Abstract
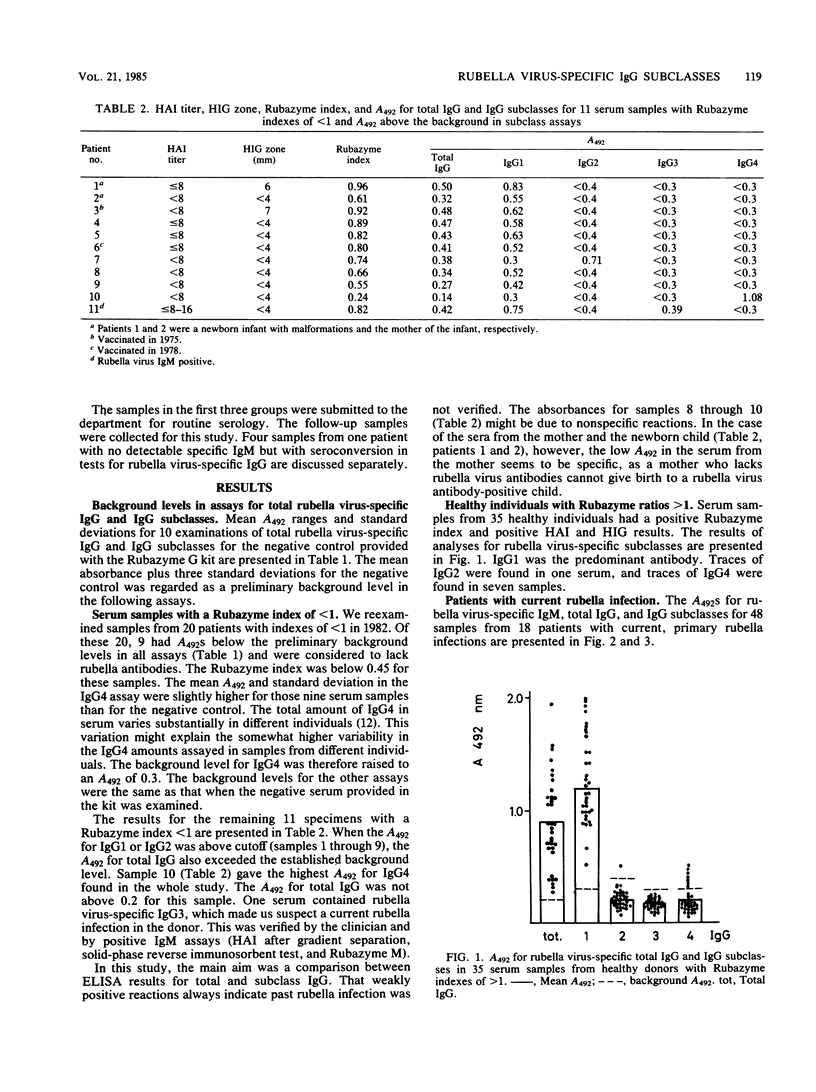
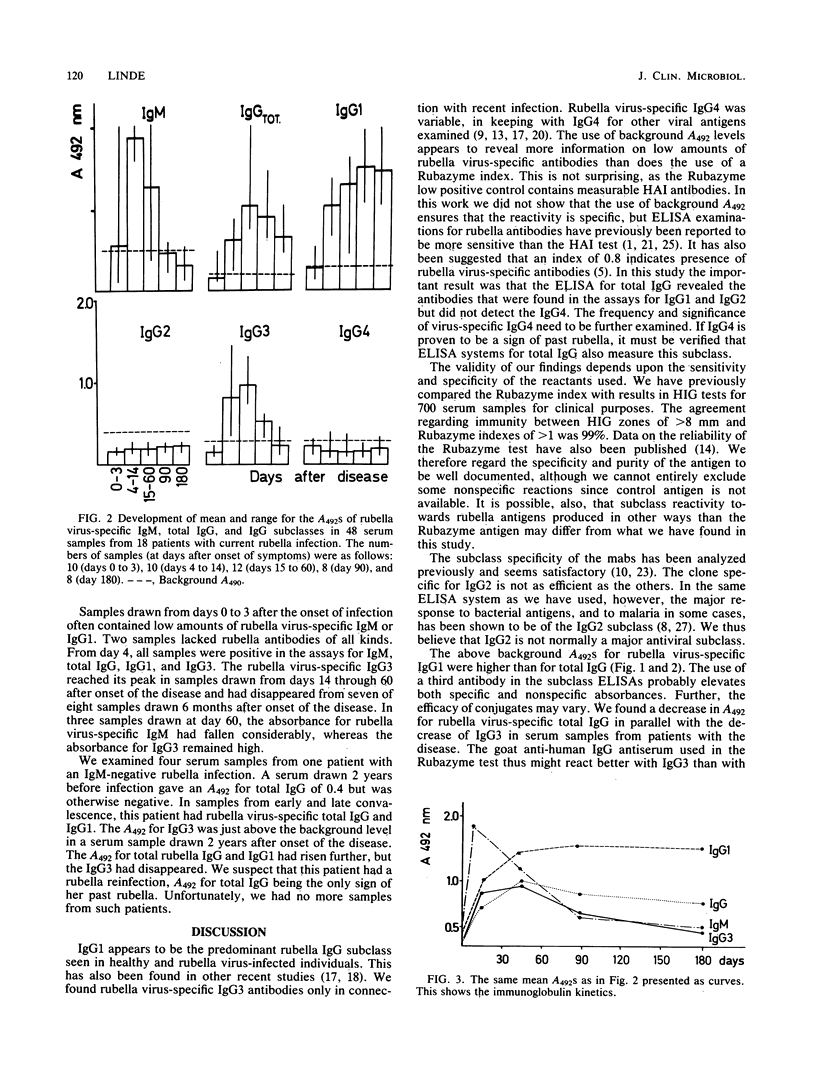
An enzyme-linked immunosorbent assay was used to study the subclass distribution of rubella virus-specific immunoglobulin G (IgG) in 97 serum samples from healthy donors and from patients with recent or remote rubella infections. Plastic beads coated with rubella antigen were incubated with test serum and then with monoclonal antibodies to the four human subclass of IgG. Rubella virus-specific IgG1 was present in all serum samples containing rubella virus-specific IgG antibodies. Rubella virus-specific IgG2 was present in 1 of 35 samples from healthy donors that also contained specific IgG1. Rubella virus-specific IgG3 was found in serum samples from patients with recent rubella infections but had disappeared by 6 months after the onset of symptoms. Rubella virus-specific IgG4 was found in low amounts in 7 of 35 samples from healthy immune donors. Of 20 serum samples that were negative by other serological techniques, 8 gave absorbances above cutoff levels in the assays for rubella virus-specific total IgG and IgG1. In 1 of 20 serum samples, the assays for total IgG and IgG2 were positive. High absorbance in the assay for rubella virus-specific IgG4 was found in one serum. This serum was negative in all other assays for rubella virus-specific antibodies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buimovici-Klein E., O'Beirne A. J., Millian S. J., Cooper L. Z. Low level rubella immunity detected by ELISA and specific lymphocyte transformation. Arch Virol. 1980;66(4):321–327. doi: 10.1007/BF01320628. [DOI] [PubMed] [Google Scholar]
- Chantler J. K., Ford D. K., Tingle A. J. Persistent rubella infection and rubella-associated arthritis. Lancet. 1982 Jun 12;1(8285):1323–1325. doi: 10.1016/s0140-6736(82)92398-4. [DOI] [PubMed] [Google Scholar]
- Cradock-Watson J. E., Ridehalgh M. K., Anderson M. J., Pattison J. R. Outcome of asymptomatic infection with rubella virus during pregnancy. J Hyg (Lond) 1981 Oct;87(2):147–154. doi: 10.1017/s0022172400069345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoyel G. A., Gaspar A., Peyramond D. Diagnosis of recent rubella virus infection by demonstration of specific immunoglobulin M antibodies: comparison of solid-phase reverse immunosorbent test with sucrose density gradient centrifugation. J Clin Microbiol. 1981 Apr;13(4):698–704. doi: 10.1128/jcm.13.4.698-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. K., Tingle A. J. Cell-mediated immune responses in patients with recurrent arthritis following rubella immunization. J Rheumatol. 1980 Mar-Apr;7(2):225–230. [PubMed] [Google Scholar]
- Grahame R., Armstrong R., Simmons N. A., Mims C. A., Wilton J. M., Laurent R. Isolation of rubella virus from synovial fluid in five cases of seronegative arthritis. Lancet. 1981 Sep 26;2(8248):649–651. doi: 10.1016/s0140-6736(81)90993-4. [DOI] [PubMed] [Google Scholar]
- Hammarström L., Granström M., Oxelius V., Persson M. A., Smith C. I. IgG subclass distribution of antibodies against S. aureus teichoic acid and alpha-toxin in normal and immunodeficient donors. Clin Exp Immunol. 1984 Mar;55(3):593–601. [PMC free article] [PubMed] [Google Scholar]
- Linde G. A., Hammarström L., Persson M. A., Smith C. I., Sundqvist V. A., Wahren B. Virus-specific antibody activity of different subclasses of immunoglobulins G and A in cytomegalovirus infections. Infect Immun. 1983 Oct;42(1):237–244. doi: 10.1128/iai.42.1.237-244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Bird P., Hardie D., Jefferis R., Ling N. R. Monoclonal antibodies (McAbs) to determinants on human gamma chains: properties of antibodies showing subclass restriction or subclass specificity. Immunology. 1982 Oct;47(2):329–336. [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Cooper M. D. New views of the immunoglobulin heavy-chain switch. Nature. 1982 Jul 22;298(5872):327–328. doi: 10.1038/298327a0. [DOI] [PubMed] [Google Scholar]
- Morell A., Roth-Wicky B., Skvaril F. Immunoglobulin G subclass restriction of antibodies against hepatitis B surface antigen. Infect Immun. 1983 Feb;39(2):565–568. doi: 10.1128/iai.39.2.565-568.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison J. R. Rubella antibody screening. J Hyg (Lond) 1982 Apr;88(2):149–153. doi: 10.1017/s0022172400070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Dennis J. Modified hemagglutination-inhibition test for rubella employing human group O erythrocytes. Appl Microbiol. 1972 Mar;23(3):471–475. doi: 10.1128/am.23.3.471-475.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siber G. R., Schur P. H., Aisenberg A. C., Weitzman S. A., Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980 Jul 24;303(4):178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- Skvaril F., Schilt U. Characterization of the subclasses and light chain types of IgG antibodies to rubella. Clin Exp Immunol. 1984 Mar;55(3):671–676. [PMC free article] [PubMed] [Google Scholar]
- Strannegård O., Grillner L., Lindberg I. M. Hemolysis-in-gel test for the demonstration of antibodies to rubella virus. J Clin Microbiol. 1975 Jun;1(6):491–494. doi: 10.1128/jcm.1.6.491-494.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist V. A., Linde A., Wahren B. Virus-specific immunoglobulin G subclasses in herpes simplex and varicella-zoster virus infections. J Clin Microbiol. 1984 Jul;20(1):94–98. doi: 10.1128/jcm.20.1.94-98.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle A. J., Yang T., Allen M., Kettyls G. D., Larke R. P., Schulzer M. Prospective immunological assessment of arthritis induced by rubella vaccine. Infect Immun. 1983 Apr;40(1):22–28. doi: 10.1128/iai.40.1.22-28.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J. J., Baringer J. R., Wolinsky J. S., Malamud N., Mednick J. P., Panitch H. S., Scott R. A., Oshiro L., Cremer N. E. Progressive rubella panencephalitis. Late onset after congenital rubella. N Engl J Med. 1975 May 8;292(19):990–993. doi: 10.1056/NEJM197505082921902. [DOI] [PubMed] [Google Scholar]
- Vartdal F., Bird P. Imprint immunofixation of IgG subclasses, using monoclonal subclass-specific antibodies. Scand J Immunol. 1983 Oct;18(4):367–370. doi: 10.1111/j.1365-3083.1983.tb01809.x. [DOI] [PubMed] [Google Scholar]
- Vartdal F., Vandvik B. Multiple sclerosis: subclasses of intrathecally synthesized IgG and measles and varicella zoster virus IgG antibodies. Clin Exp Immunol. 1983 Dec;54(3):641–647. [PMC free article] [PubMed] [Google Scholar]
- Vejtorp M. Enzyme-linked immunosorbent assay for determination of rubella IgG antibodies. Acta Pathol Microbiol Scand B. 1978 Dec;86B(6):387–392. doi: 10.1111/j.1699-0463.1978.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Vaheri A. Rubella: a method for rapid diagnosis of a recent infection by demonstration of the IgM antibodies. Br Med J. 1968 Jan 27;1(5586):221–223. doi: 10.1136/bmj.1.5586.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Berzins K., Perlmann P., Persson M. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti-P. falciparum antibodies in different sera. Clin Exp Immunol. 1983 Oct;54(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Weil M. L., Itabashi H., Cremer N. E., Oshiro L., Lennette E. H., Carnay L. Chronic progressive panencephalitis due to rubella virus simulating subacute sclerosing panencephalitis. N Engl J Med. 1975 May 8;292(19):994–998. doi: 10.1056/NEJM197505082921903. [DOI] [PubMed] [Google Scholar]


