed detectable association with Abl (Fig. 2D). In contrast, a different Cdo intracellular region-binding protein, JLP, was pulled down equally well by both GST-Cdo proteins (Fig. 2E). Taken together, these results are consistent with the conclusion that the Abl SH3 domain binds to the PXXP motif present near the Cdo carboxyl terminus.
FIG. 2.
Cdo and Abl interact through their proline-rich motif and SH3 domain, respectively. (A) Yeast cells transformed with the indicated vectors were tested for expression of β-Gal, indicative of interaction. AD, Gal4 activation domain; BD, Gal4 DNA-binding domain. (B) Lysates of 293T cells transiently transfected with GST-Cdo intracellular region (GST-Cdointra), Abl, AblΔSH2, AblΔSH3, or control (−) expression vectors, as indicated, were pulled down with glutathione-Sepharose beads and blotted with an antibody to Abl. Straight lysates were also Western blotted (WB) with antibodies to the Cdo intracellular region or Abl. (C) Lysates of 293T cells transiently transfected with Cdo, a deletion mutant form of Cdo, Abl, or control (−) expression vectors, as indicated, were immunoprecipitated (IP) with Abl antibodies and then Western blotted with an antibody to Cdo. (D) Lysates of 293T cells transiently transfected with GST-Cdointra, GST-CdointraP/A, Abl, or control (−) expression vectors, as indicated, were pulled down with glutathione-Sepharose beads and blotted with antibodies to Abl or Cdo. Straight lysates were also Western blotted with antibodies to Abl or Cdo. (E) Lysates of 293T cells transiently transfected with GST-Cdointra, GST-CdointraP/A, S epitope-tagged JLP (JLP-S), or control (−) expression vectors, as indicated, were pulled down with glutathione-Sepharose beads and blotted with antibodies to the S epitope or Cdo. Straight lysates were also Western blotted with antibodies to the S epitope or Cdo.
The Abl-binding region of Cdo is functional in myogenic differentiation.
The intracellular region deletion mutant forms of Cdo used in Fig. 2C were stably expressed in C2C12 myoblasts, and the ability of such cells to differentiate was compared to that of vector control cells and cells that stably expressed full-length Cdo. Overexpression of full-length Cdo enhanced the formation of myotubes containing large numbers of nuclei and the expression of MHC; in contrast, the expression of each deletion mutant protein at a level similar to that of the full-length protein had little effect on these parameters, relative to the vector control cells (Fig. 3A to C). Similar to the three deletion mutant proteins, stable expression of full-length Cdo harboring the P/A point mutations also had little effect on C2C12 cell differentiation (Fig. 3D to G). Therefore, the individual deletion mutant proteins and the P/A mutant protein behaved as proteins with loss-of-function mutations in this context. We previously reported that the expression of a secreted, soluble Cdo ectodomain in C2C12 cells reduced myogenesis, acting as a putative dominant negative factor (24). The difference between that result (i.e., an inhibitory effect) and the results reported here for the deletion and P/A mutant proteins (i.e., a loss-of-function effect) is likely due to a higher level of expression of the soluble construct versus the other constructs. To address this issue further, we asked whether the CdoΔ1160-1256 mutant protein (which lacks the PXXP motif) or the CdoP/A mutant protein is able to rescue the reduction in differentiation that occurs on depletion of Cdo by RNAi. A transient myogenesis assay that scores expression of MHC and myotube formation by transfectants was used (20, 25). C2C12 cells were cotransfected with control or Cdo siRNA expression vectors plus control or Cdo (wild-type or mutant protein) expression vectors. Under all conditions, a vector that drives the expression of nucleus-localized β-Gal was included to mark transfectants. Forty-eight hours after transfection, the cultures were transferred to DM, and 72 h later, the cells were double stained for β-Gal activity and for MHC expression. When transfectants fuse with nontransfected cells, many of the nuclei in the myotube become positive for β-Gal activity because the cytoplasmically translated protein diffuses within the myotube; transfection efficiencies of ∼10% are therefore used to minimize the fusion of independent β-Gal+ transfectants. Approximately 67% of double control-vector transfectants were MHC+, and these cells were scored as mononucleate (∼35% of total nuclei), containing two to four nuclei (∼21%), or containing five or more nuclei (∼11% [Fig. 3H and I]). Expression of Cdo siRNA decreased the percentage of MHC+ cells to ∼57%, with multinucleate cells representing only ∼10%, none of which were in the five-or-greater category. Coexpression with the Cdo siRNA vector of a rat Cdo cDNA that is impervious to RNAi-mediated knockdown by the mouse-specific siRNA sequence (52) resulted in a greater-than-complete phenotypic rescue of Cdo depletion, with ∼76% MHC+ cells and ∼31% of the total nuclei in the five-or-greater category. The enhancement of differentiation, relative to the double control-vector transfectants, under these conditions is most likely due to transient expression of Cdo at a level that exceeds the endogenous level. In contrast, coexpression of the CdoΔ1160-1256 mutant protein or the CdoP/A mutant protein with the Cdo siRNA vector did not rescue the inhibition of differentiation that occurred upon RNAi-mediated Cdo depletion; rather, these transfectants behaved similarly to those that received the Cdo siRNA vector plus a control vector (Fig. 3H and I). Therefore, in both stable and transient cell differentiation assays, loss or mutation of the PXXP motif resulted in a loss of function. Taken together, these results implicate multiple regions of the Cdo intracellular domain in its promyogenic function, with the PXXP sequence involved in Abl binding being one functional motif.
FIG. 3.
The Abl-binding motif of Cdo is functional in myogenic differentiation. (A) Photomicrographs of C2C12 cells that stably express the indicated Cdo proteins or a control vector (pBp) that were cultured in DM, fixed, and stained with an antibody to MHC. (B) Quantification of myotube formation by the cell lines shown in panel A. Values represent means of triplicate determinations ± 1 standard deviation. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at P < 0.01. (C) Lysates of cell lines shown in panel A were Western blotted with antibodies to Cdo, MHC, and β-tubulin as a loading control. (D) Photomicrographs of C2C12 cells that stably express the indicated Cdo proteins or a control vector (pBp) that were cultured in DM, fixed, and stained with an antibody to MHC. (E) Quantification of myotube formation by the cell lines shown in panel D. Values represent means of triplicate determinations ± 1 standard deviation. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at P < 0.01. (F) Lysates of cell lines shown in panel D were Western blotted with antibodies to Cdo and pan-cadherin as a loading control. (G) Lysates of cell lines shown in panel D were cultured for 2 or 3 days in DM (D2 and D3, respectively) and then Western blotted with antibodies to MHC and pan-cadherin as a loading control. (H) C2C12 cells were cotransfected with control or Cdo siRNA expression vectors plus Cdo or CdoP/A expression vectors and pQ-lacZ (a vector that drives expression of nucleus-localized β-Gal to mark transfectants). Differentiated cultures were double stained for β-Gal activity (blue color) and for MHC expression (brown color). (I) Quantification of C2C12 cell differentiation shown in panel H. Cultures were scored as MHC− or MHC+, with MHC+ cells further scored as having a single nucleus, two to four nuclei, or five or more nuclei. Values represent means of triplicate determinations ± 1 standard deviation. The experiment was repeated three times with similar results.
Abl promotes myogenic differentiation.
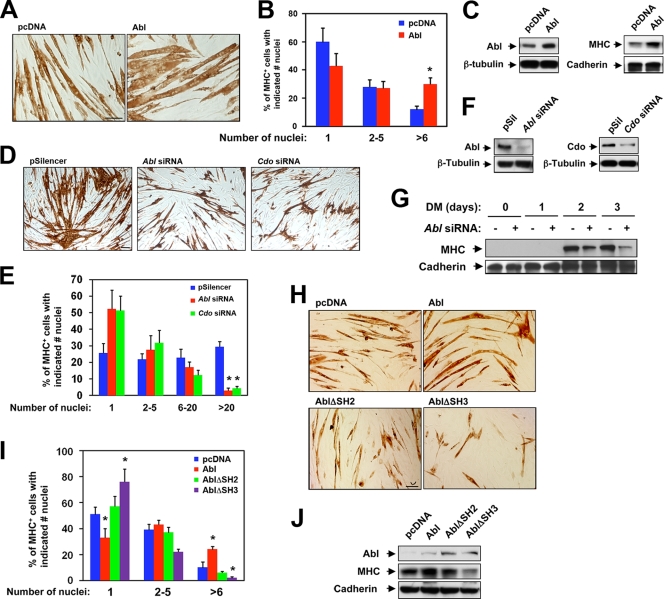
Abl has been reported to promote myogenic differentiation in a manner dependent on its cytoplasmic localization (13). As a prelude to investigating the role of the Cdo-Abl interaction in myogenesis, we confirmed Abl's promyogenic function by raising or diminishing its levels in C2C12 cells by stable overexpression or RNAi, respectively. Modest overexpression (about twofold) of Abl resulted in the formation of larger myotubes with more nuclei per myotube and higher levels of MHC production relative to vector control cells (Fig. 4A to C). The opposite effect was observed when C2C12 cells stably expressed Abl siRNA. Cells depleted of Abl formed fewer myotubes than did vector control cells, and those that were formed were thinner and contained fewer nuclei than control myotubes (Fig. 4D to F). Furthermore, Abl siRNA-expressing cells produced lower levels of MHC than did control cells at 2 and 3 days after transfer to DM (Fig. 4G). Significantly, Abl siRNA-expressing cells showed a phenotype very similar to that of Cdo RNAi-expressing cells (Fig. 4D to F) (20, 43).
FIG. 4.
Abl promotes myogenic differentiation. (A) Photomicrographs of C2C12 cells that stably express Abl or control (pcDNA) vectors that were cultured in DM, fixed, and stained with an antibody to MHC. (B) Quantification of myotube formation by the cell lines shown in panel A. Values represent means of triplicate determinations ± 1 standard deviation. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at P < 0.01. (C) Lysates of cell lines shown in panel A were Western blotted with antibodies to Abl or MHC and to β-tubulin or pan-cadherin as loading controls. (D) Photomicrographs of C2C12 cells that stably express Abl siRNA, Cdo siRNA, or control (pSilencer) vectors that were cultured in DM, fixed, and stained with an antibody to MHC. (E) Quantification of myotube formation by the cell lines shown in panel D. Values represent means of triplicate determinations ± 1 standard deviation. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at P < 0.01. (F) Lysates of cell lines shown in panel D were Western blotted with antibodies to Abl or Cdo to reveal the level of RNAi-mediated depletion and to pan-cadherin as a loading control. (G) Lysates of control and Abl siRNA cell lines shown in panel D were cultured for 1, 2, or 3 days in DM and then Western blotted with antibodies to MHC and to pan-cadherin as a loading control. (H) Photomicrographs of C2C12 cells that stably express the indicated Abl proteins or a control (pcDNA) vector that were cultured in DM, fixed, and stained with an antibody to MHC. (I) Quantification of myotube formation by the cell lines shown in panel H. Values represent means of triplicate determinations ± 1 standard deviation. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at P < 0.01. (J) Lysates of the cell lines shown in panel H were Western blotted with antibodies to Abl, MHC, and pan-cadherin as a loading control.
We next addressed some of the structural requirements for Abl function in myogenesis. Mutant Abl proteins lacking either the SH2 or the SH3 domain (designated AblΔSH2 and AblΔSH3, respectively) were stably expressed in C2C12 myoblasts, and the cells’ differentiation capabilities were tested in comparison with those of vector control cells and cells that overexpressed wild-type Abl. Similar to the results shown above, the wild-type-Abl-expressing cells displayed enhanced myogenesis with larger myotubes that contained more nuclei and elevated levels of MHC, compared to those of control cells (Fig. 4G to I). In contrast, both Abl deletion mutant proteins were impaired in promoting differentiation. The AblΔSH2 mutant protein-expressing cells differentiated similarly to control cells, despite expressing approximately twice the amount of Abl protein as the wild-type Abl-expressing cells. The AblΔSH3 mutant protein-expressing cells displayed a diminished differentiation response compared to that of control cells, with a greater percentage of mononucleate cells and few large myotubes, and a lower level of MHC production (Fig. 4H to J).
Abl kinase activity was also investigated in proliferating and differentiating myoblasts by analysis of the ability of immunoprecipitated Abl to phosphorylate MBP in vitro and by assessment of Abl phosphorylation at Y245, a regulatory site whose phosphorylation is required for full activity (5, 44). Proliferating C2C12 cells had detectable levels of Abl activity and Y245 phosphorylation, both of which increased when the cells reached near confluence (day 0, at which time the cultures were transferred to DM) (Fig. 5A). These higher levels were maintained for at least 1 day and then were reduced over the next 2 days in DM, by which time little activity was detected (Fig. 5A). This time course of Abl kinase activity and Y245 phosphorylation paralleled Abl association with Cdo (Fig. 1D and E). Consistent with this correlation, reduction of Cdo levels by RNAi led to a proportional decrease in phospho-Y245 Abl levels in both growing cells and cells at day 0 (Fig. 5B), indicating that Cdo is involved in the activation of Abl that occurs in C2C12 cells under these conditions. The observation that Cdo-Abl interaction and Abl activity and phosphorylation were highest when cells were near confluence and 1 day later suggests that these processes may be stimulated by cell-cell contact, perhaps through cadherin-based adhesion. To begin to assess this possibility, we took advantage of the fact that adhesion mediated through classical cadherins is calcium dependent and analyzed C2C12 cells that were cultured in control medium or medium supplemented with the calcium chelator EGTA. EGTA-containing cultures displayed lower levels of phospho-Y245 Abl throughout a 3-day time course than did cultures in control medium (Fig. 5C), consistent with the notion that cadherin-dependent adhesion may be involved in the Cdo-Abl interaction and Abl activation (see the Discussion for further information on this point).
FIG. 5.
Regulation of Abl kinase activity during myogenic differentiation. (A) Lysates of C2C12 cells that were proliferating in GM (G), at near confluence (0), or in DM for the indicated times were immunoprecipitated (IP) with antibodies to Abl and assayed for kinase activity with MBP as the substrate, as described in Materials and Methods. Lysates were also Western blotted with antibodies to phospho-Y245 Abl (AblY245-P), total Abl, MHC, myogenin, and β-tubulin as a loading control. (B) Lysates of C2C12 cells that stably express Cdo siRNA (+) or control (−) vectors were cultured in GM (G) or at near confluence (D0) and Western blotted with antibodies to AblY245-P, Abl, Cdo, and pan-cadherin (as a loading control). (C) Lysates of C2C12 cells in GM at near confluence (0) or in DM for the indicated times, plus or minus 1.4 mM EGTA, were Western blotted with antibodies to AblY245-P, total Abl, and pan-cadherin as a loading control. (D) Lysates from C2C12 cells that stably express Abl, kinase-deficient Abl (Abl-KD), or control (pcDNA) vectors were Western blotted with antibodies to Abl, MHC, or pan-cadherin as a loading control. (E) Photomicrographs of C2C12 cells that stably express Abl, Abl-KD, or control vectors that were cultured in DM, fixed, and stained with an antibody to MHC. (F) Quantification of myotube formation by the cell lines shown in panel E. Values represent means of triplicate determinations ± 1 standard deviation. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at P < 0.01.
To test whether Abl kinase activity is required for its ability to promote myogenesis, wild-type Abl and Abl-KD were stably expressed in C2C12 cells. As shown in the previous figures, overexpression of Abl enhanced myotube formation and production of MHC (Fig. 5D to F). Surprisingly, the Abl-KD mutant protein also promoted the formation of larger myotubes that contained more nuclei per myotube than control cells and elevated production of MHC, though myotube formation was not enhanced to the same extent as by wild-type Abl (Fig. 5D to F). These results suggest that Abl may possess both kinase-dependent and -independent functions during myogenic differentiation. Although most known Abl functions are dependent on kinase activity, there is precedent for Abl-KD possessing the ability to promote p38 MAPK activity (10, 15), which is linked to Cdo's and Abl's promyogenic function (reference 42 and see below, respectively).
Abl promotes p38 MAPK activity during myogenic differentiation.
Because Abl promotes myogenic differentiation and associates with Cdo, whose promyogenic properties are exerted mainly through p38 MAPK, the possibility that Abl participates in the regulation of p38 activity during differentiation was examined. C2C12 cells were transiently transfected with an Abl siRNA expression vector or a control vector plus a GFP expression vector to mark transfectants. Two different conditions that result in active p38 (30, 50) were tested: (i) cultures that were at high density and had not been fed with fresh medium for 48 h and (ii) cultures that had been in DM for 48 h, a midpoint in the differentiation time course. Cells were then assessed for production of pp38 by immunofluorescence. Under condition i, cells were mononucleate though some had started to elongate. Approximately 70% of the control vector-transfected cells were positive for pp38, whereas only ∼10% of the Abl siRNA-expressing cells were positive for pp38 (Fig. 6A and B). Under condition ii, most of the control vector-transfected cells had formed small, multinucleate myotubes and displayed a robust pp38 signal, whereas Abl siRNA-expressing cells were elongated but mainly mononucleate and had a visibly weaker pp38 signal; cells that expressed Cdo siRNA were similar to those that expressed Abl siRNA (Fig. 6C). To quantify this, we scored the intensity of the immunofluorescent pp38 signal in untransfected (GFP−) versus transfected (GFP+) cells on the same coverslips, with the average pp38 signal in untransfected cells set to 1.0. Control vector-transfected cells also had a pp38 signal of ∼1.0, whereas the signal for Abl siRNA-expressing cells was ∼0.25 and for Cdo siRNA-expressing cells was ∼0.2 (Fig. 6D). When a similar analysis that measured the total p38 signal was performed, there was no significant difference among untransfected cells, cells transfected with control vector, and cells transfected with the siRNA vectors (Fig. 6C and D). Consistent with analysis by microscopy, cells that expressed Abl siRNA displayed lower levels of pp38 after 2 days in DM than did control cells, as assessed by Western blotting (Fig. 6E). Therefore, C2C12 cells depleted of Abl by RNAi had a diminished capacity to activate p38 MAPK in response to differentiation-inducing culture conditions. p38 activation during myogenic differentiation in vitro is substantially, though not completely, dependent on Cdo/Bnip-2 signaling, but stress-mediated activation of p38 in myoblasts is independent of Cdo and Bnip-2 (19). As Abl is implicated in stress responses (46), we asked whether p38 activation in C2C12 cells in response to anisomycin is dependent on Abl. In contrast to differentiation-induced production of pp38, anisomycin-induced pp38 production was largely unaffected in C2C12 cells depleted of Abl by RNAi (Fig. 6F).
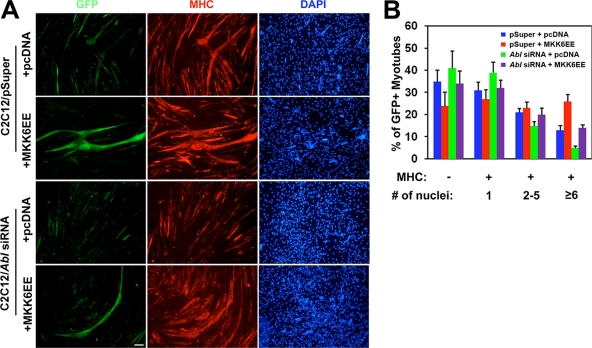
The deficient differentiation response displayed by myoblasts upon loss of Cdo is rescued by forced expression of an activated form of the immediate upstream activating kinase for p38, MKK6 (MKK6EE). To determine whether expression of MKK6EE could rescue the diminished differentiation response of cells depleted of Abl by RNAi, C2C12 cells that stably expressed Abl siRNA or control expression vectors (C2C12/Abl siRNA or C2C12/pSuper cells, respectively) were transiently transfected with pcDNA/MKK6EE or empty pcDNA expression vectors; transfected cells were marked by cotransfection of a GFP expression vector. Approximately 65% of the pcDNA-transfected C2C12/pSuper cells were MHC+ (31% mononucleate; ∼21% containing two to five nuclei; ∼13% containing six or more nuclei) (Fig. 7A and B). pcDNA-transfected C2C12/Abl siRNA cells displayed a reduction in differentiation potential, the percentage of MHC+ cells decreasing to ∼59%, with ∼14% containing two to five nuclei and ∼5% containing six or more nuclei. Expression of MKK6EE in C2C12/pSuper cells enhanced differentiation relative to that of pcDNA transfectants, with ∼76% of the GFP+ cells being MHC+ and 26% of the total nuclei in the six-or-greater category. Expression of MKK6EE in C2C12/Abl siRNA cells resulted in rescue of differentiation, the percentage of MHC+ cells and distribution of transfectants displaying one, two to five, and six or more nuclei being nearly identical between these cultures and the double control-vector cultures (Fig. 7A and B). These results are very similar to those obtained with RNAi to Cdo plus or minus MKK6EE expression (43) and suggest that Abl lies downstream of Cdo in the activation of p38. However, forced overexpression of full-length Abl did not rescue the defective differentiation program of C2C12 cells that were depleted of Cdo by RNAi (data not shown), presumably because Cdo is required for coordinated formation of the functional signaling complexes.
FIG. 7.
Expression of MKK6EE rescues the defective differentiation phenotype of Abl-depleted C2C12 cells. (A) C2C12/pSuper and C2C12/Abl siRNA cells were transfected with control (pcDNA) or MKK6EE expression vectors and a GFP expression vector to mark transfectants. Differentiated cultures were stained for MHC (red) and visualized for GFP expression. Cell nuclei were visualized by staining with DAPI (blue). (B) Quantification of the C2C12 cell differentiation shown in panel A. Cultures were scored as MHC− or MHC+, with MHC+ cells further scored as having a single nucleus, two to five nuclei, or six or more nuclei. Values represent means of triplicate determinations ± 1 standard deviation. The experiment was repeated three times with similar results.
The results described above reveal Abl to be a component of the Cdo-containing complexes that activate p38 MAPK during myogenic differentiation. These complexes also contain JLP, a scaffold protein for the p38 MAPK pathway that also binds several other signaling molecules in various contexts (e.g., the phosphatidylinositol 5-kinase PIKfyve, Gα13, and kinesin light chain 1) (19, 26, 27, 32). We therefore asked whether Abl and JLP associate. 293T cells were transiently transfected with expression vectors for S epitope-tagged JLP and Abl, and lysates were precipitated with antibodies to either the S epitope or Abl; JLP and Abl reciprocally coimmunoprecipitated (Fig. 8A and B). To assess whether these proteins interact endogenously during myogenesis, Abl was immunoprecipitated from proliferating and differentiating C2C12 myoblasts and precipitates were analyzed for the presence of JLP. While little or no association was observed in proliferating cultures, JLP coprecipitated with Abl when the cells were nearly confluent (day 0) and during 3 days of incubation in DM (Fig. 8C). This is similar to the time course of Abl interaction with Cdo and of Abl kinase activity during myoblast differentiation but somewhat more persistent. As shown in Fig. 1 and 5, Abl protein levels decreased during the differentiation time course; in contrast, JLP levels increased during cell differentiation (Fig. 8C and reference 34). While this may seem counterintuitive, it is likely that Abl and JLP play multiple roles in myoblasts and myotubes, and the alteration in their overall levels during differentiation may be unrelated to the Cdo-JLP-Abl complexes that signal early in this process.
FIG. 8.
Abl binds JLP and cooperates with JLP and Cdo to activate p38 MAPK. (A and B) Lysates of 293T cells transiently transfected with Abl, S epitope-tagged JLP, or control (−) expression vectors, as indicated, were immunoprecipitated (IP) with anti-S epitope agarose beads (A) or Abl antibodies (B) and then Western blotted (WB) with S epitope or Abl antibodies. Straight lysates were also Western blotted with these antibodies. (C) Lysates of C2C12 cells that were proliferating in GM (G), at near confluence (0), or in DM for the indicated times were immunoprecipitated with antibodies to Abl and Western blotted with JLP or Abl antibodies. Straight lysates were also Western blotted with JLP, Abl, MHC, and pan-cadherin antibodies. (D) Lysates of 293T cells transiently transfected with HA-tagged p38, S epitope-tagged JLP, Abl, Abl-KD, Cdo, or control (−) expression vectors, as indicated, were Western blotted with pp38, HA epitope (p38), S epitope (JLP), Abl, and Cdo antibodies. (E) Model of p38 MAPK activation by Cdo-containing cell surface complexes. During myogenic differentiation, Bnip-2/Cdc42 complexes and JLP/p38 complexes bind to the Cdo intracellular region, resulting in Bnip-2/Cdc42-dependent activation of p38 by a pathway whose intermediates are yet to be identified. Active p38 phosphorylates substrates that contribute to muscle-specific transcription, including Mef2, E47, and BAF60. Abl binds Cdo and JLP and promotes p38 activation and myogenesis. Activated MKK6 rescues the defective differentiation program caused by loss of Cdo, Bnip-2, or Abl, but the role of endogenous MKK6 (or MKK3) has not been established, so a question mark accompanies its position.
To ask whether these three interacting proteins (Cdo, JLP, and Abl) might collaborate in the activation of p38 MAPK, transfections in a heterologous system were used. 293T cells were transfected with a vector encoding hemagglutinin (HA)-tagged p38 and combinations of other expression vectors, and lysates were blotted with antibodies to pp38. The expression of p38 alone resulted in barely detectable levels of pp38, and the individual expression of JLP, Abl, or Cdo with p38 modestly enhanced pp38 production (Fig. 8D). Coexpression of JLP and Abl, JLP and Cdo, or Abl and Cdo increased the level of pp38 above that seen with the individually expressed proteins, and pp38 production was further increased by the simultaneous coexpression of all three proteins. Therefore, coexpression of these three mutually interacting proteins (Cdo, JLP, and Abl) produced the greatest level of signal output. Interestingly, expression of Abl-KD in place of wild-type Abl, along with Cdo and JLP, also led to enhanced production of pp38, consistent with the partial kinase-independent role for Abl observed in Fig. 5.
DISCUSSION
The mechanisms whereby extracellular cues stimulate cell differentiation are of fundamental interest, and skeletal myoblast differentiation is a highly tractable system for analysis of this area. Activation of the p38 MAPK pathway is required for myoblast differentiation, and it appears that multiple upstream signaling inputs contribute to pathway activity during this process (7, 20, 49); however, the underlying mechanisms are not well understood. We have identified multiprotein complexes based around the cell surface receptor Cdo that play a significant (though not exclusive) role in mediating p38 activation during myogenic differentiation (20). Cdo binds directly to Bnip-2 and JLP, scaffold proteins for Cdc42 and p38, respectively, and formation of these complexes results in Bnip-2/Cdc42-dependent activation of p38 bound to JLP. In this paper, it is demonstrated that the nonreceptor tyrosine kinase Abl binds both to the intracellular region of Cdo and to JLP during myogenesis and that Abl regulates p38 activity during differentiation. It has been reported that Abl accumulates in the cytoplasm of differentiating myoblasts and that cytoplasmic, but not nuclear, Abl promotes myogenic differentiation, implying that Abl interacts with other promyogenic, cytoplasmic proteins (13). Cdo and JLP are identified here as two such proteins (Fig. 8E).
In myoblasts, Cdo forms cis complexes with the cell-cell adhesion molecule N-cadherin and with the RGM and netrin receptor neogenin (21, 25); Cdo also binds the secreted morphogen Sonic hedgehog (46, 51). Analyses of Abl in myoblasts proliferating in growth medium (GM), myoblasts at near confluence (day 0, at which time many cell-cell contacts have formed but the cells have not yet been transferred into DM), and myoblasts cultured in DM over several days suggest that its activity and its involvement with Cdo-containing complexes are regulated by cell-cell contact. It is noteworthy that Abl's association with Cdo and with JLP, Abl kinase activity, and Abl Y245 phosphorylation are each relatively low in GM but at maximal levels at day 0, with maintenance through at least 1 day of culture in DM. These results suggest that cell-cell contact may be a stimulus for these processes. Consistent with this likelihood, high cell density promotes p38 activity in C2C12 cells (30, 50). Furthermore, a Cdo deletion mutant protein that is selectively deficient in N-cadherin association strongly reduces differentiation (21). Recent results indicate that N-cadherin ligation in myoblasts activates p38 in a Cdo-, JLP-, and Bnip-2-dependent manner and that Cdo, JLP, Bnip-2, and active Cdc42 accumulate at sites of N-cadherin ligation (M. Lu and R. S. Krauss, unpublished data). These results, plus the fact that inhibition of cadherin-based adhesion by calcium chelation diminished Abl Y245 phosphorylation (Fig. 5C), suggest that Abl may also be a component of such complexes.
During C2C12 cell differentiation, Abl kinase activity increases in parallel with Abl-Cdo interaction and, as assessed by phosphorylation of Abl Y245, is Cdo dependent. These results are consistent with binding of the Abl SH3 domain to the PXXP motif in Cdo, as this interaction would be predicted to place Abl in an open conformation, relieving autoinhibition and enhancing catalytic activity (1, 4, 16, 17). However, a kinase-deficient mutant form of Abl retained partial activity in promoting myogenesis and stimulating p38 MAPK activity in transfectants. This is consistent with two previous reports in which Abl overexpressed in 293 cells activated p38 and Abl-KD retained partial (10), or nearly full (15), ability to do so. We speculate that, in addition to acting as a kinase in Cdo-containing complexes, Abl may play a role as a scaffolding factor, bridging Cdo and JLP, and helping to assemble or stabilize other components of the complex that are involved in p38 activation. Coimmunoprecipitation of Cdo and JLP in differentiating myoblasts was unaffected by depletion of Abl by RNAi (data not shown), suggesting that Abl is not required to stabilize the Cdo-JLP association. It is possible that other components, or specific aspects of the dynamics, of these complexes are affected by Abl binding. Alternatively, other Abl activities, such as binding and regulation of actin structures, may contribute. These possibilities are not mutually exclusive.
In contrast to our results that reveal a partly kinase-independent function for Abl, di Bari et al. reported that kinase activity was required for Abl's ability to promote myogenesis (13). The difference between these studies is very likely related to the density at which the cells were analyzed. We have implicated Abl in a cell-cell adhesion-dependent signaling complex, whereas di Bari et al. specifically analyzed cells at low density to avoid the differentiation stimulus of cell-cell contact. The role of p38 in Abl's effects were not pursued in that study, but given that Abl is a regulator of myriad pathways and processes, it is possible that it can promote myogenesis through mechanisms in addition to the one described here.
We have previously noted that Cdo signals by a distinctive mechanism in which its intracellular region binds to scaffold proteins (Bnip-2 and JLP) that, in turn, bind multiple components of specific pathways (Cdc42 and p38 MAPK, respectively), presumably allowing tightly regulated interaction between these modules (20). This is different from the Ig/FnIII receptors that are most closely related to Cdo (e.g., Robo and DCC family receptors), which bind to enzymes and adaptor proteins (14, 39). Abl is the first enzyme found to bind directly to Cdo. Robo1 receptors, which share a five Ig plus three FnIII ectodomain topology with Cdo, also bind to Abl through a proline-rich sequence (2). However, the Abl-binding motif is evolutionarily conserved in Robo1 proteins found in Drosophila and vertebrates (as well as in certain other Robo family members) (14). In contrast, the PXXP motif in the Cdo intracellular region that binds Abl is found in mammalian, but not other known vertebrate, Cdo proteins, despite the presence of conserved amino acids that flank this motif in zebrafish and Xenopus Cdo. The intracellular regions of other members of the Cdo subfamily, including vertebrate Boc proteins and Drosophila Ihog and Boi, are unrelated to Cdo and also lack this motif (23). Therefore, the presence of an Abl-binding, PXXP motif in mammalian Cdo may represent an example of convergent evolution with Robo1 proteins; alternatively, a common ancestor may have had an Abl-binding motif, which has been selectively retained in (and whose sequence has altered between) Cdo and specific Robo receptors. The only known evolutionarily conserved properties possessed by all Cdo subfamily members are binding to hedgehog ligands and promotion of hedgehog pathway signaling, activities that do not require these proteins’ cytoplasmic tails (46, 51, 52). In contrast, Cdo's cytoplasmic tail is required for its ability to promote myogenesis. Cdo's role in myogenic differentiation has only been explored in mammalian systems; it is possible that this role has evolved more recently than its function in hedgehog signaling. Furthermore, if Cdo plays a similar role in nonmammalian vertebrate myogenesis, the mechanisms involved may be somewhat different (e.g., they may not directly involve Abl).
Acknowledgments
We thank Jean Wang for reagents and Jim Bieker and Karen Schachter for critical reading of the manuscript.
This work was supported by grants from the NIH (AR46207) and the T. J. Martell Foundation to R.S.K., by the Korea Research Foundation grant funded by the Korean government (KRF-2008-313-C00260) to J.S.K., and by the Korea Science and Engineering Foundation (KOSEF) and the Ministry of Science and Technology (MOST), Korean Government, through its National Nuclear Technology Program (S.J.L.).
Footnotes
Published ahead of print on 26 May 2009.
REFERENCES
- 1.Barilá, D., and G. Superti-Furga. 1998. An intramolecular SH3-domain interaction regulates c-Abl activity. Nat. Genet. 18280-282. [DOI] [PubMed] [Google Scholar]
- 2.Bashaw, G. J., T. Kidd, D. Murray, T. Pawson, and C. S. Goodman. 2000. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 101703-715. [DOI] [PubMed] [Google Scholar]
- 3.Bergstrom, D. A., B. H. Penn, A. Strand, R. L. Perry, M. A. Rudnicki, and S. J. Tapscott. 2002. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell 9587-600. [DOI] [PubMed] [Google Scholar]
- 4.Brasher, B. B., S. Roumiantsev, and R. Van Etten. 2001. Mutational analysis of the regulatory function of the c-Abl Src homology 3 domain. Oncogene 207744-7752. [DOI] [PubMed] [Google Scholar]
- 5.Brasher, B. B., and R. A. Van Etten. 2000. c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J. Biol. Chem. 27535631-35637. [DOI] [PubMed] [Google Scholar]
- 6.Briata, P., S. V. Forcales, M. Ponassi, G. Corte, C. Y. Chen, M. Karin, P. L. Puri, and R. Gherzi. 2005. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol. Cell 20891-903. [DOI] [PubMed] [Google Scholar]
- 7.Chen, S. E., B. Jin, and Y. P. Li. 2007. TNF-α regulates myogenesis and muscle regeneration by activating p38 MAPK. Am. J. Physiol. Cell Physiol. 292C1660-C1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, S.-Y., M.-J. Kim, C.-M. Kang, S. Bae, C.-K. Cho, J.-W. Soh, J.-H. Kim, S. Kang, H. Y. Chung, Y.-S. Lee, and S.-J. Lee. 2006. Activation of Bak and Bax through c-Abl-protein kinase Cδ-p38 MAPK signaling in response to ionizing radiation in human non-small cell lung cancer cells. J. Biol. Chem. 2817049-7059. [DOI] [PubMed] [Google Scholar]
- 9.Cole, F., W. Zhang, A. Geyra, J.-S. Kang, and R. S. Krauss. 2004. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev. Cell 7843-854. [DOI] [PubMed] [Google Scholar]
- 10.Cong, F., and S. P. Goff. 1999. c-Abl-induced apoptosis, but not cell cycle arrest, requires mitogen-activated protein kinase kinase 6 activation. Proc. Natl. Acad. Sci. USA 9613819-13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuenda, A., and P. Cohen. 1999. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 2744341-4346. [DOI] [PubMed] [Google Scholar]
- 12.de Angelis, L., J. Zhao, J. J. Andreucci, E. N. Olson, G. Cossu, and J. C. McDermott. 2005. Regulation of vertebrate myotome development by the p38 MAP kinase-MEF2 signaling pathway. Dev. Biol. 283171-179. [DOI] [PubMed] [Google Scholar]
- 13.di Bari, M. G., L. Ciuffini, M. Mingardi, R. Testi, S. Soddu, and D. Barilà. 2006. c-Abl acetylation by histone acetyltransferases regulates its nuclear-cytoplasmic localization. EMBO Rep. 7727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson, B. J., and G. F. Gilestro. 2006. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu. Rev. Cell Dev. Biol. 22651-675. [DOI] [PubMed] [Google Scholar]
- 15.Galan-Moya, E. M., J. Hernandez-Losa, C. I. Aceves Luquero, M. A. de la Cruz-Morcillo, C. Ramírez-Castillejo, J. L. Callejas-Valera, A. Arriaga, A. F. Aranburo, S. Ramón y Cajal, J. S. Gutkind, and R. Sánchez-Prieto. 2008. c-Abl activates p38 MAPK independently of its tyrosine kinase activity: implications in cisplatin-based therapy. Int. J. Cancer 122289-297. [DOI] [PubMed] [Google Scholar]
- 16.Hantschel, O., and G. Superti-Furga. 2004. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat. Rev. Mol. Cell Biol. 533-44. [DOI] [PubMed] [Google Scholar]
- 17.Harrison, S. C. 2003. Variation on an Src-like theme. Cell 112737-740. [DOI] [PubMed] [Google Scholar]
- 18.Hu, J. S., and E. N. Olson. 1990. Functional receptors for transforming growth factor-beta are retained by biochemically differentiated C2 myocytes in growth factor-deficient medium containing EGTA but down-regulated during terminal differentiation. J. Biol. Chem. 2657914-7919. [PubMed] [Google Scholar]
- 19.Ikonomov, O. C., J. Fligger, D. Sbrissa, R. Dondapati, K. Mlak, R. Deeb, and A. Shisheva. 2009. Kinesin adapter JLP links PIKfyve to microtubule-based endosome-to-trans-Golgi network traffic of furin. J. Biol. Chem. 2843750-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang, J.-S., G.-U. Bae, M.-J. Yi, Y.-J. Yang, J.-E. Oh, G. Takaesu, Y. T. Zhou, B. C. Low, and R. S. Krauss. 2008. A Cdo/Bnip-2/Cdc42 signaling pathway regulates p38α/β MAPK activity and myogenic differentiation. J. Cell Biol. 182497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang, J.-S., J. L. Feinleib, S. Knox, M. A. Ketteringham, and R. S. Krauss. 2003. Pro-myogenic members of the Ig and cadherin families associate to positively regulate differentiation. Proc. Natl. Acad. Sci. USA 1003989-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang, J.-S., M. Gao, J. L. Feinleib, P. D. Cotter, S. N. Guadagno, and R. S. Krauss. 1997. CDO: an oncogene-, serum-, and anchorage-regulated member of the Ig/fibronectin type III repeat family. J. Cell Biol. 138203-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang, J.-S., P. J. Mulieri, Y. Hu, L. Taliana, and R. S. Krauss. 2002. BOC, an Ig superfamily member, associates with CDO to positively regulate myogenic differentiation. EMBO J. 21114-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang, J.-S., P. J. Mulieri, C. Miller, D. A. Sassoon, and R. S. Krauss. 1998. CDO, a Robo-related cell surface protein that mediates myogenic differentiation. J. Cell Biol. 143403-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang, J.-S., M.-J. Yi, W. Zhang, J. L. Feinleib, F. Cole, and R. S. Krauss. 2004. Netrins and neogenin promote myotube formation. J. Cell Biol. 167493-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashef, K., C. M. Lee, J. H. Ha, E. P. Reddy, and D. N. Dhanasekaran. 2005. JNK-interacting leucine zipper protein is a novel scaffolding protein in the Gα13 signaling pathway. Biochemistry 4414090-14096. [DOI] [PubMed] [Google Scholar]
- 27.Kelkar, N., C. L. Standen, and R. J. Davis. 2005. Role of the JIP4 scaffold protein in the regulation of mitogen-activated protein kinase signaling pathways. Mol. Cell. Biol. 252733-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lluís, F., E. Ballestar, M. Suelves, M. Esteller, and P. Munoz-Canoves. 2005. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 24974-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lluís, F., E. Perdiguero, A. R. Nebreda, and P. Munoz-Canoves. 2006. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 1636-44. [DOI] [PubMed] [Google Scholar]
- 30.Lovett, F. A., I. Gonzalez, D. A. Salih, L. J. Cobb, G. Tripathi, R. A. Cosgrove, A. Murrell, P. J. Kilshaw, and J. M. Pell. 2006. Convergence of Igf2 expression and adhesion signalling via RhoA and p38 MAPK enhances myogenic differentiation. J. Cell Sci. 1194828-4840. [DOI] [PubMed] [Google Scholar]
- 31.Moresco, E. M., and A. J. Koleske. 2003. Regulation of neuronal morphogenesis and synaptic function by Abl family kinases. Curr. Opin. Neurobiol. 13535-544. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen, Q., C. M. Lee, A. Le, and E. P. Reddy. 2005. JLP associates with kinesin light chain 1 through a novel leucine zipper-like domain. J. Biol. Chem. 28030185-30191. [DOI] [PubMed] [Google Scholar]
- 33.Obenauer, J. C., L. C. Cantley, and M. B. Yaffe. 2003. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 313635-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh, J.-E., G.-U. Bae, Y.-J. Yang, M.-J. Yi, H.-J. Lee, B.-G. Kim, R. S. Krauss, and J.-S. Kang. 25 February 2009. Cdo promotes neuronal differentiation via activation of the p38 mitogen-activated kinase pathway. FASEB J. [Epub ahead of print.] doi: 10.1096/fj08-119255. [DOI] [PMC free article] [PubMed]
- 35.Perdiguero, E., V. Ruiz-Bonilla, L. Gresh, L. Hui, E. Ballestar, P. Sousa-Victor, B. Baeza-Raja, M. Jardí, A. Bosch-Comas, M. Esteller, C. Caelles, A. L. Serrano, E. F. Wagner, and P. Muñoz-Cánoves. 2007. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38α in abrogating myoblast proliferation. EMBO J. 261245-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pownall, M. E., M. K. Gustafsson, and C. P. Emerson, Jr. 2002. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 18747-783. [DOI] [PubMed] [Google Scholar]
- 37.Puri, P. L., K. Bhakta, L. D. Wood, A. Costanzo, J. Zhu, and J. Y. Wang. 2002. A myogenic differentiation checkpoint activated by genotoxic stress. Nat. Genet. 32585-593. [DOI] [PubMed] [Google Scholar]
- 38.Rampalli, S., L. Li, E. Mak, K. Ge, M. Brand, S. J. Tapscott, and F. J. Dilworth. 2007. p38 MAPK signaling regulates recruitment of Ash2L-containing methyltransferase complexes to specific genes during differentiation. Nat. Struct. Mol. Biol. 141150-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Round, J., and E. Stein. 2007. Netrin signaling leading to directed growth cone steering. Curr. Opin. Neurobiol. 1715-21. [DOI] [PubMed] [Google Scholar]
- 40.Serra, C., D. Palacios, C. Mozzetta, S. V. Forcales, I. Morantte, M. Ripani, D. R. Jones, K. Du, U. S. Jhala, C. Simone, and P. L. Puri. 2007. Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol. Cell 28200-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simone, C., S. V. Forcales, D. A. Hill, A. N. Imbalzano, L. Latella, and P. L. Puri. 2004. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 36738-743. [DOI] [PubMed] [Google Scholar]
- 42.Sirvent, A., C. Benistant, and S. Roche. 2008. Cytoplasmic signalling by the c-Abl tyrosine kinase in normal and cancer cells. Biol. Cell 100617-631. [DOI] [PubMed] [Google Scholar]
- 43.Takaesu, G., J. S. Kang, G. U. Bae, M. J. Yi, C. M. Lee, E. P. Reddy, and R. S. Krauss. 2006. Activation of p38α/β MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J. Cell Biol. 175383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanis, K. Q., D. Veach, H. S. Duewel, W. G. Bornmann, and A. J. Koleske. 2003. Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Mol. Cell. Biol. 233884-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tapscott, S. J. 2005. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 1322685-2695. [DOI] [PubMed] [Google Scholar]
- 46.Tenzen, T., B. L. Allen, F. Cole, J.-S. Kang, R. S. Krauss, and A. P. McMahon. 2006. The cell surface membrane proteins Cdo and Boc are components and targets of the hedgehog signaling pathway and feedback network in mice. Dev. Cell 10647-656. [DOI] [PubMed] [Google Scholar]
- 47.Van Etten, R. A. 1999. Cycling, stressed-out and nervous: cellular functions of c-Abl. Trends Cell Biol. 9179-186. [DOI] [PubMed] [Google Scholar]
- 48.Woodring, P. J., T. Hunter, and J. Y. Wang. 2001. Inhibition of c-Abl tyrosine kinase activity by filamentous actin. J. Biol. Chem. 27627104-27110. [DOI] [PubMed] [Google Scholar]
- 49.Wu, H., X. Wang, S. Liu, Y. Wu, T. Zhao, X. Chen, L. Zhu, Y. Wu, X. Ding, X. Peng, J. Yuan, X. Wang, W. Fan, and M. Fan. 2007. Sema4C participates in myogenic differentiation in vivo and in vitro through the p38 MAPK pathway. Eur. J. Cell Biol. 86331-344. [DOI] [PubMed] [Google Scholar]
- 50.Wu, Z., P. J. Woodring, K. S. Bhakta, K. Tamura, F. Wen, J. R. Feramisco, M. Karin, J. Y. Wang, and P. L. Puri. 2000. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 203951-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao, S., L. Lum, and P. Beachy. 2006. The ihog cell-surface proteins bind hedgehog and mediate pathway activation. Cell 125343-357. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, W., J.-S. Kang, F. Cole, M.-J. Yi, and R. S. Krauss. 2006. Cdo functions at multiple points in the Sonic hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev. Cell 10657-665. [DOI] [PubMed] [Google Scholar]