PREFACE
Mood disorders are common, chronic, recurrent mental illnesses that affect the lives of millions of individuals worldwide. To date, the monoaminergic systems (serotonergic, noradrenergic and dopaminergic) in the brain have received the greatest attention in neurobiological studies of mood disorders, and most therapeutics target these systems. However, there is growing evidence that the glutamatergic system is central to the neurobiology and treatment of these disorders. Here, we review data supporting the involvement of the glutamatergic system in mood disorder pathophysiology as well as the efficacy of glutamatergic agents in mood disorders. We also discuss exciting new prospects for the development of improved therapeutics for these devastating disorders.
Keywords: AMPA, antidepressant, bipolar disorder, depression, glutamate, NMDA
Mood disorders (major depressive disorder (MDD) and bipolar disorder (BPD)) are serious, debilitating, life-shortening illnesses that affect millions of people worldwide. The lifetime risk for any mood disorder in the United States is 20.8%, and onset typically begins in childhood or adolescence 1. Mood disorders are chronic illnesses characterized by multiple episodes of symptom exacerbation, residual symptoms between episodes, and functional impairment 2–4; the World Health Organization’s (WHO) Global Burden of Disease project ranked MDD as the fourth leading cause of disability in 1990, and predicts that it will become the second leading cause of disability worldwide by 2020 5.
Although most patients with mood disorders receive some benefit from available treatments 6, 7, the largest open label study examining the effectiveness of pharmacological treatment of MDD conducted to date (STAR*D) 7found that less than one third of patients achieved remission with an adequate trial of a standard antidepressant after up to 14 weeks of treatment. Furthermore, it was not until the completion of two antidepressant trials and nearly 24 weeks of treatment that half of the patients with MDD in the STAR*D study remitted. Similarly, many patients with BPD do not respond to existing medications 8, particularly during depressive episodes 6, 9.
A major obstacle to developing more effective treatments for mood disorders has been our limited understanding of their pathophysiology, and of the mechanisms underlying the efficacy of existing treatments. Mood disorders arise from the complex interaction of multiple genes and environmental factors, and the phenotypic expression of the disease includes not only mood disturbance, but also a constellation of cognitive, motor, autonomic, endocrine and sleep/wake abnormalities. To date, the monoaminergic (i.e., serotonergic, noradrenergic, dopaminergic) systems in the brain have received the greatest attention in neurobiological studies of mood disorders. These systems project widely to limbic, striatal and prefrontal cortical neuronal circuits that are implicated in the behavioral and visceral manifestations of mood disorders (reviewed in 10–12).
However, there are limitations to current monoamine theories related to mood disorders. For example, most antidepressants exert their initial effects by increasing the intrasynaptic levels of serotonin and/or norepinephrine. Nevertheless, meaningful improvement in core depressive symptoms emerges only after several weeks of antidepressant administration, suggesting that downstream neural adaptations rather than the elevation in synaptic monoamine levels itself are responsible for their therapeutic effects. Furthermore, although depletion of monoamines may increase the risk of mood disorders in some individuals under some circumstances, these depletions do not produce widespread clinical depression. Together, these observations suggest that monoaminergic systems do not represent a final common pathway regulating mood, but rather exert a modulatory influence (see 13 for review).
Overall, this focus on monoaminergic systems has not yet greatly advanced our understanding of the biology underlying recurrent mood disorders. Any such understanding must include an explanation for the tendency towards episodic and often profound mood disturbance that can become progressive over time. These observations suggest that although monoaminergic neurotransmitter systems play an important role in the pathophysiology and treatment of mood disorders, other systems that regulate synaptic and neural plasticity are more central to the neurobiology and treatment of these disorders 14, 15.
Research on the biological underpinnings of mood disorders has therefore begun to focus less on absolute changes in monoamines, and more on the role of neural circuits and synapses, and the processes controlling their function. Glutamate (Glu) is the major mediator of excitatory synaptic transmission in the mammalian brain 16. Under normal conditions, Glu plays a prominent role in synaptic plasticity, learning, and memory, but in pathological conditions it is known to be a potent neuronal excitotoxin, triggering either rapid or delayed neurotoxicity. The potential role of the glutamatergic system in the pathophysiology of, and treatment of, mood disorders has recently been investigated in earnest, but the available evidence suggests that abnormal activity of the glutamatergic system is likely to contribute to the impairments in synaptic and neural plasticity that are observed in patients with severe or recurrent mood disorders. Thus, numerous therapeutic strategies are being explored in an attempt to remedy the presumed impairments of Glu-mediated plasticity. Indeed, in preclinical paradigms, several of these treatments exert robust neurotrophic effects 17. Testing the efficacy and safety of these glutamatergic treatment strategies in patients with MDD and BPD may yield a better understanding of the neurobiological processes involved in these disorders, and lead to the development of improved treatments.
Physiology of the glutamatergic system
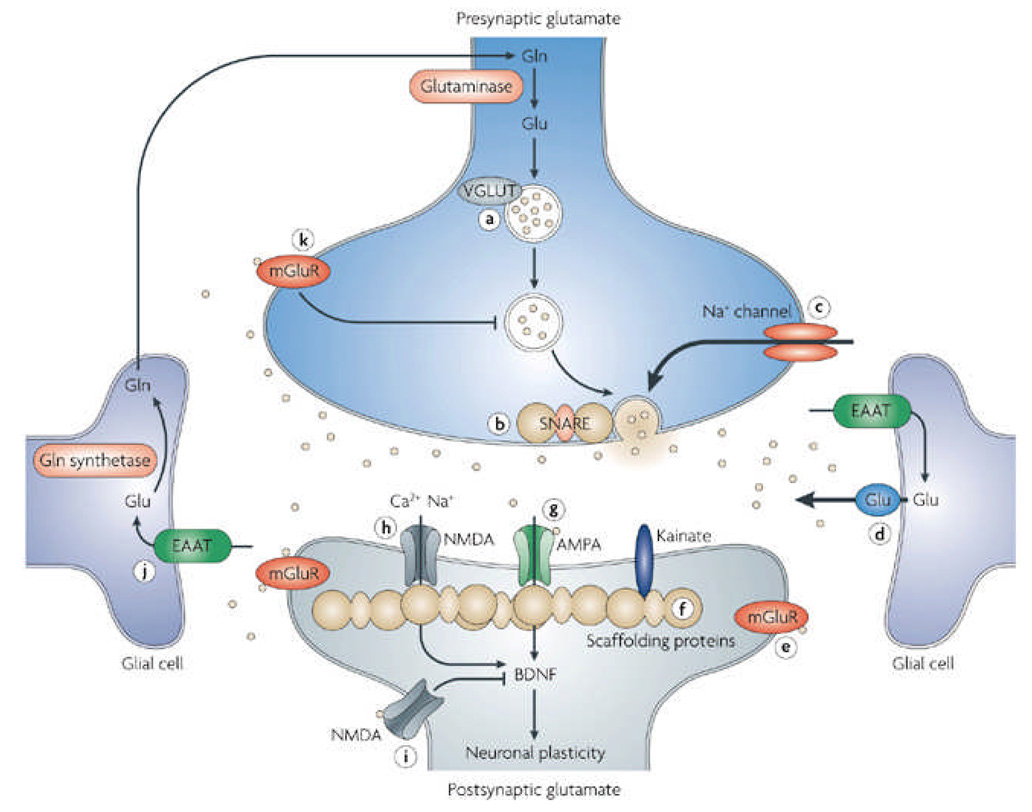
Glu can be found throughout the brain. Because tight control over glutamatergic neurotransmission is required to maintain optimal neuronal function and prevent over-activation of the system, multiple levels of regulatory processes have evolved to ensure glutamatergic excitation is maintained within narrow boundaries (Figure 1).
Figure 1. Glutamatergic neurotransmission and potential targets for drug development.
Tight physiological control is maintained over glutamatergic neurotransmission. Glutamine (Gln) is converted to glutamate (Glu) by glutaminase, though it can also be derived from the TCA cycle (not shown). Glu is packaged into presynaptic vesicles by the vesicular Glu transporter (VGLUT) proteins and released from the neuron in an activity-dependent manner through interactions with SNARE proteins. Glu is cleared from the extracellular space by excitatory amino acid transporters (EAATs) present predominantly on glia. In glial cells Glu is converted to Gln by Gln synthetase (GS). A variety of Glu receptors are present on presynaptic and postsynaptic neurons as well as on glial cells. These include both ionotropic receptors (AMPA, NMDA, and KA), as well as metabotropic receptors (mGluRs). The effect of Glu is determined by the receptor subtype, localization, and interactions with various scaffolding and signaling proteins in the postynaptic density (PSD). Activation of Glu receptors results not only in rapid ionotropic effects, but also in long-term synaptic plasticity.
Potential Targets for Drug Development numbered in the figure:
1) AMPA receptor modulation; 2) NMDA receptor modulation (a) extrasynaptic, (b) synaptic; 3) Group I metabotropic receptor modulation; 4) Voltage dependent Na+ channel modulation that regulates Glu release; 5) Group II metabotropic receptor modulation (mGluR2/3 receptor antagonists have demonstrated antidepressant activity, mGluR2/3 agonists have demonstrated axiolytic activity); 6) Facilitation of Glu clearance by EAATs; 7) Modulation of extrasynaptic Glu release. 8) PSD proteins. Theoretically, it is possible that agents capable of modifying the expression or function of PSD proteins could be used to treat mood disorders; 9) Modulation of presynaptic vesicular Glu release; 10) Modulation of presynaptic vesicular loading of Glu.
Gln=glutamine; Glu=glutamate; VGLUT=vesicular glutamate transporter; mGlu metabotropic glutamate transporter; EAAT=excitatory amino acid transporter; Ca=calcium; Na=sodium; NMDA=N-methyl-D-aspartate receptor; AMPA= α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid receptor; BDNF=brain derived neurotrophic factor; TCA=tricarboxylic acid cycle; Ac-CoA=acetyl-CoA; α-KG= α-ketoglutarate; SNARE=soluble N-ethylmaleimide sensitive factor attachment receptor complex.
In the brain, Glu can either be synthesized de novo from glucose via the Krebs cycle and the transanimation of α-oxoglutarate, or it can be recycled through the glutamate/glutamine (Glu/Gln) cycle (see below) 18. Glu is transported into synaptic vesicles by vesicular Glu transporters (VGLUTs) 19, 20, where it is stored at high concentrations, and protected from degradation before being released in a Ca2+-dependent manner into the synaptic cleft by exocytosis.
On release, Glu binds to and activates specialized ionotropic and metabotropic receptors found throughout the CNS that have wide-ranging effects on neural excitability (see BOX 1). The post synaptic density (PSD), a large supramolecular complex composed of Glu receptors, anchoring proteins, cytoskeletal proteins, and signaling proteins 21, also contributes to the regulation of Glu signaling. Glu receptors bind to several receptor-binding proteins in the PSD, including PICK1, stargazin, GRIP, membrane-associated guanylate kinases (MAGUKs), and Homer, via regions on their cytoplasmic domains. These proteins can be regulated by both post-translational splicing and phosphorylation events, and are essential for receptor trafficking, and for coupling the receptors to other scaffolding and signaling proteins.
Box 1 | Glutamate receptors
There are two major subtypes of glutamatergic receptors in the CNS: ionotropic and metabotropic. Metabotropic Glu receptors (mGluRs) are G protein-coupled receptors. Eight types have been cloned and they can be organized into three different subgroups based upon the signaling transduction pathways that they activate. Group I (mGluR1 a-d, mGluR5 a-b) act primarily through PLCβ and the activation of the IP3 and DAG second messenger systems 154. Groups II (mGluR 2 and 3) and III (mGluR4, mGluR6-8) are negatively coupled to adenylyl cyclase. Ionotropic Glu receptors are ligand-gated ion channels that open when activated by the binding of an agonist. There are three different subgroups:
AMPA Receptors
AMPA receptors mediate the fast, rapidly desensitizing excitation at most synapses, and are responsible for the initial reaction to Glu in the synapse. Their activation opens the pore permitting the inward flow of sodium, resulting in the depolarization of the neuronal membrane. The AMPA receptors comprise a homo or heteromeric complex of four subunits (GluR1-4). Because of differences in individual subunit expression, posttranscriptional modifications, and alternative splicing modifications they are functionally diverse. At mature synapses, AMPA receptors are usually co-expressed with NMDA receptors.
Kainate (KA) Receptors
KA receptors are coded by two gene families coding for the low affinity GluR5-7 subunits and the high affinity KA1 and KA2 subunits. These subunits are also subject to extensive posttranscriptional and posttranslational modification. Like AMPA receptors, KA receptors are associated with voltage-dependent channels that primarily allow for the influx of Na+ ions that mediate fast excitatory neurotransmission, but they appear to have a distinct distribution.
NMDA Receptors
NMDA receptors are believed to exist primarily as tetrameric complexes comprising two obligatory NR1 subunits and two NR2 subunits. There are at least eight splice variants of the NR1 subunit, four NR2 genes (NR2 A-D), and two NR3 subunits (NR3A and NR3B). The binding site for Glu has been found in the NR2 subunit and the site for the co-agonist glycine has been localized to the NR1 subunit.
NMDA receptors are normally blocked under resting conditions by the obstructing effects of Mg+. However, once the surrounding membrane is depolarized, these receptors may be activated by the combined binding of two molecules of Glu and two molecules of glycine or D-serine 155. Thus, NMDA receptor activation serves as a functional marker of converging excitatory input and produces excitation over longer periods of time. Synaptic NMDA receptors activate MAPK and the transcription factor cAMP- Ca2+ response element-binding protein (CREB), induce expression of the gene that encodes brain-derived neurotrophic factor (BDNF), and promote neuronal survival, whereas extrasynaptic NMDA receptors propagate opposing signals that promote cell death 156, 157.
Glu is cleared from the extracellular space via high-affinity excitatory amino acid transporters (EAATs) in neighboring glial cells, which convert Glu into glutamine (Gln) via the action of glutamine synthetase (GS). Gln is then transported back into the glutamatergic neuron where it is hydrolyzed by glutaminase back into Glu (see Figure 1). Due to the lack of degradative enzymes in the synapse, uptake by the EAATs is the primary mechanism through which the action of extracellular Glu is terminated. Failure of the EAATs to effectively clear Glu from the extracellular space results in various forms of cellular damage 22, 23 and is believed to be linked with neuropsychiatric disorders 24.
Glu in the pathophysiology of mood disorders
Abnormal function of the glutamatergic system has been implicated in the pathophysiology of many different disorders including amyotrophic lateral sclerosis (ALS), Huntington’s chorea, epilepsy, Alzheimer’s disease, schizophrenia, and anxiety disorders. Thus, dysfunction of glutamatergic neurotransmission may be a common pathophysiological mechanism, aspects of which are shared between several disorders. However, it is beyond the scope of this review to consider this extensive literature, and the reader is referred elsewhere for more information on these other disorders 25–28. This review will focus only on the relationship between glutamate neurotransmission and mood disorders.
Changes in Glu levels
Glutamatergic abnormalities have been reported in plasma 29–31, serum 32, CSF 33, 34 and brain tissue 35 of individuals afflicted with mood disorders. However, these studies are compromised by problems with medication exposure, postmortem effects on metabolism, and the inability to identify the precise source of the Glu in the peripheral tissue, thus rendering the findings difficult to interpret 36, 37. A recent postmortem study specifically controlling for the effects of postmortem interval found increased levels of Glu in the frontal cortex of patients with BPD and MDD 38.
Fortunately, in vivo measures of brain Glu content can now be made with the use of proton magnetic resonance spectroscopy (1H-MRS). Although it remains extremely difficult to assign unequivocal resonance peaks to the individual Glu resonances, a combined measure termed Glx, which predominantly reflects Glu content but also contains Gln and GABA components, has now been adopted by several groups. A growing collection of 1H-MRS brain imaging studies suggests that abnormalities do exist within the glutamatergic system of patients with mood disorders. However, the extent and direction of the changes are still to be determined, with reports of elevated Glu levels in the occipital cortex (OCC) of depressed patients, and decreased Glu levels in the anterior cingulate cortex (ACC) and possibly other frontal regions. In addition, there are reports of increased Glu content in several brain regions of individuals with BPD. While it remains difficult to draw any specific conclusions regarding the pathophysiological significance or etiology of the postmortem and imaging findings related to Glu content to date, the studies do suggest that the glutamatergic system is altered in several brain regions in association with mood disorders. Newer 13C-MRS studies capable of measuring the rate of glutamine/glutamine cycling should provide greater detail on the changes in glutamatergic function associated with mood disorders 39.
Glu receptor alterations
Several studies have found differences related to NMDA receptor expression and binding affinities between individuals with and without mood disorders. An initial report demonstrated differences in the allosteric modulation of glutamate binding through the glycine binding site on the NMDA receptor in the frontal cortex of age- and postmortem interval-matched suicide victims 40. Later studies reported decreased binding of Glu receptor antagonists in the hippocampus of eight subjects with BPD 41. Because these compounds bind to the glutamate binding site and open ion channel of the NMDA receptor, respectively, these data suggest that there is a decrease in the number of open ion channels associated with no significant change in the density of NMDA receptors in regions of the hippocampus from subjects with BPD. Using the same hippocampal tissue, McCullumsmith and colleagues (2007) recently demonstrated a coexistent decrease in NR1 and NR2A transcript expression, but no difference in NR2B expression in BPD subjects 42. This finding is consistent with an earlier report of reduced hippocampal NR1 transcript expression in individuals with mood disorders 43. A similar reduction in NMDA receptor binding and NR1 subunit expression was also reported in the superior temporal cortex of individuals with MDD and BPD 44. In sum, these studies point to a differential expression of the various NMDA receptor subunits in individuals with mood disorders.
At the genetic level, two polymorphisms of the GRIN1 gene coding for the NR1 subunit have been associated with BPD through a linkage disequilibrium study 45. Two polymorphisms in the GRIN2 gene coding for NR2B were also recently reported to be associated with BPD, especially if psychotic features were present, but no coexistent change in NR2B mRNA expression could be associated with the polymorphism in the region of the dorsolateral prefrontal cortex (DLPFC) 46. In a related study, the density of GABA interneurons that express the NMDA NR2A subunit appeared to be decreased in the ACC of subjects with BPD 47. Fewer postmortem studies have explored the association between AMPA and KA receptors and mood disorders. There is a single report of increased AMPA binding associated with a decreased GluR1 subunit expression in the striatum in BPD 48. Although no differences in AMPA or KA binding were detected in hippocampus or DLPFC, decreased levels of GluR2 were found in the DLPFC in individuals with BPD, and GluR2 and GluR3 in individuals with MDD 41, 49.
Recent postmortem studies have begun to explore whether alterations in the expression of intracellular anchoring and trafficking proteins associated with the PSD are altered in mood disorders. Decreased expression of SAP102 (a synapse-associated protein that primarily interacts with the NR2B subunit) coincided with a decrease in NR1 and NR2A subunit expression in the hippocampus of subjects with BPD 42, and has also been observed in the striatum 50 and thalamus 51 of subjects with MDD and BPD. Additional decreases in NF-L and PSD95 transcripts were observed only in BPD. PSD95 immunoreactivity was also decreased in the hippocampal dentate in BPD, but not in MDD or the orbital frontal cortex or hippocampal hilus in MDD or BPD 52. Furthermore, no differences in the expression of AMPA receptor trafficking and signaling molecules (NSF, PICK1, Stargazin, and syntenin) could be found in the DLPFC of individuals with mood disorders 49. The key findings are noted in Table 1.
Table 1.
Evidence for alterations in NMDA and AMPA/KA receptor function in mood disorders
| Target | Mood Disorder | Brain Region | Effect | Ref |
|---|---|---|---|---|
| NMDA receptor | MDD | Frontal cortex | Reduced [3H]CGP-39653 binding | 75 |
| NMDA receptor | MDD | Superior temporal cortex | NMDA receptor binding and NR1 subunit expression | 44 |
| NMDA receptor | BPD | Hippocampus | Reduced CGP-39653 and MK-801 binding and levels of NR1 and NR2A transcript | 41–43 |
| NMDA receptor | BPD | Superior temporal cortex | NMDA receptor binding and NR1 subunit expression | 44 |
| NMDA receptor | BPD | ACC | NR2A subunit decreased in GABA interneurons | 47 |
| NMDA receptor | BPD | polymorphisms in genes coding NR1 and NR2B | 46 | |
| AMPA receptor | MDD | DLPFC | Decreased levels of GluR2 and GluR3 | 41 |
| AMPA receptor | MDD | Thalamus and Striatum | Decreased expression of SAP102 | 50, 51 |
| AMPA receptor | BPD | Striatum | Increased AMPA binding is associated with decreased GluR1 subunit expression | 48 |
| AMPA receptor | BPD | DLPFC | Decreased levels of GluR2 | 49 |
| AMPA receptor | BPD | Thalamus and Striatum | Decreased expression of SAP102 | 50, 51 |
| NMDA and AMPA receptors | BPD | Hippocampus | Decrease in PSD95 immunoreactivity | 52 |
Evidence of glial cell pathology
Recent work has highlighted the importance of glial cells in the function and regulation of the glutamatergic neurotransmitter system. Although some evidence suggests that glial abnormalities are predominantly due to reduced numbers of oligodendrocytes 53, other glial cells involved in glutamate neurotransmission are also likely to be involved in glial pathology 54. Decreased glial cell number and density have been found in several brain regions in individuals with mood disorders 53, 55–62. Reduced expression of EAAT1, EAAT2, and GS has been found in two frontal brain regions in postmortem brain samples from individuals with MDD 63, and decreased levels of EAAT 3 and EAAT4 mRNA expression were found in the striatum of subjects with mood disorders 64 (reviewed in 65–67). The reduced levels of glial cell number and density in the brains of mood disorder patients are one of the most consistent pathological findings in psychiatric research. If this represents a true decrease in glial cell function it may help to explain the altered Glu content observed in several brain regions of patients with mood disorders.
Effects of mood-disorder therapeutics
Antidepressants
Pursuant to their initial finding showing that NMDA antagonists have antidepressant properties 68, seminal studies by the Skolnick laboratory demonstrated that chronic antidepressant administration (monoaminergic-based antidepressants, tricyclic antidepressants 69, 70, and electroconvulsive therapy) regulates NMDA receptor expression and function 71–75. In situ hybridization studies 76, 77 have revealed that repeated electroconvulsive shock (ECS) in rats produced an increase in expression of mRNA for GluR1, a subunit of the AMPA receptor in the dentate gyrus, and in the CA1 and CA3 cell fields of the hippocampus, areas that are believed to be critical for normal affective functioning.
Data also suggest that antidepressants upregulate AMPA receptor function by stimulating AMPA receptor subunit phosphorylation. Acute treatment with fluoxetine increases the phosphorylation of GluR1 at Ser831 and Ser845, whereas chronic dosing (19 days) with fluoxetine selectively increases the phosphorylation of GluR1 at Ser845 78. Interactions of GluR1 and GluR2/3 with proteins implicated in AMPA receptor trafficking and with scaffolding proteins appear to account for the enhanced membrane expression of AMPA receptors in the hippocampus after antidepressant treatment 79. Barbon and colleagues (2006) examined the mRNA expression of all subunits of AMPA receptors after antidepressant treatment and found that chronic treatment with fluoxetine and desipramine exerted moderate but selective effects on Glu receptor mRNA expression and RNA editing 80.
In summary, growing evidence supports the idea that antidepressants, via a cascade of time-dependent signaling changes, ultimately converge to regulate AMPA- and NMDA-mediated synaptic plasticity. Taken together, these preclinical studies suggest that reductions in NMDA receptor function are a consequence of treatment with known antidepressant medications and antidepressants and mood stabilizers regulate AMPA receptor phosphorylation and trafficking (reviewed in 81). The evidence supporting these hypotheses is briefly presented in Table 2.
Table 2.
Effects of antidepressants on the glutamatergic system
| Effect | Drug | Brain region | References |
|---|---|---|---|
| Reduced depolarizationstimulated Glu release and overflow | Several classes of antidepressants | Hippocampus and PFC | 144, 158 |
| Reduced expression or function of NMDA receptors | Several classes of antidepressants | Several brain regions | 69, 70, 72–75, 159, 160 |
| Altered expression and activation of AMPA receptors | Various antidepressants | Prefrontal cortex and hippocampus | 78–80, 114, 161, 162 |
| Increased expression of the vesicular glutamate transporter | Several antidepressant agents | Cerebral cortex | 163, 164 |
Mood stabilizers
Patients with BPD are generally treated with a class of medications known as mood stabilizers. Mood stabilizers have antimanic effects, exert prophylactic effects in preventing recurrent manic or depressive episodes, and may also possess some antidepressant properties. The prototypic agent of this class is lithium, a seemingly simple monovalent cation. A variety of anticonvulsant agents, most notably valproic acid (valproate (VPA), a substituted pentanoic acid), are also used as mood stabilizing agents 82.
In view of the evidence that excessive synaptic Glu may contribute to neuronal atrophy and loss, it is notable that chronic treatment with lithium has been shown to upregulate synaptosomal uptake of Glu in mice 83. Furthermore, chronic treatment with therapeutically relevant concentrations of lithium in cultured rat cerebellar, cortical, and hippocampal neurons protected against glutamate-induced excitotoxicity 84. The investigators report that the protection could be attributed, at least in part, to inhibition of NMDA receptor–mediated Ca2+ influx 84, 85. Chronic administration of VPA led to a dose-dependent increase in hippocampal Glu uptake capacity, as measured by uptake of [3H]glutamate into proteoliposomes, by increasing the levels of the Glu transporters EAAT1 and EAAT2 in hippocampus. Overall, chronic treatment with VPA or lithium is likely to decrease intrasynaptic Glu levels through a variety of mechanisms.
In view of the critical role of AMPA receptor trafficking in regulating various forms of plasticity, recent studies have sought to determine whether these two structurally highly dissimilar antimanic agents—lithium and VPA—affect AMPA receptor trafficking. Both have the common effect of downregulating AMPA GluR1 synaptic expression in hippocampus after prolonged treatment with therapeutically relevant concentrations, both in vitro and in vivo 86. The key findings are highlighted in Table 3.
Table 3.
Effects of lithium or VPA on the glutamatergic system
| Drug | Effect | Reference |
|---|---|---|
| Lithium | Alters VGLUT1 expression and presynapatic Glu release | 83, 163, 165 |
| Lithium | Alters Glu uptake | 166 |
| Lithium | Alters Glu receptor expression and function | 84, 85, 153, 167, 168 |
| VPA | Alters VGLUT1 expression and presynapatic Glu release | 169, 170 |
| VPA | Increases EAAT expression | 171, 172 |
| VPA | Alters Glu receptor expression and function | 89, 153, 173–178 |
Additional support for the therapeutic relevance of these data is provided by recent studies indicating that AMPA receptor antagonists attenuate several “manic-like” behaviors produced by amphetamine administration 87.
Lamotrigine is an anticonvulsant that also has mood stabilizing properties in patients with BPD. Of all the medications currently used in the treatment of mood disorders, lamotrigine probably has the most direct effects on the Glu system. There is evidence that it inhibits the release of Glu in the hippocampus of rats 88, and that it increases AMPA subunit receptor expression 89.
Glutamatergic agents in mood disorders
Several therapeutic agents that act on the glutamatergic system can be explored as potential treatments for mood disorders.
Inhibitors of glutamate release
In addition to lamotrigine, the US Food and Drug Administration-approved drug riluzole (2-amino-6-(trifluoromethoxy) benzothiazole), which is used for the treatment of ALS, has also been shown to inhibit Glu release. However, riluzole exerts a range of effects on the glutamatergic system, including AMPA receptor trafficking 89, stimulation of neurotrophic factor synthesis 90, and enhancement of Glu reuptake 91. It does not appear to directly affect NMDA or KA receptors 92. Riluzole has also been found to strongly attenuate the electrically evoked release of dopamine and norepinephrine but not serotonin; as mentioned previously, such neurotransmitters have long been implicated in the mechanism of antidepressant action 93.
Both preclinical and clinical studies indicate that riluzole has antidepressant properties 94–96. The first open label study, undertaken in depressed patients who had failed to respond to adequate trials of at least two antidepressants, found riluzole had significant antidepressant effects with 46% of trial completers meeting response criteria 94. In a subsequent study of patients with bipolar depression, augmentation of lithium with open label riluzole for eight weeks significantly improved depressive symptoms with the average total Montgomery-Asberg Depression Rating Scale scores dropping by more than 15 points by the eighth week of the study 95. Finally, in another open label study where riluzole was added to ongoing antidepressant therapy in patients with treatment-resistant MDD, patients experienced a significant improvement in depressive symptoms reflected by a nearly 10 point reduction in Hamilton depression rating scale scores after six to12 weeks 96. Overall, riluzole was well-tolerated in these trials, and interestingly, the patients who responded to riluzole tended to be in remission (i.e., almost symptom-free), suggesting that there may be a subgroup of patients for whom glutamatergic strategies have considerable value.
Although the mechanisms of lamotrigine and riluzole appear to be similar, preliminary data have also shown that patients who had previously failed to respond to lamotrigine subsequently responded to riluzole. This may be due in part to riluzole’s additional effects on Glu neurotransmission, such as enhanced Glu uptake, that are not seen with lamotrigine 91. The studies that highlight riluzole’s efficacy in subjects who were previously medication resistant are encouraging, especially because these patients usually have markedly reduced rates of response to medication. However, it will be important to confirm these findings in randomized placebo controlled trials before any firm conclusions can be drawn about the true efficacy of riluzole in the treatment of MDD.
Partial NMDA receptor agonists
D-Cycloserine (4-amino-isoxazolidin-3, DCS) is a broad spectrum antibiotic with some antidepressant properties in rodent models of depression. Early reports based on clinical observation indicated that DCS had positive effects on mood, insomnia, and appetite in depressed tuberculosis patients 97, 98. A subsequent double-blind, placebo-controlled, six-week crossover study involving 22 patients with treatment-resistant MDD found the addition of DCS at 250 mg/day to other antidepressants to be ineffective 99. However, it is possible that the doses studied were too low. It wasn’t until 1997 that it was shown that doses higher than 500 mg/day of DCS are required to elicit a neuroendocrine response (measured as an increase in plasma levels of luteinizing hormone); such a response is indicative of NMDA antagonism 100, 101. Interestingly, based on animal work showing that DCS facilitated extinction of fear when given either before or shortly after exposure to fearful cues 102, several recent clinical studies have shown that lower doses of DCS are effective in enhancing the efficacy of exposure-based therapies for anxiety disorders; no significant effect was seen when the higher dose of 500 mg/day was used 103–105.
NMDA antagonists
Memantine (1-amino-3, 5-dimethyl-adamantane) is a derivative of amantadine, a low-affinity, uncompetitive, open-channel NMDA antagonist. Memantine is well-tolerated 106, and is approved for the treatment of moderate to severe Alzheimer’s disease, where it has been shown to enhance cognition and minimize clinical deterioration in patients with Alzheimer’s disease, vascular dementia, and mixed dementia 107.
Preclinical studies have described a dose-dependent decrease in immobility time in the forced swim test (FST) in rats following administration of memantine. A synergistic effect was seen when imipramine and fluoxetine were given jointly with memantine in the FST in rats (reviewed in 65, 81). One recent paper reported that two patients with treatment-resistant BPD had improved cognition and mood stabilization with memantine 108, but an eight-week, double-blind, placebo-controlled trial of memantine in 32 patients with MDD found no significant antidepressant or anti-anxiety effects 109. It is possible, however, that antidepressant effects might have occurred with much higher doses of memantine, as suggested by another recent open-label flexible dosing study of memantine in MDD 110. Another possibility is that memantine may evince antidepressant properties by augmenting the effects of other agents.
Ketamine (dl2-(o-chlorophenyl)-2-(methylamino) cyclohexanone hydrochloride) is another NMDA antagonist 111 and a derivative of phencyclidine (PCP). Its use in humans is associated with psychotomimetic and cognitive effects that possibly preclude its chronic use in treatment 112.
Ketamine’s primary mechanism of action is blocking the NMDA receptor at the PCP site within the ionotropic channel. Simultaneously, ketamine, presumably by producing a disinhibition of GABAergic inputs and thereby enhancing the firing rate of glutamatergic neurons, increases the presynaptic release of Glu, resulting in increased extracellular levels of Glu 113. This increase in Glu release then preferentially favors AMPA receptors over NMDA receptors because the latter are blocked by ketamine; thus, the net effect of ketamine’s antidepressant effect on a cellular level is an increased glutamatergic throughput of AMPA relative to NMDA. The ability to block ketamine’s antidepressant-like effects with an AMPA antagonist suggests that enhanced AMPA throughput may be critical in producing these effects 114.
Clinically, ketamine is a popular agent for emergency department procedural sedation in children, with ample evidence to support its safety and efficacy 115. Although its psychotomimetic side effects have led to its misuse and abuse, there is no evidence that ketamine causes physical dependence in humans 116, and recent studies found no evidence that repeated, albeit limited (mostly less than four exposures), exposure to ketamine increases the risk of more severe or more protracted psychosis, perceptual changes resembling dissociation, severe emotional distress, or euphoria in healthy subjects 117 or patient populations 118.
Ketamine and related NMDA antagonists have been shown to have anxiolytic and antidepressant effects in animal models (reviewed in 65, 81) as well as antidepressant effects in humans 109, 119. One study found that seven subjects with treatment-resistant MDD showed significant improvement in depressive symptoms within 72 hours of treatment with ketamine119. Furthermore, a double-blind placebo-controlled crossover study found that a single intravenous dose of ketamine (0.5 mg/kg over 40 minutes) resulted in rapid and significant antidepressant effects in patients with treatment-resistant MDD within two hours, an effect that remained significant for seven days. Seventy-one percent of subjects met response criteria, and 29% achieved remission at 24 hours following the infusion of ketamine. Thirty-five percent of subjects maintained response to ketamine for at least one week; two of these maintained response at least two weeks. By contrast, no subject on placebo responded at one or seven days. Mild perceptual disturbances occurred in most patients, but in all cases lasted less than one hour. No serious adverse events occurred 109.
Emerging promise of novel therapeutics
AMPA potentiators
Positive modulators of AMPA receptors do not activate AMPA receptors themselves but slow the rate of receptor desensitization and/or deactivation in the presence of an agonist (see 120, 121 for review). Several modulators from various structural classes have been synthesized, including benzothiazides (e.g., cyclothiazide), the benzoyliperidines (e.g., CX-516), and the birylpropylsulfonamides (e.g., LY392098). They are being studied in cognition, anxiety, stroke, Parkinson’s disease, and MDD (reviewed in 122). In preclinical studies, Aniracetam 122, Ampalex 123, LY392098 124, LY 451616 124, 125, and S18986 126, 127 have all been found to have some antidepressant properties, such as reduction of submissive behavior. Some of these compounds have entered clinical trials but proof of their antidepressant efficacy is pending.
Subunit selective NR2B antagonists
The first generation NMDA receptor complex antagonists include ketamine, MK-801, and PCP. These blockers are potent neuroprotectants in vitro and in vivo, but are likely to produce psychotomimetic effects when used acutely. However, other more selective NMDA antagonists, especially those that act on the NR2B receptor subtype (e.g., Ifenprodil), appear to have a low liability for this outcome, particularly when used at lower doses. Unfortunately, some of these compounds also have a high affinity to alpha1-adrenoreceptors and other ion channels.
More recently, a series of oral, brain-penetrant NR2B antagonists with more selective effects are being developed. These include indole-2-carboxamides, benzimidazole-2-carboxamides, and HON0001 128–130. A recent double-blind, randomized, placebo-controlled study indicated that CP-101–606, another NR2B-specific NMDA antagonist, had statistically significant antidepressant effects in patients with treatment-resistant MDD 131. The average reduction in symptom severity was approximately two times greater in active versus placebo-treated patients. Dissociative symptoms produced by the active infusion were generally modest and resolved within eight hours.
Glial Glu transporter enhancers
Recently, there has been considerable enthusiasm in the scientific community for studying the effects of β-lactam antibiotics in neurodegenerative diseases because, unlike other classes of antibiotics, they selectively induce transcription of the gene encoding the EAAT2 (GLT1) Glu transporter 132–134. One of the β-lactam antibiotics — ceftriaxone — was found to increase expression of the gene encoding for the GLT1 receptor within a few days of its administration, and therefore might selectively modulate the glutamatergic system. Ceftriaxone has been found to have antidepressant-like effects in several animal models of depression 135. The data from this study are also consistent with the hypothesis that excessive Glu transmission might be involved in the pathophysiology of MDD.
Group I metabotropic receptor modulators
As noted above, the group I mGlu receptors play a major role in regulating postsynaptic excitability through interactions with NMDA receptors. mGluR5 receptors are an attractive target for modulating Glu neurotransmission because, in addition to being present on postsynaptic neurons, they are also present on glial cells where they appear to regulate the release of excitatory transmitters from glia; this, in turn, leads to selective stimulation of extrasynaptic NMDA receptors 136–138. Group I metabotropic receptor modulating agents, especially mGluR1/5 antagonists such as 2- methyl-6-(phenylethynyl) pyridine (MPEP) and 3-[(2-Methyl-1,3-thiazol-4-ylethynyl]pyridine (METP), have strong anxiolytic effects in preclinical animal models. More recent studies suggest that they may also possess antidepressant-like properties (see 139, 140); however, to date there are no published clinical studies demonstrating the effectiveness of this class of drug.
Group II metabotropic receptors (mGluR 2 and 3) are largely present on presynaptic membranes and on glial cells where they are believed to modulate glutamatergic neurotransmission by sensing Glu spillover and regulating transmitter release. The function of these mGlu2/3 receptors in modulating Glu neurotransmission make them extremely interesting targets for antidepressant drug development, and there is evidence suggesting that mGluR2/3-targeted drugs possess both antidepressant and anxiolytic properties (see 139, 140 for reviews).
However, whereas mGluR2/3 receptor antagonists (e.g. LY341495, MSG0039) appear to have antidepressant-like properties, mGluR2/3 receptor agonists (i.e. LY404039, LY354740) have a profile consistent with anxiolytic and antipsychotic drugs. As with the NMDA antagonists, many of the antidepressant-like effects of the mGluR2/3 receptor antagonists can be prevented by co-administration of an AMPA receptor antagonist 141, suggesting that activation of synaptic AMPA receptors may be a common pathway through which the antidepressant effects are transduced. No clinical studies have yet been conducted to explore the antidepressant efficacy of mGluR2/3 receptor antagonists 139 140.
Early phase clinical trials have recently supported the efficacy of mGluR2/3 receptor agonists in the treatment of schizophrenia 142, though some safety concerns for this class of drugs have been raised 143. If replicated in larger clinical trials, mGluR2/3 receptor agonists would represent completely novel class of therapeutics for schizophrenia.
Presynaptic packaging and Glu release inhibitors
Increasing evidence suggests that chronic antidepressant treatment results in modulation of presynaptic Glu release 144. Some evidence suggests this is the result of effects on the soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) complex that controls the structural and biochemical aspects of synaptic vesicle exocytosis 145; therefore, targeting the expression and function of these proteins has been suggested as a useful tool in the development of novel biomarkers and therapies for neuropsychiatric disorders 146.
As mentioned previously, an essential step in Glu neurotransmission is the concentration of Glu into synaptic vesicles before release from the presynaptic terminal. As a result, a series of inhibitors of Glu vesicular transporters are being developed and include amino acids and amino acid analogs, fatty acids, azo dyes, quinolines, and alkaloids. The potency with which these agents inhibit VGLUT varies considerably 147.
Recent studies have also shown the importance of the cystine-glutamate antiporter in controlling extracellular Glu content 148 and vesicular Glu release 149. N-Acetyl Cystine, an agent known to modulate the function of the cystine-glutamate antiporter has recently gained attention as a potential treatment for various neuropsychiatric disorders 150, 151, and several clinical trials are currently underway.
Conclusions
A considerable body of evidence supports the role of the Glu system in mediating synaptic plasticity as well as long-term cell growth/atrophy. Mounting evidence now also suggests that disturbances of brain and synaptic plasticity contribute to the pathological processes associated with mood disorders 152, thus providing a potential link between the glutamatergic neurotransmitter system and the pathophysiology of mood disorders. The data reviewed here suggest mood disorders are in fact associated with abnormal function and regulation of the glutamatergic neurotransmitter system. Further, emerging studies suggest that targeting glutamatergically-mediated synaptic plasticity could be an effective strategy for treating these devastating disorders.
There has, unfortunately, been little progress in developing truly novel medications for mood disorders. Although a relationship between the glutamatergic system and mood disorders was originally proposed nearly two decades ago 68, interest in the field has grown rapidly in recent years. Today, several therapies targeting this system show substantial promise for the treatment of mood disorders. Most notably, ketamine and riluzole merit continued study as putative archetypes for improved therapeutics. Ketamine is of particular interest because it has been shown to bring about rapid and relatively sustained antidepressant effects. Its rapid, robust, and consistently reproducible antidepressant effects offer a unique opportunity to better delineate the precise cellular mechanisms involved 114.
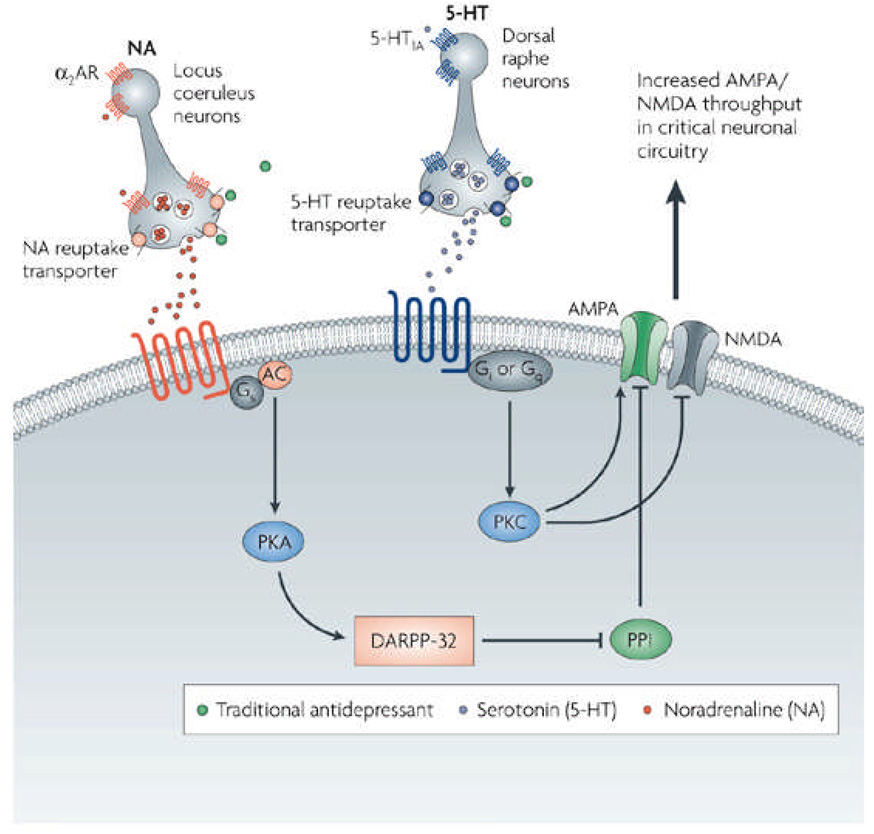
AMPA receptor stimulation appears to mediate the antidepressant-like effects of both ketamine and the group II metabotropic Glu receptor antagonist MGS0039 141, suggesting that enhanced transmission through glutamatergic AMPA receptors may provide a common mechanism of antidepressant action. In agreement with this hypothesis, chronic administration of antidepressants also enhances AMPA receptor surface levels 89, 153, and AMPA potentiators have shown antidepressant-like effects in animal models 124. We postulate that the therapeutic effects of both monoaminergic antidepressants and glutamatergic agents may be mediated by increased AMPA to NMDA throughput in critical neuronal circuits (Figure 2).
Figure 2. Antidepressants converge to regulate AMPA- and NMDA-mediated synaptic plasticity in critical neuronal circuits.
This figure depicts the complex, time-dependent regulation of intracellular signaling cascades by traditional antidepressants (green). Via their initial effects on intrasynaptic serotonin and/or norepinephrine, these agents ultimately converge to regulate AMPA and NMDA receptor trafficking, synaptic plasticity, and information processing in critical circuits. Targeting AMPA/NMDA-mediated synaptic throughput more directly may result in improved and faster-acting antidepressants. NE=norepinephrine; 5-HT=5-hydroxytryptamine (serotonin); a2-AR=a2 adrenergic autoreceptor; AMPAR=AMPA receptor; NMDAR=NMDA receptor; Gs=G protein stimulating adenylyl cyclase; AC=adenylyl cyclase; Gi or Gq=G proteins coupled to phosphoinositide turnover; PKC=protein kinase C; PKA=protein kinase A; DARPP=32= dopamine and cAMP-regulated phosphoprotein of 32 kD; PP1=protein phosphatase 1.
Although the results obtained with riluzole-like or ketamine-like drugs are still very preliminary, they provide incentive for further study of the role of Glu in mood disorders. Continued exploration of the antidepressant-like effects of glutamatergic drugs holds considerable promise for the development of new treatments for mood disorders. The fact that currently available antidepressants take weeks to achieve their full effects leaves patients particularly vulnerable to devastating symptoms and elevated risk of self-harm. Thus, any pharmacological strategy that could exert a rapid and sustained antidepressant effect within hours or even days could have a substantial beneficial impact on patients' quality of life as well as public health.
Acknowledgments
We would like to acknowledge the support of the Intramural Research Program of the National Institute of Mental Heath, the Stanley Medical Research Institute and NARSAD. NIMH K02MH076222 (GS). Ioline Henter provided outstanding editorial assistance. Drs. Sanacora and Krystal are co-sponsors of a patent application (PCTWO06108055A1) that was filed by Yale University related to the use of drugs that modulate glutamate neurotransmission for the treatment of depression. A patent application for the use of ketamine in depression has been submitted listing Drs. Manji and Zarate among the inventors. Drs. Manji and Zarate have assigned their rights on the patent to the US government.
GLOSSARY TERMS
- Synaptic plasticity
The cellular processes that result in lasting changes in the efficacy of neurotransmission. Changes in neurotransmitter levels, receptor subunit phosphorylation, surface/cellular levels of receptors, and conductance changes all regulate the strength of signal transmission at the synapse.
- Neural plasticity
Changes in intracellular signalling cascades and gene regulation that lead to modifications of synapse number and strength, variations in neurotransmitter release, remodelling of axonal and dendritic architecture and, in some areas of the CNS, the generation of new neurons. These modifications can be of short duration or long lasting.
- Major depressive disorder (MDD)
A chronic mood disorder characterized by a long-lasting depressed mood or marked loss of interest or pleasure in all or nearly all activities. MDD often affects mental efficiency, memory, appetite, and sleep habits.
- Bipolar disorder (BPD)
A mood disorder in which affected individuals alternate between states of deep depression and mania. While depression is characterized by persistent and long-term sadness or despair, mania is a mental state characterized by great excitement, flight of ideas, a decreased need for sleep, and, sometimes, uncontrollable behavior, hallucinations, or delusions.
- Glutamate/glutamine (Glu/Gln) cycle
Process through which most brain Glu is recycled. Glu released by neurons is converted to Gln in astrocytes. Gln is then transported out for re-uptake by neurons, which convert it back into Glu via the action of glutaminase.
- Psychotomimetic
Refers to a drug or substance that produces psychological or behavioral changes that resemble those of a psychotic state.
- RNA editing
Molecular processes in which the information content is altered in a RNA molecule through a chemical change in the base makeup.
- Montgomery-Asberg Depression Rating Scale
An eleven-item clinician-administered questionnaire used to rate the severity of a patient's depression.
- Hamilton Depression Rating Scale
A 21-question, multiple-choice, clinician-administered questionnaire used to rate the severity of a patient's depression.
REFERENCES
- 1.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Fagiolini A, et al. Functional impairment in the remission phase of bipolar disorder. Bipolar Disord. 2005;7:281–285. doi: 10.1111/j.1399-5618.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 3.Huxley N, Baldessarini RJ. Disability and its treatment in bipolar disorder patients. Bipolar Disord. 2007;9:183–196. doi: 10.1111/j.1399-5618.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 4.Tohen M, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160:2099–2107. doi: 10.1176/appi.ajp.160.12.2099. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 6.Rush AJ, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi MH, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 8.Judd LL, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 9.Nierenberg AA, et al. Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry. 2006;163:210–216. doi: 10.1176/appi.ajp.163.2.210. [DOI] [PubMed] [Google Scholar]
- 10.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 11.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 12.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 13.Berman RM, Krystal JH, Charney DS. In: Biology of Schizophrenia and Affective Disease. Watson SJ, editor. Washington, D.C: American Psychiatric Press; 1996. pp. 295–368. [Google Scholar]
- 14.Manji HK, Moore GJ, Rajkowska G, Chen G. Neuroplasticity and cellular resilience in mood disorders. Millennium Article. Mol Psychiatry. 2000;5:578–593. doi: 10.1038/sj.mp.4000811. [DOI] [PubMed] [Google Scholar]
- 15.Payne JL, Quiroz JA, Zarate CA, Manji HK. Timing is everything: Does the robust upregulation of noradrenergically regulated plasticity genes underlie the rapid antidepressant effects of sleep deprivation? Biol Psychiatry. 2002;52:921–926. doi: 10.1016/s0006-3223(02)01676-1. [DOI] [PubMed] [Google Scholar]
- 16.Orrego F, Villanueva S. The chemical nature of the main central excitatory transmitter: a critical appraisal based upon release studies and synaptic vesicle localization. Neuroscience. 1993;56:539–555. doi: 10.1016/0306-4522(93)90355-j. [DOI] [PubMed] [Google Scholar]
- 17.Krystal JH, et al. NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry. 1999;7:125–143. [PubMed] [Google Scholar]
- 18.Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 19.Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzog E, et al. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Peng J, et al. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- 22.Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 24.Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- 25.Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect. 1998;11:523–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- 26.Francis PT. Glutamatergic systems in Alzheimer's disease. Int J Geriatr Psychiatry. 2003;18:S15–S21. doi: 10.1002/gps.934. [DOI] [PubMed] [Google Scholar]
- 27.Cortese BM, Phan KL. The role of glutamate in anxiety and related disorders. CNS Spectr. 2005;10:820–830. doi: 10.1017/s1092852900010427. [DOI] [PubMed] [Google Scholar]
- 28.Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Prog Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH. Increased serum glutamate in depressed patients. Archiv fur Psychiatrie und Nervenkrankheiten. 1982;232:299–304. doi: 10.1007/BF00345492. [DOI] [PubMed] [Google Scholar]
- 30.Altamura CA, et al. Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry. 1993;150:1731–1733. doi: 10.1176/ajp.150.11.1731. [DOI] [PubMed] [Google Scholar]
- 31.Mauri MC, et al. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiol. 1998;37:124–129. doi: 10.1159/000026491. [DOI] [PubMed] [Google Scholar]
- 32.Mitani H, et al. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 33.Levine J, et al. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry. 2000;47:586–593. doi: 10.1016/s0006-3223(99)00284-x. [DOI] [PubMed] [Google Scholar]
- 34.Frye MA, Tsai GE, Huggins T, Coyle JT, Post RM. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry. 2006;61:162–166. doi: 10.1016/j.biopsych.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Francis PT, et al. Brain amino acid concentrations and Ca2+-dependent release in intractable depression assessed antemortem. Brain Res. 1989;494:315–324. doi: 10.1016/0006-8993(89)90600-8. [DOI] [PubMed] [Google Scholar]
- 36.Altamura C, Maes M, Dai J, Meltzer HY. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur Neuropsychopharmacol. 1995;5:71–75. doi: 10.1016/0924-977x(95)00033-l. [DOI] [PubMed] [Google Scholar]
- 37.Maes M, Verkerk R, Vandoolaeghe E, Lin A, Scharpe S. Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatr Scand. 1998;97:302–308. doi: 10.1111/j.1600-0447.1998.tb10004.x. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 39.de Graaf RA, Mason GF, Patel AB, Behar KL, Rothman DL. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16:339–357. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- 40.Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 41.Scarr E, Pavey G, Sundram S, MacKinnon A, Dean B. Decreased hippocampal NMDA, but not kainate or AMPA receptors in bipolar disorder. Bipolar Disord. 2003;5:257–264. doi: 10.1034/j.1399-5618.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 42.McCullumsmith RE, et al. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res. 2007;1127:108–118. doi: 10.1016/j.brainres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12:2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- 44.Nudmamud-Thanoi S, Reynolds GP. The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neurosci Lett. 2004;372:173–177. doi: 10.1016/j.neulet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 45.Mundo E, et al. Evidence that the N-methyl-D-aspartate subunit 1 receptor gene (GRIN1) confers susceptibility to bipolar disorder. Mol Psychiatry. 2003;8:241–245. doi: 10.1038/sj.mp.4001218. [DOI] [PubMed] [Google Scholar]
- 46.Martucci L, et al. N-methyl-D-aspartate receptor NR2B subunit gene GRIN2B in schizophrenia and bipolar disorder: Polymorphisms and mRNA levels. Schizophr Res. 2006;84:214–221. doi: 10.1016/j.schres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 48.Meador-Woodruff JH, Hogg AJ, Jr, Smith RE. Striatal ionotropic glutamate receptor expression in schizophrenia, bipolar disorder, and major depressive disorder. Brain Research Bulletin. 2001;55:631–640. doi: 10.1016/s0361-9230(01)00523-8. [DOI] [PubMed] [Google Scholar]
- 49.Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse. 2006;60:585–598. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- 50.Kristiansen LV, Meador-Woodruff JH. Abnormal striatal expression of transcripts encoding NMDA interacting PSD proteins in schizophrenia, bipolar disorder and major depression. Schizophr Res. 2005;78:87–93. doi: 10.1016/j.schres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Clinton SM, Meador-Woodruff JH. Abnormalities of the NMDA Receptor and Associated Intracellular Molecules in the Thalamus in Schizophrenia and Bipolar Disorder. Neuropsychopharmacology. 2004;29:1353–1362. doi: 10.1038/sj.npp.1300451. [DOI] [PubMed] [Google Scholar]
- 52.Toro C, Deakin JF. NMDA receptor subunit NRI and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophr Res. 2005;80:323–330. doi: 10.1016/j.schres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajkowska G, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression [comment] Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 57.Miguel-Hidalgo JJ, et al. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- 58.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 59.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 60.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder.[comment] Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 61.Webster MJ, et al. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav Immunity. 2001;15:388–400. doi: 10.1006/brbi.2001.0646. [DOI] [PubMed] [Google Scholar]
- 62.Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 63.Choudary PV, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCullumsmith RE, Meador-Woodruff JH. Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology. 2002;26:368–375. doi: 10.1016/S0893-133X(01)00370-0. [DOI] [PubMed] [Google Scholar]
- 65.Zarate CA, Quiroz J, Payne J, Manji HK. Modulators of the glutamatergic system: implications for the development of improved therapeutics in mood disorders. Psychopharmacol Bull. 2002;36:35–83. [PubMed] [Google Scholar]
- 66.Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10:808–819. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- 67.Toro CT, Hallak JE, Dunham JS, Deakin JF. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci Lett. 2006;404:276–281. doi: 10.1016/j.neulet.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 68.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 69.Sernagor E, Kuhn D, Vyklicky L, Jr, Mayer ML. Open channel block of NMDA receptor responses evoked by tricyclic antidepressants. Neuron. 1989;2:1221–1227. doi: 10.1016/0896-6273(89)90306-1. [DOI] [PubMed] [Google Scholar]
- 70.Pittaluga A, et al. Antidepressant treatments and function of glutamate ionotropic receptors mediating amine release in hippocampus. Neuropharmacology. 2007;53:27–36. doi: 10.1016/j.neuropharm.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 71.Nowak G, Trullas R, Layer RT, Skolnick P, Paul IA. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J Pharmacol Exp Ther. 1993;265:1380–1386. [PubMed] [Google Scholar]
- 72.Paul IA, Layer RT, Skolnick P, Nowak G. Adaptation of the NMDA receptor in rat cortex following chronic electroconvulsive shock or imipramine. Eur J Pharmacol. 1993;247:305–311. doi: 10.1016/0922-4106(93)90199-j. [DOI] [PubMed] [Google Scholar]
- 73.Paul IA, Nowak G, Layer RT, Popik P, Skolnick P. Adaptation of the N-methyl-D-aspartate receptor complex following chronic antidepressant treatments. J Pharmacol Exp Ther. 1994;269:95–102. [PubMed] [Google Scholar]
- 74.Skolnick P, et al. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 75.Nowak G, Legutko B, Skolnick P, Popik P. Adaptation of cortical NMDA receptors by chronic treatment with specific serotonin reuptake inhibitors. Eur J Pharmacol. 1998;342:367–370. doi: 10.1016/s0014-2999(97)01589-6. [DOI] [PubMed] [Google Scholar]
- 76.Wong ML, et al. Differential effects of kindled and electrically induced seizures on a glutamate receptor (GluR1) gene expression. Epilepsy Res. 1993;14:221–227. doi: 10.1016/0920-1211(93)90046-a. [DOI] [PubMed] [Google Scholar]
- 77.Naylor P, Stewart CA, Wright SR, Pearson RC, Reid IC. Repeated ECS induces GluR1 mRNA but not NMDAR1A-G mRNA in the rat hippocampus. Brain Res Mol Brain Res. 1996;35:349–353. doi: 10.1016/0169-328x(95)00264-s. [DOI] [PubMed] [Google Scholar]
- 78.Svenningsson P, et al. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proc Natl Acad Sci U S A. 2002;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinez-Turrillas R, Del Rio J, Frechilla D. Neuronal proteins involved in synaptic targeting of AMPA receptors in rat hippocampus by antidepressant drugs. Biochem Biophys Res Commun. 2007;353:750–755. doi: 10.1016/j.bbrc.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 80.Barbon A, et al. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry. 2006;59:713–720. doi: 10.1016/j.biopsych.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 81.Zarate CA, Jr, et al. Regulation of cellular plasticity cascades in the pathophysiology and treatment of mood disorders: role of the glutamatergic system. Ann N Y Acad Sci. 2003;1003:273–291. doi: 10.1196/annals.1300.017. [DOI] [PubMed] [Google Scholar]
- 82.Bowden CL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481–489. doi: 10.1001/archpsyc.57.5.481. [DOI] [PubMed] [Google Scholar]
- 83.Hokin LE, Dixon JF, Los GV. A novel action of lithium: stimulation of glutamate release and inositol 1,4,5 trisphosphate accumulation via activation of the N-methyl D-aspartate receptor in monkey and mouse cerebral cortex slices. Adv Enzyme Regul. 1996;36:229–244. doi: 10.1016/0065-2571(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 84.Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem. 2002;80:589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- 86.Du J, et al. Structurally dissimilar antimanic agents modulate synaptic plasticity by regulating AMPA glutamate receptor subunit GluR1 synaptic expression. Ann N Y Acad Sci. 2003;1003:378–380. doi: 10.1196/annals.1300.031. [DOI] [PubMed] [Google Scholar]
- 87.Du J, et al. The role of hippocampal GluR1 and GluR2 receptors in manic-like behaviors. J Neurosci. 2008;28:68–79. doi: 10.1523/JNEUROSCI.3080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahmad S, Fowler LJ, Whitton PS. Effects of combined lamotrigine and valproate on basal and stimulated extracellular amino acids and monoamines in the hippocampus of freely moving rats. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:1–8. doi: 10.1007/s00210-004-1008-4. [DOI] [PubMed] [Google Scholar]
- 89.Du J, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- 90.Mizuta I, et al. Riluzole stimulates nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis in cultured mouse astrocytes. Neurosci Lett. 2001;310:117–120. doi: 10.1016/s0304-3940(01)02098-5. [DOI] [PubMed] [Google Scholar]
- 91.Frizzo ME, Dall'Onder LP, Dalcin KB, Souza DO. Riluzole enhances glutamate uptake in rat astrocyte cultures. Cell Mol Neurobiol. 2004;24:123–128. doi: 10.1023/B:CEMN.0000012717.37839.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Debono MW, Le Guern J, Canton T, Doble A, Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1993;235:283–289. doi: 10.1016/0014-2999(93)90147-a. [DOI] [PubMed] [Google Scholar]
- 93.Jehle T, et al. Effects of riluzole on electrically evoked neurotransmitter release. Br J Pharmacol. 2000;130:1227–1234. doi: 10.1038/sj.bjp.0703424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zarate CA, Jr, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- 95.Zarate CA, Jr, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005;57:430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 96.Sanacora G, et al. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry. 2007;61:822–825. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crane G. Cycloserine as an antidepressant agent. Am J Psychiatry. 1959;115:1025–1026. doi: 10.1176/ajp.115.11.1025. [DOI] [PubMed] [Google Scholar]
- 98.Crane G. The psychotropic effect of cycloserine: a new use of an antibiotic. Comp Psychiatry. 1961;2:51–59. [Google Scholar]
- 99.Heresco-Levy U, et al. Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord. 2006;93:239–243. doi: 10.1016/j.jad.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 100.van Berckel BN, et al. The partial NMDA agonist D-cycloserine stimulates LH secretion in healthy volunteers. Psychopharmacology (Berl) 1998;138:190–197. doi: 10.1007/s002130050662. [DOI] [PubMed] [Google Scholar]
- 101.van Berckel BN, et al. Behavioral and neuroendocrine effects of the partial NMDA agonist D-cycloserine in healthy subjects. Neuropsychopharmacology. 1997;16:317–324. doi: 10.1016/S0893-133X(96)00196-0. [DOI] [PubMed] [Google Scholar]
- 102.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 103.Ressler KJ, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 104.Guastella AJ, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Kushner MG, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 106.Reisberg B, et al. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63:49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 107.Reisberg B, et al. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 108.Teng CT, Demetrio FN. Memantine may acutely improve cognition and have a mood stabilizing effect in treatment-resistant bipolar disorder. Rev Bras Psiquiatr. 2006;28:252–254. doi: 10.1590/s1516-44462006000300020. [DOI] [PubMed] [Google Scholar]
- 109.Zarate CA, Jr, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 110.Ferguson JM, Shingleton RN. An open-label, flexible-dose study of memantine in major depressive disorder. Clin Neuropharmacol. 2007;30:136–144. doi: 10.1097/WNF.0b013e3180314ae7. [DOI] [PubMed] [Google Scholar]
- 111.Harrison NL, Simmonds MA. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985;84:381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zarate CA, Charney DS, Manji HK. Searching for rational anti-N-methyl-D-aspartate treatment for depression. Arch Gen Psychiatry. 2007;64:1100–1101. doi: 10.1001/archpsyc.64.9.1099. [DOI] [PubMed] [Google Scholar]
- 113.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maeng S, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 115.Green SM, et al. Intravenous ketamine for pediatric sedation in the emergency department: safety profile with 156 cases. Acad Emerg Med. 1998;5:971–976. doi: 10.1111/j.1553-2712.1998.tb02773.x. [DOI] [PubMed] [Google Scholar]
- 116.Britt GC, McCance-Katz EF. A brief overview of the clinical pharmacology of "club drugs". Subst Use Misuse. 2005;40:1189–1201. doi: 10.1081/JA-200066730. [DOI] [PubMed] [Google Scholar]
- 117.Perry EB, Jr, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 2007;192:253–260. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- 118.Carpenter WTJ. The schizophrenia ketamine challenge study debate. Biol Psychiatry. 1999;46:1081–1091. doi: 10.1016/s0006-3223(99)00194-8. [DOI] [PubMed] [Google Scholar]
- 119.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 120.Bleakman D, Lodge D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology. 1998;37:1187–1204. doi: 10.1016/s0028-3908(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 121.Borges K, Dingledine R. AMPA receptors: molecular and functional diversity. Prog Brain Res. 1998;116:153–170. doi: 10.1016/s0079-6123(08)60436-7. [DOI] [PubMed] [Google Scholar]
- 122.Black MD. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacology (Berl) 2005;179:154–163. doi: 10.1007/s00213-004-2065-6. [DOI] [PubMed] [Google Scholar]
- 123.Knapp RJ, et al. Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. Eur J Pharmacol. 2002;440:27–35. doi: 10.1016/s0014-2999(02)01338-9. [DOI] [PubMed] [Google Scholar]
- 124.Li X, et al. Antidepressant-like actions of an AMPA receptor potentiator ( LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 125.Bai F, Bergeron M, Nelson DL. Chronic AMPA receptor potentiator ( LY451646) treatment increases cell proliferation in adult rat hippocampus. Neuropharmacology. 2003;44:1013–1021. doi: 10.1016/s0028-3908(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 126.Lauterborn J, Lynch G, Vanderklish P, Arai A, CM G. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lauterborn J, et al. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- 128.Suetake-Koga S, et al. In vitro and antinociceptive profile of HON0001, an orally active NMDA receptor NR2B subunit antagonist. Pharmacol Biochem Behav. 2006;84:134–141. doi: 10.1016/j.pbb.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 129.Borza I, et al. Selective NR1/2B N-methyl-D-aspartate receptor antagonists among indole-2-carboxamides and benzimidazole-2-carboxamides. J Med Chem. 2007;50:901–914. doi: 10.1021/jm060420k. [DOI] [PubMed] [Google Scholar]
- 130.Liverton NJ, et al. Identification and characterization of 4-methylbenzyl 4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate, an orally bioavailable, brain penetrant NR2B selective N-methyl-D-aspartate receptor antagonist. J Med Chem. 2007;50:807–819. doi: 10.1021/jm060983w. [DOI] [PubMed] [Google Scholar]
- 131.Preskorn S, et al. Annual Conference of the American Psychological Association 154; San Diego, California. 2007. [Google Scholar]
- 132.Brown RH., Jr Amyotrophic lateral sclerosis--a new role for old drugs. N Engl J Med. 2005;352:1376–1378. doi: 10.1056/NEJMcibr050274. [DOI] [PubMed] [Google Scholar]
- 133.Miller TM, Cleveland DW. Medicine. Treating neurodegenerative diseases with antibiotics. Science. 2005;307:361–362. doi: 10.1126/science.1109027. [DOI] [PubMed] [Google Scholar]
- 134.Rothstein JD, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 135.Mineur YS, Picciotto MR, Sanacora G. Antidepressant-like effects of ceftriaxone in male C57BL/6J mice. Biol Psychiatry. 2006;61:250–252. doi: 10.1016/j.biopsych.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 136.D'Ascenzo M, et al. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 138.Lee Y, Gaskins D, Anand A, Shekhar A. Glia mechanisms in mood regulation: a novel model of mood disorders. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-006-0652-4. [DOI] [PubMed] [Google Scholar]
- 139.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 140.Witkin JM, Marek GJ, Johnson BG, Schoepp DD. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- 141.Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005;1042:92–98. doi: 10.1016/j.brainres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 142.Patil ST, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 143.Dunayevich E, et al. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301531. [August 22. Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 144.Bonanno G, et al. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci. 2005;25:3270–3279. doi: 10.1523/JNEUROSCI.5033-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang M, Yang Y, Dong Z, Cao J, Xu L. NR2B-containing N-methyl-D-aspartate subtype glutamate receptors regulate the acute stress effect on hippocampal long-term potentiation/long-term depression in vivo. Neuroreport. 2006;17:1343–1346. doi: 10.1097/01.wnr.0000227994.07799.6c. [DOI] [PubMed] [Google Scholar]
- 146.Lesch KP, Schmitt A. Antidepressants and gene expression profiling: how to SNARE novel drug targets. Pharmacogenomics J. 2002;2:346–348. doi: 10.1038/sj.tpj.6500150. [DOI] [PubMed] [Google Scholar]
- 147.Thompson CM, et al. Inhibitor of the glutamate vesicular transporter (VGLUT) Curr Med Chem. 2005;12:2041–2056. doi: 10.2174/0929867054637635. [DOI] [PubMed] [Google Scholar]
- 148.Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lafleur DL, et al. N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology (Berl) 2006;184:254–256. doi: 10.1007/s00213-005-0246-6. [DOI] [PubMed] [Google Scholar]
- 151.LaRowe SD, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 152.Carlson PJ, Singh JB, Zarate CA, Jr, Drevets WC, Manji HK. Neural circuitry and neuroplasticity in mood disorders: insights for novel therapeutic targets. NeuroRx. 2006;3:22–41. doi: 10.1016/j.nurx.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Du J, et al. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci. 2004;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Conn PJ. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann N Y Acad Sci. 2003;1003:12–21. doi: 10.1196/annals.1300.002. [DOI] [PubMed] [Google Scholar]
- 155.Balazs R, Bridges RJ, Cotman CW. Excitatory amino acid transmission in health and disease. USA, New York: Oxford University Press; 2005. [Google Scholar]
- 156.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 157.Ivanov A, et al. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Michael-Titus AT, Bains S, Jeetle J, Whelpton R. Imipramine and phenelzine decrease glutamate overflow in the prefrontal cortex--a possible mechanism of neuroprotection in major depression? Neuroscience. 2000;100:681–684. doi: 10.1016/s0306-4522(00)00390-0. [DOI] [PubMed] [Google Scholar]
- 159.White G, Lovinger DM, Peoples RW, Weight FF. Inhibition of N-methyl-D-aspartate activated ion current by desmethylimipramine. Brain Res. 1990;537:337–339. doi: 10.1016/0006-8993(90)90381-k. [DOI] [PubMed] [Google Scholar]
- 160.Boyer PA, Skolnick P, Fossom LH. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci. 1998;10:219–233. doi: 10.1007/BF02761776. [DOI] [PubMed] [Google Scholar]
- 161.Song I, et al. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 162.Stoll L, Seguin S, Gentile L. Tricyclic antidepressants, but not the selective serotonin reuptake inhibitor fluoxetine, bind to the S1S2 domain of AMPA receptors. Arch Biochem Biophys. 2007;458:213–219. doi: 10.1016/j.abb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 163.Moutsimilli L, et al. Selective cortical VGLUT1 increase as a marker for antidepressant activity. Neuropharmacology. 2005;49:890–900. doi: 10.1016/j.neuropharm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 164.Tordera RM, Pei Q, Sharp T. Evidence for increased expression of the vesicular glutamate transporter, VGLUT1, by a course of antidepressant treatment. J Neurochem. 2005;94:875–883. doi: 10.1111/j.1471-4159.2005.03192.x. [DOI] [PubMed] [Google Scholar]
- 165.Dixon JF, Los GV, Hokin LE. Lithium stimulates glutamate "release" and inositol 1,4,5-trisphosphate accumulation via activation of the N-methyl-D-aspartate receptor in monkey and mouse cerebral cortex slices. Proc Natl Acad Sci U S A. 1994;91:8358–8362. doi: 10.1073/pnas.91.18.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dixon JF, Hokin LE. Lithium stimulates accumulation of second-messenger inositol 1,4,5-trisphosphate and other inositol phosphates in mouse pancreatic minilobules without inositol supplementation. Biochem J. 1994;304:251–258. doi: 10.1042/bj3040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma J, Zhang GY. Lithium reduced N-methyl-D-aspartate receptor subunit 2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by PSD-95 in rat hippocampus following cerebral ischemia. Neurosci Lett. 2003;348:185–189. doi: 10.1016/s0304-3940(03)00784-5. [DOI] [PubMed] [Google Scholar]
- 168.Karkanias NB, Papke RL. Lithium modulates desensitization of the glutamate receptor subtype gluR3 in Xenopus oocytes. Neurosci Lett. 1999;277:153–156. doi: 10.1016/s0304-3940(99)00878-2. [DOI] [PubMed] [Google Scholar]
- 169.Kang TC, et al. Valproic acid reduces enhanced vesicular glutamate transporter immunoreactivities in the dentate gyrus of the seizure prone gerbil. Neuropharmacology. 2005;49:912–921. doi: 10.1016/j.neuropharm.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 170.Cunningham MO, Woodhall GL, Jones RS. Valproate modifies spontaneous excitation and inhibition at cortical synapses in vitro. Neuropharmacology. 2003;45:907–917. doi: 10.1016/s0028-3908(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 171.Ueda Y, Willmore LJ. Molecular regulation of glutamate and GABA transporter proteins by valproic acid in rat hippocampus during epileptogenesis. Exp Brain Res. 2000;133:334–339. doi: 10.1007/s002210000443. [DOI] [PubMed] [Google Scholar]
- 172.Hassel B, Iversen EG, Gjerstad L, Tauboll E. Up-regulation of hippocampal glutamate transport during chronic treatment with sodium valproate. J Neurochem. 2001;77:1285–1292. doi: 10.1046/j.1471-4159.2001.00349.x. [DOI] [PubMed] [Google Scholar]
- 173.Loscher W. Effects of the antiepileptic drug valproate on metabolism and function of inhibitory and excitatory amino acids in the brain. Neurochem Res. 1993;18:485–502. doi: 10.1007/BF00967253. [DOI] [PubMed] [Google Scholar]
- 174.Zeise ML, Kasparow S, Zieglgansberger W. Valproate suppresses N-methyl-D-aspartate-evoked, transient depolarizations in the rat neocortex in vitro. Brain Res. 1991;544:345–348. doi: 10.1016/0006-8993(91)90078-a. [DOI] [PubMed] [Google Scholar]
- 175.Ko GY, Brown-Croyts LM, Teyler TJ. The effects of anticonvulsant drugs on NMDA-EPSP, AMPA-EPSP, and GABA-IPSP in the rat hippocampus. Brain Res Bull. 1997;42:297–302. doi: 10.1016/s0361-9230(96)00268-7. [DOI] [PubMed] [Google Scholar]
- 176.Turski L. [The N-methyl-D-aspartate receptor complex. Various sites of regulation and clinical consequences] Arzneimittelforschung. 1990;40:511–514. [PubMed] [Google Scholar]
- 177.Steppuhn KG, Turski L. Modulation of the seizure threshold for excitatory amino acids in mice by antiepileptic drugs and chemoconvulsants. J Pharmacol Exp Ther. 1993;265:1063–1070. [PubMed] [Google Scholar]
- 178.Kunig G, et al. Inhibition of [3H]alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid [AMPA] binding by the anticonvulsant valproate in clinically relevant concentrations: an autoradiographic investigation in human hippocampus. Epilepsy Res. 1998;31:153–157. doi: 10.1016/s0920-1211(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 179.Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006;31:1659–1674. doi: 10.1038/sj.npp.1300920. [DOI] [PubMed] [Google Scholar]