Abstract
Chronic alcohol abuse impairs both alveolar epithelial and macrophage function, and renders individuals susceptible to acute lung injury, pneumonia, and other serious lung diseases. Zinc deficiency, which is known to impact both epithelial and immune cell functions, is also associated with alcohol abuse. In this study, chronic alcohol ingestion (6 wk) in rats altered expression of key zinc transporters and storage proteins in the small intestine and the lung, and decreased zinc levels in the alveolar compartment. Zinc supplementation of alveolar epithelial monolayers derived from alcohol-fed rats in vitro, or of the diets of alcohol-fed rats in vivo, restored alveolar epithelial barrier function, and these improvements were associated with salutary changes in tight junction protein expression and membrane localization. In parallel, dietary zinc supplementation increased intracellular zinc levels, GM-CSF receptor expression, and bacterial phagocytic capacity in the alveolar macrophages of alcohol-fed rats. Together, these studies implicate zinc deficiency as a novel mechanism mediating alcohol-induced alveolar epithelial and macrophage dysfunction. Importantly, these findings argue that dietary supplementation can overcome alcohol-induced zinc deficiency and restore alveolar epithelial and macrophage function, and therefore could be an effective treatment for the susceptible alcoholic lung phenotype.
Keywords: GM-CSF, phagocytosis, tight junctions, zinc transporters, metallothionein
CLINICAL RELEVANCE
Alcohol-induced zinc deficiency within the alveolar space could have severe consequences for lung health, and zinc supplementation may improve the functional integrity of alveolar epithelial cells and macrophages.
Chronic alcohol abuse produces a myriad of defects within the lung and renders the affected individual susceptible to pneumonia (1) and acute lung injury (2, 3). Our research group has determined that chronic alcohol ingestion in experimental animals as well as in otherwise healthy individuals has profound effects on airway epithelial and macrophage function (4–9). Specifically, the epithelial barrier within the small gas-exchanging airways, the alveoli, is compromised. In parallel, the alveolar macrophage does not phagocytose bacteria normally. In combination, the alveolar epithelium and macrophage in the alcoholic lung are poorly prepared to respond to inflammatory stresses and/or invasion by infectious organisms.
Although the precise mechanisms by which alcohol abuse impairs both alveolar epithelial and macrophage function are still under investigation, recent progress has been made. For example, we recently determined that chronic alcohol ingestion in an experimental model decreases GM-CSF receptor expression in both the alveolar epithelium and the alveolar macrophage (6, 10) and, in parallel, decreases GM-CSF–dependent barrier formation in the alveolar epithelium (10) and bacterial phagocytic function in the alveolar macrophage (6). Importantly, treating alcohol-fed rats with recombinant GM-CSF via the upper airway normalizes lung epithelial barrier function and decreases endotoxin-induced lung injury (11), and restores alveolar macrophage innate immune functions (6). More recently, we determined that chronic alcohol ingestion significantly alters the expression and/or membrane localization of several key proteins that form the tight junctions between the alveolar epithelial cells and limit paracellular permeability, and that GM-CSF treatment has salutary effects on tight junction protein expression (12). However, the fundamental mechanisms by which alcohol interferes with GM-CSF signaling, alveolar epithelial tight junction protein expression, and/or alveolar macrophage host immune responses within the alveolar space remain undiscovered.
Zinc is an abundant and critical micronutrient; two families of transporters as well as storage/carrier proteins tightly regulate its tissue, cellular, and organellar distribution (13). It is a co-factor for at least 300 known enzymes including several key antioxidants, and is vital for the function of thousands of transcription factors; as such it is critically involved in cell growth, differentiation, and function (14, 15). Although zinc deficiency is known to impact function in the ciliated epithelium within the upper airway (14, 16), there is very little known about zinc homeostasis within the alveolar space. Further, although alcohol abuse has long been associated with zinc deficiency, the mechanisms for and the potential impact of this zinc deficiency have not been systematically examined. Based on the evolving understanding of the critical role of zinc in maintaining normal epithelial and immune function (13, 16, 17), alcohol-induced zinc deficiency within the alveolar space would have severe consequences for lung health (16).
In search of a common mechanism underlying the myriad effects of chronic alcohol ingestion on alveolar epithelial and macrophage function, we sought to investigate whether or not alcohol caused significant zinc deficiency in our experimental rat model, and if so, could it be unified mechanistically with our previous findings in experimental and clinical studies that have characterized the susceptible alcoholic lung phenotype.
MATERIALS AND METHODS
Alcohol Feeding
Adult Male Sprague-Dawley rats (initial weights 150–200 g; Charles River Laboratory, Wilmington, MA) were fed the Lieber-DeCarli liquid diet (Research Diets, New Brunswick, NJ) containing either alcohol (ethanol; 36% of total calories) or an isocaloric substitution with maltin-dextrin ad lib for 6 weeks as previously published (6, 10, 18). Zinc acetate (10, 100, or 500 mg/L) was added to the alcohol diets of some rats; each mg of zinc acetate provides approximately 0.3 mg of elemental zinc. In this model, rats typically consume approximately 50 ml of the liquid diet per day and achieve final weights of 300 to 350 g; therefore, the zinc supplementation ranged from approximately 0.5 mg/kg/day to approximately 5 mg/kg/day to approximately 25 mg/kg/day of elemental zinc. We chose this range because the recommended dietary supplementation for individuals with zinc deficiency is approximately 3 mg/kg/day (15). All work was performed with the approval of the Institutional Use and Care of Animals Committee (IACUC) at the Atlanta VAMC.
Bronchoalveolar Lavage and Isolation of Alveolar Macrophages
After pentobarbital anesthesia (100 mg/kg intraperitoneally), a tracheotomy tube was placed and rat lungs were first lavaged with 5 ml sterile cold PBS (pH 7.4). The recovered (∼ 3.5 ml) fluid was stored in aliquots at −70°C for measurement of zinc levels as described below; there were no significant differences in the volume recovered from any of the experimental groups, so zinc levels were not corrected for dilution. To isolate macrophages, lungs were lavaged four times with 10 ml of sterile cold PBS (pH 7.4). The recovered lavage solution was centrifuged at 1,500 rpm for 7 minutes, and the cell pellet resuspended in sterile media for functional studies. This procedure routinely yields cells that are more than 98% viable by Trypan Blue exclusion test. Diff-Quick (IMEB, Inc., San Marcos, CA) and CD-32 staining showed that over 95% were alveolar macrophages.
Measurement of Zinc Levels
Zinc levels in the bronchoalveolar lavage fluid and in the isolated alveolar macrophages and epithelial cells were measured using a zinc-specific dye FluoZin-3 (Invitrogen, Carlsbad, CA), a recently developed fluorochrome with a high affinity for zinc (Kd 15 nM) and little affinity for calcium or magnesium (19, 20). This free acid form is not membrane permeable and is mainly used to detect zinc release in extracellular spaces such as lavage fluid. FluoZin-3 (500 nM) was added to the first aliquot of lavage fluid, and resulting fluorescence was measured in a fluorescence plate reader. The excitation and emission wavelengths for FluoZin-3 are 494 and 516, respectively. A standard curve for zinc acetate was generated with each run and the zinc values in the unknown samples were extrapolated from this curve. To keep the zinc contamination to a minimum level, Milli-Q water (Millipore, Billerica, MA), reagent-grade reagents, sterile glassware, and sterile plasticware were used. The membrane permeable form, FluoZin-3AM, was used for staining intracellular zinc in alveolar macrophages and epithelial cells. FluoZin-3AM is routinely used to measure zinc in many different cell types (21, 23). Cells were stained for 1 hour at room temperature and resulting fluorescence was measured using FACScan flow cytometer (Becton Dickinson, San Jose, CA). To correlate cellular function with zinc levels, GM-CSF receptor expression and phagocytosis were measured in the alveolar macrophages as we have published previously (6).
RNA Isolation and Real-Time PCR
Total RNA was extracted from alveolar macrophages, alveolar epithelial cells, and the duodenal portion of the small intestine using Qiagen RNeasy Mini Kit (Valencia, CA) and treated with Qiagen DNase I to eliminate contaminating genomic DNA. Reverse transcription was performed with 1 μg of total RNA in total volume of 25 μl per reaction in Bio-Rad iScript cDNA synthesis kit (Hercules, CA). Real-time PCR was carried on the Bio-Rad iCycler. Amplification was preformed in Bio-Rad iQ SYBR green supermix containing specific primers and with denaturing at 95°C for 20 seconds, annealing at 58°C for 20 seconds, and extension at 20 seconds. Aliquots of cDNA were diluted 10- to 10,000-fold to generate relative standard curves to which sample cDNA was compared. Standards and samples were run in triplicate. QuantumRNA class II 18S primers were purchased from Ambion (Austin, TX). PCR amplicons from all species were normalized for the amount of 18S in the same cDNA sample, which was also standardized on a dilution curve from the cDNA samples. Dilution curves showed that the real-time PCR efficiency was greater than 95% for all genes analyzed. Real-time SYBR green dissociation curves showed one species of amplicon for each primer set. All the primers (Table 1) were designed in our laboratory and were obtained from Invitrogen.
TABLE 1.
PCR PRIMERS USED IN THIS STUDY
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| MT1rat | 5′-GAACTGCAAATGCACCTCCT | 5′-ACTTGTCCGAGGCACCTTT |
| MT2rat | 5′-ACAGATGGATCCTGCTCCTG | 5′-CACTTGTCCGAAGCCTCTTT |
| ZNT4mouse | 5′-TGCCGTCCTCTACTTGCTTT | 5′-TAGGCGATGAAATCCAAAGG |
| ZIP4mouse | 5′-CTTGGCTCTAGGCAAACCTG | 5′-AGTGTGGCCAGGTAATCGTC |
| ZIP1mouse | 5′-GGTCTCTCTCTGCCAGTTTTCG | 5′-CAGCATTAAGGAGGCAGAGG |
| ZO-1rat | 5′- AGCGAAGCCACCTGAAGATA | 5′- GATGGCCAGCAGGAATATGT |
| Occludinrat | 5′- AATTCCCGAATCCCAATTTC | 5′- AAGGGCAGAGTGGACTGAGA |
| MT1human | 5′-GCAAATGCAAAGAGTGCAAA | 5′-ATGGGTCAGGGTTGTATGGA |
| ZNT4human | 5′-TCTGGGTGTGAACGTAACCA | 5′-GATGGGGTCAGCAATCTTGT |
Flow Cytometric Detection of Membrane Receptor Expression
Membrane and intracellular expression of GM-CSF receptors on alveolar macrophages were measured by an established protocol we have published previously (6). Briefly, cells were incubated for 30 minutes at room temperature with rabbit polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) to either the rat GM-CSF receptor α or β subunit, or to an isotype-matched control antibody. Cells were washed to remove unbound antibody followed by 30 minutes of incubation at room temperature with secondary anti-rabbit antibody conjugated to fluorescein isothiocyanate (FITC). Cells were washed with PBS and were kept in the dark at 4°C until analyzed. The labeled cells were analyzed by FACScan flow cytometer (Becton Dickinson, San Jose, CA). Data are expressed as the mean channel fluorescence for positive cells in each group. In some experiments, the expression of metallothionein and the zinc transporters ZIP4 and ZNT4 was measured in fresh alveolar macrophages using polyclonal antibodies and appropriate FITC-conjugated secondary antibodies.
Alveolar Macrophage Bacterial Phagocytosis
Alveolar macrophages were isolated from control- and alcohol-fed rats and incubated for 1 hour with FITC-labeled Staphylococcus aureus (Wood strain without protein A; Molecular Probes, Eugene, OR) in a 10:1 ratio; after incubation cells were vigorously washed several times with PBS. Extracellular fluorescence was quenched by addition of trypan blue as described (22) and FITC-labeled bacteria containing cells were measured by flow cytometry. The phagocytic index was calculated as follows: (% positive cells x mean channel fluorescence)/100. Phagocytosis images were obtained by a microscope equipped with epifluorescence (Leica Microsystems GmbH, Wetzlar, Germany).
Isolation of Alveolar Epithelial Cells
Alveolar epithelial cells from control-fed and ethanol-fed rats were isolated using our established protocol (4, 10). Briefly, rats were anesthetized and lungs were removed en bloc after tracheostomy. Lungs were lavaged with 40 ml PBS (pH 7.4) to remove alveolar macrophages. Lungs were perfused with buffer to remove blood cells, and then filled with elastase solution to dissociate cells from lungs. The lung tissue was minced in a solution containing DNase I and newborn calf serum. The finely minced lung tissue was successively filtered through 100-μM and 20-μM nylon mesh. The cell suspension was plated on bacteriologic plastic plates coated with IgG to remove remaining alveolar macrophages. After 1 hour of incubation at 37°C the nonadherent type II epithelial cells were carefully removed. Cells obtained by this method were always more than 95% viable by Trypan Blue exclusion.
Epithelial Cell Lines
We used the L2 rat lung epithelial cell line (ATCC, Manassas, VA) and the Caco-2 human intestinal epithelial cell line (ATCC). To examine a direct connection between zinc deficiency and epithelial permeability, we cultured L2 cells with a Zn chelator, N,N,N′,N′-tetrakis-(2-pyridyl-methyl) ethylenediamine (TPEN) in vitro. The monolayers remained adherent and intact after 5 μM TPEN treatment for 48 hours. To examine the direct effects of alcohol on intestinal epithelial zinc status, Caco-2 cells were cultured overnight with 1% ethanol in the presence or absence of 10 μM zinc acetate. Cells were more than 96% viable after these treatments.
Epithelial Permeability Assay
Primary alveolar epithelial cells or L2 cells were plated onto a Transwell plate and cultured for 8 days. The barrier function was assessed by placing 14C-sucrose on the apical side of each monolayer. After 2 hours, 10 μl of media was sampled from the basolateral side and counted in the scintillation counter. Permeability was defined as the fraction of the initial radioactivity placed on the apical surface that appeared on the basolateral surface of the monolayer and expressed as a percentage.
Statistics
Data are presented as mean ± SEM. Data analysis was done by ANOVA with Student-Newman-Keuls test for group comparison and were considered statistically significant at a P value of < 0.05.
RESULTS
Chronic Alcohol Ingestion Decreases Zinc Levels in the Alveolar Epithelial Lining Fluid, and this Effect Is Abrogated by Dietary Zinc Supplementation
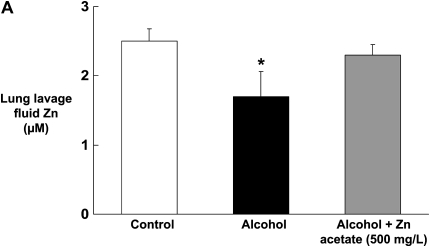
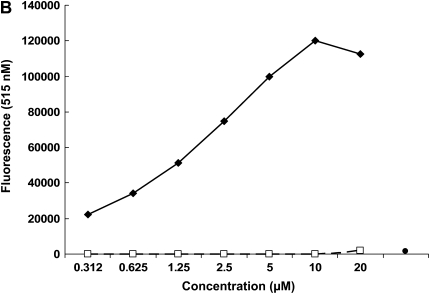
We first determined the effect of chronic alcohol ingestion on the levels of zinc in the alveolar epithelial lining fluid (as reflected by the levels in the lung lavage fluid), as this represents the pool of zinc bioavailability within the alveolar space, particularly for the alveolar macrophage. As shown in Figure 1A, chronic alcohol ingestion significantly (P < 0.05) decreased zinc levels in the lung lavage fluids of alcohol-fed rats by approximately 40% when compared with control-fed rats. We next determined whether or not dietary zinc supplementation could increase the levels of zinc in the alveolar space of alcohol-fed rats. Importantly, and as shown in Figure 1A, dietary zinc supplementation (500 mg/L of zinc acetate) restored zinc levels in the lavage fluids of alcohol-fed rats when compared with control-fed rats. As detailed in Materials and Methods, in this study we measured zinc levels using the zinc-specific dye FluoZin-3 (Invitrogen). To confirm that FluoZin-3 is indeed specific for zinc, we spiked normal saline separately with zinc, copper, calcium, or iron, at concentrations ranging from 0.312 to 20 μM (to span the concentrations of zinc in the lavage fluids in Figure 1A), and then analyzed each sample with FluoZin-3. As shown in Figure 1B, FluoZin-3 fluorescence increased in a linear fashion across the range of concentrations and appeared to saturate at 10 μM. In contrast, there was no detectable fluorescence with 20 μM concentration of calcium or iron (not shown), and only a slight fluorescence above baseline when copper was added at concentrations as high as 20 μM. It is possible that higher concentrations of calcium will be present in the airway liquid lining. However, even at a calcium concentration of 1 mM, the fluorescence was essentially at the baseline (Figure 1B). Therefore, the performance of FluoZin-3 in these studies is consistent with published studies showing that it is a highly specific indicator of zinc concentrations in biological samples.
Figure 1.
Chronic alcohol ingestion decreases zinc levels in the alveolar epithelial lining fluid, and this effect is abrogated by dietary zinc supplementation. Shown in A are the zinc concentrations (μM) in the lung lavage fluids of rats fed a control diet, an alcohol diet, or an alcohol diet supplemented with zinc acetate (500 mg/L) for 6 weeks. Zinc concentrations were determined using the zinc-specific fluorescent dye, FluoZin-3 (Invitrogen), and the standard curve for the assay is shown in B. As shown in A, chronic alcohol ingestion significantly (P < 0.05) decreased zinc levels in the lung lavage fluids of alcohol-fed rats when compared with control-fed rats. In contrast, alcohol-fed rats whose diets were supplemented with zinc had the same (P > 0.05) levels of zinc in their lavage fluids as control-fed rats. Each value represents the mean ± SEM of four or more determinations. The zinc levels in the lavage fluids of control-fed rats whose diets were supplemented with zinc acetate (500 mg/L) were not significantly increased (data not shown). As shown in the standard curve in B, Fluozin-3 fluorescence was linear over a range of zinc concentrations that spanned the concentrations measured in the lung lavage fluids in A. In contrast, there was no detectable fluorescence with iron (not shown), and only a slight fluorescence above baseline when copper was added at concentrations as high as 20 μM, or when calcium was added at a concentration of 1 mM as shown. Therefore, the performance of FluoZin-3 in these studies is consistent with published studies showing that it is a highly specific indicator of zinc concentrations in biological samples. *P < 0.05 compared with control-fed rats. Symbols in B: diamonds, Zn; squares, Cu; circle, Ca (1 mM).
Chronic Alcohol Ingestion Alters Gene Expression of Zinc Transporters in the Small Intestine
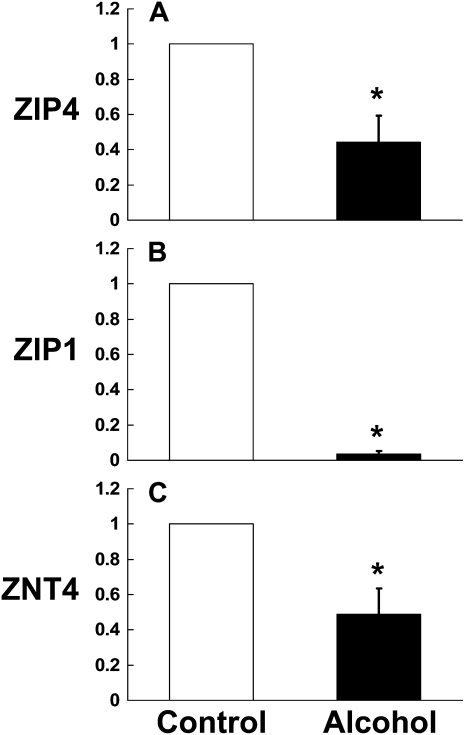
Although it is well recognized that alcohol abuse is associated with zinc deficiency, the mechanisms are unknown. The general assumption has been that zinc deficiency in alcoholics is caused by a poor diet, and this may well be a factor. However, in our experimental model chronic alcohol ingestion significantly decreased zinc levels in the alveolar space despite a nutritionally complete diet. Therefore, we speculated that alcohol could interfere with the transporters in the small intestine that mediate dietary zinc absorption. To our knowledge, the effects of chronic alcohol ingestion on zinc transporters in the intestine have not previously been examined. To address this question, we isolated the duodenum from control-fed and alcohol-fed rats. We first determined the relative expression of the zinc importing transporters, ZIP4 and ZIP1, by real-time PCR. ZIP4 is the principal importer of dietary zinc on the apical surface of enterocytes, so we determined its expression as well as that of ZIP1 for comparison. As shown in Figure 2, chronic alcohol ingestion significantly decreased (P < 0.05) the relative gene expressions of ZIP4 and ZIP1 in the duodenum when compared with control-fed rats. These results suggest that alcohol may directly interfere with dietary zinc absorption. In parallel, the gene expression of the zinc transporter, ZNT4, which is localized to the basolateral side of intestinal cells and is responsible for exporting zinc out of the intestine into the circulation, was also significantly decreased (P < 0.05) by chronic alcohol ingestion (Figures 2A–2C). This latter finding is consistent with the observed inhibitions of ZIP4 and ZIP1 expression by alcohol, as ZNTs are known to be down-regulated in a zinc-deficient environment in an effort by cells to retain zinc, and is further evidence that chronic alcohol ingestion interferes with the transport of dietary zinc from the intestinal lumen across the intestinal epithelium.
Figure 2.
Chronic alcohol ingestion decreases the relative expression of the zinc transporter proteins ZIP4, ZIP1, and ZNT4 in the small intestine. Gene expression of the zinc transporters (A) ZIP4, (B) ZIP1, and (C) ZNT4 in the small intestine (duodenum) of control-fed and alcohol-fed rats were determined by real time PCR. The average relative gene expression normalized to 18S was determined by the comparative method (2−ΔΔCt), with the target gene expression in tissue from control-fed rats set as 1 in each case. Each value represents the mean ± SEM of at least three separate tissue samples. *P < 0.05 compared with control.
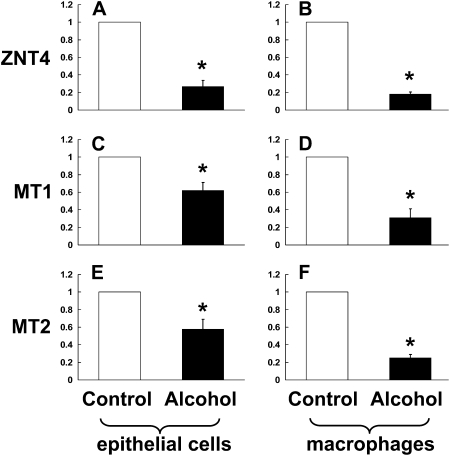
In Parallel, Chronic Alcohol Ingestion Alters Gene Expression of the Zinc Transporter, ZNT4, and of the Zinc Storage Protein, Metallothionein, in the Alveolar Epithelial Cells and Macrophages
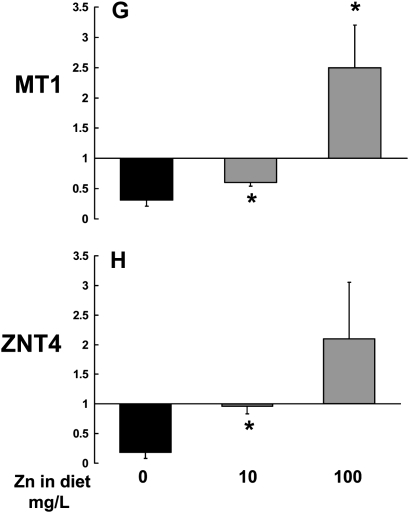
As chronic alcohol ingestion significantly lowered zinc levels in the alveolar space (Figure 1A), and altered gene expression of zinc transporters in the small intestine (Figure 2), we next examined the relative gene expression of ZNT4 and metallothionein in the alveolar epithelial cells and macrophages. Specifically, we reasoned that if alcohol-induced malabsorption of dietary zinc is the primary cause of the decreased zinc levels in the alveolar space, then the cells within this environment should decrease ZNT4 expression in an attempt to limit export and thereby preserve intracellular zinc levels. Further, if intracellular zinc pools are indeed decreased by alcohol, then the cellular levels of metallothionein, the principal zinc-storage protein, should likewise be decreased. Consistent with these predictions and as shown in Figures 3A–3F, chronic alcohol ingestion significantly decreased (P < 0.05) the relative gene expressions of ZNT4, metallothionein-1 (MT1) and metallothionein-2 (MT2) in the alveolar epithelial cells and in the alveolar macrophages when compared with control-fed rats. Importantly, and as shown in Figures 3G and 3H, dietary zinc supplementation (10 or 100 mg/L of zinc acetate) increased the zinc status of alveolar macrophages from alcohol-fed rats by up-regulating the relative gene expressions of MT1 and ZNT4. We also examined whether or not zinc supplementation could improve the intracellular zinc status in the gut epithelium. We exposed Caco-2 intestinal epithelial cells to a noncytotoxic concentration of ethanol (1%) overnight with or without 10 μM zinc. Ethanol significantly (P < 0.05) decreased the relative gene expressions of MT1 and ZNT4 by 60% and 25%, respectively, and an addition of 10 μM zinc to these cultures increased the expressions of MT1 and ZNT4 by 200% and 160%, respectively, consistent with the effects observed in the lung cells (data not shown as figures). These data support our observation that ethanol decreases and simultaneous addition of zinc increases the zinc status of the cells.
Figure 3.
Chronic alcohol ingestion decreases the relative gene expression of ZNT4, metallothionein-1 (MT1), and metallothionein-2 (MT2) in the alveolar epithelial cells and alveolar macrophages. Gene expression of (A and B) the zinc transporter ZIP4, and of the zinc storage proteins (C and D) MT1 and (E and F) MT2 in freshly isolated alveolar epithelial cells and macrophages from control-fed and alcohol-fed rats. G and H show the restoration of MT1 and ZNT4 gene expression when the diets of alcohol-fed rats (solid bars) were supplemented with zinc (10 or 100 mg/L of zinc acetate; shaded bars). The average relative gene expression normalized to 18S was determined by the comparative method (2−ΔΔCt), with the target gene expression in cells from control-fed rats set as 1 in each case. Each value represents the mean ± SEM of at least four separate cell isolations. *P < 0.05 compared with control.
Zinc Bioavailability Correlates with Alcohol-Induced Alveolar Epithelial Barrier Dysfunction
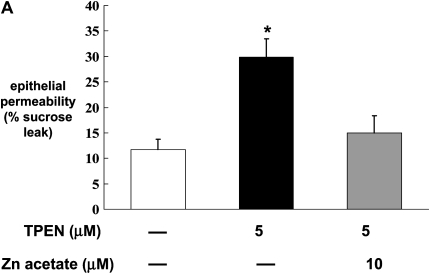
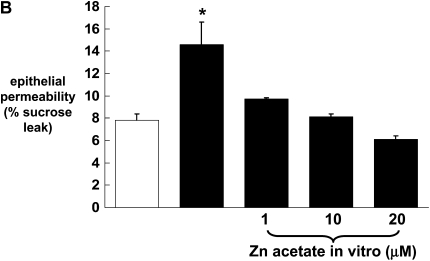
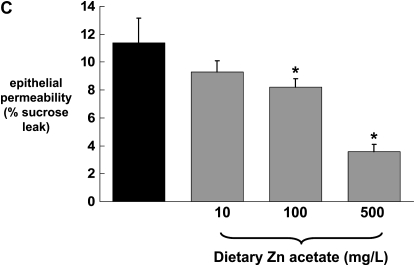
Previously we have published our findings that chronic alcohol ingestion causes significant alveolar epithelial barrier disruption, as reflected by increased paracellular permeability in vitro and in vivo (4). To determine whether or not the alcohol-induced changes in zinc levels and zinc transporters could be related to alcohol-induced barrier dysfunction in the alveolar epithelium, we first established monolayers with the rat alveolar epithelial cell line (L2), and then treated them with the zinc chelator TPEN. Monolayers were incubated with or without TPEN (5 μM) before examining epithelial barrier integrity, as reflected by paracellular leak of radiolabeled sucrose as we have used previously (4). As shown in Figure 4A, epithelial monolayers treated with TPEN had a significant increase (P < 0.05) in paracellular permeability compared with untreated monolayers. Evidence that this permeability was due to zinc depletion and not to a nonspecific toxicity of TPEN is also shown in Figure 4A; specifically, when TPEN-treated monolayers were incubated with zinc acetate, the barrier function was restored to that of untreated monolayers. We extended these studies and treated primary alveolar epithelial monolayers derived from alcohol-fed rats with zinc acetate to determine if this could reverse the effects of chronic alcohol ingestion on barrier function. As shown in Figure 4B, primary alveolar epithelial monolayers derived from alcohol-fed rats had a significant increase (P < 0.05) in paracellular permeability compared with monolayers derived from control-fed rats, consistent with our previously published studies (4). Importantly, when these monolayers were incubated with zinc acetate, their paracellular permeability was restored to the same levels as that of monolayers from control-fed rats. Consistent with the results in Figure 4A, treatment with zinc acetate was equally effective at concentrations of 1, 10, and 20 μM. These results suggest that zinc deficiency is a reversible cause of alcohol-induced alveolar epithelial barrier dysfunction. To extend these findings in vitro further, we next prepared alveolar epithelial monolayers derived from alcohol-fed rats in which some of the animals had their diets supplemented with zinc acetate at concentrations of 10, 100, or 500 mg/L. In these experiments, the monolayers were not treated with zinc in vitro. As shown in Figure 4C, dietary zinc supplementation of 100 or 500 mg/L significantly improved (P < 0.05) alveolar epithelial barrier function, with the greater effect seen with the 500 mg/L supplementation.
Figure 4.
Zinc bioavailability affects barrier function in alveolar epithelial monolayers. In A, monolayers derived from a rat lung epithelial cell line (L2) were cultured for 48 hours with or without the zinc chelator TPEN (5 μM) and with or without zinc acetate (10 μM). *P < 0.05 compared with no TPEN treatment. In B, monolayers were derived from alveolar epithelial cells freshly isolated from control-fed (open bars) and alcohol-fed (solid bars) rats and then cultured for 8 days with or without zinc acetate (1, 10, or 20 μM) added to the cultures every 48 hours with fresh media. *P < 0.05 compared with control-fed rats. In C, monolayers were derived from alveolar epithelial cells freshly isolated from alcohol-fed rats (solid bars) and alcohol-fed rats whose diets were supplemented with zinc acetate (10, 100, or 500 mg/L; shaded bars), and then cultured for 8 days without any additional zinc added to the media. * P < 0.05 compared with alcohol-fed rats. In all experiments in A, B, and C, monolayer permeability was assessed by quantifying the transepithelial flux of radiolabeled sucrose in 2 hours as described in Materials and Methods. In each case, data shown are the mean ± SEM of four determinations.
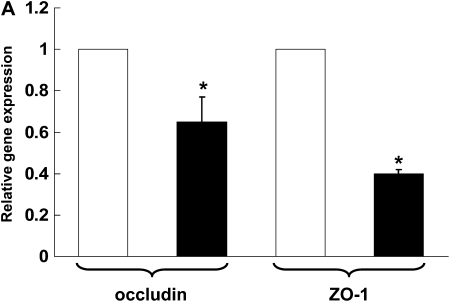
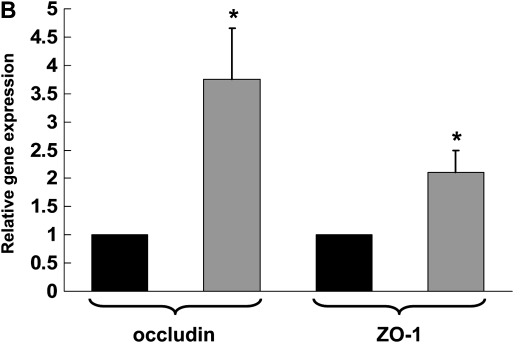
Dietary Zinc Supplementation Increases the Gene and the Protein Expression of the Tight Junction Proteins, Occludin and ZO-1, in the Alveolar Epithelial Cells of Alcohol-Fed Rats
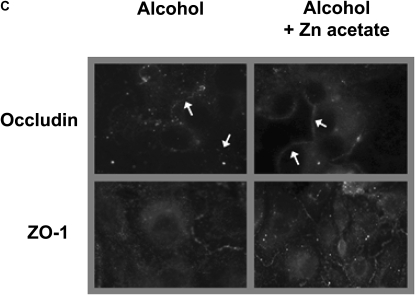
Recently we determined that chronic alcohol ingestion significantly alters the expression of several key proteins that mediate tight junction assembly and function within the alveolar epithelium, findings that provided a plausible mechanism for the previously observed effects of alcohol on lung epithelial permeability (4). As zinc supplementation improved alveolar epithelial barrier function in alcohol-fed rats, it was logical to next determine if zinc supplementation had any significant effects on the expression of tight junction components. In this study we assessed the expression of occludin, a tight junction protein that spans the cell membrane, and of ZO-1, a scaffolding protein that is essential for normal tight junction assembly and function. As shown in Figure 5A, chronic alcohol ingestion significantly decreased (P < 0.05) the relative gene expressions of both occludin and ZO-1 in alveolar epithelial cells (relative expression was normalized to that of cells from control-fed rats for occludin and ZO-1). Interestingly, and as shown in Figure 5B, dietary zinc supplementation (based on the results in Figure 4 we used the 500 mg/L concentration) significantly increased (P < 0.05) the relative gene expressions of occludin and ZO-1 in the alveolar epithelial cells of alcohol-fed rats (relative expression was normalized to that of cells from alcohol-fed rats for occludin and ZO-1). In parallel, we examined alveolar epithelial monolayers derived from alcohol-fed rats with or without dietary zinc supplementation and assessed the relative localization of occludin and ZO-1 proteins into the plasma membranes by immunofluorescent cytochemistry. Representative images are shown in Figure 5C; and, consistent with the results in Figure 5B, zinc supplementation appeared to improve the relative localizations of both occludin and ZO-1 into the intercellular junctions of alveolar epithelial cells in these monolayers. Together, the results in Figure 5 provide novel evidence that alcohol-induced alveolar epithelial barrier dysfunction is mediated through changes in zinc bioavailability.
Figure 5.
Dietary zinc supplementation mitigates the effects of chronic alcohol ingestion on the expression of the tight junction occludin and ZO-1 in the alveolar epithelium. Alveolar epithelial cells were freshly isolated from control-fed rats, alcohol-fed rats, and alcohol-fed rats whose diets were supplemented with zinc acetate (500 mg/L). Gene expression of the tight junction transmembrane protein, occludin, and of the tight junction scaffolding protein, ZO-1, were determined by real-time PCR. The average relative gene expression normalized to 18S was determined by the comparative method (2−ΔΔCt), with the target gene expression in cells from control-fed rats set as 1 in A and from alcohol-fed rats set as 1 in B. A shows the relative gene expression of occludin and ZO-1 in cells from control-fed rats (open bars) versus alcohol-fed rats (solid bars), and B shows the relative gene expression of occludin and ZO-1 in cells from alcohol-fed rats (solid bars) versus cells from alcohol-fed rats supplemented with dietary zinc (shaded bars). Each value represents the mean ± SEM of three to four separate isolated cell preparations. In parallel, freshly isolated alveolar epithelial cells from alcohol-fed rats and alcohol-fed rats supplemented with dietary zinc were cultured for 8 days to form monolayers and then occludin and ZO-1 protein localization were assessed qualitatively by fluorescent immunocytochemistry as described in Materials and Methods. Representative images are shown in C. Epithelial monolayers from alcohol-fed rats showed decreased overall staining for both occludin and ZO-1, with the most striking abnormality being discontinuous staining in the cell membranes. In contrast, epithelial monolayers from alcohol-fed rats supplemented with dietary zinc had more intense and continuous staining of occludin and ZO-1 in cell membranes. (Arrows in the occludin images are provided to highlight these findings.) (A) *P < 0.05 compared with control-fed rats; (B) *P < 0.05 compared with alcohol-fed rats.
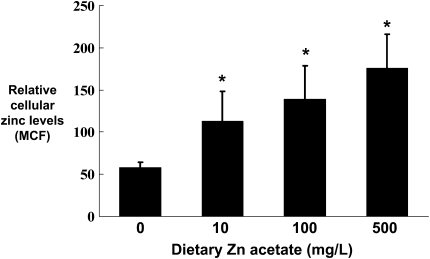
Dietary Zinc Supplementation Increases Intracellular Zinc Levels in Alveolar Macrophages and Epithelial Cells from Alcohol-Fed Rats
As dietary zinc supplementation restored zinc levels in the alveolar space of alcohol-fed rats (Figure 1), and normalized alveolar epithelial barrier function (Figure 4C), we next asked whether or not this treatment could increase intracellular zinc levels and innate immune function of the alveolar macrophage. As shown in Figure 6, dietary zinc supplementation significantly increased (P < 0.05) the relative intracellular concentrations of zinc in alveolar macrophages from alcohol-fed rats; in these experiments, all three dietary concentrations tested (10, 100, or 500 mg/L) increased intracellular zinc levels. Although the relative uptake of zinc by alveolar macrophages and epithelial cells in vitro was similar, the relative uptake of zinc by these cells in vivo was slightly different. Specifically, zinc uptake by alveolar macrophages in vivo was greater (∼ 300%, as quantified by mean channel fluorescence) as compared with the uptake by alveolar epithelial cells (∼ 140%). However, as dietary zinc restored function to both cell types (Figure 6 shows data only for alveolar macrophages), this relative difference in zinc uptake in vivo may not be biologically significant.
Figure 6.
Dietary zinc supplementation during chronic alcohol ingestion increases intracellular zinc levels in alveolar macrophages. Alveolar macrophages were isolated from alcohol-fed rats and from alcohol-fed rats whose diets were supplemented with zinc acetate (10, 100, or 500 mg/L) and then stained with the zinc-specific membrane-permeable dye FluoZin-3 AM (Invitrogen). The cells were then analyzed by flow cytometry and the relative intracellular zinc levels determined by measuring the mean channel fluorescence (MCF). Each value represents the mean ± SEM of four to six rats in each group. Alveolar macrophages isolated from control-fed rats whose diets were supplemented with zinc acetate (10, 100, or 500 mg/L) had no significant increase (P > 0.05) in their intracellular zinc levels (not shown). *P < 0.05 compared with alcohol diet without zinc supplementation.
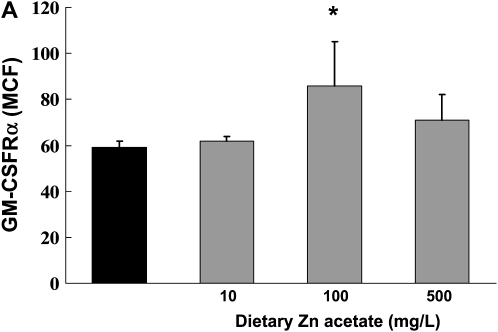
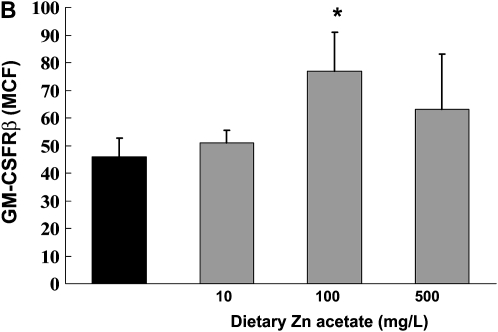
In Parallel, Dietary Zinc Supplementation Increases Expression of both GM-CSF Receptor Subunits and Restores Bacterial Phagocytic Function in Alveolar Macrophages from Alcohol-Fed Rats
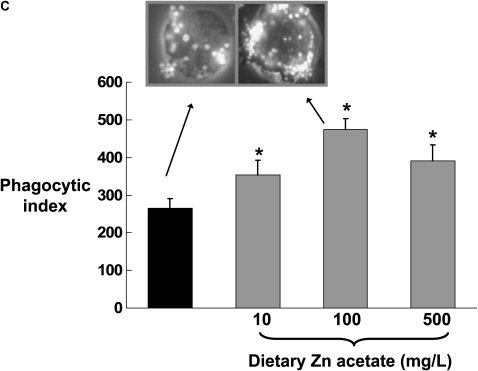
Previously we have published our findings that chronic alcohol ingestion decreases GM-CSF signaling in the alveolar macrophage, as reflected by decreased cell membrane expression of the GM-CSF receptor (both the α binding subunit and the β signaling subunit), and by decreased expression and nuclear localization of the GM-CSF master transcription factor, PU.1 (6). This defect in GM-CSF signaling appears to mediate the effects of alcohol ingestion on alveolar macrophage innate immune functions. Therefore, we speculated that alcohol-induced zinc deficiency could possibly be related to the previously recognized effects of alcohol on GM-CSF signaling. To test this speculation, we first assessed expression of the GM-CSF receptor α and β subunits in alveolar macrophages from alcohol-fed rats whose diets were supplemented with zinc. As shown in Figures 7A and 7B, dietary zinc supplementation increased expression of both GM-CSF receptor subunits, although this effect was only significant (P < 0.05) at the 100 mg/L concentration. In parallel, we determined the effects of dietary zinc supplementation on alveolar macrophage bacterial phagocytic capacity, as assessed by measuring the phagocytic indices of freshly isolated macrophages in vitro. As shown in Figure 7C, all three dietary concentrations of zinc acetate (10, 100, or 500 mg/L) significantly increased (P < 0.05) the bacterial phagocytic indices of alveolar macrophages from alcohol-fed rats, with the greatest effect appearing to be at the 100 mg/L concentration, consistent with the effects on GM-CSF receptor expression in Figures 7A and 7B. Together, the results depicted in Figures 6 and 7 indicate that, as with alveolar epithelial barrier function, dietary zinc supplementation preserved alveolar macrophage innate immune function in the face of chronic alcohol ingestion. These results are intriguing and are similar to our recent findings that ex vivo zinc supplementation of alveolar macrophages from HIV-1 transgenic rats restored zinc levels, GM-CSF receptor expression, and phagocytosis (23).
Figure 7.
Dietary zinc supplementation during chronic alcohol ingestion increases GM-CSF receptor expression and bacterial phagocytosis capacity in alveolar macrophages. Alveolar macrophages were isolated from alcohol-fed rats (solid bars) and from alcohol-fed rats whose diets were supplemented with zinc acetate (10, 100, or 500 mg/L; shaded bars) and then analyzed for GM-CSF receptor expression by flow cytometry as described in Materials and Methods. A and B show mean channel fluorescence for the α binding subunit (GM-CSFRα) and the β signaling subunit (GM-CSFRβ), respectively. In parallel, alveolar macrophages from each isolate were incubated with FITC-labeled inactive Staphylococcus aureus and then analyzed for phagocytic function by flow cytometry as described in Materials and Methods and expressed as the phagocytic index (% positive cells × mean channel fluorescence)/100). Shown in C are the phagocytic indices for each group. The insets are representative merged images of an alveolar macrophage (phase contrast) with ingested FITC-labeled bacteria in the alcohol-fed group and the alcohol-fed group supplemented with 100 mg/L of zinc acetate. For all of the data shown in A–C, each value is the mean ± SEM of four rats in each group. Dietary zinc supplementation had no significant effect (P > 0.05) on phagocytic function of alveolar macrophages from control-fed rats (not shown). *P < 0.05 compared with alcohol diet without zinc supplementation.
DISCUSSION
In this study we determined that chronic alcohol ingestion for 6 weeks in an experimental rat model alters the expression of key zinc transporters in the small intestine and in the alveolar epithelium and alveolar macrophages. In parallel, and likely as a consequence, chronic alcohol ingestion significantly decreases the bioavailability of zinc within the alveolar space. This alcohol-induced zinc deficiency has enormous consequences on alveolar epithelial barrier function as well as on alveolar macrophage innate immune function. Specifically, we determined that zinc depletion in vitro reproduces the effects of chronic alcohol ingestion on alveolar epithelial barrier function, and that zinc supplementation, either directly to cells in vitro or in the diets of alcohol-fed rats in vivo, reverses these effects. Further, dietary zinc supplementation significantly increased expression of two key tight junction proteins occludin and ZO-1 in the alveolar epithelium of alcohol-fed rats, suggesting that zinc bioavailability influences tight junction expression and/or assembly and therefore may mediate the alcohol-induced alveolar epithelial permeability we have identified previously (4). In addition to these novel findings regarding zinc and alveolar epithelial barrier function, we also determined that dietary zinc supplementation increases expression of the GM-CSF receptor and enhances bacterial phagocytosis in alveolar macrophages from alcohol-fed rats. Overall, this study provides provocative evidence that zinc depletion within the alveolar space mediates both the alveolar epithelial and alveolar macrophage dysfunction characterizing the alcoholic lung phenotype that renders alcoholics susceptible to both acute lung injury and pneumonia. If these findings ultimately prove to be relevant in the clinical situation, then dietary zinc supplementation would be predicted to decrease the incidence or at least the severity of these devastating lung diseases in these vulnerable individuals.
Zinc is an essential micronutrient, present primarily in protein-rich foods, that is absorbed by the intestinal epithelium in the duodenum and the ileum. Within the human body there are approximately 2 to 3 g of zinc distributed in a nonuniform pattern in cells and extracellular fluids (13). The small intestine absorbs approximately 3 to 4 mg/day, and therefore current recommended daily allowances range from 5 mg/day for infants to 16 to 19 mg/day for lactating women (13). Zinc is stored and/or used in two relatively distinct pools; the first is the fixed pool of zinc that is bound to metalloproteins. This fixed pool constitutes approximately 80 to 90% of total body zinc and turns over very slowly (13, 15). The second pool is the labile pool, which includes free or loosely bound zinc that is available for exchange among cells and organelles (13, 15). This pool is much more susceptible to depletion during periods of dietary zinc deficiency. As free zinc can be toxic to some proteins and enzymes, the labile pool is largely bound to zinc-carrying proteins; prominent among these are the metallothioneins, a family of cysteine-rich proteins that bind zinc and other metals (13, 24). The movement of zinc across plasma and organellar membranes is tightly regulated by zinc transporters. There are two general classes; the solute carrier-39 (SLC39) transporter family includes a subfamily of ZRT/IRT-related proteins (known as ZIPs) that import zinc into the cytosol, either from the extracellular space or from an organellar compartment (13, 15). The second is the solute carrier-30 (SLC30) or cation diffusion facilitator (CDF) transporter family, which includes the ZNT subfamily (13, 15). These transporters export zinc from the cytosol, either into specific organelles or extracellularly such as into the alveolar epithelial lining fluid. At least 14 different ZIPs (ZIP1 to ZIP14) and at least 10 different ZNTs (ZNT1 to ZNT10) have been identified and are distributed in a tissue-specific and cell-specific manner (13, 15). For example, the rare human disease acrodermatitis enteropathica (AE) has been shown to involve a genetic mutation in ZIP4, which is richly expressed in the apical surface of enterocytes in the small intestine where dietary zinc is absorbed (15). Within this tissue, ZIP4 expression is inversely correlated with dietary zinc; specifically, its expression increases when dietary zinc is deficient, and when dietary zinc is high its expression wanes (15). In addition, divalent cation transporter-1 (DCT-1) may contribute to zinc, copper, and iron absorption in the enterocyte although the magnitude of this potential contribution is unknown (13). Once imported into the enterocyte, zinc is coupled to cysteine-rich intestinal protein (CRIP), which carries it to the basolateral surface, where it is exported into the circulation by ZNT1 and carried by albumin and other proteins to distal organs (13). When dietary zinc levels are high, there can be some transporter-independent absorption through a paracellular route, and affected individuals with AE, who suffer from a typical rash and immune deficiencies, can be effectively treated with dietary zinc supplements (15). Therefore, it is not surprising in this study that dietary zinc supplementation could overcome the alcohol-induced zinc deficiency associated with decreased expression of ZIP4. Our results do suggest that the dietary concentration of 100 mg/L of zinc acetate, corresponding to approximately 5 mg/kg/day in these alcohol-fed rats, appears to be in the optimal range. This is consistent with the dosing of dietary supplements in zinc-deficient humans (15), and suggests that the findings in this experimental model may well translate to the clinical setting.
Zinc is implicated in many aspects of immune function, including T and B lymphocytes and innate immunity (25). In addition, zinc has structural and catalytic roles in more than 300 metalloenzymes, including alcohol dehydrogenase (26), and regulates many cellular processes, including signal transduction and gene expression (17). Although considerable information is available on the role of zinc in the brain, the liver, the immune system, and the musculoskeletal system, relatively fewer studies have focused on its role in the lungs. One notable exception is work by Zalewski and colleagues demonstrating a critical role for zinc in maintaining the health of the large airways, and how zinc deficiency may contribute to the pathophysiology of reactive airway diseases such as asthma (13, 14, 16, 17, 27). It was recently reported (28) that human ZIP 8 is up-regulated at the onset of inflammation, thereby importing zinc into the cells and facilitating cytoprotection of the lung epithelial cells. In another study, Bao and Knoell had reported (29) that zinc protects the lung epithelium during inflammatory stress. They observed that zinc deprivation combined with proinflammatory cytokines accelerated caspase-3 activation, leading to increased paracellular leak across monolayers of lung epithelial cultures. Zinc also plays a role in the regulation of cellular glutathione (29) and protects cell membranes from oxidative damage (30). This is particularly intriguing, as we have published extensively on the role of oxidant stress and glutathione depletion in experimental models of chronic alcohol ingestion and in human subjects with alcohol abuse (5, 7, 31–34). Therefore, alcohol-induced zinc deficiency may play a central role in the oxidant stress and consequent cellular dysfunction that has been shown to characterize the alcoholic lung phenotype. This seems plausible as zinc deficiency produces widespread abnormalities, but is particularly detrimental to epithelial cells and the immune system.
Worldwide, dietary zinc deficiency is common and contributes to disease burdens in many areas, most notably sub-Saharan Africa and Southeast Asia (15). In the United States, dietary zinc deficiency is less common but remains problematic in individuals with either a zinc-poor diet or with intestinal abnormalities that limit zinc absorption. Importantly, chronic alcohol abuse has a strong association with zinc deficiency. However, whether this zinc deficiency is due to poor dietary intake, an inhibition of zinc absorption, or an increase in loss of zinc from the gut has not been determined (15). This study provides important new evidence that chronic alcohol ingestion interferes with zinc absorption in the small intestine and transport to distal organs, in this case the lung. To our knowledge, this is the first report on the effects of chronic alcohol ingestion on zinc absorption in the gut, its bioavailability within the alveolar space, and how this modulates alveolar epithelial and macrophage function. An important next step will be to determine how chronic alcohol ingestion alters the expression of zinc transporters in the gut and in distal organs such as the lung. For example, these effects could be directly related to the alcohol molecule itself, to one of its metabolites such as acetaldehyde, or to a more indirect consequence such as oxidant stress. In parallel, these new observations raise important questions as to how zinc bioavailability is mechanistically linked to the alterations in GM-CSF receptor expression and GM-CSF–dependent cellular functions within the alveolar space of the alcoholic lung. As zinc is a co-factor for hundreds of enzymes and is a critical component of transcription factors, the possibilities are numerous. However, the salutary effects of dietary zinc supplementation in this study are impressive and may reflect that alcohol exerts its effects through multiple zinc-sensitive mechanisms.
In summary, we report that chronic alcohol ingestion in an otherwise nutritionally complete diet, and in the absence of other biological stresses, alters the expression of zinc transporters within the gut and the lung and decreases zinc bioavailability within the alveolar space. Further, dietary zinc supplementation restores alveolar zinc levels and improves alveolar epithelial barrier function and alveolar macrophage immune function. If these findings ultimately translate to the clinical setting, they suggest that dietary zinc supplementation could have profound effects on restoring lung health in alcoholics and could thereby decrease the morbidity and mortality of pneumonia and acute lung injury in these vulnerable individuals.
This work was supported by NIH P50 AA 013757, R01 AA 11660, T32 AA 013528, and a Merit Review by the Department of Veterans Affairs.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0209OC on December 23, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med 1995;155:1649–1654. [DOI] [PubMed] [Google Scholar]
- 2.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA 1996;275:50–54. [PubMed] [Google Scholar]
- 3.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 2003;31:869–877. [DOI] [PubMed] [Google Scholar]
- 4.Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol 2000;279:L127–L135. [DOI] [PubMed] [Google Scholar]
- 5.Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 1998;101:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, Brown LA, Guidot DM. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony- stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol 2005;175:6837–6845. [DOI] [PubMed] [Google Scholar]
- 7.Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol 2007;292:L813–L823. [DOI] [PubMed] [Google Scholar]
- 8.Burnham EL, Brown LA, Halls L, Moss M. Effects of chronic alcohol abuse on alveolar epithelial barrier function and glutathione homeostasis. Alcohol Clin Exp Res 2003;27:1167–1172. [DOI] [PubMed] [Google Scholar]
- 9.de Wit M, Best AM, Gennings C, Burnham EL, Moss M. Alcohol use disorders increase the risk for mechanical ventilation in medical patients. Alcohol Clin Exp Res 2007;31:1224–1230. [DOI] [PubMed] [Google Scholar]
- 10.Joshi PC, Applewhite L, Mitchell PO, Fernainy K, Roman J, Eaton DC, Guidot DM. GM-CSF receptor expression and signaling is decreased in lungs of ethanol-fed rats. Am J Physiol Lung Cell Mol Physiol 2006;291:L1150–L1158. [DOI] [PubMed] [Google Scholar]
- 11.Pelaez A, Bechara RI, Joshi PC, Brown LA, Guidot DM. Granulocyte/macrophage colony- stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol 2004;286:L106–L111. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez AL, Koval M, Fan X, Guidot DM. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol 2007;41:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murgia C, Lang CJ, Truong-Tran AQ, Grosser D, Jayaram L, Ruffin RE, Perozzi G, Zalewski PD. Zinc and its specific transporters as potential targets in airway disease. Curr Drug Targets 2006;7:607–627. [DOI] [PubMed] [Google Scholar]
- 14.Tudor R, Zalewski PD, Ratnaike RN. Zinc in health and chronic disease. J Nutr Health Aging 2005;9:45–51. [PubMed] [Google Scholar]
- 15.Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, Fazel N. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007;56:116–124. [DOI] [PubMed] [Google Scholar]
- 16.Zalewski PD, Truong-Tran AQ, Grosser D, Jayaram L, Murgia C, Ruffin RE. Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets. A review. Pharmacol Ther 2005;105:127–149. [DOI] [PubMed] [Google Scholar]
- 17.Zalewski PD. Zinc metabolism in the airway: basic mechanisms and drug targets. Curr Opin Pharmacol 2006;6:237–243. [DOI] [PubMed] [Google Scholar]
- 18.Velasquez A, Bechara RI, Lewis JF, Malloy J, McCaig L, Brown LA, Guidot DM. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res 2002;26:1245–1251. [DOI] [PubMed] [Google Scholar]
- 19.Stojanovic A, Stitham J, Hwa J. Critical role of transmembrane segment zinc binding in the structure and function of rhodopsin. J Biol Chem 2004;279:35932–35941. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Bertoglio BA, Gee KR, Kay AR. The zinc indicator FluoZin-3 is not perturbed significantly by physiological levels of calcium or magnesium. Cell Calcium 2008;44:422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasse H, Ober-Blobaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol 2008;181:6491–6502. [DOI] [PubMed] [Google Scholar]
- 22.Sahlin S, Hed J, Rundquist I. Differentiation between attached and ingested immune complexes by a fluorescence quenching cytofluorometric assay. J Immunol Methods 1983;60:115–124. [DOI] [PubMed] [Google Scholar]
- 23.Joshi P, Raynor R, Fan X, Guidot DM. HIV-1-transgene expression in rats decreases alveolar macrophage zinc levels and phagocytosis. Am J Respir Cell Mol Biol 2008;39:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truong-Tran AQ, Ruffin RE, Zalewski PD. Visualization of labile zinc and its role in apoptosis of primary airway epithelial cells and cell lines. Am J Physiol Lung Cell Mol Physiol 2000;279:L1172–L1183. [DOI] [PubMed] [Google Scholar]
- 25.Prasad AS. Discovery of human zinc deficiency: impact on human health. Nutrition 2001;17:685–687. [DOI] [PubMed] [Google Scholar]
- 26.Caballeria J, Gimenez A, Andreu H, Deulofeu R, Pares A, Caballeria L, Ballesta AM, Rodes J. Zinc administration improves gastric alcohol dehydrogenase activity and first-pass metabolism of ethanol in alcohol-fed rats. Alcohol Clin Exp Res 1997;21:1619–1622. [PubMed] [Google Scholar]
- 27.Truong-Tran AQ, Carter J, Ruffin R, Zalewski PD. New insights into the role of zinc in the respiratory epithelium. Immunol Cell Biol 2001;79:170–177. [DOI] [PubMed] [Google Scholar]
- 28.Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol 2008;294:L1127–L1136. [DOI] [PubMed] [Google Scholar]
- 29.Bao S, Knoell DL. Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am J Physiol Lung Cell Mol Physiol 2006;291:L1132–L1141. [DOI] [PubMed] [Google Scholar]
- 30.Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med 2004;37:1182–1190. [DOI] [PubMed] [Google Scholar]
- 31.Brown LA, Harris FL, Bechara R, Guidot DM. Effect of chronic ethanol ingestion on alveolar type II cell: glutathione and inflammatory mediator-induced apoptosis. Alcohol Clin Exp Res 2001;25:1078–1085. [PubMed] [Google Scholar]
- 32.Brown LA, Harris FL, Guidot DM. Chronic ethanol ingestion potentiates TNF-alpha-mediated oxidative stress and apoptosis in rat type II cells. Am J Physiol Lung Cell Mol Physiol 2001;281:L377–L386. [DOI] [PubMed] [Google Scholar]
- 33.Guidot DM, Roman J. Chronic ethanol ingestion increases susceptibility to acute lung injury: role of oxidative stress and tissue remodeling. Chest 2002;122:309S–314S. [DOI] [PubMed] [Google Scholar]
- 34.Moss M, Guidot DM, Wong-Lambertina M, Ten HT, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 2000;161:414–419. [DOI] [PubMed] [Google Scholar]