Abstract
The blue-blindness (tritanopia) of the human foveola normally goes unnoticed but can be directly visualized by having observers view a flickering, monochromatic, short-wavelength field. The blue scotoma appears as a tiny dark spot in central vision, the visibility of which depends upon the wavelength of the field and the temporal frequency of modulation. Comparisons of fading times as a function of flicker frequency for the blue scotoma, foveal afterimages and optically stabilized images indicate a common time course, consistent with the hypothesis that perceptual filling-in of the foveal blue scotoma reflects the operation of neural processes similar to those involved in fading and regeneration of stabilized images.
Keywords: Scotoma, Foveal blue-blindness, Perceptual filling-in, Stabilized images
1. Introduction
Looking at the sky on a clear day, one sees a perfectly homogeneous blue color. Yet, the central human fovea contains no short-wavelength-sensitive (S-) cone receptors (König, 1894; Willmer & Wright, 1945; Wald, 1967), so one might expect to see a tiny dark spot in the center of the visual field bouncing around as the eyes move across the sky. This foveal S-cone, or blue, scotoma is invisible to the observer, possibly because of neural interpolation or filling-in processes (Gerrits & Vendrik, 1970; Williams, MacLeod, & Hayhoe, 1981; Spillmann & Werner, 1996) by which the perception of blue mediated by neighboring areas is assigned to the central fovea. The foveola is devoid of S-cones (tritanopic), while the immediate surround has a high density of regularly spaced S-cones (Williams et al., 1981; Curcio et al., 1991). It is currently debated, however, whether perceptual filling-in of a scotoma is caused by active neural processes, or whether the brain simply ignores the corresponding region because of the absence of information (Churchland & Ramachandran, 1996; Dennett, 1996; Pessoa, Thompson, & Noë, 1998; Spillmann & deWeerd, in press). The present study suggests neural filling-in of the foveal blue scotoma.
As a scotoma is fixed on the retina, it may be compared to a stabilized retinal image. By analogy with stabilized retinal images, the blue scotoma should become visible by having the observer view a short-wavelength field flickering at a suitable frequency (Fiorentini & Ercoles, 1960). The theoretical rationale behind this prediction is based on the transient nature of the neural response and the antagonistic organization of the receptive fields of neurons in the retina and LGN, which, when temporally modulated, generate transient contrast activity across the border between adjacent regions of different luminance (Gerrits & Vendrik, 1970; Magnussen & Glad, 1975; DeValois, Webster, DeValois, & Lingelbach, 1986; Fiorentini, Baumgartner, Magnussen, Schiller, & Thomas, 1990). Following Barlow and Sparrock (1964), we further assume that sensitivity differences in the mosaic of retinal receptors mimic differences in luminance in a stabilized image, i.e. the activation of S-cones in the foveola and its immediate surround are different, while the ratio of M- and L-cones is constant over this region of retina (Nerger & Cicerone, 1992). In other words, we assume that the neural effects of sensitivity difference caused either by local bleaching of the retinal pigments (as in the case of afterimages) or by the absence of receptors in well-defined regions (as in the case of the foveal blue scotoma) are comparable to the neural effects of a luminance pattern optically stabilized on the retina. In the present study, we provide evidence for this view, showing that temporally modulated, monochromatic short-wavelength fields counteract the perceptual filling-in process and make the foveal blue scotoma directly visible to the observer as a tiny dark spot in the center of the visual field.
2. Methods
Subjects sat in a dark booth and steadied themselves using a combined chin-forehead rest. The right eye was centered relative to the 2 mm exit pupil of a three-channel Maxwellian view system that was employed for stimulus presentation. A 1000 W Xenon arc lamp powered by an Oriel Universal power supply was used in conjunction with a Jobin Yvon monochromator (8 nm half-band width) and Schott interference filters (10 nm half band width). Only one channel was used in the experiments reported here. It contained an 8 deg monochromatic disk comprising wavelengths that were varied randomly from 410 to 510 nm in 10 nm steps. An electromagnetic shutter provided square-wave flicker with 100% modulation depth and frequencies varying between 0.1 and 10 Hz.
Stimuli were set to equal brightness by adjusting a neutral density wedge according to a step-by-step procedure (in both directions). Individual stimuli were presented for 1 s, followed by a 3 s interval and a 1 s comparison stimulus. It should be noted that unlike the spectral efficiency function measured by heterochromatic flicker photometry (Eisner & MacLeod, 1980), the spectral efficiency for brightness is strongly dependent on S-cone signals to a chromatic pathway (Guth, Donley, & Marrocco, 1969; Wagner & Boynton, 1972; Bauer & Röhler, 1977). Field luminance at 470 nm was 79 trolands, time-average luminance 39.5 trolands, as measured with a Spectra Scan 704 photometer, and computed according to the method of Westheimer (1966).
In control experiments with a single observer, the measurements with equal brightness stimuli were repeated with equal luminance stimuli keyed to 450 nm and 460 nm. The results were similar to those obtained with equal brightness stimuli and are not reported.
Two types of psychophysical measurements were obtained. In one series of measurements, we assessed the visibility of the blue scotoma by having observers rate the perceived strength or salience of the dark spot on a 10-point scale where a value of ‘one’ meant that the phenomenon was not visible. In a second series of measurements, we determined the time to fade of the blue scotoma. Fading was timed by the observer with the aid of an electronic stopwatch; the watch was operated by the observer and started the moment the dark spot became visible and stopped when it disappeared, merging into the homogeneous background. The time was read off by the experimenter.
3. Results
3.1. Demonstration of the foveal blue-scotoma
The foveal blue scotoma is most easily observed with a homogenous 450 nm monochromatic background modulated in a square-wave fashion at temporal frequencies of 1–2 Hz. Under these conditions, the scotoma appears as a small, colorless dark spot with irregular, ragged borders in central vision. The dark spot is clearly visible for several seconds before it fades. We have demonstrated this to a number of subjects, all of whom have readily perceived the entoptic phenomenon and spontaneously provided similar phenomenological descriptions.
Size estimates were made by inserting a reticle in the collimated part of the optical path; the subject projected the foveal dark spot onto the scale and read off the number of divisions subtended by the spot. Measurements were obtained from four observers whose estimations of spot size varied between 24 and 32 arcmin visual angle. The size determinations may be slight overestimations of the area subtended by the dark spot, as they may be influenced by the involuntary eye movements associated with fixation.
To evaluate the possible relations between the foveal dark-spot and the well-known entoptic phenomena of Haidinger’ brushes and Maxwell’s spot, both of which are interpreted as reflecting the macular pigmentation (Miles, 1954; Spencer, 1967; Nussbaum, Pruett, & Delori, 1981), we produced the foveal dark spot and Haidinger’s brushes simultaneously by inserting a rotating polarizer in the optical path. Haidinger’s brushes appeared as a rotating spindle-shaped figure subtending a visual angle of 2–5 deg, but the polarizer produced no rotation of the much smaller foveal dark spot, and with slow flicker, the dark spot faded after a few seconds, leaving Haidinger’s brushes clearly visible. Thus, the two phenomena are functionally separable.
3.2. Effect of wavelength on visibility of the blue scotoma
We next measured the spectral dependence of visibility by having subjects rate the perceived strength of the foveal dark spot on a 10-point scale. Three subjects provided 10 judgments each of the strength or salience of the spot at a frequency of 1 Hz at selected background wavelengths randomly presented at 10 nm intervals between 400 nm and 510 nm. Data were collected in two experimental sessions.
The results are shown in Fig. 1, where the left panel plots rating as a function of wavelength for each observer separately. Individual results are quite stable. The perceived strength of the dark spot peaks at 450 nm and falls sharply towards shorter and longer wavelengths; at field wavelengths beyond 410 nm and 500 nm, the dark spot is no longer visible. In the right panel, the averaged results for the three observers are plotted in terms of log relative rating values and compared to the relative spectral sensitivity function of the short-wavelength cones (Stockman & Sharpe, 2000). There is a good correspondence between the two functions.
Fig. 1.
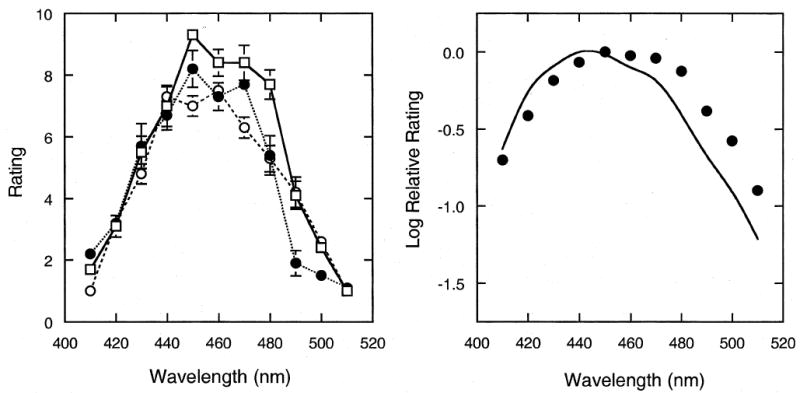
Rating of the perceived strength or salience of the foveal blue-scotoma as a function of the wavelength of a temporally 1 Hz square-wave modulated, monochromatic field adjusted to equal brightness. The left panel shows the results for individual observers. Error bars denote ± 1 standard error of the mean. The right panel shows the results averaged over three observers, plotted in terms of log relative ratings and compared to the relative spectral sensitivity function of the short-wavelength cones.
A similar picture emerged from a second experiment where fading time was used as an indicator of visibility. We reasoned that if the strength of the transient neural border activity induced by flicker stimulation is related to the difference in sensitivity between adjacent regions, and the resistance to fading is related to this activity (Magnussen & Torjussen, 1974; Gerling & Spillmann, 1987), the time-to-fade of the foveal dark spot should vary with the background spectral wavelength, for a fixed frequency of temporal modulation. Fading time was measured as the time between the appearance of the foveal dark spot upon onset of the flicker stimulus and its subsequent disappearance. Each observer was tested five times at each wavelength in random order, in two different sessions, with flicker frequency set to 2 Hz.
Absolute fading times varied between observers, but the shape of the function relating fading time to wavelength was similar for all observers. The individual results are shown in the left panel of Fig. 2, the grand means are shown in the right panel, plotted in terms of log relative fading time. The results are similar to those for visibility rating, fading time following the sensitivity function of the S-cones (right panel) with a maximum at 450 nm for two observers and 460 for one observer (left panel). The correspondence is quite good, considering that a precise match between spectral sensitivity and fading times, or between sensitivity and rating scale values, is not expected. The correlation coefficients between the rated strength of the foveal dark spot and the fading time were 0.84, 0.88 and 0.93 for individual observers.
Fig. 2.
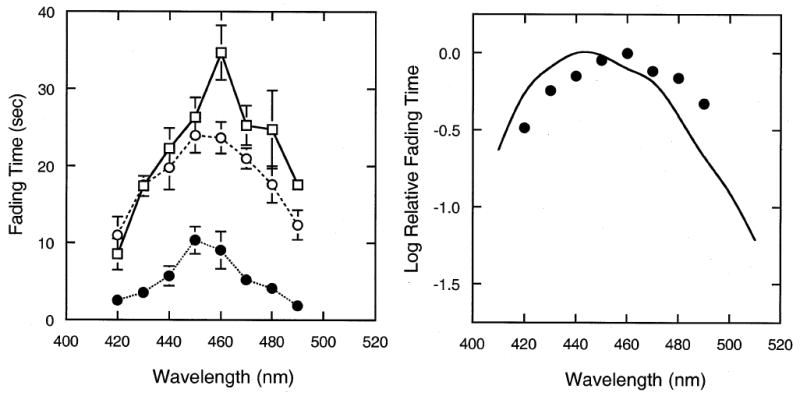
Perceptual fading of the foveal blue scotoma. The time required for the foveal blue-scotoma to fade is plotted as a function of the wavelength of a monochromatic field, square-wave modulated at 2 Hz. Left panel shows the results for individual observers. Error bars denote ± 1 standard error of the mean. The right panel shows the results averaged over observers and plotted as log relative fading time; curve as in Fig. 1.
3.3. Effect of flicker frequency on visibility of the blue scotoma
To test the analogy between filling-in of the foveal blue scotoma and fading of stabilized retinal images, we determined the effect of flicker frequency on the visibility of the foveal dark-spot, using time-to-fade as a visibility criterion. From these measurements, we should be able to make a direct comparison of fading time of the foveal dark spot and the results from analogous experiments on optical image stabilization and visual afterimages reported in the literature.
For frequencies below 1 Hz, there was a range where fading of the blue scotoma was not observed, or observed only occasionally: at each stimulus onset, the foveal dark-spot was activated and remained visible during the complete light phase of the flicker cycle. At even lower frequencies (0.1 Hz), the dark spot behaved as in continuous light, was transiently visible (for 2–3 s) at the light-onset but faded during the light phase. Quantitative measurements with flicker were therefore carried out for frequencies between 1 and 10 Hz with a 450 nm background. The upper panel of Fig. 3 shows the mean results for three observers, together with comparable results for optically stabilized images and negative afterimages. The results for optical image stabilization are taken from Fiorentini and Ercoles (1960) (tables 7 and 8; the data points in Fig. 3 are the means of two observers) and represent the visibility of a foveally viewed dark line stimulus, subtending 30 arcmin, as a function of the flicker frequency of the background field, modulated in a square-wave fashion with a mean luminance of 3.5 cd/m2. This is a lower background luminance than in the present experiments; however, experiments on flicker and stabilized-image visibility have shown that the shape of the function is largely invariant of both modulation amplitude and luminance (Tulunay-Keesey, 1969).
Fig. 3.
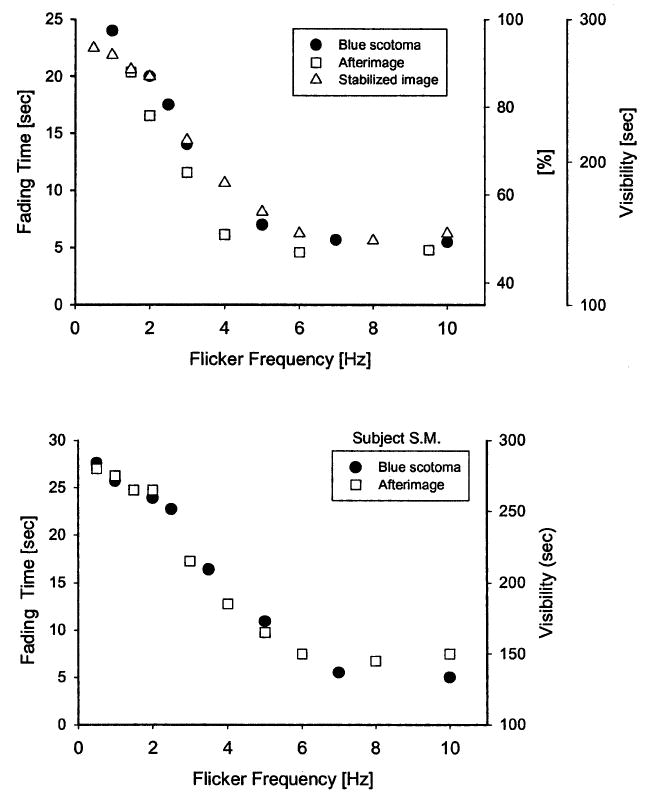
Comparison of fading characteristics of the foveal blue scotoma, optically stabilized images and visual afterimages. In the upper panel, filled symbols and left ordinate represent the time-to-fade for the foveal blue scotoma as a function of the frequency of luminance modulation of a monochromatic (450 nm) field. The means of three observers are based on 10 measurements at each frequency. Open squares and right ordinate (labeled ‘sec’) show the visibility of a flash-induced foveal afterimage, replotted from Magnussen and Torjussen (1974, fig. 1). Means of two observers. Open triangles and right ordinate (labeled ‘%’) show the visibility of the stabilized image of a foveally viewed line stimulus, based on data reported by Fiorentini and Ercoles (1960, tables 7 and 8). The lower panel shows afterimage and blue scotoma results for observer SM, who served in both experiments.
The afterimage data are taken from Magnussen and Torjussen (1974), who measured the fading of foveal afterimages induced by a high-intensity photoflash. The afterimage subtended 10 × 40 arcmin and was observed against a white background whose luminance was modulated in a square-wave fashion with a time-average luminance of 2.8 cd/m2. Fig. 3 shows the duration of the afterimage, means of two observers; parallel curves were found for a second measure, summing up the periods of visibility before the afterimage finally faded (not shown). As one observer (SM) served in both the afterimage and the present experiment, we are able to make an intra-individual comparison, and the results for this observer are shown in the lower panel of Fig. 3. Despite the quarter of a century separating the two sets of measurements, the correspondence is excellent. These comparisons strongly suggest common mechanisms of fading and visibility.
4. Discussion
The size and shape of the foveal dark spot observed under the present conditions agree with estimates of the foveal blue scotoma derived from the retinal sensitivity profile for short-wavelength light and the anatomical distribution of cones. Detailed psychophysical measurements have produced a sensitivity map of the S-cone mechanism in the central fovea forming an irregular, 20 arcmin region of low sensitivity surrounded by steep, ragged valleys and peaks (Williams et al., 1981), and anatomical studies corroborate these findings by showing a 20–25 arcmin S-cone free zone with an irregular spacing of S-cones on the foveal flanks (Curcio et al., 1991).
The function relating the visibility of the foveal blue scotoma to the wavelength of the flickering field is in good correspondence with the sensitivity of the S-cones. Measurements of the spectral sensitivity of an S-cone pathway in both normals and blue-cone monochromats indicate a plateau in sensitivity in the region of 430–450 nm (Stockman, Sharpe, & Fach, 1999). The visibility of the blue scotoma in the shorter wavelength region would be additionally affected by absorption in the ocular media that enters the psychophysical measurements progressively below 450 nm (Ruddock, 1972; Stockman et al., 1999), and this may account for the rapid fall-off of the visibility of the scotoma (Fig. 2, left panel).
A second factor that reduces the sensitivity of the fovea to short-wavelength light is the yellow pigmentation of the macula, which has a density spectrum peaking at 460 nm (Bone, Landrum, & Cains, 1992) and high concentration in the foveola (Werner, Donnelly, & Kliegl, 1987; Hammond, Wooten, & Snodderly, 1997). The macular pigment is believed to be responsible for the entoptic phenomena of Haidinger’s brushes and Maxwell’s spot (Nussbaum et al., 1981). Our control experiment showing that the visibility of the foveal dark-spot was independent of the conditions producing Haidinger’s brushes would appear to rule out macular pigmentation as the basis of the foveal dark-spot, but the macular pigmentation might serve to enhance the phenomenon. It is also possible that, conversely, some of the observations reported on the Maxwell spot, and thus attributed to macular pigmentation, may actually refer to the blue scotoma: Maxwell’s spot is most easily observed with a dichroic filter that transmits long-wave and short-wave light and typically appears as a circular spot with an overall diameter of approximately 2.5–3 deg visual angle with a small homogeneous center region subtending about 30 arcmin, surrounded by one or two bands or rings of a different color and intensity (Miles, 1954; Spencer, 1967). The bull’s-eye appearance of the phenomenon does not fit the smooth density gradient of the macular pigmentation distribution (Werner et al., 1987; Hammond et al., 1997), and one of the earliest systematic studies of the phenomenon concluded that the center region of the Maxwell spot ‘cannot be explained by reduction of transmitted light through macular pigment but more probably represents a distinctive cone population’ (Miles, 1954, p. 37). It is thus conceivable that the well-defined center region of Maxwell’s spot and other reports of small Maxwell spots (Williams et al., 1981) under different viewing conditions are observations of the foveal blue scotoma.
As the region of the foveal blue scotoma is defined by differences in relative sensitivity to light rather than blindness, it is more related to local bleaching of photo-pigments and/or neural adaptation than to scotomata caused by lesions of the retina or central visual pathways, or to the blind spot. The processes involved are therefore more akin to those involved in stabilized image fading and related effects such as the Krauskopf (1963) and Craik–O’Brien– Cornsweet (Cornsweet, 1970) illusions, and so-called artificial scotomas (Ramachandran & Gregory, 1991; DeWeerd, Desimone, & Ungerleider, 1998). The comparisons of Fig. 3 are consistent with this view, showing very similar curves relating visibility to flicker frequency for foveal afterimages, stabilized images and the blue scotomas, and suggest that perceptual filling-in of the foveal blue scotoma is an active neural process that involves the same neural mechanisms by which optically stabilized retinal images fade from view. We conclude that adaptation or inhibition of border and contrast subserving processes is the likely basis of perceptual filling-in of the foveal blue-scotoma.
There is also evidence that the possibility of directly perceiving a scotoma is not confined to the naturally occurring blue-scotoma or the artificial, functional scotoma represented by negative afterimages. In some cases, a scotoma caused by a lesion can be made visible with the aid of dynamic textured backgrounds (Aulhorn & Köst, 1988; Aulhorn, Schiefer, & Herzau, 1990; Churchland & Ramachandran, 1996; Bachmann & Fahle, 2000). Such psychophysical procedures could form a test of filling-in as an active process as opposed to an ignored region. It is tempting to speculate that when the perceptual filling-in can be counteracted—by flicker, motion or other measures—an active neural process is in operation. The foveal blue scotoma is an example of such active filling-in.
Acknowledgments
This research was supported by the Alexander-von-Humboldt Stiftung (S.M. and J.S.W.), the Norwegian Research Council (S.M.), and the German Research Council (L.S.). We thank R. Teichmann for serving as observer, and B.R. Hammond, S. Grossberg and D.R. Williams for comments.
References
- Aulhorn F, Köst G. Rauschfeldkampimetrie. Eine neuartige perimetrische Untersuchungsmethode. Klinische Monatsblätter für Augenheilkunde. 1988;192:284–288. doi: 10.1055/s-2008-1050114. [DOI] [PubMed] [Google Scholar]
- Aulhorn F, Schiefer U, Herzau V. Wahrnehmung des Blinden Flecks bei der Rauschfeldkampimetrie. Ein zusätzliches diagnostisches Kriterium bei Papillenveränderungen Fortschritte der Ophthalmologie. 1990;87:516–520. [PubMed] [Google Scholar]
- Bachmann G, Fahle M. Component perimetry: a fast method to detect visual field defects caused by brain lesions. Investigative Ophthalmology & Visual Science. 2000;41:2870–2886. [PubMed] [Google Scholar]
- Barlow HB, Sparrock JMB. The role of afterimages in dark adaptation. Science. 1964;144:1309–1314. doi: 10.1126/science.144.3624.1309. [DOI] [PubMed] [Google Scholar]
- Bauer HD, Röhler R. Brightness generation in the human visual system. Colour-brightness: a contribution of cortical colour channels to brightness sensation. Vision Research. 1977;17:1211–1216. doi: 10.1016/0042-6989(77)90156-0. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vision Research. 1992;32:105–110. doi: 10.1016/0042-6989(92)90118-3. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Ramachandran VS. Filling-in: why Dennett is wrong. In: Akins K, editor. Perception. Oxford: Oxford University Press; 1996. pp. 132–157. [Google Scholar]
- Cornsweet T. Visual perception. London: Academic Press; 1970. [Google Scholar]
- Curcio CA, Allen KA, Sloan KR, Lerea CL, Hurley JB, Klock IB, Milam AH. Distribution and morphology of human cone receptors stained with anti-blue opsin. Journal of Comparative Neurology. 1991;312:610–624. doi: 10.1002/cne.903120411. [DOI] [PubMed] [Google Scholar]
- Dennett D. Seeing is believing—or is it? In: Akins K, editor. Perception. Oxford: Oxford University Press; 1996. pp. 158–172. [Google Scholar]
- DeValois RL, Webster MA, DeValois KK, Lingelbach B. Temporal properties of brightness and color induction. Vision Research. 1986;26:887–897. doi: 10.1016/0042-6989(86)90147-1. [DOI] [PubMed] [Google Scholar]
- DeWeerd P, Desimone R, Ungerleider LG. Perceptual filling-in: a parametric study. Vision Research. 1998;38:2721–2734. doi: 10.1016/s0042-6989(97)00432-x. [DOI] [PubMed] [Google Scholar]
- Eisner A, MacLeod DIA. Blue-sensitive cones do not contribute to luminance. Journal of the Optical Society of America. 1980;70:121–123. doi: 10.1364/josa.70.000121. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Baumgartner G, Magnussen S, Schiller P, Thomas JP. The perception of brightness and darkness. Relations to neuronal receptive fields. In: Spillmann L, Werner J, editors. Visual perception. The neurophysiological foundations. New York: Academic Press; 1990. pp. 129–161. [Google Scholar]
- Fiorentini A, Ercoles AM. Vision with stabilized images and intermittent illumination. Atti Fondatione Georgio Ronchi. 1960;15:618–633. [Google Scholar]
- Gerling J, Spillmann L. Duration of visual afterimages on modulated backgrounds: postreceptoral processes. Vision Research. 1987;27:521–527. doi: 10.1016/0042-6989(87)90038-1. [DOI] [PubMed] [Google Scholar]
- Gerrits HJM, Vendrik AJH. Simultaneous contrast, filling-in process and information processing in man’s visual system. Experimental Brain Research. 1970;11:411–440. doi: 10.1007/BF00237914. [DOI] [PubMed] [Google Scholar]
- Guth SL, Donley NJ, Marrocco RT. On luminance additivity and related topics. Vision Research. 1969;9:537–575. doi: 10.1016/0042-6989(69)90019-4. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. Journal of the Optical Society of America A. 1997;14:1187–1196. doi: 10.1364/josaa.14.001187. [DOI] [PubMed] [Google Scholar]
- König A. ber den menschlichen Sehpurpur und seine Bedeutung für das Sehen. Sitzungsberichte der Preussischen Akademie der Wissenschaften. 1894;30:577–598. [Google Scholar]
- Krauskopf J. Effect of retinal image stabilization on the appearance of heterochromatic targets. Journal of Optical Society of America. 1963;53:741–744. doi: 10.1364/josa.53.000741. [DOI] [PubMed] [Google Scholar]
- Magnussen S, Glad A. Temporal frequency characteristics of spatial interaction in human vision. Experimental Brain Research. 1975;23:519–528. doi: 10.1007/BF00234919. [DOI] [PubMed] [Google Scholar]
- Magnussen S, Torjussen T. Sustained visual afterimages. Vision Research. 1974;14:743–744. doi: 10.1016/0042-6989(74)90074-1. [DOI] [PubMed] [Google Scholar]
- Miles WR. Comparison of functional and structural areas in the human fovea. I Method of entopic plotting. Journal of Neurophysiology. 1954;17:22–38. doi: 10.1152/jn.1954.17.1.22. [DOI] [PubMed] [Google Scholar]
- Nerger JL, Cicerone CM. The ratio of L cones to M cones in the human parafoveal retina. Vision Research. 1992;32:879–888. doi: 10.1016/0042-6989(92)90030-m. [DOI] [PubMed] [Google Scholar]
- Nussbaum JJ, Pruett RC, Delori FC. Macular pigment. The first 200 years. Retina. 1981;1:296–310. [PubMed] [Google Scholar]
- Pessoa L, Thompson E, Noë A. Finding out about filling-in: a guide to perceptual completion for visual science and the philosophy of perception. Behavioral and Brain Sciences. 1998;21:723–747. doi: 10.1017/s0140525x98001757. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Gregory RL. Perceptual filling-in of artificially induced scotomas in human vision. Nature. 1991;350:699–702. doi: 10.1038/350699a0. [DOI] [PubMed] [Google Scholar]
- Ruddock KH. Light transmission through the ocular media and macular pigment and its significance for psychophysical investigation. In: Jameson D, Hurvich LM, editors. Visual psychophysics. In: Handbook of sensory physiology. 4. VII. Berlin: Springer; 1972. pp. 455–483. [Google Scholar]
- Spencer JA. An investigation of Maxwellís spot. British Journal of Physiological Optics. 1967;24:103–147. [PubMed] [Google Scholar]
- Spillmann L, deWeerd P. Surface completion: psychophysics and neurophysiology of texture fading and filling-in. In: Pessoa L, deWeerd P, editors. Filling-in: from perceptual completion to skill learning. Oxford: Oxford University Press; in press. [Google Scholar]
- Spillmann L, Werner JS. Long-range interaction in visual perception. Trends in Neurosciences. 1996;19:428–434. doi: 10.1016/0166-2236(96)10038-2. [DOI] [PubMed] [Google Scholar]
- Stockman A, MacLeod DIA, Johnson NE. Spectral sensitivities of human cones. Journal of the Optical Society of America A. 1993;10:2491–2521. doi: 10.1364/josaa.10.002491. [DOI] [PubMed] [Google Scholar]
- Stockman A, Sharpe LT. The spectral sensitivities of the middle- and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vision Research. 2000;40:1711–1737. doi: 10.1016/s0042-6989(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Stockman A, Sharpe LT, Fach C. The spectral sensitivity of human short-wavelength sensitive cones derived from thresholds and color matches. Vision Research. 1999;39:2901–2927. doi: 10.1016/s0042-6989(98)00225-9. [DOI] [PubMed] [Google Scholar]
- Tulunay Keesy U. Visibility of a stabilized target as a function of the frequency and amplitude of luminance variation. Journal of the Optical Society of America. 1969;39:604–610. doi: 10.1364/josa.59.000604. [DOI] [PubMed] [Google Scholar]
- Wagner G, Boynton RM. Comparison of four methods of heterochromatic photometry. Journal of the Optical Society of America. 1972;62:1508–1515. doi: 10.1364/josa.62.001508. [DOI] [PubMed] [Google Scholar]
- Wald G. Blue-blindness in the normal fovea. Journal of the Optical Society of America. 1967;57:1289–1303. doi: 10.1364/josa.57.001289. [DOI] [PubMed] [Google Scholar]
- Werner JS, Donnelly SK, Kliegl R. Aging and human macular pigment density; appended with translations from the work of Max Schultze and Ewald Hering. Vision Research. 1987;27:257–268. doi: 10.1016/0042-6989(87)90188-x. [DOI] [PubMed] [Google Scholar]
- Westheimer G. The Maxwellian view. Vision Research. 1966;6:669–682. doi: 10.1016/0042-6989(66)90078-2. [DOI] [PubMed] [Google Scholar]
- Williams DR, MacLeod DIA, Hayhoe MM. Punctate sensitivity of the blue-sensitive mechanism. Vision Research. 1981;21:1357–1375. doi: 10.1016/0042-6989(81)90242-x. [DOI] [PubMed] [Google Scholar]
- Willmer EN, Wright WD. Colour sensitivity of the fovea centralis. Nature. 1945;156:119. [Google Scholar]


