Abstract
Objective
To evaluate deaths from AIDS-defining malignancies (ADM) and non-AIDS-defining malignancies (nADM) in the D:A:D Study and to investigate the relationship between these deaths and immunodeficiency.
Design
Observational cohort study.
Methods
Patients (23 437) were followed prospectively for 104 921 person-years. We used Poisson regression models to identify factors independently associated with deaths from ADM and nADM. Analyses of factors associated with mortality due to nADM were repeated after excluding nADM known to be associated with a specific risk factor.
Results
Three hundred five patients died due to a malignancy, 298 prior to the cutoff for this analysis (ADM: n=110; nADM: n=188). The mortality rate due to ADM decreased from 20.1/1000 person-years of follow-up [95% confidence interval (CI) 14.4, 25.9] when the most recent CD4 cell count was <50 cells/μl to 0.1 (0.03, 0.3)/1000 person-years of follow-up when the CD4 cell count was more than 500 cells/μl; the mortality rate from nADM decreased from 6.0 (95% CI 3.3, 10.1) to 0.6 (0.4, 0.8) per 1000 person-years of follow-up between these two CD4 cell count strata. In multivariable regression analyses, a two-fold higher latest CD4 cell count was associated with a halving of the risk of ADM mortality. Other predictors of an increased risk of ADM mortality were homosexual risk group, older age, a previous (non-malignancy) AIDS diagnosis and earlier calendar years. Predictors of an increased risk of nADM mortality included lower CD4 cell count, older age, current/ex-smoking status, longer cumulative exposure to combination antiretroviral therapy, active hepatitis B infection and earlier calendar year.
Conclusion
The severity of immunosuppression is predictive of death from both ADM and nADM in HIV-infected populations.
Keywords: AIDS-defining malignancies, D:A:D study, HIV infection, mortality, non-AIDS-defining malignancies
Introduction
The use of combination antiretroviral therapy (cART) to treat HIV infection has led to changes in the causes of death in HIV-infected individuals in industrialized countries [1-3]. Over time, the risk that infected individuals will experience other comorbidities has increased, as a consequence of concomitant infections, the aging process and the high level of established risk factors for cardiovascular diseases (CVD)/cancers. These comorbidities may also result from prolonged exposure to cART or direct effects of HIV or both [1,2,4-7]. In this context, though the incidence of AIDS-defining malignancies (ADM) has declined, as with other AIDS events [3], non-AIDS-defining malignancies (nADM) are now an important cause of mortality in HIV-infected individuals [1,2,8]; the risk of nADM increases with age in uninfected individuals [9]. The incidence of several nADM is higher among HIV-infected individuals than among the general population in the same geographic area [10]. Several studies that have assessed the standardized incidence ratio of nADM have shown no differences in pre-antiretroviral therapy (ART) and ART calendar times, though many biases might affect these analyses [11-13].
Little is known about mortality from malignancies in the cARTera or risk factors for this. Therefore, we described the rates of death from ADM and nADM and investigated possible risk factors for these deaths, including immunodeficiency.
Methods
The D:A:D study
The D:A:D study is an observational study formed by the collaboration of 11 HIV cohorts. The primary aim is to establish whether the use of cART is associated with an increased risk of CVD. The current analysis includes data from 23 437 HIV-positive patients monitored at 188 clinics in Europe, the United States and Australia. The D:A:D study methodology has been detailed elsewhere [7]. Patients were under active follow-up at the time of study enrolment (December 1999-April 2001) and were followed prospectively with data obtained during regular outpatient visits. Information on CVD and deaths is provided in real time; each endpoint is validated and coded centrally. Deaths were classified using the ‘Coding of Death in HIV’ (CoDe) System [14] that includes detailed information on all causes of death and known comorbidities prior to death. The immediate, contributing and underlying causes of death are identified for all deaths with sufficient information available. Here, we focused on cancer diagnoses coded as the underlying cause of death.
Study endpoints
Deaths from ADM were those in which the underlying cause of death was Kaposi’s sarcoma; non-Hodgkin’s lymphoma (NHL), either systemic or of the brain; or cervical cancer. Deaths from nADM were those in which the underlying cause of death was cancer of the lung, anal canal, remaining gastrointestinal tract (gastrointestinal or liver), urogenital tract, upper airways (oral, nasopharynx, larynx), hematological (excluding NHL) or other sites (breast, central nervous system, sarcoma, skin, other).
Statistical analyses
The analytical approach was similar to that used previously [15]. Individuals were followed prospectively from enrolment in D:A:D until death, 6 months after the individual’s last clinic visit or 1st February 2005, whichever occurred first. This censoring strategy (predefined for all D:A:D analyses) is based on the usual time by which a patient would be expected to reattend clinic, and information on death would become available to clinicians through routine means. As some cohorts obtain mortality data from death registries, following all patients until death, including those who had been lost to follow-up, could introduce differential censoring between those who did and did not die.
Each person’s follow-up was divided into a series of consecutive 1-month periods, and the individual’s status (sex, risk group, ethnicity, most recent CD4 cell count, HIV RNA level, age, smoking status, previous AIDS, hepatitis B/C and cART exposure with categories as described previously [14]) was determined at the start of each.
Factors associated with death from each type of malignancy were identified from Poisson regression models (GENMOD procedure, SAS software, version 9.1). Factors associated with each outcome in univariable analyses (P<0.1) were included in multivariable regression models that also included adjustment for cohort and calendar year. When constructing these models, all continuous variables were initially categorized and rate ratios examined to see if it was appropriate to combine consecutive categories or include the variables as continuous covariates or both. These analyses, together with a concern for a biologically plausible relationship, suggested that the latest CD4 cell count would be most appropriately included as a continuous log2-transformed covariate. Thus, the estimated rate ratios relate to a doubling in the latest CD4 cell count. These initial analyses also suggested that only a high (>10 000 copies/ml) HIV RNA level was associated with ADM mortality (with no relationship between latest HIV RNA level and nADM mortality). Visual assessment of the rate ratios suggested that though the risk of ADM mortality was higher in those exposed to cART than in unexposed individuals, there was no trend with additional exposure. Thus, cART exposure was incorporated into these analyses as a binary variable. In contrast, compared with unexposed individuals, those exposed to cART for less than 1, 1-2, 2-3 and 3-4 years had higher but similar death rates from nADM, whereas those exposed for 4-5, 5-6 and more than 6 years had higher (but again similar) death rates. Thus, for analyses of nADM mortality, cART exposure was recategorized as none, less than 4 years and at least 4 years of exposure.
Sensitivity analyses considered whether the nadir CD4 cell count or the cumulative duration of immunosuppression (the total time spent with a CD4 cell count <200 cells/μl) provided additional prognostic information about the risk of ADM/nADM mortality to that already provided by the latest CD4 cell count. In these analyses, models were initially fitted that included only the duration of immunosuppression or nadir CD4 cell count (and all other confounders); the models were then refit after additionally controlling for the latest CD4 cell count. These analyses were restricted to the subgroup of patients exposed to cART. Additional sensitivity analyses considered the impact of lagging CD4 measurements by 3 and 12 months, and the association between the latest CD4 cell count and nADM mortality in different calendar periods, all with similar findings (results not reported). Finally, the list of malignancies considered to be non-AIDS related was modified to remove events known to be associated with specific risk factors, and analyses were repeated to see whether the relationships varied.
Results
A total of 305 patients in the D:A:D study died from ADM (n=112) or nADM (n=193). The ADMs leading to death included 82 NHL, 28 Kaposi’s sarcomas and two cervical cancers; the most frequent nADM leading to death was lung cancer (n=62), followed by gastrointestinal cancers (n=25), hematological cancers (n=22), anal cancers (n=20), urogenital cancers (n=18), liver cancers (n=16) and cancer of the upper airways (n=10). Other nADM were reported for the remaining 20 deaths.
The characteristics of patients dying from each type of malignancy are reported in Table 1. The large majority of individuals were men; patients dying from nADM were older [median age 52 (range, 32-79) versus 43 (range, 23-67) years], were less likely to have a prior (non-malignancy) AIDS diagnosis (49.2 versus 80.4%), had a higher nadir [median 87 cells/μl (range, 0-581) versus 30 cells/μl (range, 0-445)] and higher latest [median 211 cells/μl (range, 1-1183) versus 75 cells/μl (range, 0-671)] CD4 cell count.
Table 1. Characteristics of patients dying from AIDS-defining malignancies (ADM) and non-AIDS-defining malignancies (nADM).
| Death due to |
||||
|---|---|---|---|---|
| ADM | nADM | Other causes | Patients remaining alive | |
| N | 112 | 193 | 1328 | 21 804 |
| Men [n (%)] | 99 (88.4) | 163 (84.5) | 1049 (79.0) | 16 477 (75.6) |
| Risk group | ||||
| Homosexual | 68 (60.7) | 104 (53.9) | 479 (36.1) | 9936 (45.6) |
| IDU | 13 (11.6) | 38 (19.7) | 474 (35.7) | 4184 (19.2) |
| Heterosexual | 16 (14.3) | 35 (18.1) | 204 (15.4) | 5814 (26.7) |
| Other/not known | 15 (13.4) | 16 (8.3) | 171 (12.9) | 1870 (8.6) |
| Race [n (%)] | ||||
| White | 50 (44.6) | 90 (46.6) | 674 (50.8) | 10 316 (47.3) |
| Black | 9 (8.0) | 17 (8.8) | 183 (13.8) | 2204 (10.1) |
| Other | 6 (5.4) | 2 (1.0) | 32 (2.4) | 708 (3.3) |
| Not known | 47 (42.0) | 84 (43.5) | 439 (33.1) | 8576 (39.3) |
| Age at death or at last follow-up (years) | 43 (23-67) | 52 (32-79) | 44 (22-85) | 43 (19-93) |
| Prior (non-malignancy) AIDS event | 90 (80.4) | 95 (49.2) | 700 (52.7) | 5709 (26.2) |
| Nadir CD4 cell count (cells/μl) | 30 (0-445) | 87 (0-581) | 70 (0-1150) | 184 (0-2013) |
| Peak HIV RNA (log10 copies/ml) | 5.4 (1.7-6.9) | 5.0 (1.7-6.8) | 5.3 (1.7-7.0) | 4.9 (1.7-7.9) |
| Cumulative duration of immunosuppression (years) | 2.6 (0-9.7) | 1.4 (0-12.8) | 1.5 (0-15.3) | 0.1 (0-16.9) |
| Exposure to cART [n (%)] | ||||
| Never received cART | 8 (7.1) | 6 (3.1) | 136 (10.2) | 2283 (10.5) |
| Receiving cART at time of deatha | 53 (47.3) | 118 (61.1) | 666 (50.2) | 15 090 (69.3) |
| Previous exposure but not receiving at time of deatha | 51 (45.5) | 69 (35.8) | 526 (39.6) | 4431 (20.3) |
| Cumulative exposure to cART at time of death (years)a,b | 3.9 (0.1-9.6) | 4.5 (0.0-8.8) | 3.6 (0.0-9.2) | 6.0 (0.0-14.0) |
| Latest CD4 cell count (cells/μl) [median (range)] | ||||
| All patients | 75 (0-671) | 211 (1-1183) | 182 (0-2484) | 479 (0-2864) |
| Receiving cART at time of deatha | 107 (1-671) | 222 (1-1183) | 215 (0-1466) | 480 (0-2670) |
| Not receiving cART at time of deatha | 39 (0-620) | 173 (3-963) | 160 (0-2484) | 473 (0-2864) |
| Latest HIV RNA (log10 copies/ml) | ||||
| All patients | 3.8 (1.7-6.3) | 2.3 (1.7-6.0) | 3.7 (1.7-6.9) | 1.7 (1.7-7.9) |
| Receiving cART at time of death | 2.8 (1.7-6.3) | 1.9 (1.7-5.7) | 2.7 (1.7-6.9) | 1.7 (1.7-6.9) |
| Not receiving cART at time of death | 4.6 (1.7-5.9) | 2.7 (1.7-6.0) | 4.3 (1.7-6.8) | 3.2 (1.7-7.9) |
cART, Combination antiretroviral therapy; IDU, injection drug users.
Classified at last clinic visit for those remaining alive.
Among those ever exposed to cART.
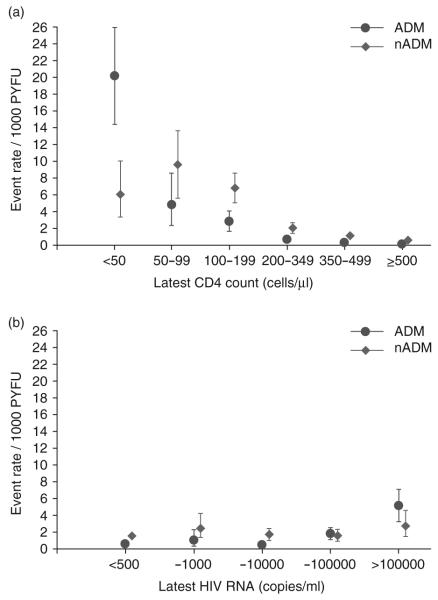
Patients contributed 104 921 person-years of follow-up (PYFU) to the analysis; the median follow-up was 4.6 years [interquartile range (IQR) 4.4, 4.9], with an average annual rate of loss to follow-up of less than 3%. Seven of the 305 malignancies (two ADM, five nADM) occurred after the end of follow-up and were excluded from subsequent analyses. Thus, the overall mortality rates from ADM and nADM were 1.1 [95% confidence interval (CI) 0.9-1.2] and 1.8 (95% CI 1.5-2.1)/1000 PYFU, respectively. Mortality rates from ADM and nADM, stratified by the latest CD4 cell count (Fig. 1a) and latest HIV RNA (Fig. 1b), are shown in Fig. 1. The mortality rate of ADM decreased from 20.1 (95% CI 14.4-25.9)/1000 PYFU while the latest CD4 cell count was <50 cells/μl to 0.1 (95% CI 0.03-0.3)/1000 PYFU while the CD4 cell count was >500 cells/μl. A similar, though less pronounced, relationship with the latest CD4 cell count was also seen for deaths from nADM with the mortality rate dropping from 6.0 (95% CI 3.3-10.1)/1000 PYFU to 0.6 (95% CI 0.4-0.8)/1000 PYFU between the same two CD4 cell count strata. Mortality rates for nADM were higher than those for ADM in all but the lowest latest CD4 cell count stratum (<50 cells/μl). The associations between the latest HIV RNA level and mortality from ADM/nADM (Fig. 1b) were not as strong as those seen with the latest CD4 cell count.
Fig. 1.
Rates of mortality from AIDS-defining malignancies (ADM) and non-AIDS-defining malignancies (nADM) (with 95% CI) stratified by (a) latest CD4 cell count and (b) latest HIV RNA. ADM, AIDS-defining malignancies; PYFU, personyears of follow-up.
In multivariable regression analysis (right-hand column, Table 2), the latest CD4 cell count remained a strong predictor of ADM mortality, whereas the relationship with the latest HIV RNA level became nonsignificant. A two-fold higher (i.e., doubling) CD4 cell count was associated with an approximate halving in ADM mortality (adjusted rate ratio 0.53, 95% CI 0.48-0.59). Other independent predictors of a higher risk of ADM mortality were homosexual risk group, older age, a previous (non-malignancy) AIDS diagnosis and earlier calendar year. Replacement of the latest CD4 cell count with the nadir CD4 cell count led to similar conclusions, but the nadir CD4 cell count was a weaker predictor of ADM mortality. Among patients who had received cART, both the latest CD4 cell count and nadir CD4 cell counts were independently associated with ADM mortality. However, in these analyses, a low latest CD4 cell count (adjusted rate ratio per two-fold higher 0.43, 95% CI 0.36-0.51), but a high nadir CD4 cell count (1.31 per two-fold higher, 95% CI 1.08-1.58) were predictive of ADM mortality, suggesting that those at highest risk were those whose CD4 cell count had remained low on cART, or whose count had risen but subsequently fallen. The duration of immunosuppression was strongly associated with ADM mortality (adjusted rate ratio per year of immunosuppression 1.15, 95% CI 1.10-1.20) but became non-significant after further adjusting for the latest CD4 cell count (rate ratio per year of immunosuppression 1.02, 95% CI 0.93-1.11).
Table 2. Results from unadjusted and adjusted analyses of factors associated with mortality due to AIDS-defining malignancies.
| Unadjusted |
Adjusteda |
|||||
|---|---|---|---|---|---|---|
| Rate ratio | 95% CI | P value | Rate ratio | 95% CI | P value | |
| Latest CD4 cell count (per log2 higher) | 0.51 | 0.48-0.54 | 0.0001 | 0.53 | 0.48-0.59 | 0.0001 |
| Latest HIV RNA<10 000 copies/ml | 4.33 | 2.97-6.30 | 0.0001 | 0.90 | 0.52-1.53 | 0.69 |
| Sex/risk | ||||||
| Male homosexual | 1 | 1 | ||||
| Male heterosexual | 0.70 | 0.38-1.30 | 0.52 | 0.27-0.99 | ||
| Female heterosexual | 0.19 | 0.07-0.51 | 0.25 | 0.09-0.71 | ||
| Male IDU | 0.45 | 0.22-0.90 | 0.38 | 0.18-0.79 | ||
| Female IDU | 0.44 | 0.16-1.20 | 0.51 | 0.18-1.44 | ||
| Men/women/other/not known | 1.19 | 0.68-2.08 | 0.0001 | 0.70 | 0.39-1.28 | 0.006 |
| Age (per 5 years older) | 1.09 | 0.99-1.19 | 0.09 | 1.14 | 1.03-1.26 | 0.02 |
| Previous AIDSb | 7.16 | 4.81-10.66 | 0.0001 | 2.85 | 1.82-4.47 | 0.0001 |
| Any exposure to cART | 1.96 | 0.96-4.03 | 0.07 | 1.21 | 0.51-2.86 | 0.65 |
| Hepatitis B status | ||||||
| Negative | 1 | 1 | ||||
| Positive - active | 1.90 | 1.07-3.40 | 1.71 | 0.94-3.09 | ||
| Positive - inactive | 0.54 | 0.30-0.99 | 0.65 | 0.32-1.31 | ||
| Vaccinated | 0.58 | 0.14-2.37 | 0.86 | 0.20-3.69 | ||
| Not known | 0.86 | 0.52-1.44 | 0.02 | 0.95 | 0.50-1.79 | 0.24 |
| Calendar year | ||||||
| 1999-2001 | 1 | 1 | ||||
| 2002 | 0.72 | 0.45-1.15 | 0.84 | 0.52-1.36 | ||
| 2003 | 0.43 | 0.25-0.77 | 0.48 | 0.26-0.86 | ||
| 2004/2005 | 0.34 | 0.18-0.63 | 0.0003 | 0.41 | 0.22-0.78 | 0.005 |
cART, combination antiretroviral therapy; CI, confidence interval; IDU, injecting drug user.
Adjusted for all the variables listed in the model and for cohort.
Excludes AIDS malignancies.
Similar analyses were conducted for nADM mortality (Table 3), with an increased risk of nADM mortality in those with lower CD4 cell counts and those who were older but a lower risk in more recent calendar years. Mortality from nADM was higher in current and prior smokers and in those with active hepatitis B virus infection. Furthermore, there was a strong relationship with cumulative exposure to cART, with those not exposed to cART having a risk of nADM mortality that was 62% lower than that among individuals exposed for less than 4 years, and those with at least 4 years of exposure having a 76% increased risk compared with those exposed for less than 4 years. Adjustment for the nadir CD4 cell count instead of the latest CD4 cell count led to similar conclusions, with a weaker association with the nadir CD4 cell count. However, among patients who had received cART, there was no independent association between the nadir CD4 cell count and nADM mortality (adjusted rate ratio 1.10, 95% CI 0.97-1.24) after adjusting for the latest CD4 cell count (adjusted rate ratio 0.56, 95% CI 0.50-0.63). Similarly, a significant relationship with the duration of immunosuppression (1.12 per year, 95% CI 1.08-1.16) in a model that adjusted for the other identified risk factors was removed after adjusting for the latest CD4 cell count.
Table 3. Results from unadjusted and adjusted analyses of factors associated with mortality due to non-AIDS-defining malignancies.
| Unadjusted |
Adjusteda |
|||||
|---|---|---|---|---|---|---|
| Rate ratio | 95% CI | P value | Rate ratio | 95% CI | P value | |
| Latest CD4 cell count (per log2 higher) | 0.64 | 0.60-0.68 | 0.0001 | 0.61 | 0.57-0.66 | 0.0001 |
| Sex/risk group | ||||||
| Male homosexual | 1 | 1 | ||||
| Male heterosexual | 0.80 | 0.50-1.28 | 0.72 | 0.44-1.19 | ||
| Female heterosexual | 0.42 | 0.24-0.74 | 0.86 | 0.48-1.55 | ||
| Male IDU | 0.88 | 0.58-1.35 | 1.38 | 0.87-2.21 | ||
| Female IDU | 0.71 | 0.37-1.37 | 1.58 | 0.80-3.12 | ||
| Men/women/other/not known | 0.77 | 0.45-1.33 | 0.04 | 0.57 | 0.32-1.01 | 0.07 |
| Age (per 5 years older) | 1.48 | 1.39-1.57 | 0.0001 | 1.60 | 1.49-1.72 | 0.0001 |
| Previous AIDSb | 2.42 | 1.81-3.23 | 0.0001 | 1.30 | 0.95-1.78 | 0.10 |
| Smoking status | ||||||
| Current smoker | 1.81 | 1.21-2.70 | 2.89 | 1.86-4.48 | ||
| Ex-smoker | 1.88 | 1.17-3.04 | 2.01 | 1.22-3.32 | ||
| Never smoked | 1 | 1 | ||||
| Not known | 1.39 | 0.86-2.26 | 0.01 | 1.16 | 0.61-2.22 | 0.0001 |
| Exposure to cART | ||||||
| None | 0.23 | 0.09-0.57 | 0.38 | 0.15-0.95 | ||
| <4 years | 1 | 1 | ||||
| ≥4 years | 1.62 | 1.20-2.18 | 0.0001 | 1.76 | 1.25-2.47 | 0.0001 |
| Hepatitis B status | ||||||
| Negative | 1 | 1 | ||||
| Positive - active | 2.51 | 1.60-3.92 | 1.81 | 1.14-2.88 | ||
| Positive - inactive | 1.17 | 0.80-1.72 | 0.93 | 0.59-1.47 | ||
| Vaccinated | 0.43 | 0.11-1.74 | 0.46 | 0.11-1.92 | ||
| Not known | 1.31 | 0.89-1.91 | 0.002 | 0.60 | 0.36-1.00 | 0.004 |
| Calendar year | ||||||
| 1999-2001 | 1 | 1 | ||||
| 2002 | 0.90 | 0.62-1.31 | 0.71 | 0.49-1.05 | ||
| 2003 | 0.93 | 0.64-1.34 | 0.61 | 0.41-0.90 | ||
| 2004/2005 | 0.52 | 0.33-0.82 | 0.02 | 0.30 | 0.19-0.48 | 0.0001 |
ADM, AIDS-defining malignancy; cART, combination antiretroviral therapy; CI, confidence interval; IDU, injecting drug user.
Adjusted for all the variables listed in the table and for cohort.
Excluding AIDS malignancies.
Finally, the list of non-AIDS-related malignancies was modified to remove malignancies associated with specific risk factors, and analyses were repeated to determine whether the relationships varied (Table 4). After exclusion of lung cancers, the relationship between nADM mortality and smoking status was weakened whereas that with active HBV infection disappeared after excluding cases of hepatocellular cancer. In contrast, the relationship between the latest CD4 cell count and nADM mortality remained after excluding either anal cancers or Hodgkin’s lymphoma (Table 4), and the relationship remained similar whether nADMs with an underlying viral cause (anal cancers, Hodgkin’s lymphoma and liver cancers: adjusted rate ratio per two-fold higher 0.58, 95% CI 0.50-0.66) or no underlying viral cause (all other nADM 0.63, 95% CI 0.58-0.69) were considered.
Table 4. Relationship between mortality due to non-AIDS-defining malignancies and specific risk factors after excluding cancers already known to be associated with these risk factors.
| Including all nADMa |
Excluding nADMs associated with risk factor |
|||||
|---|---|---|---|---|---|---|
| Rate ratio | 95% CI | P value | Rate ratio | 95% CI | P value | |
| Relationship with smoking status after excluding lung cancers | ||||||
| Current smoker | 2.89 | 1.86-4.48 | 1.70 | 1.03-2.81 | ||
| Ex-smoker | 2.01 | 1.22-3.32 | 1.38 | 0.78-2.45 | ||
| Never smoked | 1 | 1 | ||||
| Not known | 1.16 | 0.61-2.22 | 0.0001 | 0.72 | 0.34-1.54 | 0.06 |
| Relationship with hepatitis B status after excluding hepatocellular carcinomas | ||||||
| Negative | 1 | 1 | ||||
| Positive - active | 1.81 | 1.14-2.88 | 1.28 | 0.74-2.20 | ||
| Positive - inactive | 0.93 | 0.59-1.47 | 1.10 | 0.68-1.76 | ||
| Vaccinated | 0.46 | 0.11-1.92 | 0.55 | 0.13-2.28 | ||
| Not known | 0.60 | 0.36-1.00 | 0.004 | 0.51 | 0.29-0.91 | 0.04 |
| Relationship with latest CD4 cell count after excluding anal cancers | ||||||
| Latest CD4 cell count (per log2 higher) | 0.61 | 0.57-0.66 | 0.0001 | 0.62 | 0.57-0.68 | 0.0001 |
| Relationship with latest CD4 cell count after excluding Hodgkin’s lymphoma | ||||||
| Latest CD4 cell count (per log2 higher) | 0.61 | 0.57-0.66 | 0.0001 | 0.62 | 0.57-0.67 | 0.0001 |
Discussion
In this large prospective study, we found that the latest CD4 cell count is strongly associated with the risk of death from both ADM and nADM. This finding has been confirmed by a recently published meta analysis on the incidence of cancers in HIV/AIDS and transplant recipients, which revealed the importance of immunosuppression [16].
Non-AIDS events have become an important cause of mortality [1,2] as individuals with HIV infection survive to older ages and have started to suffer from similar agerelated diseases to the HIV-uninfected population.
The coding systems currently used to classify non-AIDS causes of death among HIV-uninfected individuals do not adequately capture all causes in HIV-infected individuals. The CoDe system provides a standardized approach for collecting data on and reviewing causes of death in HIV-infected individuals. The D:A:D study was instrumental in initiating and implementing this system, and all deaths reported to D:A:D are now reported using this tool [14].
Not surprisingly, given the high uptake of cART, we observed a higher rate of death from nADM than from ADM. Lung cancer is the most common fatal nADM in our study, concordant with the high prevalence of smoking among individuals in D:A:D and the HIV-infected population [17-19].
We have previously reported a close correlation between the CD4 cell count and non-AIDS-related deaths [15,20]. Here, we extend these analyses to deaths from nADM; our results are consistent with reports from a population-based study of HIV-infected individuals in New York City [8]. An important finding from our analyses is that nADM mortality was higher than ADM mortality in all patients other than those with very low (<50 cells/μl) CD4 cell counts. Interestingly, our analyses suggested that mortality rates from nADM appear to peak in those with a CD4 cell count in the range 50-199 cells/μl. Unfortunately, we do not have enough evidence to say whether this finding reflects a genuine reduction in risk of fatal nADM in the lowest CD4 strata or is simply a consequence of random variability or the more aggressive nature of other diseases that are more common in those with the lowest CD4 cell counts or both.
Age is also a strong predictor of death from malignancies, suggesting that, at similar CD4 cell counts, older patients are at greater risk of dying from malignancy. This may be a consequence of the higher incidence of cancers in older individuals, as well as an increased likelihood of death in older individuals, irrespective of the cause. However, this finding may also reflect reduced immune system activity in the elderly that is not fully captured in our models by the CD4 cell count [21,22]. Interestingly, the HIV RNA level was only weakly associated with ADM mortality and was not independently associated with nADM mortality. Correlations between death from ADM and nADM with CD4 cell counts have also been observed in other cohort studies [23], and a recent report has suggested that incomplete viral suppression during HAART is a strong predictor of NHL [24].
It is heartening that the risk of dying from either ADM or nADM has decreased in more recent calendar years. Given that cancer-screening procedures have not changed greatly, this decreased incidence might be the result of more effective preventive measures, improved management of malignancies when they occur or a delayed effect of cART due to the long incubation periods of some malignancies. An increasing proportion of HIV-infected individuals in D:A:D have stopped smoking in more recent years [25], but the decline in nADM mortality remained significant after adjusting for smoking status. Although other reports have described an increased incidence of nADM in more recent years, these studies generally included individuals followed both before and after cART introduction; thus, these findings may reflect the reduction in AIDS-related mortality in the cARTera, which may leave the population of HIV-infected individuals at risk of non-AIDS events [26,27].
Surprisingly, nADM mortality was related to cART exposure, whereas a weak association with ADM mortality disappeared after adjustment for other factors. These findings might simply reflect the fact that individuals taking effective cART are less likely to die from ADM and, as a result, may be more likely to die from nADM. However, our estimates of mortality from nADM should not be affected by this ‘competing risk’, unless the underlying risk factor profile of patients has also changed for the worse. If anything, the risk factor profile of these patients (who are all cART -treated with higher CD4 cell counts) would suggest that the risk of nADM mortality should be lower in this group. Furthermore, this potential bias should also apply to other non-AIDS-related causes of death, but previous analyses [15] have not identified such a relationship. A more likely explanation is that after diagnosis of a nADM, cART may be started quickly to enhance the immune system in preparation for the detrimental effects of antineoplastic drugs. In contrast, patients dying from ADM may be more likely to have stopped or never received cART (in fact, the results shown in Table 1 would support this explanation). Finally, we cannot rule out the possibility that our results may reflect a genuine adverse effect of long-term cART exposure - some antiretrovirals, particularly nucleoside reverse transcriptase inhibitors (NRTI), may be carcinogenic with prolonged exposure, and some NRTIs may have inhibitory effects on DNA-polymerase beta [28,29].
As expected from other reports [2,17], smoking was a strong risk factor for deaths from lung cancer. After exclusion of lung cancer from the definition of nADM, the relationship between smoking status and nADM mortality was weakened. As lung cancer is the most frequent nADM leading to death in HIV-infected individuals, preventive campaigns to reduce smoking targeted toward the HIV population are required. In a similar way, the association between active HBV infection and nADM mortality was removed after exclusion of liver cancers from the nADM definition. Campaigns to increase uptake of HBV vaccination among HIV-infected individuals and adequate treatment for chronic hepatitis B are needed, as the incidence of fatal liver cancer is increased among HIV-infected individuals [29], and liver disease now represents a major cause of death in these individuals [4,15]. Contrary to expectations, we did not find any association between hepatitis C virus (HCV) infection and nADM mortality; previous findings from the D:A:D study [15] have shown that the cause of death in HCV-coinfected patients is often decompensated cirrhosis rather than hepatocellular carcinoma [30]. Thus, though HCV infection may be a risk factor for the development of liver cancer, it may have a less important role as a prognostic marker for outcome.
An increased incidence of anal cancers in HIV-infected individuals has been reported, mainly associated with infection with high-risk subtypes of human papillomavirus, longer duration of HIV infection and severe immunosuppression [31,32]. However, even after removing anal cancers from our definition of nADM, or other cancers known to be related to immunosuppression, the relationship between nADM mortality and the latest CD4 cell count remained evident, confirming the impact of severe immunodepression on the risk of death from nADM.
The present study has the strengths of its large size, broad geographical representation and rigorous coding of causes of death. Nonetheless, it has some limitations. We do not collect information on non-fatal malignancies; our analyses are thus restricted to fatal events. Furthermore, factors associated with death from a malignancy may be risk factors either because they help to induce the disease or accelerate its course or both, that is, lower CD4 cell count may have increased risk of the event or of event-related death. As a general ‘rule-of-thumb’, it is usually recommended that to avoid the possibility of model overfitting, there should be at least 10 endpoints for each covariate considered. Our final multivariable models therefore included a large number of covariates relative to the number of endpoints witnessed. However, our findings surrounding most of the covariates considered were as expected from the published literature. Furthermore, our findings relating to the latest CD4 cell count were relatively unchanged between the univariable and multivariable models, suggesting that this finding was unlikely to be a consequence of an overfitted model. Finally, these results, consisting of a cohort of patients in Europe, United States and Australia should not be generalized to second and third world countries.
In conclusion, we found that the severity of immunosuppression is predictive of the risk of dying from a malignancy. Thus, improvements to patients’ immune systems following the use of cART may be expected to have a positive impact on the risk of death from nADM, underlining the importance of HIV treatment strategies that aim to prevent immunodeficiency.
Acknowledgements
The study has been presented at the 14th Conference on Retroviruses and Opportunistic Infections, Los Angeles, February 2007 as oral (abstract no. 84).
This study was supported by the Oversight Committee for The Evaluation of Metabolic Complications of HAART, a collaborative committee with representation from academic institutions, the European Agency for the valuation of Medicinal Products, the Food and Drug Administration, the patient community and all pharmaceutical companies with licensed anti-HIV drugs in the US market: Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, Pfizer, and Hoffman-LaRoche.
This study was also supported by a grant (CURE/97-46486) from the Health Insurance Fund Council, Amstelveen, the Netherlands, to the AIDS Therapy Evaluation Project Netherlands (ATHENA); by a grant from the Agence Nationale de Recherches sur le SIDA (Action Coordonnée no.7, Cohortes), to the Aquitaine Cohort; by the Commonwealth Department of Health and Ageing and a grant from the Australian National Council on AIDS, Hepatitis C and Related Diseases’ Clinical Trials and Research Committee, to the Australian HIV Observational Database (AHOD); by grants from the Fondo de Investigación Sanitaria (FIS 99/0887) and Fundación para la Investigación y la Prevención del SIDA en Espanã (FIPSE 3171/00), to the Barcelona Antiretroviral Surveillance Study (BASS); by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants 5U01AI042170-10 and 5U01AI046362-03), to the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). The European Commission BIOMED 1 (CT94-1637) and BIOMED 2 (CT97-2713), the 5th Framework (QLK2-2000-00773) and the 6th Framework (LSHP-CT-2006-018632) were the primary sponsors of the EuroSIDA study. Unrestricted grants were also provided by Bristol-Myers Squibb, GlaxoSmithKline, Roche, Gilead, Pfizer, Merck and Co., Tibotec and Boehringer Ingelheim. The participation of centers from Switzerland was supported by a grant from the Swiss Federal Office for Education and Science (EuroSIDA); by an unrestricted educational grant from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GSK, Pfizer, Janssen-Cilag, Italy, to the Italian Cohort Naive to Antiretrovirals (ICONA Foundation); and by a grant from the Swiss National Science Foundation, to the Swiss HIV Cohort Study (SHCS).
Members of the Writing committee: Antonella d’Arminio Monforte (Clinic of Infectious and Tropical Diseases, San Paolo Hospital, University of Milan, Milan), MD, DMSc; Donald Abrams (Department of Hematology-Oncology, San Francisco General Hospital, San Francisco), MD; Christian Pradier (Centre Hospitalier Universitaire, Hopital de l’Archet, Nice), Rainer Weber (University Hospital Zurich, Zurich), MD; Peter Reiss (Academic Medical Center, Amsterdam), MD, PhD; Fabrice Bonnet (INSERM E0338 and U593, Victor Segalen-Bordeaux 2 University, Bordeaux), MD; Ole Kirk (Copenhagen HIV Program, University of Copenhagen, Copenhagen), MD, DMSc; Matthew Law (National Centre in HIV Epidemiology and Clinical Research, Sydney), PhD; Stephane De Wit (Centre Hospitalier Universitaire Saint-Pierre, Brussels), MD, PhD; Nina Friis-Møller (Copenhagen HIV Program, University of Copenhagen, Copenhagen), MD, PhD; Andrew N. Phillips (Royal Free and University College, London), PhD; Caroline A. Sabin (Royal Free and University College, London), PhD; Jens D. Lundgren (Copenhagen HIV Program, University of Copenhagen, Copenhagen) MD, DMSc.
All members of the writing group have participated in discussions on the design of the study, the choice of statistical analyses and interpretation of findings and have been involved in the preparation and review of the article.
D:A:D Steering Committee consists of persons with symbol ‘asterisk’ against their names given below (symbol # indicates chair) and S. Collins, E. Loeliger, R. Tressler, I. Weller.
D:A:D Central Coordination: N. Friis-Møller, S.W. Worm, C.A. Sabin, A Sjøl (verification of primary endpoint), J.D. Lundgren.
D:A:D data managers: A. Sawitz (coordinator), M. Rickenbach, P. Pezzotti, E. Krum, L. Gras, E. Balestre, A. Sundström, B. Poll, E. Fontas, F. Torres, K. Petoumenos, J. Kjær.
The members of the 11 cohorts are as follows:
Athena (AIDS Therapy Evaluation Project Netherlands):
Central coordination: F. de Wolf, S. Zaheri, L. Gras.
Participating physicians (with their cities): W. Bronsveld, M.E. Hillebrand-Haverkort (Alkmaar); J.M. Prins, J.C. Bos, J.K.M. Eeftinck Schattenkerk, S.E. Geerlings, M.H. Godfried, J.M.A. Lange, F.C. van Leth, S.H. Lowe, J.T.M. van der Meer, F.J.B. Nellen, K. Pogány, T. van der Poll, P. Reiss*, T.A. Ruys, Sankatsing, R. Steingrover, G. van Twillert, M. van der Valk, M.G.A. van Vonderen, S.M.E Vrouenraets, M. van Vugt, F.W.M.N. Wit, A. van Eeden, J.H. ten Veen, P.S. van Dam, J.C. Roos, K. Brinkman, P.H.J. Frissen, H.M. Weigel, J.W. Mulder, E.C.M. van Gorp, P.L. Meenhorst, A.T.A. Mairuhu, J. Veenstra, S.A. Danner, M.A. Van Agtmael, F.A.P. Claessen, R.M. Perenboom, A. Rijkeboer, M. van Vonderen (Amsterdam); C. Richter, J. van der Berg, R. van Leusen (Arnhem); R. Vriesendorp, F.J.F. Jeurissen, R.H. Kauffmann, E.L.W. Koger, HAGA (Den Haag); B. Bravenboer (Eindhoven); C.H.H. ten Napel, G.J. Kootstra (Enschede); H.G. Sprenger, W.M.A.J. Miesen, R. Doedens, E.H. Scholvinck (Groningen); R.W. ten Kate (Haarlem); D.P.F. van Houte, M. Polee (Leeuwarden); F.P. Kroon, van den Broek, J.T. van Dissel, E.F. Schippers (Leiden); G. Schreij, S. van de Geest, A. Verbon (Maastricht); P.P. Koopmans, M. Keuter, F. Post, A.J.A.M. van der Ven (Nijmegen); M.E. van der Ende, I.C. Gyssens, M. van der Feltz, J.G. den Hollander, S. de Marie, J.L. Nouwen, B.J.A. Rijnders, T.E.M.S. de Vries (Rotterdam); J.R. Juttmann, C. van de Heul, M.E.E. van Kasteren, St. Elisabeth (Tilburg); M.M.E. Schneider, M.J.M. Bonten, J.C.C. Borleffs, P.M. Ellerbroek, I.M. Hoepelman, C.A.J.J. Jaspers, I. Schouten, C.A.M. Schurink (Utrecht); W.L. Blok, A.A. Tanis (Vlissingen); P.H.P. Groeneveld (Zwolle).
Aquitaine (France):
Scientific committee: R. Salamon (chair), J. Beylot, M. Dupon, M. Le Bras, J.L. Pellegrin, J.M. Ragnaud.
Central coordination: F. Dabis*, G. Chêne, H. Jacqmin-Gadda, R. Thiébaut, S. Lawson-Ayayi, V. Lavignolle, E. Balestre, M.J. Blaizeau, M. Decoin, A.M. Formaggio, S. Delveaux, S. Labarerre, B. Uwamaliya, E. Vimard, L. Merchadou, G. Palmer, D. Touchard, D. Dutoit, F. Pereira, B. Boulant.
Participating physicians (with their cities): J. Beylot, P. Morlat, N. Bernard, M. Bonarek, F. Bonnet, B. Coadou, P. Gelie, D. Jaubert, C. Nouts, D. Lacoste, M. Dupon, H. Dutronc, G. Cipriano, S. Lafarie, I. Chossat, J.Y. Lacut, B. Leng, J.L. Pellegrin, P. Mercié, J.F. Viallard, I. Faure, P. Rispal, C. Cipriano, S. Tchamgoué, M. Le Bras, F. Djossou, D. Malvy, J.P. Pivetaud, J.M. Ragnaud, D. Chambon, C. De La Taille, T. Galperine, S. Lafarie, D. Neau, A. Ochoa, C. Beylot, M.S. Doutre, J.H. Bezian, J.F. Moreau, J.L. Taupin, C. Conri, J. Constans, P. Couzigou, L. Castera, H. Fleury, M.E. Lafon, B. Masquelier, I. Pellegrin, P. Trimoulet, F. Moreau, C. Mestre, C. Series, A. Taytard (Bordeaux).
AHOD (Australian HIV Observational Database, Australia):
Central coordination: M. Law*, K. Glenday and K. Petoumenos (Sydney, New South Wales).
Participating physicians (with their cities, states): J. Anderson, P. Cortissos, A. Mijch, K. Watson, N. Roth, J. Nicolson (Melbourne, Victoria); M. Bloch, S. Agrawal, T. Franic, D. Baker, R. Vale, A. Carr, D. Cooper, M. Lacey, K. Hesse (Sydney, New South Wales); J. Chuah, D. Lester, W. Fankhauser (Gold Coast, Queensland), S. Mallal, C. Forsdyke, S. Bulgannawar (Perth, Western Australia).
BASS (Spain):
Central coordination: G. Calvo*, F. Torres, S. Mateu (Barcelona).
Participating physicians (with their cities): P. Domingo, M.A. Sambeat, J. Gatell, E. Del Cacho, J. Cadafalch, M. Fuster (Barcelona); C. Codina, G. Sirera, A. Vaqué (Badalona).
The Brussels St Pierre Cohort (Belgium): N. Clumeck, S. De Wit*, M. Gerard, K. Kabeya, D. Konopnicki, A. Libois, M.C. Payen, B. Poll, Y. Van Laethem.
CPCRA (USA):
Central coordination: J. Neaton, G. Bartsch, W.M. ElSadr*, E. Krum, G. Thompson, D. Wentworth.
Participating physicians (with their cities and states): R. Luskin-Hawk (Chicago, Illinois); E. Telzak (Bronx, New York); W.M. El-Sadr (Harlem, New York); D.I. Abrams (San Francisco, California); D. Cohn (Denver, Colorado); N. Markowitz (Detroit, Michigan); R. Arduino (Houston, Texas); D. Mushatt (New Orleans, Louisiana); G. Friedland (New Haven, Connecticut); G. Perez (Newark, New Jersey); E. Tedaldi (Philadelphia, Pennsylvania); E. Fisher (Richmond, Virginia); F. Gordin (Washington, DC); L.R. Crane (Detroit, Michigan); J. Sampson (Portland, Oregon); J. Baxter (Camden, New Jersey).
EuroSIDA (multinational):
Central coordination: O. Kirk*, C.H. Olsen, A. Mocroft, A.N. Phillips*, J.D. Lundgren*#.
Participating countries and physicians (with their cities): Austria - N. Vetter (Vienna); Belarus - I. Karpov, A. Vassilenko (Minsk); Belgium - N. Clumeck, S. De Wit, B. Poll (Brussels); R. Colebunders (Antwerp); Czech Republic - L. Machala, H. Rozsypal (Prague); D. Sedlacek (Plzen); Denmark - J. Nielsen, T. Benfield, J. Gerstoft, T. Katzenstein, A.B. E. Hansen, P. Skinhøj (Copenhagen); C. Pedersen (Odense); Estonia - K. Zilmer (Tallinn); France - C. Katlama, J.-P. Viard, P-M Girard (Paris); T. Saint-Marc, P. Vanhems (Lyon); C. Pradier (Nice); F. Dabis (Bordeaux); Germany - M. Dietrich, C. Manegold, J. van Lunzen, H.-J. Stellbrink (Hamburg); S. Staszewski, M. Bieckel (Frankfurt); F.D. Goebel (Münich); G. Fätkenheuer (Cologne); J. Rockstroh (Bonn); R.E. Schmidt (Hannover); Greece - J. Kosmidis, P. Gargalianos, H. Sambatakou, J. Perdios, G. Panos, A. Filandras (Athens); Hungary - D. Banhegyi (Budapest); Ireland - F. Mulcahy (Dublin); Israel - I. Yust, M. Burke, D. Turner (Tel Aviv); S. Pollack, J. Hassoun (Haifa); Z. Sthoeger (Rehovot); S. Maayan (Jerusalem); Italy - S. Vella, A. Chiesi (Rome); C. Arici (Bergamo); R. Pristerá (Bolzano); F. Mazzotta, A. Gabbuti (Florence); R. Esposito, A. Bedini (Modena); A. Chirianni, E. Montesarchio (Naples); V. Vullo, P. Santopadre, P. Narciso, A. Antinori, P. Franci, M. Zaccarelli (Rome); A. Lazzarin, A. Castagna, A. d’Arminio Monforte (Milan); Latvia - L. Viksna (Riga); Lithuania - S. Chaplinskas (Vilnius); Luxembourg - R. Hemmer, T. Staub (Luxembourg); Netherlands - P. Reiss (Amsterdam); Norway - J. Bruun, A. Maeland, V. Ormaasen (Oslo); Poland - B. Knysz, J. Gasiorowski (Wroclaw); A. Horban (Warsaw); D. Prokopowicz, A. Wiercinska-Drapalo (Bialystok); A. Boron-Kaczmarska, M. Pynka (Szczecin); M. Beniowski, E. Mularska (Chorzow); H. Trocha (Gdansk); Portugal - F. Antunes, K. Mansinho, F. Maltez (Lisbon); Romania - D. Duiculescu, V. Babes, A. Streinu-Cercel (Bucarest); Russia - E. Vinogradova, A. Rakhmanova (St. Petersburg); Serbia & Montenegro - D. Jevtovic (Belgrade); Slovakia - M. Mokráš, D. Staneková (Bratislava); Spain - J. GonzálezLahoz, M. Sanchez-Conde, T. García-Benayas, L. Martin-Carbonero, V. Soriano (Madrid); B. Clotet, A. Jou, J. Conejero, L. Ruiz, C. Tural (Badalona); J.M. Gatell, J.M. Miró, L. Zamora (Barcelona); Sweden -A. Blaxhult, A. Karlsson, P. Pehrson (Stockholm); Switzerland - B. Ledergerber, R. Weber (Zürich); P. Francioli, A. Telenti (Lausanne); B. Hirschel, V. Soravia-Dunand (Geneve); H. Furrer (Bern); Ukraine - E. Kravchenko, N. Chentsova (Kyiv); United Kingdom - M. Fisher (Brighton); R. Brettle (Edinburgh); S. Barton, A.M. Johnson, D. Mercey, M. Murphy, M.A. Johnson, J. Weber, G. Scullard (London).
HivBivus (Sweden):
Central coordination: L. Morfeldt*, G. Thulin, A. Sundström.
Participating physicians (with their cities): B.Åkerlund (Huddinge); K. Koppel, A. Karlsson (Stockholm); L. Flamholc, C. Håkangård (Malmö). ICONA Foundation (Italy):
Central coordination: A. d’Arminio Monforte*.
Steering Committee: A. Ammassari, A. Antinori, F Maggiolo, C. Balotta, P. Bonfanti, M. Capobianchi, A. Castagna, F. Ceccherini-Silberstein, A. Cozzi-Lepri, A. d’Arminio Monforte, A. De Luca, C. Gervasoni, E. Girardi, S. Lo Caputo, R. Murri, C. Mussini, M. Puoti, C. Torti.
Governing Body
M. Moroni (Chair), G. Carosi, R. Cauda, F. Chiodo, A. d’Arminio Monforte, G Di Perri, M. Galli, R. Iardino, G. Ippolito, A. Lazzarin, F Mazzotta, R. Panebianco, G. Pastore, C.F. Perno.
Participating physicians and centers:
Italy: M. Montroni, G. Scalise, A. Costantini, A. Riva (Ancona); U. Tirelli, F. Martellotta (Aviano-PN); G. Pastore, N. Ladisa (Bari); F. Suter, F. Maggiolo (Bergamo); F. Chiodo, V. Colangeli, C. Fiorini (Bologna); G. Carosi, G. Cristini, C. Torti, C. Minardi, D. Bertelli (Brescia); T. Quirino (Busto Arsizio); P.E. Manconi, P. Piano (Cagliari); E. Pizzigallo, M. D’Alessandro (Chieti); G. Carnevale, A. Zoncada (Cremona); F. Ghinelli, L. Sighinolfi (Ferrara); F. Leoncini, F. Mazzotta, M. Pozzi, S. Lo Caputo (Firenze); B. Grisorio, S. Ferrara (Foggia); G. Pagano, G. Cassola, A. Alessandrini, R. Piscopo (Genova); F. Soscia, L. Tacconi (Latina); A. Orani, P. Perini (Lecco); D. Tommasi, P. Congedo (Lecce); F. Chiodera, P. Castelli (Macerata); M. Moroni, A. Lazzarin, G. Rizzardini, L. Caggese, A. d’Arminio Monforte, A. Galli, S. Merli, C. Pastecchia, M.C. Moioli (Milano); R. Esposito, C. Mussini (Modena); A. Gori, S. Cagni (Monza), N. Abrescia, A. Chirianni, C.M. Izzo, M. De Marco, R. Viglietti, E Manzillo (Napoli); C. Ferrari, P. Pizzaferri (Parma); G. Filice, R. Bruno (Pavia); G. Magnani, M.A. Ursitti (Reggio Emilia); M. Arlotti, P. Ortolani (Rimini); R. Cauda, M. Andreoni, A. Antinori, G. Antonucci, P. Narciso, V. Tozzi, V. Vullo, A. De Luca, M. Zaccarelli, R. Acinapura, P. De Longis, M.P. Trotta, M. Lichtner, F. Carletti (Roma); M.S. Mura, M. Mannazzu (Sassari); P. Caramello, G. Di Perri, G.C. Orofino, M. Sciandra (Torino); E. Raise, F. Ebo (Venezia); G. Pellizzer, D. Buonfrate (Vicenza).
The Nice Cohort (France):
Central Coordination: C. Pradier*, E. Fontas, C. Caissotti.
Participating Physicians: P. Dellamonica, L. Bentz, E. Bernard, F. De Salvador-Guillouet, J. Durant, V. Mondain-Miton, I. Perbost, B. Prouvost-Keller, P. Pugliese, V. Rahelinirina, P.M. Roger, F. Vandenbos. SHCS (Swiss HIV Cohort Study, Switzerland): M. Battegay, E. Bernasconi, J. Böni, H. Bucher, Ph. Bürgisser, S. Cattacin, M. Cavassini, R. Dubs, M. Egger, L. Elzi, P. Erb, M. Fischer, M. Flepp, A. Fontana, P. Francioli (President of the SHCS), H.J. Furrer, M. Gorgievski, H. Günthard, B. Hirschel, L. Kaiser, C. Kind, Th. Klimkait, U. Lauper, B. Ledergerber, M. Opravil, F. Paccaud, G. Pantaleo, L. Perrin, J.-C. Piffaretti, M. Rickenbach, C. Rudin P. Schmid, J. Schüpbach, R. Speck, A. Telenti, A. Trkola, P. Vernazza, R. Weber*, S. Yerly.
References
- 1.Mocroft A, Brettle R, Kirk O, Blaxhult A, Parkin JM, Antunes F, et al. for the EuroSIDA study group Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS. 2002;16:1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 2.Lewden C, Salmon D, Morlat P, Jougla E, Bonnet F, Hériprete L, et al. Causes of death among human immunodeficiency viruses (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–130. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Salmon-Ceron D, Lewden C, Morlat P, Bévilacqua S, Jougla E, Bonnet F, et al. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42:799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 6.Casau NC. Perspective on HIV infection and aging: emerging research on the horizon. Clin Infect Dis. 2005;41:855–863. doi: 10.1086/432797. [DOI] [PubMed] [Google Scholar]
- 7.The Data Collection on Adverse Events of anti-HIV drugs (DAD) Study Group. Writing Committee. Friis-Moller N, Sabin C, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. [Google Scholar]
- 8.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute . SEER Cancer Statistic Review, 1975-2002. National Cancer Institute; Bethesda, Maryland: 2005. [Google Scholar]
- 10.Patel P, Hanson D, Sullivan P, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Annals Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 11.Clifford GM, Polesel J, Rickenbach M, Del Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 12.Herida M, Mary-Krause M, Kaphan R, Cadranel J, Poizot-Martin I, Rabaud C, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21:3447–3453. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 13.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 14.Olsen CH, Friis-Møller N, d’Arminio Monforte A, Chene G, Davey R, De Wit S, et al. for the CoDe Working Group Pilot of the CoDe (Coding of Death) project - a standardized approach to code causes of death in HIV infected individuals; 10th European AIDS Conference/EACS; Dublin. Nov 2005. [Google Scholar]
- 15.The D:A:D Study. Writing Committee. Weber R, Sabin C, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human Immunodeficiency Virus. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 16.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 17.Engels EA, Goedert JJ. Human immunodeficiency virus/acquired immunodeficiency syndrome and cancer: past, present, and future. J Natl Cancer Inst. 2005;97:407–409. doi: 10.1093/jnci/dji085. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Cigarette smoking among adults - United States 2000. MMWR Morb Mortal Wkly Rep. 2002;51:642–645. [PubMed] [Google Scholar]
- 19.Kirk GD, Merlo C, O’Driscoll P, Metha SH, Galay N, Vlahov D, et al. HIV infection is associated with an increased risk of lung cancer, independent of smoking. Clin Infect Dis. 2007;45:103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber R, Friis-Moller N, Sabin C, Reiss P, d’Arminio Monforte A, Dabis F, et al. HIV and non-HIV-related deaths and their relationship to immunodeficiency: the D:A:D study [abstract 595]; Presented at 12th Conference on Retroviruses and Opportunistic Infections; Boston. February 2005. [Google Scholar]
- 21.Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, et al. Shortage of circulating naïve CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- 22.Bestilny LJ, Gill MJ, Mody CH, Riabowol KT. Accelerated replicative senescence of the peripheral immune system induced by HIV infection. AIDS. 2000;14:771–780. doi: 10.1097/00002030-200005050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Bruyand M, Thiebaut R, Lawson-Ayayi S, Joly P, Sasco A, Pellegrin JL, et al. Groupe d’Epidémiologie Clinique du SIDA en Aquitaine (GECSA) Immunodeficiency and risk of AIDS-defining and non-AIDS-defining cancers: ANRS CO3 Aquitaine Cohort, 1998 to 2006 [abstract 15]; Program and abstracts of the 15th Conference on Retroviruses and Opportunistic Infections; Boston. 3-6 February 2008. [Google Scholar]
- 24.Zoufaly A, Stellbrink HJ, an der Heiden M, Kollan C, Van Lunzen J, Hamouda O, Clinsurv Study Group Insufficient virus suppression during HAART is a strong predictor for the development of AIDS related Lymphoma: the German CLINSURV Cohort [abstract 16]; 15th Conference on Retroviruses and Opportunistic Infections; Boston. 3-6 February 2008. [Google Scholar]
- 25.Sabin C, Morfeldt L, Friis-Moller N, Rickenbach M, Reiss P, d’Arminio Monforte A, et al. on behalf of the D:A:D study group Changes over time in antiretroviral therapy (ART) use and risk factors for cardiovascular disease (CVD) in the D:A:D: study; Program and abstracts of the 12th Conference on Retro-viruses and Opportunistic Infections [abstract 866]; Boston. February 2005. [Google Scholar]
- 26.Allardice GM, Hole DJ, Brewster GH, Boyd J, Goldberg DJ. Incidence of malignant neoplasms among HIV-infected persons in Scotland. Br J Cancer. 2003;89:505–507. doi: 10.1038/sj.bjc.6601139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olivero OA, Tejera AM, Fernandez JL, Taylor BJ, Das S, Divi RL, Poirier MC. Zidovudine induces S-phase arrest and cell cycle gene expression changes in human cells. Mutagenesis. 2005;20:139–146. doi: 10.1093/mutage/gei019. [DOI] [PubMed] [Google Scholar]
- 28.Carter MM, Torres SM, McCash L, Yu M, Walker VE. Relative mutagenic potencies of several nucleoside analogs, alone or in drug pairs, at the HPRT and TK loci of human TK6 lymphoblastoid cells. Environ Mol Mutagen. 2007;48:239–247. doi: 10.1002/em.20282. [DOI] [PubMed] [Google Scholar]
- 29.Murillas J, Del Rio M, Riera M, Vaquer P, Salas A, Leyes M, et al. Increased incidence of hepatocellular carcinoma (HCC) in HIV-1 infected patients. Eur J Intern Med. 2005;16:113–115. doi: 10.1016/j.ejim.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Merchante N, Giron-Gonzalez JA, Gonzalez-Serrano M, Torre-Cisneros J, Garcia-Garcia JA, Arizcorreta A, et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS. 2006;20:49–57. doi: 10.1097/01.aids.0000198087.47454.e1. [DOI] [PubMed] [Google Scholar]
- 31.Burgi A, Brodine S, Wegner S, Milazzo M, Wallace MR, Spooner K, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005;104:1505–1511. doi: 10.1002/cncr.21334. [DOI] [PubMed] [Google Scholar]
- 32.Frisch M, Biggar RJ, Engels EA, Goedert JJ, for the AIDS-Cancer Match Registry Study Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]