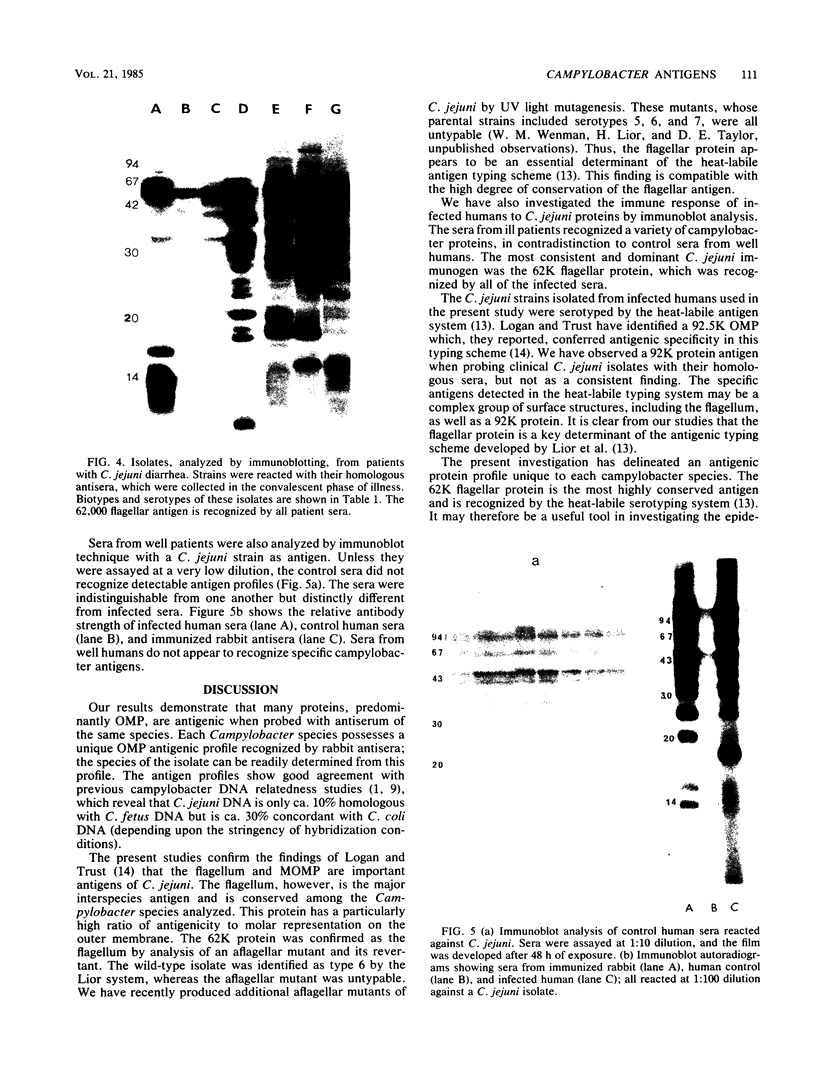
Abstract
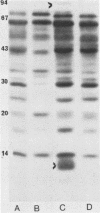
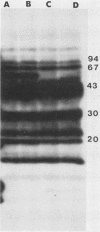
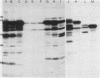
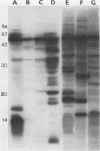
Outer membrane proteins of Campylobacter jejuni and other campylobacter species were analyzed for their antigenic potentials by immunoblotting. Polypeptides were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred electrophoretically, and reacted with rabbit antisera to C. jejuni. Each Campylobacter species analyzed demonstrated a unique outer membrane protein antigenic profile; interspecies antigen sharing was observed to be compatible with the degree of DNA relatedness between the species. The most highly conserved outer membrane protein antigen was the flagellum (molecular weight, 62,000). An aflagellate mutant was found to be untypable with the heat-labile system, in contrast to its parental isolate. The immunogenic potentials of C. jejuni proteins were examined by immunoblot analysis of sera from infected humans. Sera of convalescent patients, reacted with their homologous C. jejuni isolates, recognized a variety of campylobacter proteins. The most consistent immunogen in human infection was the flagellar protein. Patient sera assayed by the immunoblot technique were easily distinguished from control sera, which did not recognize specific campylobacter antigens. These findings suggest that the campylobacter flagellar protein is an essential determinant of the heat-labile antigen typing scheme and is the dominant immunogen recognized during C. jejuni infections in humans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belland R. J., Trust T. J. Deoxyribonucleic acid sequence relatedness between thermophilic members of the genus Campylobacter. J Gen Microbiol. 1982 Nov;128(11):2515–2522. doi: 10.1099/00221287-128-11-2515. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., LaForce F. M., Wilson N. A., Wang W. L. Reservoirs for human campylobacteriosis. J Infect Dis. 1980 May;141(5):665–669. doi: 10.1093/infdis/141.5.665. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Bokkenheuser V. Vibrio fetus infection in man. I. Ten new cases and some epidemiologic observations. Am J Epidemiol. 1970 Apr;91(4):400–409. doi: 10.1093/oxfordjournals.aje.a121150. [DOI] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Lahita R. G., Winn W. C., Jr, Roberts R. B. Campylobacteriosis in man: pathogenic mechanisms and review of 91 bloodstream infections. Am J Med. 1978 Oct;65(4):584–592. doi: 10.1016/0002-9343(78)90845-8. [DOI] [PubMed] [Google Scholar]
- Karmali M. A., Fleming P. C. Campylobacter enteritis. Can Med Assoc J. 1979 Jun 23;120(12):1525–1532. [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Penner J. L., Fleming P. C., Williams A., Hennessy J. N. The serotype and biotype distribution of clinical isolates of Campylobacter jejuni and Campylobacter coli over a three-year period. J Infect Dis. 1983 Feb;147(2):243–246. doi: 10.1093/infdis/147.2.243. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lior H., Woodward D. L., Edgar J. A., Laroche L. J., Gill P. Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors. J Clin Microbiol. 1982 May;15(5):761–768. doi: 10.1128/jcm.15.5.761-768.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983 Nov;42(2):675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Structural and antigenic heterogeneity of lipopolysaccharides of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1984 Jul;45(1):210–216. doi: 10.1128/iai.45.1.210-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Pearson A. D. The identification of outer membrane proteins and flagella of Campylobacter jejuni. J Gen Microbiol. 1984 May;130(5):1201–1208. doi: 10.1099/00221287-130-5-1201. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980 Dec;12(6):732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis - the first five years. J Hyg (Lond) 1982 Oct;89(2):175–184. doi: 10.1017/s0022172400070704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., De Grandis S. A., Karmali M. A., Fleming P. C. Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother. 1981 May;19(5):831–835. doi: 10.1128/aac.19.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]